Abstract
The acid-activated proton channel formed by the influenza M2 protein is important for the lifecycle of the virus. A single histidine (His), His37, in the transmembrane domain (M2TM) of the protein is responsible for pH activation and proton selectivity of the channel. Recent studies suggested three models for how His37 mediates proton transport: a shuttle mechanism involving His37 protonation and deprotonation, a hydrogen-bonded (H-bonded) imidazole-imidazolium dimer model, and a transporter model involving large protein conformational changes in synchrony with proton conduction. Using magic-angle-spinning (MAS) solid-state NMR, we examined the proton exchange and backbone conformational dynamics of M2TM in a virus-envelope-mimetic membrane. At physiological temperature and pH, 15N NMR spectra show fast exchange of the imidazole 15N between protonated and unprotonated states. To quantify the proton exchange rates, we measured the 15N T2 relaxation times and simulated them for chemical-shift exchange and fluctuating N-H dipolar fields under 1H decoupling and MAS. The exchange rate is 4.5×105 s−1 for Nδ1 and 1.0×105 s−1 for Nε2, which are approximately synchronized with the recently reported imidazole reorientation. Binding of the antiviral drug, amantadine, suppressed both proton exchange and ring motion, thus interfering with the proton transfer mechanism. By measuring the relative concentrations of neutral and cationic His as a function of pH, we determined the four pKa values of the His37 tetrad in the viral membrane. Fitting the proton current curve using the charge-state populations from these pKa’s, we obtained the relative conductance of the five charge states, which showed that the +3 channel has the highest time-averaged unitary conductance. At physiologically relevant pH, 2D correlation spectra indicate that the neutral and cationic histidines do not have close contacts, ruling out the H-bonded dimer model. Moreover, a narrowly distributed non-ideal helical structure coexists with a broadly distributed ideal helical conformation without interchanging on the sub-10 ms timescale, thus excluding the transporter model in the viral membrane. These data support the shuttle mechanism of proton conduction, whose essential steps are His-water proton exchange, facilitated by imidazole ring reorientations.
Introduction
The M2 protein of the influenza virus forms a proton-selective tetrameric channel that allows virus entry into host cells and prevents premature conformational changes of hemagglutinin by maintaining the high pH of the trans-Golgi network1,2. M2 also mediates membrane scission and virus budding from the host cell3,4. During virus entry, M2 is activated by the low pH of the endosome and conducts 101–104 protons per second into the virion5,6, which causes uncoating and release of the ribonucleoprotein into the host cell. A single residue, His37, in the transmembrane (TM) domain (Fig. 1a) is responsible for pH activation and proton selectivity of the channel7,8. The channel is irreversibly inhibited by the antiviral drugs amantadine (Amt) and rimantadine9, which bind specifically to the TM pore10–13. The high-resolution structure of the M2 transmembrane domain has been extensively studied by solid-state NMR10,14,15, X-ray crystallography11,16 and solution NMR17 under a range of pH and drug binding conditions.
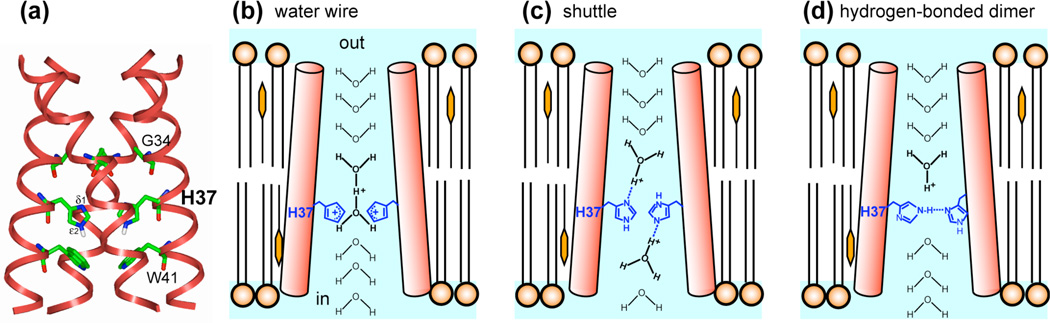
Fig. 1.
Contending models of the proton conduction mechanism of the influenza M2 proton channel. (a) High-resolution (1.65 Å) crystal structure of tetrameric M2TM at an intermediate pH of ~6.5 (PDB: 3LBW). Key residues, His37, Trp41, and Gly34, are shown as sticks. (b–d) Schematic illustrations of three proton conduction models. For clarity only two of the four helices are depicted. (b) The water wire model posits that protons are conducted exclusively by water molecules without involving His37, which only functions to open the pore constriction at this juncture. A Zundel cation (H5O2+) is depicted here as an example of a possible conducting species48. The actual water molecules may not be in single file and likely have orientational disorder. (c) The shuttle model posits that protons bind and unbind His37, and the conformational changes of His37 required to regenerate the initial state for the next proton relay represent the rate-limiting step of proton conduction. (d) The hydrogen-bonded dimer model posits that a neutral His37 forms a strong hydrogen bond with a cationic His37 at intermediate pH. The breakage of this hydrogen bond by a hydronium ion causes His37-Trp41 interaction and subsequent proton transfer to the virus interior.
Two general mechanisms of proton conduction mechanism have been proposed for M2. The water wire model posits that protons are conducted solely by the Grotthuss mechanism18 via a continuous column of H-bonded water molecules, when electrostatic repulsion of several cationic His37’s removes the pore constriction at this site (Fig. 1b)19–21. His37 plays a passive role in this model and is not directly involved in proton transfer. A second model posits that protons bind and unbind His37 with every conduction event, causing His37 conformational changes during the process (Fig. 1c)22. This “shuttle” model was proposed to explain the measured deuterium isotope effect, pH dependence of the proton current, and saturation conductance of the channel6,22. Recently, we reported low-pH specific His37 sidechain reorientation23, whose minimum energy barrier of 60 kJ/mol is consistent with the proton conduction barrier of ~100 kJ/mol obtained from functional assays5, supporting the shuttle model. On the other hand, the energy barrier for purely water-mediated proton transport in M2 was calculated to be less than 42 kJ/mol20, suggesting that protons are not solely conducted by a water wire. These results suggest that His37 shuttles protons into the virion by imidazole protonation and deprotonation, facilitated by ring reorientation.
Concurrently, two other His-based proton conduction models were also proposed. The H-bonded dimer model, developed from MD simulations of His37 structure and interaction with Trp4115, suggests a low-barrier H-bond between Nδ1 of a neutral His and Nε2H of a cationic His in the +2 tetrad (Fig. 1d). The third protonation event, with a pKa of 6.3 based on 15N NMR of DMPC/DMPG-bound M2TM24, is hypothesized to disrupt this imidazole-imidazolium dimer and establish cation-π interactions between His37 and Trp41. Subsequent conformational fluctuations are envisioned to break this cation-π interaction, exposing Nε2H to the C-terminal water and causing proton transfer15. Finally, a transporter model was proposed in which M2TM backbone alternates between two or more conformations with different water accessibilities to the exterior and interior of the virus, and the motion is synchronized with proton transport25,26. This model was based on experimental11,16,17 and MD simulated25 M2 structures, which show pH-dependent changes of the tilt angles of the N- and C-terminal halves of the TM helix.
Evaluating these proton conduction models is important not only for influenza virology but also for understanding other ion channels such as voltage-gated proton channels27. In this study, we investigate the proton exchange of His37 at physiologically relevant pH, temperature and membrane composition using MAS solid-state NMR. 15N chemical shift spectra show definitively that His37 protonate and deprotonate at rates of ~105 s−1 without forming imidazole-imidazolium dimers. We determined the four acid dissociation constants in the virus-mimetic (VM) membrane28, from which the relative conductance of the different charge states of the channel are estimated. These data show that His37-water proton transfer, facilitated by imidazole ring reorientation, is the essential proton transport mechanism of M2.
Materials and Methods
Membrane sample preparation
The TM domain (residues 22–46, SSDPLVVAASIIGILHLILWILDRL) of the M2 protein of the Udorn strain of the influenza A virus was synthesized (PrimmBiotech, Cambridge, MA) using Fmoc solid-phase protocols and purified to >95% purity. The peptide was uniformly 13C, 15N-labeled at Gly34, His37 and Ile39 or Ile42.
A virus-envelope-mimetic membrane mixture containing 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE), egg sphingomyelin (SM) and cholesterol (Chol) was prepared at an SM : DPPC : DPPE : Chol molar ratio of 28 : 21 : 21 : 3028. The VM mixture resembles the virus-envelope lipid composition, and enhances the spectral resolution of M2TM compared to model phosphocholine bilayers. Importantly, the VM membrane mixture also immobilizes the uniaxial diffusion of the four-helix bundle28–30, thus enabling detection of sidechain motions. SM was dissolved in chloroform/methanol (5:1) solution, then mixed with DPPC, DPPE and cholesterol in chloroform. The solution was dried with a stream of nitrogen gas, suspended in cyclohexane and lyophilized. The dry lipid powder was resuspended in 1 mL buffer solution of desired pH, votexed, and freeze-thawed eight times to create uniform lipid vesicles. M2TM powder was codissolved with the detergent octyl-β-D-glucoside (OG) in 1 mL of the same buffer at an OG concentration of 20 mg/mL. The pH of the M2TM/OG solution was adjusted with ~100 µL diluted HCl or NaOH solution to the desired pH. The solution was then mixed with 1 mL lipid vesicle solution, vortexed for 2 hours and dialyzed with a 3.5-kDa molecular weight cutoff against 1 L buffer at 4°C for 3 days to remove the detergent. The protein-lipid precipitant usually appeared after one day. The proteoliposome solution was centrifuged at 150,000 g and 6°C for 4 hours to yield a membrane pellet with a hydration level of ~40 wt%. The final protein : lipid molar ratio was 1 : 15. The pellet was packed into 4 mm MAS rotors for solid-state NMR experiments.
Membrane samples were prepared at five pH values from 8.5 to 4.5. The pH 8.5 sample was prepared using a Tris buffer (10 mM Tris, 1 mM EDTA, and 0.01 mM NaN3). The pH 7.0 sample was prepared using a phosphate buffer (10 mM NaH2PO4/Na2HPO4, 1 mM EDTA, and 0.01 mM NaN3). The pH 6.0 sample was prepared using a Bis-Tris buffer (10 mM Bis-Tris, 1 mM EDTA, and 0.01 mM NaN3). The pH 5.2 and pH 4.5 samples were prepared using a citrate buffer (10 mM citric acid/sodium citrate, 1 mM EDTA, and 0.01 mM NaN3). We also prepared two Amt-bound M2TM samples at pH 6.0 and 5.2, by directly titrating 5–7 µL of Amt-containing solution into the NMR rotor to achieve a final drug : tetramer ratio of 4 : 1. All buffer pH’s were measured at ambient temperature, and the pH of the supernatants after centrifugation was checked and verified to be within 0.1 unit of the desired pH.
Solid-state NMR spectroscopy
Solid-state NMR experiments were carried out on a Bruker DSX-400 MHz spectrometer (9.4 Tesla) and an AVANCE 600 MHz (14.1 Tesla) spectrometer (Karlsruhe, Germany) using 4 mm triple-resonance MAS probes. Typical radiofrequency (rf) pulse lengths were 5 µs for 13C, 6–7 µs for 15N and 3.5–4 µs for 1H. 13C chemical shifts were referenced to the α-Gly CO signal at 176.49 ppm on the TMS scale, and 15N chemical shifts were referenced to the 15N signal of N-acetyl-valine at 122.0 ppm on the liquid ammonia scale.
1D 15N cross polarization (CP) MAS spectra were measured using 1H spin lock field strengths of ω1/2π = 50 kHz. Unprotonated nitrogens, whose concentrations at different pH values are important for determining the His37 pKa’s, generally have lower CP efficiencies than the protonated nitrogens due to their weak 1H-15N dipolar couplings. To maximize the unprotonated 15N signal, we used a relatively long CP contact time of 3 ms and optimized the Hartman-Hahn condition by using the model compound N-t-Boc-proline, which contains an unprotonated 15N. While the optimal CP contact time for maximizing the unprotonated 15N signal is likely longer than 3 ms, longer contact times will reduce the protonated 15N intensity due to 1H T1ρ relaxation. Thus, 3 ms was chosen as a compromise. For the pKa analysis, where quantitative intensity ratios of the NH and N peaks were required, we used neutral His as the model compound to calibrate the scaling factor κ between the NH and N intensities.
15N T2 relaxation times were measured at 308 K under 7 kHz MAS using a Hahn-echo sequence with a 12 µs 180° pulse. The 1H decoupling field strength was 62.5 kHz during the echo delays for all samples to ensure that the motion was probed on the same timescale.
Two-dimensional (2D) 1H-driven 13C spin diffusion experiments with a DARR31 mixing time of 40–60 ms were conducted under 7–10 kHz MAS. 2D 15N-13C correlation spectra were measured using a REDOR-based pulse sequence32 for 13C-15N polarization transfer. The experiments were conducted at 273 K or 243 K under 7–10 kHz MAS. A typical 13C-15N recoupling time of 0.6 ms was used to obtain one-bond 15N-13C cross peaks. 2D 13C-1H dipolar-chemical-shift (DIPSHIFT) correlation experiments33 were carried out under 3.7, 3.9 and 5 kHz MAS at 308 K. 1H homonuclear decoupling was achieved using the MREV-8 sequence34 at 3.7 and 3.9 kHz MAS and the FSLG sequence35 at 5.0 kHz MAS. The 1H 90° pulse length in these homonuclear decoupling sequences was 4 µs. The t1 time-domain data were fit to obtain the apparent 13C-1H dipolar couplings, then divided by the scaling factor of the homonuclear decoupling sequence, which is 0.47 for MREV-8 and 0.577 for FSLG, to obtain the true couplings.
To measure dipolar order parameters, both the homonuclear decoupling scaling factor and the rigid-limit coupling have uncertainties that can affect the order parameter values. Thus we measured the product of these two factors using the crystalline model peptide formyl-Met-Leu-Phe (f-MLF). Using the theoretical scaling factors for MERV-8 and FSLG, we obtained apparent rigid-limit values of 22.7 kHz for the Cα-Hα coupling and high order parameters of 0.95–1.0 for the f-MLF backbone. Thus, we used these scaling factors and rigid-limit couplings to extract the His37 order parameters.
Numerical simulations of 15N T2 relaxation under exchange and 1H decoupling
15N T2 relaxation was simulated using the exchange-matrix formalism36 involving an exchange matrix, a chemical shift matrix and a dipolar coupling matrix. The 2 × 2 exchange matrix π↔ has diagonal elements of −k and off-diagonal elements of +k = 1/2τC. The diagonal chemical-shift and dipolar frequency matrices have diagonal elements of
| (1a) |
| (1b) |
Here, ωiso,n is the isotropic chemical shift frequency of site n, and
| (2a) |
| (2b) |
The coefficients C1,NH,n and C2,NH,n are defined similarly in terms of the dipolar coupling constants δNH,n, which include a factor of 2π.
Time was incremented in steps of the dipolar-decoupling pulse spacing tps = 1/2ν1 with the radio-frequency field strength ν1 = γB1/2π. The inversion and refocusing of the isotropic chemical shift evolution by the π-pulse (i.e. the Hahn echo) is reproduced by inverting the sign of the chemical-shift frequency matrix. Similarly, continuous-wave (CW) dipolar decoupling is approximated by dipolar-frequency inversion by a sequence of 180° pulses spaced by tps:
| (3) |
thus giving the same average nutation angle as CW decoupling. To speed up the calculation, the products of exponential matrices were evaluated for one rotation period, tr, and the Kth powers of these product matrices were then used to calculate the signal after 2K rotor periods. The T2 relaxation time was obtained from the condition S(T2) = e−1 = 0.37.
The location of the T2 minimum and the change of T2 with the exchange rate k for purely dipolar modulations under pulsed decoupling are as predicted by Rothwell and Waugh for CW decoupling37, while the depth of the T2 minimum agrees within 20%. In the simulations, we have increased the dipolar coupling strength by 10% to match the Rothwell-Waugh curve.
With the frequency and exchange matrices in the same exponents in eq. (3), changes in chemical shift and dipolar frequencies occur concomitantly, as in reality. Curves for individual processes, including pure chemical exchange, MAS effect on chemical shift anisotropy, pure dipolar modulation under heteronuclear decoupling, were obtained by setting all other coupling constants or shift differences to zero, but otherwise retaining MAS and decoupling.
The chemical shift anisotropy (CSA) is contained in ωCS,nn in eq. (1). The CSA is found to have little effect on the overall T2 relaxation times. This is expected from the theory of MAS, which refocuses the moderate CSA-induced dephasing of the magnetization within a relatively short time, the rotor period. Similarly, the chemical shift asymmetry parameter η has no detectable effect on the total T2 relaxation time and has been set to zero in eq. (1) to simplify the simulations. The chemical-shift change due to deprotonation is approximated as a change in the chemical shift anisotropy parameter δCSA by 2π × 2 kHz (50 ppm). For powder averaging, the angle β in eq. (2) was varied in 15° steps.
Results
15N NMR spectra indicate His37 protonation and deprotonation at physiological pH and temperature
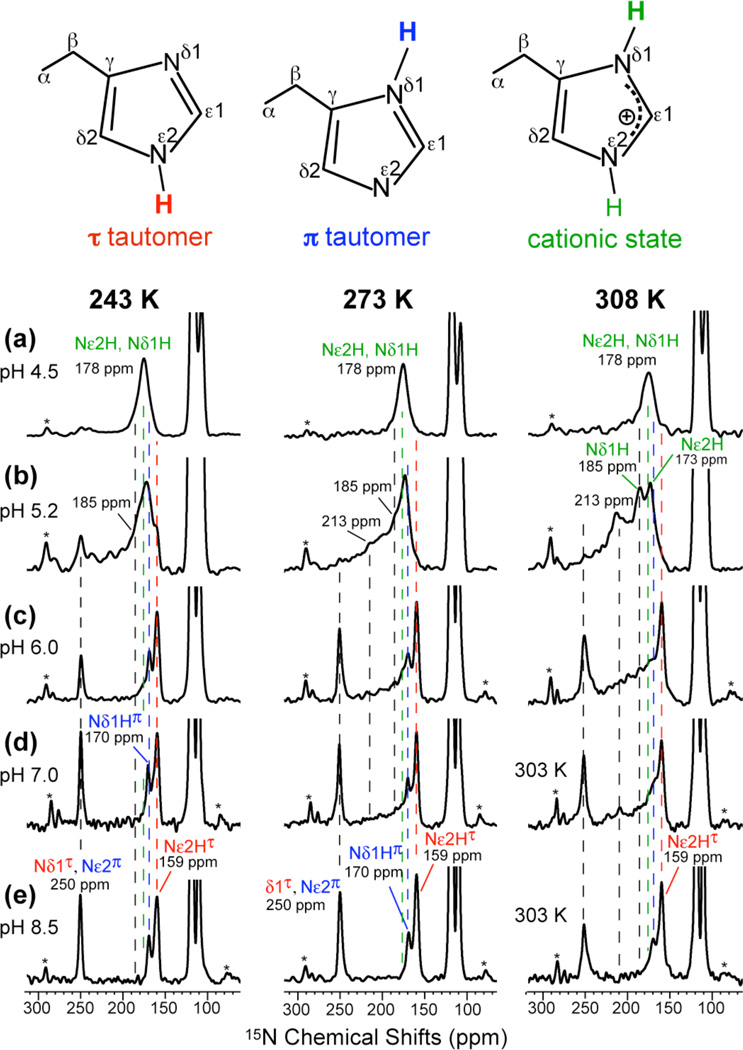
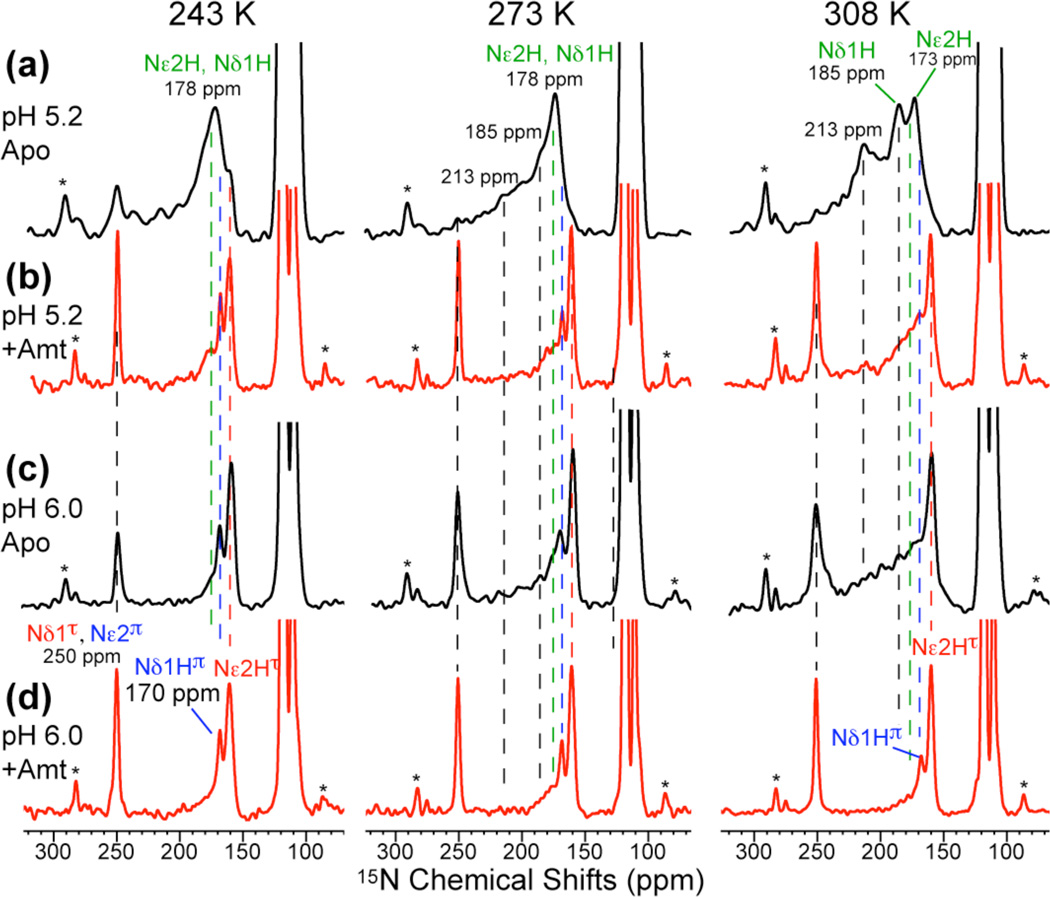
The 15N and 13C MAS spectra of His37-labeled M2TM in the mildly acidic pH range of 7 – 5 are shown in Fig. 2 and Fig. S1 and compared with spectra at pH 8.5 and 4.5.23 The 15N chemical shifts are highly sensitive to the protonation state of the imidazole: unprotonated Nδ1 in the τ tautomer and Nε2 in the π tautomer of neutral His resonate at ~250 ppm while protonated 15N in cationic and neutral His resonate at 160–190 ppm. 2D correlation spectra (Fig. 3) distinguished the protonated Nε2H (176 ppm) and Nδ1H (180 ppm) of cationic His from the protonated Nε2H(τ) and Nδ1H(π) of neutral His at 159 and 170 ppm, respectively. At low temperature, the 15N spectra (Fig. 2, left column) show the expected trend of higher cationic His intensity and lower neutral His intensity with decreasing pH. More importantly, at 303–308 K and between pH 5.2 and 7.0, the 15N spectra reveal chemical exchange of imidazole nitrogens. At pH 5.2, the 250-ppm peak present at 243 K disappeared above 273 K and a new 213-ppm peak appeared at 308 K (Fig. 2b). This frequency is half way between the unprotonated (250 ppm) and protonated (178 ppm) 15N chemical shifts, indicating that it results from equal-population exchange between the two species. Thus, the imidazole rings undergo protonation and deprotonation at 308 K, at a rate much faster than the 15N chemical shift difference (Δν) of ~70 ppm or ~3000 s−1. The protonation and deprotonation events can be attributed to His-water proton exchange but are inconsistent with His-His hydrogen bonding, as we show below. Between Nδ1 and Nε2, the more likely candidate for the 213-ppm exchange peak is Nδ1 (Fig. 4a), because chemical shift assignment23 indicates that Nε2 has higher proton affinities than Nδ1 in His37. Nε2 is the main protonated nitrogen at high pH (Fig. 2e) and preserves its protonated chemical shift (159-ppm) from pH 8.5 to 6 before moving downfield at lower pH. Previous bond length measurements indicated a short Nε2-H bond length of 1.03 Å at pH 8.523, also supporting the high proton affinity of Nε2.
Fig. 2.
15N CP-MAS spectra of His37-labeled M2TM as a function of pH and temperature. Peak assignments for the Nε2-protonated τ tautomer (red), Nδ1-protonated π tautomer (blue), and cationic His (green), whose structures are shown at the top, are obtained from 2D correlation spectra. (a) pH 4.5. (b) pH 5.2. (c) pH 6.0. (d) pH 7.0. (e) pH 8.5. Spectra were measured at 243 K, 273 K, and 308 or 303 K. Note the exchange intensities at pH 5.2 and 6.0 above 273 K. Asterisks indicate MAS sidebands.
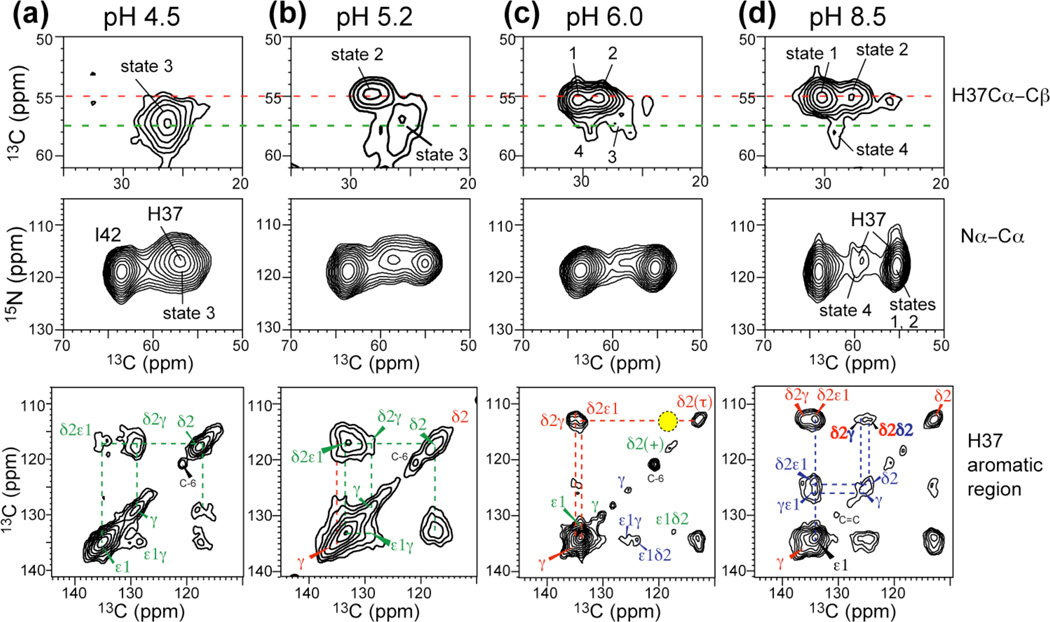
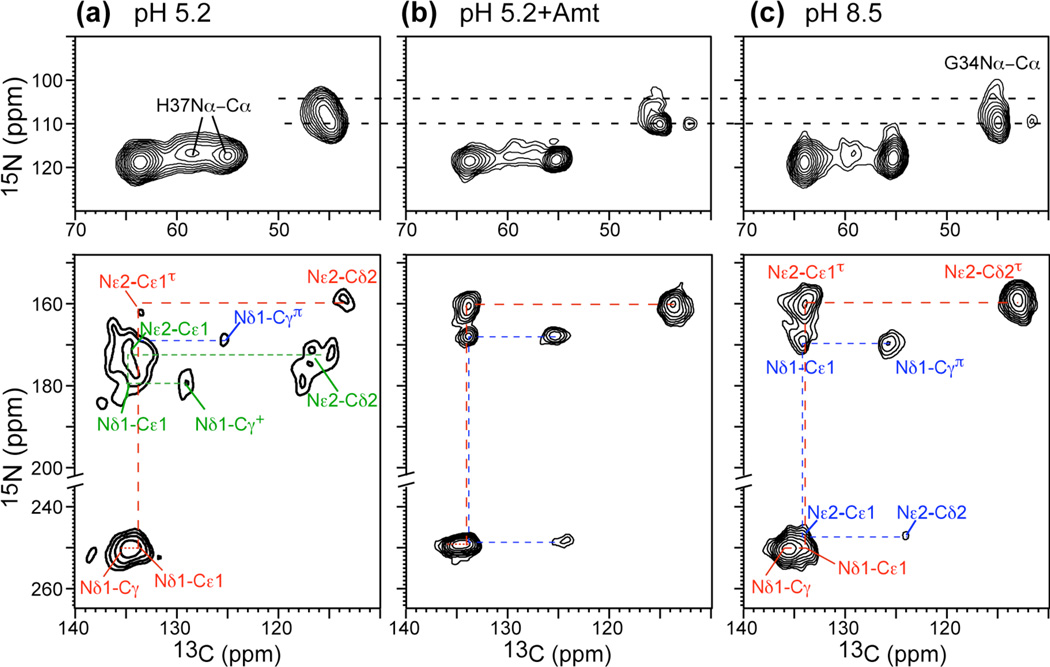
Fig. 3.
2D 13C-13C and 15N-13C correlation spectra of His37 at 273 K or 243 K as a function of pH. (a) pH 4.5. (b) pH 5.2. (c) pH 6. (d) pH 8.5. States 1–4 denote different backbone conformations41. 13C-13C 2D correlation spectra were measured with mixing times of 40 ms or 60 ms. No neutral-cationic Cδ2(τ)-Cδ2(+) cross peak was observed (yellow shade) at pH 6.0. A 300 ms spectrum (Fig. S3a) confirms the lack of imidazole-imidazolium contact.
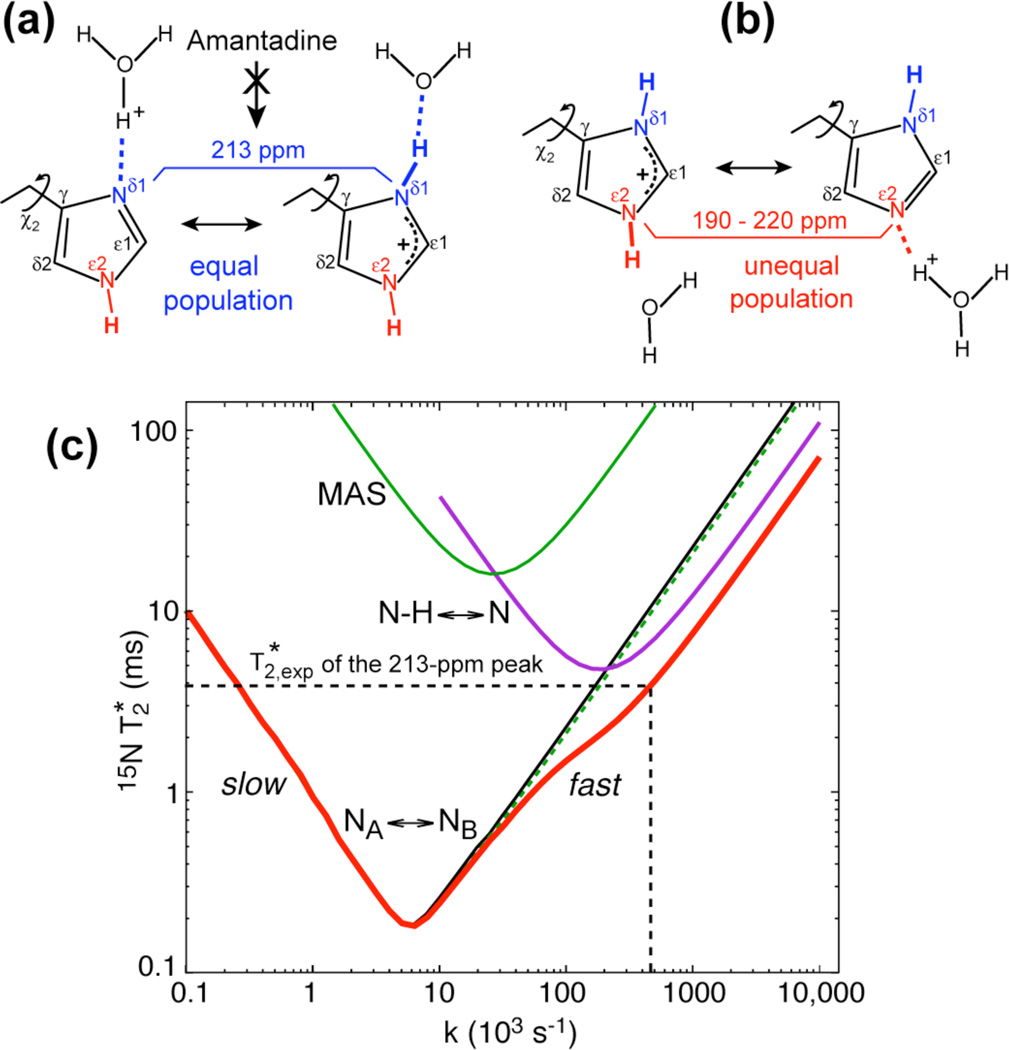
Fig. 4.
Proton exchange models of His37 and simulations of 15N T2 to determine the exchange rates. (a) Equal-population Nδ1-Nδ1H exchange, which is suppressed by the drug. (b) Unequal-population Nε2H-Nε2 exchange. (c) Dependence of 15N exchange-induced on the exchange rate k. Black line: exchange of isotropic chemical shifts only (NA ↔ NB). Green curve at top center: exchange of anisotropic chemical shifts (ΔδCSA/2π = 2 kHz) under 7 kHz MAS. Green dashed line: exchange of isotropic (Δν = 3 kHz) and anisotropic chemical shifts under MAS. Purple curve at top right: N-H dipolar modulation (ΔδNH/2π= 9 kHz) under 63 kHz 1H decoupling (N-H ↔ N). Thick red line: full simulation combining all three effects.
In addition to the 213-ppm exchange peak, we also observed a broad intensity band from 190 to 220 ppm for pH 7 to 5.2 at high temperature. The broadening could be inhomogeneous due to a distribution of neutral to charged His ratios, [His]/[HisH+], or homogeneous due to exchange at rates comparable to the 15N isotropic chemical shift difference. We measured 15N T2 relaxation times to distinguish these two possibilities (Fig. S2): at pH 5.2, the broad band has a T2 of 1.7 ms, indicating a homogeneous linewidth of 4.7 ppm, which is much narrower than the 30-ppm span of the broad peak. Thus, most of the line broadening is inhomogeneous in nature. The center of mass of the broad band is closer to the protonated than the unprotonated chemical shift, suggesting that the equilibrium is shifted to the protonated state. Since Nε2 has a higher proton affinity, we attribute this broad band to Nε2 exchange (Fig. 4b).
Not all Nδ1 and Nε2 participate in exchange: the 308 K spectrum at pH 5.2 show residual intensities at 173 ppm and 185 ppm, indicating that some nitrogens retain their protons most of the time. We attribute the 173-ppm peak to non-exchanging Nε2H and the 185-ppm peak to non-exchanging Nδ1H.
To test whether the 15N exchange peaks result from H-bonding between neutral and cationic His, as proposed in the dimer model15,24, we measured 2D 13C-13C correlation spectra at pH 6, where both neutral and cationic His are present (Fig. 3c) and where most channels are in the +2 state (see below). If strong H-bonded dimers exist, cross peaks between neutral and charged sidechains are expected. The Cδ2 peak is well resolved between the neutral τ tautomer (112 ppm) and cationic imidazolium (118 ppm), thus providing a good probe of intermolecular contacts. The dimer model15 predicts a Cδ2(τ) to Cδ2(+) distance of 5.9 Å, which is within the distance reach of 13C spin diffusion. Fig. 3c and S3 show that Cδ2(τ)-Cδ2(+) correlation is absent at mixing times from 40 ms to 300 ms. In contrast, inter-tautomer Cδ2(τ)-Cδ2(π) cross peaks were detected at pH 8.5 within as short as 40 ms (Fig. 3d), confirming that 13C spin diffusion is able to detect close intermolecular contacts within the His37 tetrad. Thus, the 2D spectra exclude the H-bonded dimer model.
Quantification of proton exchange rates through 15N T2 relaxation under 1H decoupling
To quantify the NH-N exchange rate, we first considered two-site exchange averaging only isotropic chemical shifts. In this scenario, the exchange rate k can be determined from the exchange-induced 15N T2 relaxation time, , and the isotropic shift difference Δν according to 38. For the 213-ppm exchange peak at pH 5.2, we measured an 15N T2 of 2.8 ms while the non-exchanging backbone amides have T2’s of ~10 ms (Fig. S2, Table S1). Thus, the exchange-induced is 3.9±0.5 ms. With a chemical shift difference of ~3000 Hz, the rate based on the two-site isotropic-shift exchange model is thus 1.8 × 105 s−1.
However, for chemical exchange in solids under 1H dipolar decoupling, we need to consider the fact that exchange-induced fluctuations of the 15N-1H dipolar coupling interfere with coherent averaging by 1H decoupling. Similarly, fluctuations of the 15N chemical shift anisotropy (CSA) interfere with time averaging by MAS. We performed numerical simulations to take into account all these effects quantitatively. An analytical theory for part of this problem, heteronuclear dipolar T2 relaxation under 1H decoupling, has been described by Rothwell and Waugh37. However, the theory was derived for non-spinning solids assuming isotropic rotational diffusion driving relaxation, instead of two-site exchange in a solid under MAS. To modify that theory for our purpose, we start with equation (7) of Rothwell and Waugh for the time-dependent change in the density operator ρ:
| (4) |
where γI and γS are the gyromagnetic ratios of the I-spin (1H) and the heteronuclear spin S (15N), and is a Wigner rotation matrix with an angular dependence (3cos2 β(t)−1)/2. In addition to removing the non-secular terms, we have added an ensemble average over the terms and moved the distance-dependent term into the integral over τ, because the internuclear distance r fluctuates during deprotonation and protonation of the nitrogen. Next, we define a frequency correlation function
| (5) |
The time evolution of the density operator is related to the correlation function as:
| (6) |
The correlation function decays exponentially towards its asymptotic value Cω(∞) with a time constant τC:
| (7) |
The constant term in equation (7), Cω(∞), can be disregarded in the following since it contributes only to the spectral density at ω = 0, not ω1.
For exchange between two equally populated sites with frequencies ωA and ωB, the correlation function at τ = 0 is . The frequency at long τ is (ωA + ωB)/2, thus Cω (∞) = 〈(ωA + ωB)2/4〉 (For a more stringent derivation, see Supporting Information). Therefore, equation (6) can be rewritten as
| (8) |
Assuming that the N-H vectors in sites A and B are parallel, or that ωB is negligibly small, we can factorize
| (9) |
where the dipolar coupling constants are and β is the angle between the N-H vector and the external field B0. Since the term corresponding to equation (9) in the Rothwell-Waugh theory37 for isotropic rotation diffusion is , the prefactor is the only modification to the Rothwell-Waugh equations. This factor carries through to their equation 10, which in the current context reads
| (10) |
For δA = 2π × 10 kHz for an N-H bond and δB= 2π × 1 kHz for an unprotonated N,
| (11) |
Thus, the dipolar relaxation rate is five times smaller than the value obtained if incorrectly applying the original Rothwell-Waugh theory to the histidine nitrogen undergoing proton exchange. The five-fold difference arises essentially because half of the time, the nitrogen is not protonated and thus has a weak coupling to protons.
Fig. 4c shows the calculated 15N as a function of the exchange rate k, where all three effects, isotropic chemical shift exchange, CSA modulation under MAS, and N-H dipolar modulation under 1H decoupling, are considered separately and in combination. The curves for these processes have minima when the exchange rate is half the relevant frequency ωX, i.e. the isotropic-shift difference 2πΔν, the MAS frequency ωr, and the 1H dipolar decoupling field strength ω1, as expected for ωXτmin ≈ 1 and k = 1/2τ. Over a wide range of motional rates, the 3-kHz isotropic-shift exchange is the dominant relaxation mechanism. The effects of the 10-kHz fluctuating N-H dipolar fields are limited by the sufficiently large (63-kHz) 1H decoupling field strength, while the effects of the small (~2 kHz) 15N CSA fluctuations are strongly suppressed by 7-kHz MAS. The global minimum occurs when k matches the isotropic shift difference, while the T2-contribution from exchange dynamics interfering with 1H decoupling is minor because the nitrogen is protonated only about half the time. The effects of MAS are quite negligible, and the total relaxation rate is quite well approximated as the simple sum of the relaxation rates due to chemical shift exchange and dipolar modulation. The measured value of 3.9 ms for the 213-ppm peak translates to a proton exchange rate of 4.5 ± 1.0 × 105 s−1. A second solution in the slow regime, ~250 s−1, can be excluded by motional narrowing of the 15N spectra, which requires an exchange rate much faster than 3000 s−1. For the broad exchange band assigned to Nε2, the measured values at pH 5.2 and 6.0 are 0.9–2.0 ms (Table S1), which correspond to exchange rates of 1.0 ± 0.5 × 105 s−1. These 105 s−1 rates are about an order of magnitude larger than the proton exchange rates of buffered solutions at similar pH39. This difference is expected because the diffusion-limited exchange should be facilitated by the confinement of His37 in the small cavity of the channel.
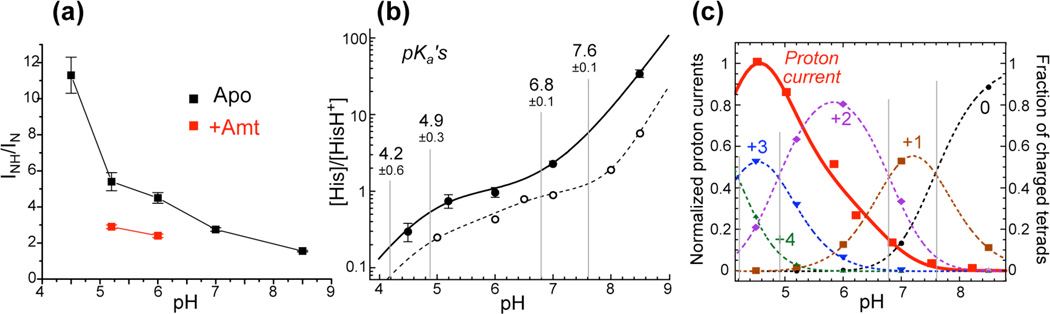
Proton dissociation constants of the His37 tetrad in M2 bound to the virus-mimetic membrane
The 15N NMR spectra not only reveal the kinetics of proton exchange, but also give rich information on the protonation equilibria of the His37 tetrad in the virus-mimetic membrane. We integrated the protonated and unprotonated 15N signals at 243 K. The intensity ratios INH/IN directly indicate the concentration ratio of neutral to cationic histidines as a function of pH, from which the four equilibrium constants can be calculated (Supporting Information, equations S4 and S5). The intensity ratios were measured at five different pH values for four unknown pKa’s, thus the problem is over-determined. The 243 K 15N spectra (Fig. 2, left column) were used for peak integration because the low-temperature intensities are minimally affected by motional averaging. We assume that the temperature dependence of histidine pKa values can be neglected because it should be largely canceled by the temperature dependence of the buffer pKa, which has similar trend and magnitude. Fig. 5a plots the INH/IN ratios as a function of pH, and the corresponding [His]/[HisH+] ratios are shown in Fig. 5b. Fitting the latter yielded four pKa’s for the His37 tetrad in the viral-membrane bound M2TM: 7.6 ± 0.1, 6.8 ± 0.1, 4.9 ± 0.3, and 4.2 ± 0.6 (gray vertical lines), where the error bars include both systematic uncertainties in the sample pH and the propagated random uncertainties in the intensity ratios (Table S2). For comparison, the pKa’s of the peptide in DMPC/DMPG bilayers have been reported24 as 8.2, 8.2, 6.3 and < 5 based on a different set of measured [His]/[HisH+] values (Fig. 5b, dotted line). Therefore, compared to the previous model membrane result, the four histidines protonate at lower pH and the first two protonation steps are resolved in the cholesterol-rich VM membrane. The methods for extracting pKa’s differ between the two studies. We obtained [His]/[HisH+] ratios from the relative intensities of unprotonated and protonated 15N signals, which are resolved by more than 70 ppm. The different 1H-15N cross polarization efficiencies of N and NH signals were taken into account by calibration using a model compound (Fig. S4) so that the intensity ratio accurately reflects the relative amounts of unprotonated and protonated nitrogens. In comparison, the previous model-membrane study24 obtained [His]/[HisH+] ratios by deconvolution of the broad protonated 15N peak into multiple components, which had large uncertainties due to significant exchange broadening of these peaks at the temperature of the experiments. Apart from experimental uncertainties, the pKa differences may also reflect real differences in the protonation equilibria of M2TM in the two lipid membranes: the anionic DMPG used in the previous study may increase the local concentration of protons on the bilayer surface, thus increasing the apparent pKa values.
Fig. 5.
Protonation equilibria of the His37 tetrad in viral-membrane bound M2TM. (a) INH/IN without drug (black) and with drug (red). (b) [His]/[HisH+] ratios (filled circles) as a function of pH and the best-fit curve (solid line), resulting in the four pKa’s. For comparison, the [His]/[HisH+] ratios reported previously for DMPC/DMPG-bound M2TM (open circles and dashed line) are also shown24. (c) Proton currents of Udorn M2 (red squares)7 and normalized populations of all five charge states (symbols and dotted lines) as a function of pH. Best fit of the proton currents (red line) was obtained using relative time-averaged conductance of 1.9±0.2, 5.5±0.7 and 0.4±1.6 for the +2, +3 and +4 channels and 0 for +1 and neutral channels.
To relate the His pKa’s to electrophysiological data, we fit the proton currents of Udorn and Weybridge M2 (Table S3)7,40 as the population-weighted sum of the time-averaged unitary currents. We assume that the equilibrium populations of the four charge states, which are fully determined by the pKa’s, are applicable to the kinetic condition under which the proton currents are measured. The charge-state populations Ni of the channels are related to the acid dissociation constants according to
| (12) |
where the normalization constant is Σ= [H+]4 + [H+]3K1 + [H+]2K1K2 + [H+]K1K2K3 + K1K2K3K4. Fig. 5c plots the populations of the five charge states of the channel as a function of pH. As expected, the more cationic tetrads are populated at lower pH, and the intersections of the population curves of two neighboring charge states correspond to the pKa’s (gray vertical lines).
The proton current at a certain pH is the product of the electrochemical potential, the number of channels Ni for the charge state i, the open probability Po,i of that state, and the unitary conductance gi:
| (13) |
The electrochemical potential, which provides the driving force for proton conduction, is the difference between the applied voltage V and the Nernst potential EH. The latter results from the pH difference between the inside and outside of the membrane, EH = 2.303 RT(pHin − pHout)/F, where R is the gas constant, T is temperature, and F is the Faraday constant. The product of the unitary conductance and the open probability is the time-averaged single-channel conductance g̅i,
| (14) |
We fit two sets of proton current data. Wang et al7 reported proton currents of Udorn M2 using an applied voltage of −120 mV, pHin = 7.5, and pHout from 4.5 to 8.2. Chizhmakov et al40 reported the chord conductance g = I/(V − EH) for the Weybridge strain of M2 using an applied voltage of −60 mV, pHin = 7.4, and pHout from 4.0 to 7.2 (Table S3). The latter dataset can be fit using the equation
| (15) |
Fig. 5c shows the Udorn M2 proton current data (red squares) as a function of pH. The current reaches maximum at the lowest pH of 4.5 while being close to zero above neutral pH. Qualitatively, this already indicates that the neutral and +1 tetrads, which are predominantly populated above pH 7, conduct few protons, while the +3 channels, whose population curve has a very similar pH dependence as the functional curve with a maximum near pH 4.5, are chiefly responsible for proton conduction. Quantitative fitting of the proton current curve using the charge-state populations gave relative g̅i values of 1.9 ± 0.2, 5.5 ± 0.7 and 0.4 ± 1.6 for the +2, +3 and +4 channels and 0 for the neutral and +1 states, confirming that the +3 channel has the highest time-averaged unitary conductance. Importantly, these relative g̅i values are obtained without a priori assumptions of whether a given charge state conducts or not. The same qualitative trend is observed by fitting the conductance of Weybridge M240, yielding relative g̅i values of 0.47 ± 0.07, 1.41 ± 0.24 and 0.70 ± 0.25 for the +2, +3 and +4 states (Fig. S5a). Both sets of proton current data indicate that the +2 state has 2–3 fold smaller, but non-vanishing, proton fluxes compared to the +3 state (Fig. S5b). The time-averaged unitary conductance of the +4 channel has large uncertainties due to the difficulty of measuring whole-cell currents at the lowest pH.
pH-dependent backbone conformational changes
2D 13C-13C and 15N-13C correlation spectra (Fig. 3) revealed not only His37 sidechain structure but also backbone conformational changes as a function of pH. In general, the His37 Cα chemical shift increased while the Cβ chemical shift decreased with decreasing pH, indicating that the His37 backbone became more helical41. Four backbone conformations can be identified in the whole pH range. At pH 8.5 three non-ideal helical conformations with distinct Cα, Cβ and 15N chemical shifts were resolved (Fig. 3d), whereas at low pH the peaks are broadened and shifted to more ideal helical chemical shifts (Fig. 3a). We designate these conformations as states 1–4, of which states 1–3 were already reported recently41. These states do not correlate in a simple way to the charge states of the channel, since M2TM conformation is also affected by the lipid membrane. At pH 5.2, the Cα-Cβ cross peaks indicate that the non-ideal helical state 2 coexists with the broadly distributed ideal helical state 3 without fast exchange between them, since the two peaks appear at their limiting frequencies from 273 K (Fig. 3b) to 308 K (Fig. S3b). Given the 13C chemical shift differences of ~300 Hz, any conformational exchange must be much slower than 300 s−1. Decreasing the pH to 4.5 left only the broad state-3 peak, which has a shorter 15N T2 than the other conformations (Table S1), indicating that the low-pH peptide undergoes small-amplitude fluctuations and samples a broad conformational space. The fact that the average conformation of the low-pH peptide is more ideally helical suggests that the splayed-open packing of the C-terminal half of the helix in the low-pH crystal structure11 is present in bilayers, even though the crystal structure was determined using detergent solubilized M2TM. Taken together, the 2D spectra indicate that multiple backbone conformations exist from pH 8.5 to 4.5, but no fast interconversion occurs between the two main conformations at physiological acidic pH on the sub-10 ms timescale in the VM membrane.
Effects of Amt binding on His37 proton exchange and motion
If His37-water proton exchange is essential for proton conduction, then Amt binding should suppress this exchange and reestablish the INH/IN ratios. Indeed, the 15N spectra of drug-bound M2TM at pH 6.0 and 5.2 (Fig. 6) show a significant increase of the 250-ppm intensity, suppression of the 213-ppm peak, and reduction of the 190–220 ppm intensity. 2D 15N-13C correlation spectra confirm that the drug-bound peptide at pH 5.2 has similar conformation as the apo peptide at pH 8.5 (Fig. 7), indicating that Amt binding, although occurring at Ser3110, inhibits Nδ1 protonation at His37. Recent water-protein 1H spin diffusion experiments showed that Amt binding dehydrated the N-terminal pore and reduced the water-exposed surface area of the protein by two fold42. Thus, inhibition of His37 protonation likely results from reduced access to water. The effect of Amt on the Nε2H–Nε2 exchange is weaker, as the 190–220 ppm intensity is partially retained (Fig. 6b). This is consistent with the presence of water C-terminal to His37 as seen in both SSNMR data23 and the 1.65 Å crystal structure16. When Nε2 is unprotonated, Nδ1 must be protonated, giving the π tautomer. Indeed, the 170-ppm peak of the π tautomer is stronger in the drug-bound spectra than the apo spectra. The higher intensity of the 250-ppm peak (Fig. 6b, d), which corresponds to a pKa reduction, is also consistent with previous results of DMPC/DMPG-bound M2TM43. Amt binding at pH 5.2 also perturbed Gly34 conformation, by shifting it to a less helical state, as seen in the 2D 15N-13C correlation spectra (Fig. 7b)41, indicating that the low-pH drug-complexed peptide adopts a conformation similar to the kinked helix of the apo peptide at high pH.
Fig. 6.
15N CP-MAS spectra of His37 in apo (a, c) and drug-bound (b, d) M2TM. (a, b) pH 5.2. (c, d) pH 6.0 at 243 K, 273 K and 308 K. Drug binding increased the unprotonated peak intensity at 250 ppm and reduced the exchange intensities.
Fig. 7.
2D 15N-13C correlation spectra of His37 and Gly34-labeled M2TM without and with Amt at 243 K. (a) pH 5.2 without drug. (b) pH 5.2 with Amt. (b) pH 8.5 without drug. Top row: backbone N-Cα region. Bottom row: imidazole sidechain region. The drug-bound spectrum at pH 5.2 resembles the drug-free spectrum at pH 8.5.
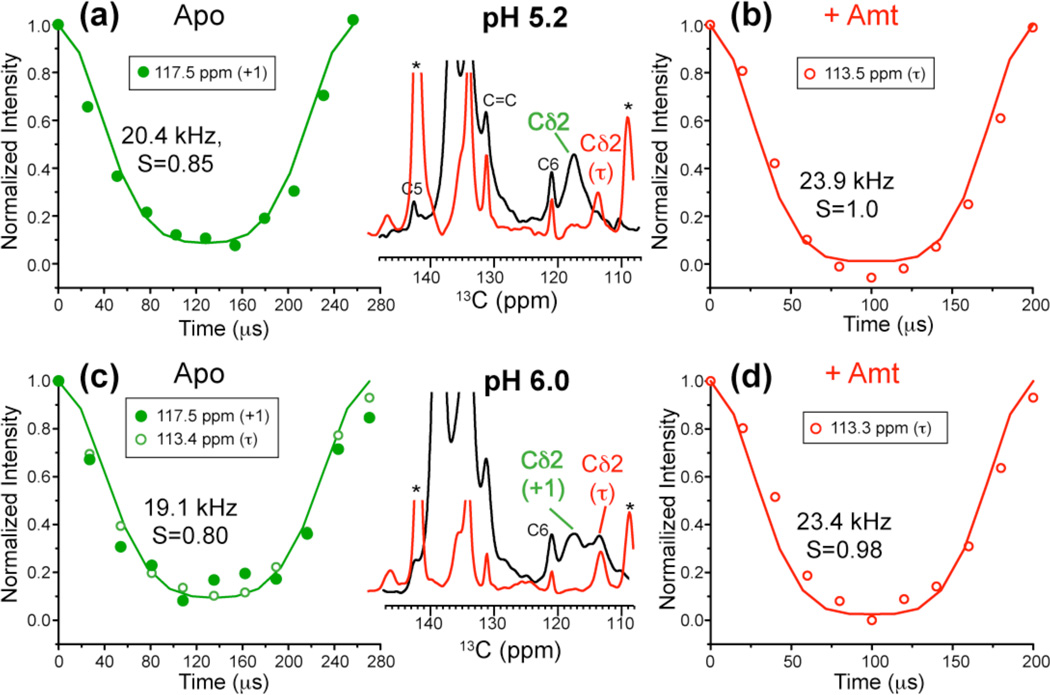
In the drug-free low-pH channel, the His37 sidechain undergoes small-angle reorientations23 that were proposed to orient the imidazole nitrogens to water molecules for proton transfer. To test whether this motion persists in the presence of the drug, we measured Cδ2-H dipolar couplings of the drug-bound M2TM at pH 5.2 and 6.0. Indeed, the Cδ2-H dipolar coupling reached rigid limit at 308 K in the Amt-bound state, in contrast to the apo state at the same pH (Fig. 8). Thus, the imidazolium ring motion is inhibited by Amt.
Fig. 8.
His37 sidechain dynamics at mildly acidic pH and 308 K from Cδ2-H dipolar couplings. (a) Apo peptide at pH 5.2. (b) Amt-bound peptide at pH 5.2. (c) Apo peptide at pH 6. (d) Amt-bound peptide at pH 6. The aromatic regions of the 13C spectra of the drug-free (black) and drug-bound (red) samples are compared in the middle column. The data were measured under 3.9 and 3.7 kHz MAS for (a, c) and under 5 kHz MAS for (b, d). The best-fit C–H dipolar coupling value and the corresponding bond order parameter are indicated for each curve.
Discussion
These 15N NMR data, measured at physiological temperature, pH and membrane composition, provide rich insights into the proton conduction mechanism of the influenza M2 channel. The imidazole nitrogens protonate and deprotonate at rates of 105 s−1, averaging the 15N isotropic chemical shifts. These rates are determined from CP-MAS spectra, which have rarely been used for quantitative kinetic analysis of systems undergoing chemical exchange because the analysis has been perceived as complex and difficult: in addition to the stochastic chemical exchange process, there are two separate coherent averaging processes present, dipolar decoupling and magic angle spinning, on time scales that are not sufficiently separate to make the problem easily reducible. Our novel analysis overcomes this difficulty and takes all these dynamic processes into account.
The 105 s−1 proton exchange rate is about an order of magnitude larger than the proton transfer rates in buffered solutions39, indicating that His37 confinement in the water-filled pore speeds up the diffusion-limited proton transfer. The His37 proton exchange rate is consistent with the ring reorientation rate estimated from motionally averaged dipolar couplings23, supporting the proposal that ring reorientation is synchronized with and facilitates proton transfer. The measured proton exchange rate is significantly larger than the time-averaged unitary proton flux of M2 from whole-cell patch-clamp and liposome experiments5,6,44. This is expected, because the NMR-detected exchange rate includes futile exchanges, where a proton may be transferred to and from His37 repeatedly without exiting the channel, which is likely in part due to the action of the gating residue Trp4145,46. It should be noted that the reported open probabilities and unitary conductance of M2 vary widely, because M2 is not a voltage- or ligand-gated channel and its conductance is too small to be measured accurately.
The His37 protonation and deprotonation require H-bonding with water: Amt binding in the N-terminal pore, which dehydrates the channel42, completely suppressed the 213-ppm exchange peak (Fig. 6b). In comparison, the Nε2 exchange intensity is partially retained in the presence of the drug, consistent with the retention of water C-terminal to His3723. The water-His37 exchange agrees well with the observed water clusters above and below the His37 tetrad in the pH 6.5 crystal structure16.
The absence of neutral-cationic histidine cross peaks in the 2D spectra (Fig. 3c) rules out the existence of strong H-bonds between histidines. In addition, the H-bonded dimer model requires the His37 χ2 angle to be 90°15, which contradicts the measured χ2 angle of 180° in the lipid bilayer by SSNMR23 and in the detergent by crystallography16. The 180° χ2 angle points Nδ1 to the N-terminus of the pore, supporting H-bonding with water rather than to a neighboring His.
The four pKa’s for the viral-membrane bound M2TM yielded the populations of the five charge states as a function of pH. Using these populations, we fit the proton conductance from two independent functional studies7,40 to obtain the relative conductance of the charge states. Both fits indicate that the +3 channel has the highest time-averaged unitary conductance and is thus chiefly responsible for M2’s proton channel activity (Fig. 5c). The high g̅+3 can be due to a large open probability, a high intrinsic unitary conductance, or a combination of both. The +2 channel has lower g̅, but its population is the largest in the physiological pH range, thus it may be important for proton storage before full channel activation, as proposed recently16. The +4 state has smaller g̅ than the +3 channel, even though it must have the highest open probability46, suggesting that its intrinsic conductance, g, is low. This is reasonable because the +4 tetrad exerts the largest repulsion with the proton, which should cause a higher energy barrier for proton transport than the +3 state, as predicted by calculations20.
In the virus-mimetic membrane, M2TM does not exhibit large-amplitude backbone conformational interconversion on the sub-10 ms timescale at physiological pH, contradicting the transporter model25,26. Instead, we observed broad 15N linewidths and short T2’s for the state that dominates at low pH (state 3), suggesting that the functionally relevant motions are small-amplitude fluctuations around a relatively ideal helical conformation. Although the VM membrane is relatively ordered due to the presence of cholesterol and sphingomyelin, the TM peptide retains its drug binding ability in this membrane47, thus the lack of large-scale transport-synchronized backbone motion cannot be attributed to the lack of conformational flexibility of the helical bundle. Instead, our data suggest a different type of protein backbone motion, entailing much smaller amplitudes, for proton transport.
In conclusion, these solid-state NMR data provide definitive evidence of His37 protonation and deprotonation at physiological acidic pH. The 1.0 – 4.5 × 105 s−1 proton transfer between His and water is synchronized with imidazole ring reorientations. The +3 channel has the highest time-averaged unitary conductance, defining the activated channel. We suggest that Trp41, through interactions with His37, reduces the high His37 proton exchange rate to the much smaller time-averaged proton flux. The lack of close contact between neutral and charged histidines rules out the H-bonded dimer model, while the lack of large-amplitude backbone conformational changes in the VM membrane modifies the transporter model. These findings support the shuttle mechanism, where protons from water molecules bind and unbind His37 on the microsecond timescale, facilitated by ring reorientation and small-amplitude backbone fluctuations.
Supplementary Material
Acknowledgement
This work was funded by NIH grant GM088204 and NSF grant MCB-0543473.
Footnotes
Supporting Information Available
Additional equations of correlation function, pKa analysis, tables of 15N T2, histidine intensity quantification, proton current data, and NMR spectra. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Cady SD, Luo WB, Hu F, Hong M. Biochemistry. 2009;48:7356–7364. doi: 10.1021/bi9008837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinto LH, Lamb RA. J. Biol. Chem. 2006;281:8997–9000. doi: 10.1074/jbc.R500020200. [DOI] [PubMed] [Google Scholar]
- 3.Rossman JS, Jing X, Leser GP, Lamb RA. Cell. 2010;142:902–913. doi: 10.1016/j.cell.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroeder C, Heider H, Möncke-Buchner E, Lin TI. Eur. Biophys. J. 2005;34:52–66. doi: 10.1007/s00249-004-0424-1. [DOI] [PubMed] [Google Scholar]
- 5.Lin TI, Schroeder C. J. Virol. 2001;75:3647–3656. doi: 10.1128/JVI.75.8.3647-3656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mould JA, Li HC, Dudlak CS, Lear JD, Pekosz A, Lamb RA, Pinto LH. J. Biol. Chem. 2000;275:8592–8599. doi: 10.1074/jbc.275.12.8592. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Lamb RA, Pinto LH. Biophys. J. 1995;69:1363–1371. doi: 10.1016/S0006-3495(95)80003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balannik V, Carnevale V, Fiorin G, Levine BG, Lamb RA, Klein ML, Degrado WF, Pinto LH. Biochemistry. 2010;49:696–708. doi: 10.1021/bi901799k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Takeuchi K, Pinto LH, Lamb RA. J. Virol. 1993;67:5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cady SD, Schmidt-Rohr K, Wang J, Soto CS, DeGrado WF, Hong M. Nature. 2010;463:689–692. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, Soto CS, Tereshko V, Nanda V, Stayrook S, DeGrado WF. Nature. 2008;451:596–599. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jing X, Ma C, Ohigashi Y, Oliveira FA, Jardetzky TS, Pinto LH, Lamb RA. Proc. Natl. Acad. Sci. USA. 2008;105:10967–10972. doi: 10.1073/pnas.0804958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg MR, Casarotto MG. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13866–13871. doi: 10.1073/pnas.1002051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cady SD, Mishanina TV, Hong M. J. Mol. Biol. 2009;385:1127–1141. doi: 10.1016/j.jmb.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma M, Yi M, Dong H, Qin H, Peterson E, Busath D, Zhou HX, Cross TA. Science. 2010;330:509–512. doi: 10.1126/science.1191750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acharya A, Carnevale V, Fiorin G, Levine BG, Polishchuk A, Balannick V, Samish I, Lamb RA, Pinto LH, DeGrado WF, Klein ML. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15075–15080. doi: 10.1073/pnas.1007071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnell JR, Chou JJ. Nature. 2008;451:591–595. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agmon N. Chem. Phys. Lett. 1995;244:456–462. [Google Scholar]
- 19.Sansom MSP, Kerr ID, Smith GR, Son HS. Virology. 1997;233:163–173. doi: 10.1006/viro.1997.8578. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Wu Y, Voth GA. Biophys. J. 2007;93:3470–3479. doi: 10.1529/biophysj.107.105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada A, Miura T, Takeuchi H. Biochemistry. 2001;40:6053–6060. doi: 10.1021/bi0028441. [DOI] [PubMed] [Google Scholar]
- 22.Pinto LH, Dieckmann GR, Gandhi CS, Papworth CG, Braman J, Shaughnessy MA, Lear JD, Lamb RA, DeGrado WF. Proc. Natl. Acad. Sci. USA. 1997;94:11301–11306. doi: 10.1073/pnas.94.21.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu F, Luo W, Hong M. Science. 2010;330:505–508. doi: 10.1126/science.1191714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J, Fu R, Nishimura K, Zhang L, Zhou HX, Busath DD, Vijayvergiya V, Cross TA. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6865–6870. doi: 10.1073/pnas.0601944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khurana E, Dal Peraro M, DeVane R, Vemparala S, DeGrado WF, Klein ML. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1069–1074. doi: 10.1073/pnas.0811720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polishchuk AL, Lear JD, Ma C, Lamb RA, Pinto LH, DeGrado WF. Biochemistry. 2010;49:10061–10071. doi: 10.1021/bi101229m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decoursey TE. Physiol. Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 28.Luo W, Cady SD, Hong M. Biochemistry. 2009;48:6361–6368. doi: 10.1021/bi900716s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cady SD, Hong M. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1483–1488. doi: 10.1073/pnas.0711500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cady SD, Hong M. J. Biomol. NMR. 2009;45:185–196. doi: 10.1007/s10858-009-9352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takegoshi K, Nakamura S, Terao T. Chem. Phys. Lett. 2001;344:631–637. [Google Scholar]
- 32.Hong M, Griffin RG. J. Am. Chem. Soc. 1998;120:7113–7114. [Google Scholar]
- 33.Munowitz MG, Griffin RG, Bodenhausen G, Huang TH. J. Am. Chem. Soc. 1981;103:2529–2533. [Google Scholar]
- 34.Rhim W-K, Elleman DD, Vaughan RW. J. Chem. Phys. 1973;59:3740–3749. [Google Scholar]
- 35.Bielecki A, Kolbert AC, Levitt MH. Chem. Phys. Lett. 1989;155:341–346. [Google Scholar]
- 36.Abragam A. Principles of Nuclear Magnetism. Oxford: Clarendon Press; 1961. [Google Scholar]
- 37.Rothwell WP, Waugh JS. J. Chem. Phys. 1981;74:2721–2732. [Google Scholar]
- 38.Harris RK. Nuclear Magnetic Resonance Spectroscopy: a physicochemical view. Harlow, England: Longman Scientic & Technical; 1986. [Google Scholar]
- 39.Luz Z, Meiboom S. J. Am. Chem. Soc. 1963;85:3923–3925. [Google Scholar]
- 40.Chizhmakov IV, Geraghty FM, Ogden DC, Hayhurst A, Antoniou M, Hay AJ. J. Physiol. 1996;494:329–336. doi: 10.1113/jphysiol.1996.sp021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu F, Luo W, Cady SD, Hong M. Biochim. Biophys. Acta. 2011;1808:415–423. doi: 10.1016/j.bbamem.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo W, Hong M. J. Am. Chem. Soc. 2010;132:2378–2384. doi: 10.1021/ja9096219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu J, Riqiang F, Cross TA. Biophys. J. 2007;93:276–283. doi: 10.1529/biophysj.106.102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moffat JC, Vijayvergiya V, Gao PF, Cross TA, Woodbury DJ, Busath DD. Biophys. J. 2008;94:434–445. doi: 10.1529/biophysj.107.109082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Y, Zaitseva F, Lamb RA, Pinto LH. J. Biol. Chem. 2002;277:39880–39886. doi: 10.1074/jbc.M206582200. [DOI] [PubMed] [Google Scholar]
- 46.Zhou HX. Biophys. J. 2011;100:912–921. doi: 10.1016/j.bpj.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cady SD, Wang T, Hong M. J. Am. Chem. Soc. 2011;133:11572–11579. doi: 10.1021/ja202051n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smondyrev AM, Voth GA. Biophys. J. 2002;83:1987–1996. doi: 10.1016/S0006-3495(02)73960-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.