Abstract
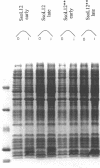
Overexpression of the Sulfolobus solfataricus L12 ribosomal protein gene in E.coli cells yielded two products of different size. If the E.coli cells carrying the overexpression plasmid were induced in the early stage of bacterial growth, the smaller of the two products was almost exclusively produced. However, induction in a late stage of bacterial growth yielded the larger product in significant excess. The larger protein was identified as the translation product of the entire SsoL12 gene, while the smaller product was a N-terminally shortened version of the L12 protein (sh-SsoL12), starting with a N-terminal methionine at position 22 of the coded protein and continuing with the predicted protein sequence. Position 22 is an isoleucine in the complete SsoL12 protein sequence, coded by an AUA codon. A subclone (SsoL12**) of the SsoL12 gene containing overexpression plasmid, lacking the regular AUG start codon and the putative Shine Dalgarno sequence, was constructed to determine if E.coli ribosomes could initiate at this AUA codon. During overexpression the SsoL12** construct yielded exclusively the sh-SsoL12 product in significant amounts. An AUA start codon has never been found before in a natural message. However, experiments utilizing site directed mutagenesis to generate AUA start codons showed that this codon can be functional for initiation in prokaryotes and eukaryotes. The findings presented in this paper show that AUA acts as an initiation codon in a natural message expressed in a heterologous organism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D., Hedgpeth J., Selzer G. B., Epstein R. H. Temperature-sensitive mutation in the initiation codon of the rIIB gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1979 Feb;76(2):700–704. doi: 10.1073/pnas.76.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Johnston H. M., Roth J. R. DNA sequence changes of mutations altering attenuation control of the histidine operon of Salmonella typhimurium. J Mol Biol. 1981 Feb 5;145(4):735–756. doi: 10.1016/0022-2836(81)90312-0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S. Translation initiation at non-AUG triplets in mammalian cells. J Biol Chem. 1989 Mar 25;264(9):5031–5035. [PubMed] [Google Scholar]
- Singer B. S., Gold L. A mutation that confers temperature sensitivity on the translation of rIIB in bacteriophage T4. J Mol Biol. 1976 May 25;103(3):627–646. doi: 10.1016/0022-2836(76)90221-7. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- de Smit M. H., van Duin J. Secondary structure of the ribosome binding site determines translational efficiency: a quantitative analysis. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7668–7672. doi: 10.1073/pnas.87.19.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]