Abstract
Rationale
Evidence for a relationship between cigarette smoking and Attention-Deficit/Hyperactivity Disorder (ADHD) has prompted investigations into nicotinic treatments for this disorder. Impulsivity is a hallmark of ADHD and is measured in the laboratory as behavioral inhibition (BI) using the Stop Signal Task (SST). Acute nicotine improves SST performance in adolescents and young adults with ADHD and impaired baseline SST performance, raising questions about the role of nicotinic acetylcholine receptor function in BI. The specificity of this effect to those with ADHD, the component processes of the SST affected by nicotine, and the effects of nicotinic antagonism are yet unknown.
Objectives
This study investigated the effects of both a nicotinic receptor agonist and antagonist on the SST and choice reaction time task (CRT) in highly impulsive (HI) and control (CTRL) subjects.
Methods
This was a within-subjects, double blind study of: 7 mg transdermal nicotine, 20 mg oral mecamylamine and placebo. Subjects were recruited into HI (n=11) and CTRL (n=14) groups based on both SST and clinical criteria.
Results
BI was significantly improved by nicotine compared to placebo in the HI group and impaired by mecamylamine in the CTRL group. Reaction time on the SST was improved by nicotine compared to placebo in the CTRL group and was unchanged in both groups on the CRT.
Conclusions
These findings demonstrate nicotinic modulation of BI in subjects with both normal and disordered baseline performance. The effects on BI are consistent with cholinergic enhancement of signal detection processes and/or modulation of noradrenaline by nicotine.
Keywords: Cholinergic, nicotine, mecamylamine, ADHD, stop signal task, impulsivity, behavioral inhibition, reaction time, acetylcholine
Introduction
Interest in the role of nicotinic cholinergic receptor function in Attention–Deficit/Hyperactivity Disorder (ADHD) has arisen from converging evidence for the relationship between smoking and ADHD (Lambert et al. 1987; Mick et al. 2002; Milberger et al. 1998; Pomerleau et al. 1995; Schmitz et al. 2006; Kollins et al. 2005) leading to the proposal that persons with ADHD may use cigarette smoking as self-medication (Conners et al. 1996; Gehricke et al. 2007; Potter et al. 2006).
It is well known that nicotine improves cognition, including attention, in psychiatric and healthy volunteer populations (Levin et al. 2006; Singh et al. 2004). Clinical trials have shown reduced ADHD symptoms following administration of nicotine (Conners et al. 1996; Gehricke et al. 2009; Levin et al. 1996; Shytle et al. 2002) or novel nicotinic agonists (Biederman et al. 2006; Wilens et al. 1999). Specific studies of nicotinic effects on cognition in ADHD are limited, but indicate that nicotine reduces variability in reaction time (Conners et al. 1996; Levin et al. 1996) and behavioral inhibition (Potter and Newhouse 2004; 2008) in young adults with ADHD.
Behavioral inhibition (BI), defined as the ability to delay or withhold a pre-potent response (Barkley 1997) is a widely used behavioral characteristic in studies of ADHD (Willcutt et al. 2005), as well as other psychiatric and substance use disorders. The time to inhibit a pre-potent response, the Stop Signal Reaction Time (SSRT) can be measured using the Stop Signal Task (SST; Logan 1994). In this task subjects perform a primary choice reaction task while withholding their response when an infrequent auditory tone, the “stop” signal, occurs (See Logan 1994 for details). This task has advantages over traditional Go-NOGO tasks, including that automatic inhibition is unlikely to occur (Verbruggen and Logan 2008) and that the speed of inhibition is estimated. Impaired SST performance (abnormally long SSRT) is seen in many clinical populations including ADHD and substance use disorders (Barkley 1997; Fillmore and Rush 2002; Monterosso et al. 2005; Oosterlaan and Sergeant 1998; Tannock 1998). It has been hypothesized that impaired BI underlies broad deficits in executive function (Barkley 1997; Fillmore and Rush 2002; Oosterlaan and Sergeant 1998; Vogel-Sprott et al. 2001), contributing to clinical impairments in these disorders.
While the beneficial effect of nicotine on BI has important treatment implications, our understanding of nicotinic receptor function and BI is still preliminary. First, it is unknown if the effect of nicotine is specific to response inhibition or improves performance more generally on the SST. GO-RT and SSRT have been shown to be stochastically independent (Aron et al. 2007; Band et al. 2003; Bedard et al. 2002; Logan 1994) and are hypothesized to be modulated by distinct monoamine systems (Bari et al. 2009). Our previous work with nicotine has found positive effects on SSRT without changes in GO-RT (Potter and Newhouse 2004; 2008). However, as these were small studies of subjects with impaired SSRT at baseline. Thus greater clarification of the effects of nicotine on SST performance is warranted. Second, nicotine has baseline dependent effects on many cognitive functions (Perkins 1999) and the presence of these effects on the SST is unexplored. Finally, there has been no work done to examine the effects of nicotinic antagonism on SST performance. Knowing the effect of cholinergic system disruptions will advance our understanding of the role of nicotinic cholinergic receptor system function in BI in both impaired and healthy populations.
To address these questions, the present study examined the effects of acute nicotinic stimulation and blockade on SST performance in two groups of non-smokers; highly impulsive (HI) young adults with ADHD and impaired baseline SST, and healthy controls (CTRL) with normal SST performance. This approach allowed us to use a clinically relevant yet precisely defined behavioral phenotype to assess the effects of both agonism and antagonism of nicotinic cholinergic receptors. We hypothesized that nicotine would improve SSRT in the HI group, consistent with our past research, and that mecamylamine would impair SSRT in both groups. We further hypothesized that nicotine would improve GO-RT in the CTRL group, a finding consistent with many years of research on the effects of nicotine on reaction time in healthy volunteers (Bates et al. 1994; Kerr et al. 1991; Levin et al. 1998; Mancuso et al. 2001; Sherwood et al. 1992), but not in the HI group, as we have seen in our previous work (Potter and Newhouse 2004; 2008). This study also included a Choice Reaction Time (CRT) task to dissociate drug effects on simple reaction time from those on the process of behavioral inhibition. We hypothesized that NIC would improve the recognition component of reaction time in both subject groups reflecting enhanced attention.
Methods and Materials
Design Overview
This was an acute, single dose, within-subjects, double-blind study with the following drug conditions: 1) 7 mg transdermal nicotine (NIC) administered for 45 minutes, 2) 20 mg oral mecamylamine (MEC) and 3) placebo (PLC). Each drug was administered on a separate study day (randomly assigned) and study days occurred at least 48 hours and no more than 10 days apart. This study was approved by the Institutional Review Board at the University of Vermont and conducted in accordance with all relevant regulations. The primary outcome measure was performance on the SST.
Subjects
Twelve high impulsive (HI) and fifteen control (CTRL) young adults (age 18 – 24) completed this study. A total of 59 subjects were recruited by flyers and provided written informed consent prior to screening. Thirty two (32) screen fails were due to: personal/scheduling conflicts (n=12), SST performance criteria (n=7), medical contraindication (n=5), psychiatric co-morbidity (n=4), use of nicotine (n=2), co-morbid learning disability (n=1), recreational use of psychostimulants (n=1), or did not meet ADHD diagnostic criteria (n=1). Two (2) subjects were missing SST data due to computer malfunction and were excluded from all analyses; leaving 11 in the HI and 14 in the CTRL groups.
HI subjects met full DSM-IV-TR criteria for ADHD-Combined Type (with no co-morbid disorders) and performed at least 1.5 standard deviations (SD) above the mean norm SSRT in the reference age range (Williams et al. 1999). CTRL subjects had no DSM-IV-TR Axis I diagnoses, and performed within 1 SD of the mean norm SSRT. Psychiatric disorders were assessed using the SCID (First et al. 1996) and the behavior disorder supplement for the K-SADS-PL (Kaufman et al. 1997), with all interviews conducted by trained clinicians (MS or Ph.D.). Subjects were all non-smokers defined by no current smoking (last 3 months) and never a period of daily smoking. No subjects met criteria for current alcohol or drug dependence. Four (4) subjects (1 HI, 3 CTRL) met criteria for alcohol abuse in the past 12 months. The Wechsler Abbreviated Scales of Intelligence (WASI; Wechsler 1999) was administered to estimate IQ. Demographic characteristics of the participants who completed the study are presented in Table 1.
Table 1.
Subject demographics at the time of screening. CTRL control group; HI high impulsive group; FSIQ full scale intelligence quotient; ACC accuracy; GO-RT go reaction time; STDEV standard deviation of go reaction time; SSD stop signal delay; SSRT stop signal reaction time.
| HI (n=11) | CTRL (n=14) | |
|---|---|---|
| n (%) or Mean±SD | ||
| Female | 8 (73) | 7 (50) |
| Age (years) | 19.7±02.1 | 20.1±01.6 |
| FSIQ | 115.8±08.3 | 114.0±07.0 |
| Stop Signal Task | msec | |
| ACC (%) | 96.7±2.8 | 97.9±4.0 |
| GO-RT | 493.5±89.1 | 493.7±116.3 |
| STDEV * | 124.7±47.7 | 91.1±31.6 |
| SSD * | 185.2±110.4 | 294.8±125.1 |
| SSRT * | 308.2±84.4 | 198.9±49.0 |
p<.05 different between HI and CTRL by t-test.
The status of medication treatment for ADHD was assessed at screening. Eight (8) subjects were taking psychostimulants and 1 was taking atomoxetine. Psychostimulants were washed out for 48 hours (at least 12 half lives) and atomoxetine for 7 days before each study day. This length of washout is consistent with previous studies of the effects of acute drug administration on cognition in ADHD (Aron et al. 2003; DeVito et al. 2009; Potter and Newhouse 2008).
Drug Administration
Drugs were administered using a double-blind, double-placebo procedure in which subjects were administered both transdermal and oral medication/placebos on each study day. A transdermal nicotine patch (7 mg Nicoderm CQ) or matching placebo (obtained from 1-800-PATCHES) was placed on the subject’s upper back. Oral mecamylamine (Inversine, purchased from Targacept Inc.) or placebo (lactose) was administered in blinded capsules. Dose selection and timing were determined by prior studies showing that 45 minutes of a 7 mg transdermal nicotine patch produces reliable cognitive effects without intolerable side effects in non-smokers (Potter and Newhouse 2004; 2008), and that 20 mg of mecamylamine produces cognitive deficits with acceptable side effects at 150 minutes (Newhouse et al. 1994; Dumas et al. 2009).
Study Day Procedures
Subjects fasted after midnight before each study day. Confirmation of non-smoking status (expired CO <10 ppm) and a negative urine pregnancy test were required prior to drug administration. Oral capsules were administered at approximately 08:30 am. The transdermal patch was applied 105 minutes later, and was removed after 45 minutes. The SST and Choice Reaction Time task (CRT) were administered as part of a cognitive battery (including assessment of delay aversion, working memory, and risk taking; results not reported here). Following cognitive testing participants completed behavior ratings.
Stop Signal Task (SST) (Logan 1984)
This is a computer-administered test of behavioral inhibition (see Logan et al. 1984 for full task details). Participants respond to two equally probable “go” signals (the letters X and O) on a computer screen and are instructed not to respond if an auditory signal (the “stop” signal) is present. Subjects completed 4 blocks of 64 trials each, 25% of which were stop trials. The task began with a 250 msec delay between the go and stop signals (Stop Signal Delay, SSD), which was dynamically adjusted by 50 msec after every stop trial to approximate a .5 probability of successful inhibition (Logan et al 1984). SSRT was calculated by subtracting the average SSD from the mean GO-RT (Logan et al. 1984). Subjects completed a training session between screening and the first drug challenge day to stabilize task performance.
Choice Reaction Time Task (CRT)
The CRT from the Milford Test Battery (Hindmarch 1984) was administered. This simple reaction time task has an array of LED lights each associated with a light sensitive diode (LSD) used for response keys. Subjects place their index finger on the “home” LSD and respond to one of six “target” LSDs in the array by moving their index finger to the LSD associated with the target light. This allows total reaction time to be broken into a recognition component (time to remove index finger from home LSD) and a motor component (time from removing finger from home LSD to covering the target LSD). This task has been used in many studies of nicotinic drugs (i.e. Dumas et al. 2008).
Behavioral Ratings
In the Physical Symptom Checklist (PSCL) (Van Kammen and Murphy 1975) subjects rate 22 symptoms (nausea, headache, itchiness etc.) on a four point scale in reference to their experiences that morning. This measure has been used extensively to assess physical symptoms in response to acute drug challenges (McNair et al. 1971; Potter and Newhouse 2008; Van Kammen and Murphy 1975). In the Profile of Mood States (POMS; McNair et al. 1971) participants rate a list of 65 adjectives of mood and/or physical well being on a five point scale. The POMS yields cluster scores for vigor, tension, depression, anger, fatigue and difficulty concentrating as well as a composite Total Mood Disturbance score which is calculated by summing the six factor scores with the vigor score negatively weighted.
Data Processing and Analysis
SST data was excluded for any block (64 trials) where the probability of responding to the stop signal was <.32 or >.68, per previous methods (Potter and Newhouse 2008). From the total data set (n=300 blocks) 2 blocks were excluded from CTRL and 3 blocks from HI subjects. On the CRT, median reaction times were analyzed to minimize the effect of rare extreme values within a subject. The group level distribution of the CRT data was normal and did not necessitate transformation.
Repeated measures mixed model ANOVAs were used to determine differences related to drug and group on the dependent variables. An a priori alpha level of .05 was used. Significant interactions were explored using t-tests with a Tukey-Kramer adjustment to correct for multiple comparisons where appropriate. Effect size (Cohen’s d; Cohen 1977) was calculated for contrasts of interest using the (Morris and DeShon 2002) equation to account for within subjects correlation.
Results
Stop Signal Task
Group Differences and Task Variable Intercorrelations
At screening, expected group differences were seen including significantly higher SSRT [t(23) = 9.18, p<.001], lower SSD [t(23) = −2.97, p=.007], and larger STDEV [t(23) = 4.75, p<.001] in the HI group (Table 1). GO-RT and SSRT were not significantly correlated across four task blocks in either the CTRL [r(192) =.06, p=.391] or HI [r(157) = .13, p=.110] group supporting independent consideration of GO-RT and SSRT.
Training Effects
Examination of performance differences at screening compared to the PLC study day (the training effect) was carried out using mixed model ANOVAs. Since the order of drug administration was randomized and counterbalanced, the length of time reflected in this comparison varies between subjects. A significant main effect of Training on SSRT was found, with all subjects improving during the training period. The Group X Training interaction was significant for SSRT [F(1,23) = 18.12, p<.001] with the HI group showing greater change [t(23) = −8.95, p<.001, 67.09±11.1 msec] than the CTRL group [t(23) = −3.98, p<.001, 25.29±5.4 msec], (see training data in Table 1). There were no significant training effects on GO-RT or accuracy.
Drug Effects
Stop Signal Task
Both groups of subjects had high (>95%) accuracy at all drug conditions, however there was a significant [F(2,23) = 26.97, p<.001] Drug X Group interaction with post-hoc contrasts indicating that HI subjects had significantly [t(23) = −3.21, p=.004] lower accuracy (96.5%) compared to CTRL subjects (98.9%) on the PLC day.
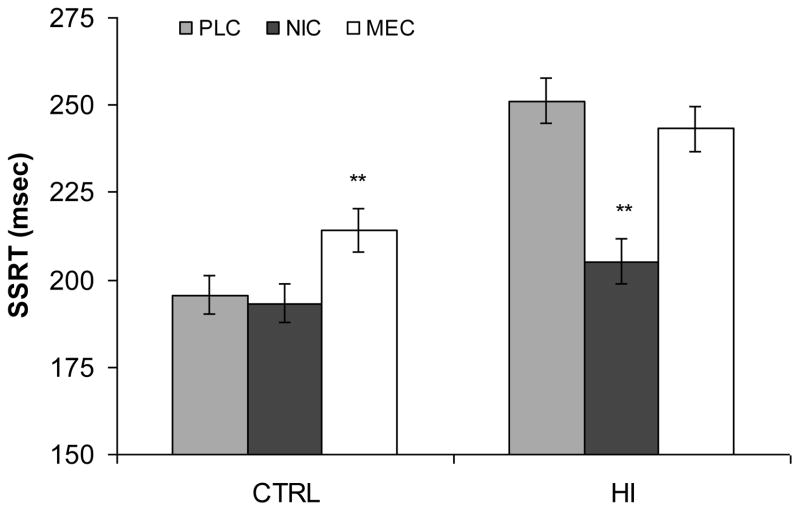
A significant Drug X Group interaction was found [F(2,23) = 13.07, p<.001] on SSRT. In the HI group NIC improved inhibition compared to PLC (205.2 msec and 251.2 msec, respectively) [t(23) = −6.90, p<.001, d=.93, 95%CI[−30.9, 36.8]] and MEC produced no change (243.2 msec). In the CTRL group NIC did not significantly [t(23) = −0.40, p=.695] affect SSRT (193.3 msec) and MEC impaired inhibition compared to PLC (214.2 msec and 195.6 msec, respectively) [t(23) = 3.09, p=.005, d=−1.39, 95%CI[−21.3, 23.2]] (Figure 1).
Fig. 1.
Stop Signal Reaction Time (SSRT) in msec by Group and Drug (LS Means): PLC placebo; NIC nicotine (7 mg); MEC mecamylamine (20 mg); CTRL control group; HI high impulsive group; ** p<.01 different from PLC of same group, error bars are standard error around the mean.
GO-RT changes were seen in a significant Drug X Group interaction [F(2,23) = 3.80, p=.037] with NIC shortening GO-RT compared to PLC in the CTRL group [t(23) = −3.50, p=.002, d=.62, 95%CI[−47.0, 41.4]] and having no effect in the HI group [t(23)=0.34, p=.737], Table 2). MEC was not significantly different from PLC in either group.
Table 2.
Stop Signal Task (SST) performance (msec) across Drug conditions by Group. LS Means±SE; PLC placebo; NIC nicotine (7 mg); MEC mecamylamine (20 mg); CTRL control group; HI high impulsive group; GO-RT go reaction time; STDEV standard deviation of go reaction time; SSD stop signal delay; ACC accuracy.
| HI | CTRL | |||||
|---|---|---|---|---|---|---|
| PLC | NIC | MEC | PLC | NIC | MEC | |
| GO-RT | 454.6±20.1 | 458.5±19.3 | 471.8±19.5 | 444.0±17.9 | 406.8±17.4* | 451.2±17.4 |
| STDEV | 85.5±5.0 | 82.3±5.9 | 96.2±5.7* | 54.5±4.5 | 52.6±5.0 | 81.4±5.4* |
| SSD | 200.6±12.5 | 269.7±10.2* | 262.2±10.7* | 249.3±10.9 | 233.1±9.6* | 260.1±9.4 |
| ACC | 96.5±0.6 | 98.1±0.6* | 97.4±0.6* | 98.9±0.5 | 97.5±0.5* | 98.1±0.5* |
p<.05 different from PLC of same group.
There was a significant Drug X Group interaction [F(2,23) = 16.98, p<.001] on SSD. In the HI group both NIC [t(23) = 5.65, p<.001] and MEC [t(23) = 5.18, p<.001] resulted in longer SSD than PLC. In the CTRL group NIC [t(23) = −2.40, p=.025] was associated with a shorter SSD compared to PLC and MEC did not significantly [t(23)=1.0, p=.329] affect SSD (Table 2).
On the STDEV of GO-RT, there was a main effect [F(2,23) = 23.12, p<.001] of Drug. MEC was associated with a larger STDEV than either PLC [t(23) = 5.85, p<.001] or NIC [t(23) = 5.98, p<.001] (Table 2).
Choice Reaction Time Task (CRT)
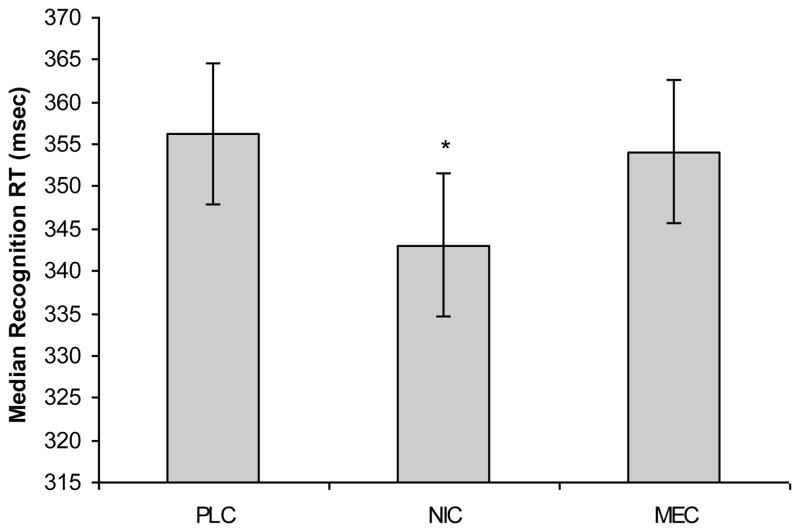
There were no significant Drug or Group related effects on overall reaction time, or on the motor component of reaction time on this task (Table 3). Analysis of recognition reaction time (median) showed a significant [F(2,39) = 4.57, p=.017] main effect of Drug with NIC producing shorter recognition reaction times than PLC (Figure 2). The Drug X Group interaction for recognition reaction time was not significant [F(2,39)=.43, p=.650] (Table 3).
Table 3.
Choice Reaction Time Task (CRT) performance (msec) across Drug conditions by Group. Medians±SE; PLC placebo; NIC nicotine (7 mg); MEC mecamylamine (20 mg); CTRL control group; HI high impulsive group.
| HI | CTRL | |||||
|---|---|---|---|---|---|---|
| PLC | NIC | MEC | PLC | NIC | MEC | |
| Recognition | 369.1±12.4 | 352.08±12.8 | 367.1±12.8 | 343.6±11.1 | 334.0±11.1 | 341.1±11.1 |
| Motor | 275.6±18.7 | 275.0±19.7 | 281.6±19.7 | 250.4±16.8 | 259.4±16.9 | 253.4±17.2 |
| Total | 657.8±28.3 | 635.3±29.4 | 662.3±29.4 | 601.4±25.4 | 602.2±25.5 | 599.9±25.5 |
Fig. 2.
Median Recognition Reaction Time in msec by Drug (LS Means): PLC placebo; NIC nicotine (7 mg); MEC mecamylamine (20 mg); * p<.05 different from PLC; error bars are standard error around the median.
Behavior Ratings
There were no significant drug-related effects on the total score on the PSCL. Examination of individual items using Tukey-Kramer adjustment for multiple comparisons found significant Drug X Group interactions for tired [F(2,25) = 9.24, p=.001] and heart pounding [F(2,25) = 4.07, p=.030]. Both effects were driven by changes in the HI group with significant decreases in tired rating [t(25) = −4.72, adjusted p=.001] associated with NIC and lower rating of heart pounding [t(25) = −4.00, adjusted p=.008] associated with MEC compared to PLC. Main effects of drug were seen for headache [F(2,25) = 3.88, p=.034], itching [F(2,25) = 5.21, p<.001] and nausea [F(2,25) = 3.94, p=.033]. MEC was associated with increased headache [t(25) = 0.77, adjusted p=.04] and NIC was associated with increased itching [t(25) = 5.21, adjusted p<.001] and nausea [t(25) = 2.80, adjusted p=.03] compared to PLC.
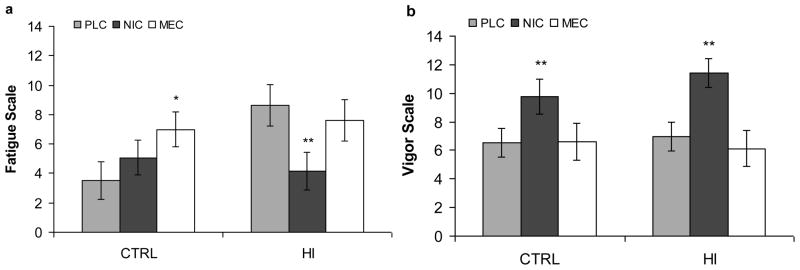
There were no significant drug related differences from PLC on the Tension, Anger, Confusion, or Total Mood Disturbance cluster scores of the POMS. A significant Drug X Group interaction was seen on Fatigue [F(2,25) = 5.28, p=.012] with NIC significantly [t(25) = −3.25, adjusted p=.0343] decreasing fatigue in the HI group (Figure 3a), and a trend [t(25) = 2.94, adjusted p=.07] for MEC increasing fatigue compared to PLC in the CTRL group. There was a significant main effect [F(2,25) = 25.01, p<.001] of Drug on Vigor. NIC was associated with increased vigor in both the HI [t(25) = 4.92, adjusted p<.001] and CTRL [t(25) = 1.99, adjusted p=.003] groups compared to PLC (Figure 3b). An ANCOVA to determine the effect of the drug conditions on SSRT after accounting for changes in Fatigue ratings was carried out. Fatigue did not significantly (p>.95) contribute to the model, and did not alter the pattern of the drug effects.
Fig. 3.
Profile of Mood States (POMS) cluster scores by Group and Drug (LS Means): a Fatigue and b Vigor; PLC placebo; NIC nicotine (7 mg); MEC mecamylamine (20 mg); CTRL control group; HI high impulsive group; ** p<.01 different from PLC of same group, * p<.05 different from PLC of same group, error bars are standard error around the mean.
Discussion
This study found clear effects of nicotinic system manipulations on the SST in both groups of subjects supporting the hypothesis that nicotinic acetylcholine receptor function modulates BI in both impaired and healthy populations. Important group differences were found with the HI subjects showing improved SSRT without changes in GO-RT following NIC, a finding that is consistent with our prior work in ADHD (Potter and Newhouse 2004) and with the finding of no significant change in overall median reaction time in either group on the CRT task. The CTRL group showed impaired SSRT following MEC without impaired GO-RT, a novel finding. Both drugs were well tolerated and there is no evidence that physical or behavioral side effects contributed to the pattern of results.
Although the mechanism for these findings cannot be definitively determined from this investigation, our findings fit well into existing theories of basal forebrain cholinergic system function and cognition. It has been proposed that rapid cholinergic transients occur in the presence of infrequent but salient environmental cues and serve to interrupt ongoing neural activity to improve signal detection (Sarter et al. 2009a; b). This mechanism fits with our findings on both the SST and the recognition component of the CRT and is additionally consistent with a robust literature (Bucci et al. 1998; Chiba et al. 1995; Sarter et al. 2003; Sarter et al. 2005) demonstrating that cholinergic stimulation improves detection and attention to salient environmental stimuli.
It is well know that nicotine modulates the function of other neurotransmitter systems including both dopamine and norepinephrine (see Mansvelder and McGehee 2002; Picciotto 2003 for review). Behavioral studies have found that psychostimulants improve SST performance in both humans and rodents (de Wit et al. 2000; Boonstra et al. 2005; see Winstanley et al. 2006 for review) with impaired baseline performance. In addition, studies have revealed that psychostimulant effects are not specific to the stopping process. For example, it has been reported that amphetamine administration improves reaction time to the “go” signal in humans (Bedard et al. 2003; Eagle et al. 2009) and blockade of dopamine reuptake with GBR-12909 produces faster GO-RT but does not affect SSRT in rodents (Eagle et al. 2009; Overtoom et al. 2003) leading to the proposal that dopaminergic effects on the SST are primarily related to changes in the go process (Bari et al. 2009). The results of our study are not consistent with the notion of nicotinic modulation of dopamine as the primary mechanism, as there were no consistent effects on GO-RT with either MEC or NIC in the SST and no overall effects on the CRT. However NIC did improve GO-RT in the CTRL group which is consistent with past literature suggesting dopaminergic modulation by nicotinic stimulation as a mechanism for the changes in reaction time (Quik and Jeyarasasingam 2000; Wonnacott 1997).
A small literature exists demonstrating noradrenergic contributions to inhibition, and our findings are consistent with these studies. Atomoxetine has been associated with improvements in SSRT in humans (Chamberlain et al. 2009; Chamberlain et al. 2006) and rodents (Robinson et al. 2008) that are not rate dependent. Atomoxetine increases levels of catecholamines (Bymaster et al. 2002) as well as acetylcholine and histamine (Horner et al. 2007; Tzavara et al. 2006). These complex neurochemical effects limit the specificity of these findings. However, results of the current study are consistent with atomoxetine studies, suggesting nicotinic modulation of noradrenaline as a possible mechanism for the effects on SSRT.
The dissociable effects of nicotinic manipulations on the stop versus go processes are of particular interest. The separability of these processes in the SST was proposed by Logan and colleagues (Logan et al. 1984) and is supported by empirical research (Logan et al. 1984; De Jong et al. 1995; De Jong et al. 1990), functional brain imaging (Aron and Poldrack 2006), neurochemical investigations (Bari et al. 2009) and by the results presented here.
It is evident that the reciprocal neurochemical manipulations used in this study (agonism and antagonism) have differing effects in the two subject groups. One appealing explanation for this is the known rate dependent effects of nicotine across many cognitive domains (Dews 1977). Principles of rate dependency would predict that for SSRT HI subjects would show larger response to NIC and CTRL subjects would show larger response to MEC due to differing baselines. The findings on SSRT are consistent with this explanation although the absence of an improvement in SSRT following NIC in the CTRL group may simply reflect a measurement issue (“floor” effect) as HI subjects were unimpaired at baseline.
Extending the principles of rate dependency to GO-RT, similar results for both subject groups would be expected given their statistically equivalent baseline reaction time on the SST and CRT. Interestingly, in the SST the CTRL subjects, but not the HI subjects had shorter GO-RTs in response to nicotine. Building from the hypothesis that changes in GO-RT are related to dopaminergic function in this task (Bari et al. 2009), it may be that the HI subjects, experience dopaminergic inefficiency related to ADHD such that the modulation of dopamine produced by NIC is not sufficient to improve GO-RT in these subjects. Alternately, increased variability in performance may interfere with the ability to accurately measure changes in simple reaction time in HI subjects.
Limitations
Issues of generalizability are inherent to our highly specific phenotype, however they do not diminish the relevance of these results to the neurochemical modulation of BI through nicotinic receptors, and the findings remain relevant to clinical disorders characterized by impulsivity. This study had subjects with above average IQ, and the HI subjects were young adults with ADHD who are known to minimize their symptoms (Fischer et al. 2007). In addition there was a large sex imbalance (only 3 males in the HI group). A recent meta-analysis (Balint et al. 2009) concluded that there is generally greater cognitive impairment in males than females with ADHD. The underrepresentation of males in the HI group may underestimate the size of the effects seen in this study. It remains undetermined if there are sex differences in nicotinic modulation of inhibition.
Another factor is the history of stimulant medication use in the HI group. There is little literature addressing differences in the effects of drugs between chronically medicated and medication naïve ADHD subjects. However, it has been suggested that in ADHD, abnormal brain activity during BI is due to the disorder and not related to history of stimulant pharmacotherapy (Rubia et al. 2005).
Finally, this study found a training effect on SSRT between screening and PLC. Although the effect was greater in the HI group, their performance remained significantly impaired relative to the CTRL group, making regression to the mean an unlikely explanation for these findings. We are therefore confident in the effects of nicotine on SSRT in the HI group. Given the training effect in the HI subjects it is possible that their performance stabilized such that it was resistant to declines associated with MEC.
Conclusions
The results of this study support the hypothesis that nicotinic acetylcholine receptor function modulates BI in subjects with normal and impaired baseline performance and/or psychopathology. The strategy of using both a nicotinic agonist and antagonist allowed for the measurement of changes in BI in both groups of subjects. These data further support the proposed separability of neurotransmitter effects on the go and stop processes. Nicotinic effects were seen primarily on the stop process which may be related to cholinergic transients improving signal detection on the SST and reflected in improved recognition reaction time on the CRT. Future studies using functional brain imaging may help to understand the mechanisms by which nicotine modulates BI. These findings have implications for the development of novel pharmacotherapies targeting nicotinic acetylcholine receptors for ADHD and other disorders characterized by impulsive behavior.
Acknowledgments
This work was supported by: GCRC M01-00109, K23MH079216 and R03MH073573 to AP and R21MH069670 to DB. This experiment was conducted in compliance with all applicable laws of the United States of America.
References
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–8. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–4. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. Journal of Neuroscience. 2006;26:2424–33. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint S, Czobor P, Komlosi S, Meszaros A, Simon V, Bitter I. Attention deficit hyperactivity disorder (ADHD): gender- and age-related differences in neurocognition. Psychol Med. 2009;39:1337–45. doi: 10.1017/S0033291708004236. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 2003;112:105–42. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 2009;205:273–83. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bates T, Pellett O, Stough C, Mangan G. Effects of smoking on simple and choice reaction time. Psychopharmacology (Berl) 1994;114:365–8. doi: 10.1007/BF02244860. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. J Abnorm Child Psychol. 2003;31:315–27. doi: 10.1023/a:1023285614844. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Dev Neuropsychol. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone S, Hammerness P, Surman C, Harpold T, Dougherty M, Aleardi M, Spencer T. A double-blind comparison of galantamine hydrogen bromide and placebo in adults with attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychopharmacol. 2006;26:163–6. doi: 10.1097/01.jcp.0000204139.20417.8a. [DOI] [PubMed] [Google Scholar]
- Boonstra AM, Kooij JJ, Oosterlaan J, Sergeant JA, Buitelaar JK. Does methylphenidate improve inhibition and other cognitive abilities in adults with childhood-onset ADHD? J Clin Exp Neuropsychol. 2005;27:278–98. doi: 10.1080/13803390490515757. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci. 1998;18:8038–46. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Muller U, Rubia K, Del Campo N, Craig K, Regenthal R, Suckling J, Roiser JP, Grant JE, Bullmore ET, Robbins TW, Sahakian BJ. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–5. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–3. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. J Neurosci. 1995;15:7315–22. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences (revised ed.) Academic Press; 1977. [Google Scholar]
- Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD. Strategies and mechanisms in nonselective and selective inhibitory motor control. J Exp Psychol Hum Percept Perform. 1995;21:498–511. doi: 10.1037//0096-1523.21.3.498. [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: the control of response processes. J Exp Psychol Hum Percept Perform. 1990;16:164–82. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000;114:830–7. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Clark L, Kent L, Dezsery AM, Turner DC, Aitken MR, Sahakian BJ. Methylphenidate improves response inhibition but not reflection-impulsivity in children with attention deficit hyperactivity disorder (ADHD) Psychopharmacology (Berl) 2009;202:531–9. doi: 10.1007/s00213-008-1337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews PB. Rate-dependency hypothesis. Science. 1977;198:1182–3. doi: 10.1126/science.563103. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Saykin AJ, McDonald BC, McAllister TW, Hynes ML, Newhouse PA. Nicotinic versus muscarinic blockade alters verbal working memory-related brain activity in older women. American Journal of Geriatric Psychiatry. 2008;16:272–82. doi: 10.1097/JGP.0b013e3181602a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Lehmann O, Theobald DE, Pena Y, Zakaria R, Ghosh R, Dalley JW, Robbins TW. Serotonin depletion impairs waiting but not stop-signal reaction time in rats: implications for theories of the role of 5-HT in behavioral inhibition. Neuropsychopharmacology. 2009;34:1311–21. doi: 10.1038/npp.2008.202. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–73. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition @CID-I/P, Version 2.0) New York State Psychiatric Institute; 1996. [Google Scholar]
- Fischer M, Barkley RA, Smallish L, Fletcher K. Hyperactive children as young adults: driving abilities, safe driving behavior, and adverse driving outcomes. Accid Anal Prev. 2007;39:94–105. doi: 10.1016/j.aap.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Gehricke JG, Hong N, Whalen CK, Steinhoff K, Wigal TL. Effects of transdermal nicotine on symptoms, moods, and cardiovascular activity in the everyday lives of smokers and nonsmokers with attention-deficit/hyperactivity disorder. Psychol Addict Behav. 2009;23:644–55. doi: 10.1037/a0017441. [DOI] [PubMed] [Google Scholar]
- Gehricke JG, Loughlin SE, Whalen CK, Potkin SG, Fallon JH, Jamner LD, Belluzzi JD, Leslie FM. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine Tob Res. 2007;9(Suppl 4):S523–36. doi: 10.1080/14622200701685039. [DOI] [PubMed] [Google Scholar]
- Hindmarch I. Psychological performance models as indicators of the effects of hypnotic drugs on sleep. Psychopharmacology Suppl. 1984;1:58–68. doi: 10.1007/978-3-642-69659-6_4. [DOI] [PubMed] [Google Scholar]
- Horner WE, Johnson DE, Schmidt AW, Rollema H. Methylphenidate and atomoxetine increase histamine release in rat prefrontal cortex. Eur J Pharmacol. 2007;558:96–7. doi: 10.1016/j.ejphar.2006.11.048. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data.[see comment] Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Sherwood N, Hindmarch I. Separate and combined effects of the social drugs on psychomotor performance. Psychopharmacology (Berl) 1991;104:113–9. doi: 10.1007/BF02244564. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Archives of General Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS, Sassone D, Sandoval J. Persistence of hyperactivity symptoms from childhood to adolescence and associated outcomes. Am J Orthopsychiatry. 1987;57:22–32. doi: 10.1111/j.1939-0025.1987.tb03505.x. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology (Berl) 1998;140:135–41. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: a users guide to the stop signal paradigm. In: Dagenbach DCTH, editor. Inhibitory processes in attention, memory and language. Academic Press; San Diego: 1994. [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–91. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Mancuso G, Lejeune M, Ansseau M. Cigarette smoking and attention: processing speed or specific effects? Psychopharmacology. 2001;155:372–378. doi: 10.1007/s002130000678. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–17. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. Educational and Industrial Testing Service; 1971. [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry. 2002;41:378–85. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Milberger S, Faraone SV, Biederman J, Chu MP, Wilens T. Familial risk analysis of the association between attention-deficit/hyperactivity disorder and psychoactive substance use disorders. Arch Pediatr Adolesc Med. 1998;152:945–51. doi: 10.1001/archpedi.152.10.945. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–7. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–25. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Sergeant JA. Response inhibition and response re-engagement in attention-deficit/hyperactivity disorder, disruptive, anxious and normal children. Behavioural brain research. 1998;94:33–43. doi: 10.1016/s0166-4328(97)00167-8. [DOI] [PubMed] [Google Scholar]
- Overtoom CC, Verbaten MN, Kemner C, Kenemans JL, van Engeland H, Buitelaar JK, van der Molen MW, van der Gugten J, Westenberg H, Maes RA, Koelega HS. Effects of methylphenidate, desipramine, and L-dopa on attention and inhibition in children with Attention Deficit Hyperactivity Disorder. Behav Brain Res. 2003;145:7–15. doi: 10.1016/s0166-4328(03)00097-4. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Baseline-dependency of nicotine effects: a review. Behavioural Pharmacology. 1999;10:597–615. doi: 10.1097/00008877-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Nicotine as a modulator of behavior: beyond the inverted U. Trends Pharmacol Sci. 2003;24:493–9. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–8. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2004;176:182–94. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacology, Biochemistry & Behavior. 2008;88:407–17. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA, Bucci DJ. Central nicotinic cholinergic systems: a role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behav Brain Res. 2006;175:201–11. doi: 10.1016/j.bbr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Quik M, Jeyarasasingam G. Nicotinic receptors and Parkinson's disease. Eur J Pharmacol. 2000;393:223–30. doi: 10.1016/s0014-2999(99)00888-2. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–37. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162:1067–75. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiology of Learning & Memory. 2003;80:245–56. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Research - Brain Research Reviews. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009a;78:658–67. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat Rev Neurosci. 2009b;10:383–90. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz M, Denardin D, Laufer Silva T, Pianca T, Hutz MH, Faraone S, Rohde LA. Smoking during pregnancy and attention-deficit/hyperactivity disorder, predominantly inattentive type: a case-control study. J Am Acad Child Adolesc Psychiatry. 2006;45:1338–45. doi: 10.1097/S0890-8567(09)61916-X. [DOI] [PubMed] [Google Scholar]
- Sherwood N, Kerr JS, Hindmarch I. Psychomotor performance in smokers following single and repeated doses of nicotine gum. Psychopharmacology (Berl) 1992;108:432–6. doi: 10.1007/BF02247416. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Wilkinson BJ, Sanberg PR. A pilot controlled trial of transdermal nicotine in the treatment of attention deficit hyperactivity disorder. World J Biol Psychiatry. 2002;3:150–5. doi: 10.3109/15622970209150616. [DOI] [PubMed] [Google Scholar]
- Singh A, Potter A, Newhouse P. Nicotinic acetylcholine receptor system and neuropsychiatric disorders. IDrugs. 2004;7:1096–103. [PubMed] [Google Scholar]
- Tannock R. Attention Deficit Hyperactivity Disorder: Advances in Cognitive, Neurobiological, and Genetic Research. Journal of child psychology and psychiatry, and allied disciplines. 1998;39:65–99. [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Overshiner CD, Davis RJ, Perry KW, Wolff M, McKinzie DL, Witkin JM, Nomikos GG. Procholinergic and memory enhancing properties of the selective norepinephrine uptake inhibitor atomoxetine. Mol Psychiatry. 2006;11:187–95. doi: 10.1038/sj.mp.4001763. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and d-amphetamine by lithium carbonate treatment. Psychopharmacologia. 1975;44:215–24. doi: 10.1007/BF00428897. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci. 2008;12:418–24. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Sprott M, Easdon C, Fillmore M, Finn P, Justus A. Alcohol and behavioral control: cognitive and neural mechanisms. Alcohol Clin Exp Res. 2001;25:117–21. [PubMed] [Google Scholar]
- Wechsler DS. Wechsler Abbreviated Scales of Intelligence. The Psychological Corporation; 1999. [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M, Polisner D. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–7. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–46. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35:205–13. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006;16:106–14. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–8. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]