Abstract
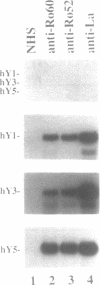
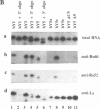
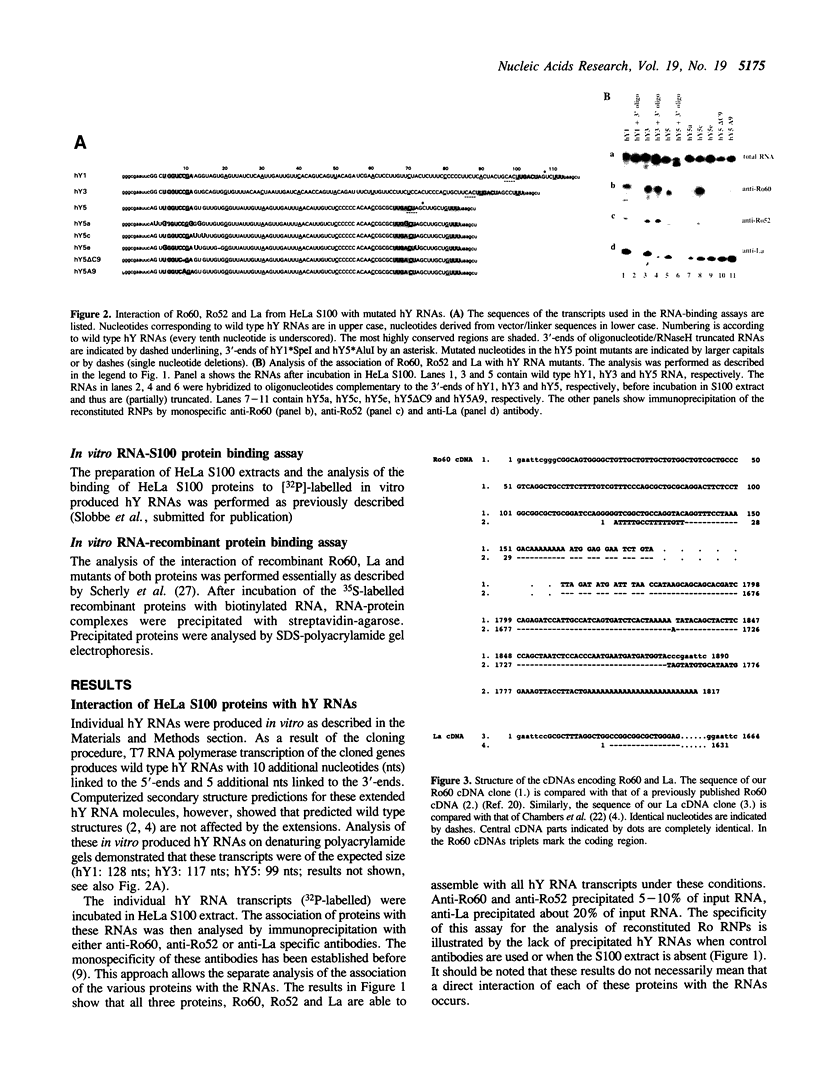
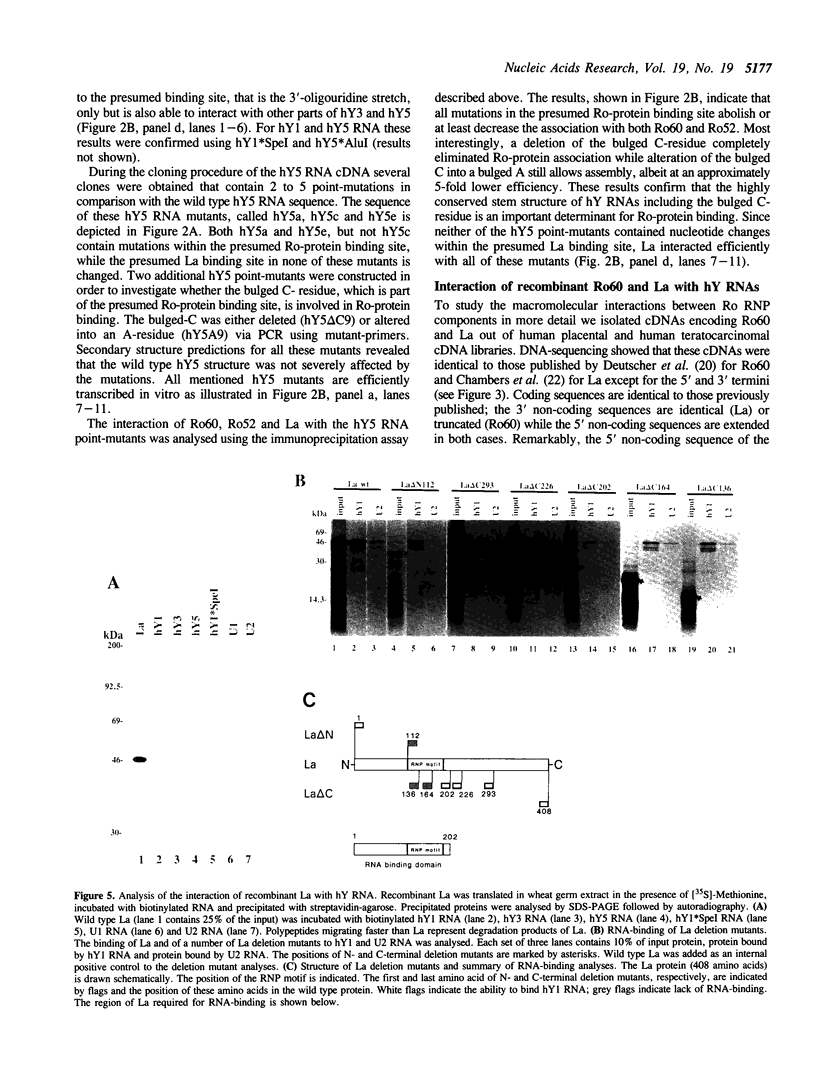
The interactions between Ro and La proteins and hY RNAs have been analysed. The binding site for the 60 kDa Ro protein on hY RNAs is shown to be the terminal part of the base paired stem structure, which contains the most highly conserved sequence among hY RNAs. The bulged C-residue within this region plays an important role in the recognition by this protein. The same regions of hY RNAs are essential for the association of the 52 kDa Ro protein with the RNAs, strongly suggesting that the 60 kDa Ro protein is required for the 52 kDa Ro protein to bind, presumably via protein-protein interactions, to Ro RNPs. The binding site for the La protein on hY RNAs is shown to be the oligouridylate stretch near the 3'-end of the RNAs, which is also recognized when additional nucleotides flank this motif at the 3'-side. Additional sequence elements in hY3 and hY5, but not in hY1, are bound by the La protein as well. Deletion mutagenesis showed that the RNP motif, previously identified in many ribonucleoprotein (RNP) proteins and in some cases shown to be almost sufficient for the interaction with RNA, of both the 60 kDa Ro and the La protein are not sufficient for the interaction with hY RNAs. Substantial parts of these proteins flanking the RNP motif are needed as well. It is likely that they stabilize the correct conformation of the RNP motif for RNA binding.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Ben-Chetrit E., Chan E. K., Sullivan K. F., Tan E. M. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988 May 1;167(5):1560–1571. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Chetrit E., Gandy B. J., Tan E. M., Sullivan K. F. Isolation and characterization of a cDNA clone encoding the 60-kD component of the human SS-A/Ro ribonucleoprotein autoantigen. J Clin Invest. 1989 Apr;83(4):1284–1292. doi: 10.1172/JCI114013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990 Apr 25;265(12):6513–6516. [PubMed] [Google Scholar]
- Boire G., Craft J. Biochemical and immunological heterogeneity of the Ro ribonucleoprotein particles. Analysis with sera specific for the RohY5 particle. J Clin Invest. 1989 Jul;84(1):270–279. doi: 10.1172/JCI114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire G., Craft J. Human Ro ribonucleoprotein particles: characterization of native structure and stable association with the La polypeptide. J Clin Invest. 1990 Apr;85(4):1182–1190. doi: 10.1172/JCI114551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyon J. P., Slade S. G., Chan E. K., Tan E. M., Winchester R. Effective separation of the 52 kDa SSA/Ro polypeptide from the 48 kDa SSB/La polypeptide by altering conditions of polyacrylamide gel electrophoresis. J Immunol Methods. 1990 May 25;129(2):207–210. doi: 10.1016/0022-1759(90)90440-7. [DOI] [PubMed] [Google Scholar]
- Chambers J. C., Kenan D., Martin B. J., Keene J. D. Genomic structure and amino acid sequence domains of the human La autoantigen. J Biol Chem. 1988 Dec 5;263(34):18043–18051. [PubMed] [Google Scholar]
- Chan E. K., Hamel J. C., Buyon J. P., Tan E. M. Molecular definition and sequence motifs of the 52-kD component of human SS-A/Ro autoantigen. J Clin Invest. 1991 Jan;87(1):68–76. doi: 10.1172/JCI115003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. K., Sullivan K. F., Tan E. M. Ribonucleoprotein SS-B/La belongs to a protein family with consensus sequences for RNA-binding. Nucleic Acids Res. 1989 Mar 25;17(6):2233–2244. doi: 10.1093/nar/17.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher S. L., Harley J. B., Keene J. D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9479–9483. doi: 10.1073/pnas.85.24.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989 Mar;8(3):851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. The RNA binding protein La influences both the accuracy and the efficiency of RNA polymerase III transcription in vitro. EMBO J. 1989 Mar;8(3):841–850. doi: 10.1002/j.1460-2075.1989.tb03445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O., Billings P. B. Characterization of the La (SS-B) antigen from several mammalian sources. J Immunol. 1984 Sep;133(3):1397–1403. [PubMed] [Google Scholar]
- Itoh K., Itoh Y., Frank M. B. Protein heterogeneity in the human Ro/SSA ribonucleoproteins. The 52- and 60-kD Ro/SSA autoantigens are encoded by separate genes. J Clin Invest. 1991 Jan;87(1):177–186. doi: 10.1172/JCI114968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Nucleotide sequence of 4.5S RNA (C8 or hY5) from HeLa cells. Biochem Biophys Res Commun. 1982 Sep 16;108(1):363–370. doi: 10.1016/0006-291x(82)91875-7. [DOI] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Eukaryotic small ribonucleoproteins. Anti-La human autoantibodies react with U1 RNA-protein complexes. J Biol Chem. 1984 Feb 10;259(3):1929–1933. [PubMed] [Google Scholar]
- Mamula M. J., O'Brien C. A., Harley J. B., Hardin J. A. The Ro ribonucleoprotein particle: induction of autoantibodies and the detection of Ro RNAs among species. Clin Immunol Immunopathol. 1989 Sep;52(3):435–446. doi: 10.1016/0090-1229(89)90158-x. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Silverman E. D., Laxer R. M., Bentur L., Isacovics B., Hardin J. A. Human monoclonal anti-La antibodies. The La protein resides on a subset of Ro particles. J Immunol. 1989 Nov 1;143(9):2923–2928. [PubMed] [Google Scholar]
- Mathews M. B., Francoeur A. M. La antigen recognizes and binds to the 3'-oligouridylate tail of a small RNA. Mol Cell Biol. 1984 Jun;4(6):1134–1140. doi: 10.1128/mcb.4.6.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau G., Lin N., Stutz F., Clarkson S. Functional analyses of the Xenopus La protein. Mol Biol Rep. 1990;14(2-3):51–51. doi: 10.1007/BF00360412. [DOI] [PubMed] [Google Scholar]
- Nagai K., Oubridge C., Jessen T. H., Li J., Evans P. R. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature. 1990 Dec 6;348(6301):515–520. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- O'Brien C. A., Harley J. B. A subset of hY RNAs is associated with erythrocyte Ro ribonucleoproteins. EMBO J. 1990 Nov;9(11):3683–3689. doi: 10.1002/j.1460-2075.1990.tb07580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., Slobbe R. L., Van Venrooij W. J. Structure and function of La and Ro RNPs. Mol Biol Rep. 1990;14(2-3):43–48. doi: 10.1007/BF00360410. [DOI] [PubMed] [Google Scholar]
- Query C. C., Bentley R. C., Keene J. D. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell. 1989 Apr 7;57(1):89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Rader M. D., O'Brien C., Liu Y. S., Harley J. B., Reichlin M. Heterogeneity of the Ro/SSA antigen. Different molecular forms in lymphocytes and red blood cells. J Clin Invest. 1989 Apr;83(4):1293–1298. doi: 10.1172/JCI114014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Delling U., Chen C. H., Rosen C. A., Sonenberg N. A bulge structure in HIV-1 TAR RNA is required for Tat binding and Tat-mediated trans-activation. Genes Dev. 1990 Aug;4(8):1365–1373. doi: 10.1101/gad.4.8.1365. [DOI] [PubMed] [Google Scholar]
- Sachs A. B., Davis R. W., Kornberg R. D. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol Cell Biol. 1987 Sep;7(9):3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D., Boelens W., Dathan N. A., van Venrooij W. J., Mattaj I. W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B'' and their cognate RNAs. Nature. 1990 Jun 7;345(6275):502–506. doi: 10.1038/345502a0. [DOI] [PubMed] [Google Scholar]
- Scherly D., Boelens W., van Venrooij W. J., Dathan N. A., Hamm J., Mattaj I. W. Identification of the RNA binding segment of human U1 A protein and definition of its binding site on U1 snRNA. EMBO J. 1989 Dec 20;8(13):4163–4170. doi: 10.1002/j.1460-2075.1989.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Turner D. H., Sugimoto N., Freier S. M. RNA structure prediction. Annu Rev Biophys Biophys Chem. 1988;17:167–192. doi: 10.1146/annurev.bb.17.060188.001123. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. Genes for two small cytoplasmic Ro RNAs are adjacent and appear to be single-copy in the human genome. Cell. 1983 Mar;32(3):735–744. doi: 10.1016/0092-8674(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. N., Uhlenbeck O. C. Role of a bulged A residue in a specific RNA-protein interaction. Biochemistry. 1987 Dec 15;26(25):8221–8227. doi: 10.1021/bi00399a030. [DOI] [PubMed] [Google Scholar]