Abstract
Uromodulin, also known as Tamm-Horsfall protein, is a glycoprotein expressed exclusively by renal tubular cells lining the thick ascending limb of the loop of Henle. Although the physiologic functions of this protein remain elusive, significant progress has been made over the last decade that highlights the importance of uromodulin in the pathophysiology of various diseases, such as medullary cystic kidney disease, urinary tract infections, and nephrolithiasis. Meanwhile, there is a renewed interest in the role of uromodulin in kidney injury, both acute and chronic. In this article, we review the existing evidence that supports a role for uromodulin in acute kidney injury (AKI), chronic kidney disease (CKD), and renal inflammation. Contrary to the conventional view of uromodulin as an instigator in kidney injury, new data from uromodulin knockout mice reveal a protective role for this protein in AKI, possibly through down-regulating interstitial inflammation. In CKD, uromodulin excretion, when adjusted for kidney function, is increased; the significance of this remains unclear. Although it has been suggested that uromodulin exacerbates progressive kidney injury, we propose that the elevation in uromodulin secretion is instead reactive to injury, and reflects an increase of uromodulin in the renal parenchyma where it slows the injury process.
Index words: Inflammation, urinary casts, biomarker, tubular cross-talk, UMOD
Background
Tamm-Horsfall protein was discovered by Igor Tamm and Frank Horsfall in 1950 when they precipitated a protein from the urine that inhibited hemagglutination of viruses 1–2. This glycoprotein is exclusively expressed in the kidney, specifically by cells lining the thick ascending limb of the nephron 3–4. In 1985, Muchmore and Decker isolated a protein named uromodulin from the urine of pregnant women that had immunosuppressive effects on T cells in vitro 5. Shortly thereafter, Pennica et al demonstrated by amino acid sequencing that the uromodulin and Tamm-Horsfall protein are in fact identical 6. Recent discoveries have underscored the importance of uromodulin (encoded by the UMOD gene) as a regulatory protein in health 7–8 and in various conditions, such as medullary cystic kidney disease 9, glomerulocystic kidney disease 10, urinary tract infections 11–12, nephrolithiasis 13, and acute kidney injury 14–15. More recently, polymorphisms in the UMOD gene have been strongly linked to chronic kidney disease 16, further raising the interest in the role of this protein in progressive kidney injury 17. There are previous comprehensive reviews that discussed broadly the biology and role of uromodulin in various diseases 18–21. In the present review, we focus on the potential role of uromodulin in kidney injury, both acute and chronic, in light of more recent in vivo work based on uromodulin knockout and transgenic mice. We will also discuss how the measurement of urinary uromodulin can be optimized for use as a biomarker for kidney disease.
Case Vignette
Over the course of 13 years, a 52 year white man with type 2 diabetes mellitus, hypertension, atrial fibrillation and hyperlipidemia underwent serial measurements of his kidney function and urine albumin levels, as well as two 24 hour urine collections (Table 1). At the beginning of the follow up period, he had normal kidney function and normoalbuminuria. Urine collection at that time showed 105.2 mg of protein excreted per 24 hours. The patient was maintained throughout this period on multiple medications, including an angiotensin-converting enzyme inhibitor, a diuretic, and a beta-blocker. His diabetes management was challenging, requiring combination therapy with insulin, metformin, and glipizide. His Hemoglobin A1C trend is also shown in table 1. In the last five years, he developed progressive microalbuminuria, which stabilized at an albumin-creatinine ratio of 48.7 mg/g during year 13. A repeat 24 hour-urine collection 11 years after the initial collection showed 238.0 mg protein excreted. His kidney function remained preserved.
Table 1.
Laboratory values of the case vignette
| Year | SCr (mg/dl) | eGFR (ml/min/1.73m2)* | HbA1c (%) | ACR (mg/g) | 24-h Protein Excretion (mg) |
|---|---|---|---|---|---|
| 1 | 0.9 | 93.5 | - | - | 105.2 |
| 2 | 0.9 | 93.1 | 9.9 | 0 | - |
| 3 | 0.9 | 92.8 | 8.8 | 9.8 | - |
| 4 | 1.0 | 81.9 | 9.7 | - | - |
| 5 | 0.9 | 92.1 | 10.8 | 15.8 | - |
| 6 | 0.9 | 91.8 | 10.7 | 16.0 | - |
| 7 | 0.9 | 91.5 | 9.2 | 15.4 | - |
| 8 | 0.9 | 91.2 | 10.3 | 24.0 | - |
| 9 | 0.9 | 90.9 | 12.0 | 29.9 | - |
| 10 | 0.9 | 90.6 | 10.8 | 43.0 | - |
| 11 | 0.9 | 90.3 | 9.6 | 96.1 | - |
| 12 | 0.8 | 103.1 | 8.7 | 69.9 | 238.0 |
| 13 | 0.9 | 89.7 | 8.6 | 48.7 | - |
Note: Conversion factors for units: SCr in mg/dl to μmol/L, x88.4; eGFR in mL/min/1.73m2 to mL/s/1.73m2, x0.01667.
Abbreviations: ACR, albumin-creatinine ratio; SCr, Serum Creatinine; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c.
eGFR calculated using the 4-variable MDRD [Modification of Diet in Renal Disease] Study equation.
Although uromodulin was not measured in this patient, this protein accounts for the majority of the urinary protein in healthy individuals. Therefore, we expect that the first 24 hour urine collection consisted predominantly of uromodulin. Can the baseline levels of uromodulin predict the susceptibility of patients to acute or chronic kidney injury? As we discuss below, diabetic nephropathy at an early stage can increase the urinary excretion of uromodulin. This may be reflected in the subsequent increase in the 24 hour urinary excretion of protein, which may not be solely accounted for by the development of microalbuminuria. What is the significance of increased uromodulin on the pathogenesis of kidney disease? Does it predict deterioration of kidney function?
Pathogenesis
Uromodulin synthesis and secretion
Uromodulin is an 80–90 kDa protein 5–6, 18, 22 expressed solely in the thick ascending limb (TAL), 3–4 with no production in the macula densa cells 23. It contains several epidermal growth factor-like domains and a zona pellucida domain, and is heavily glycosylated (30% of molecular weight) 18, 22. Within the TAL, uromodulin is predominantly apically targeted, a process facilitated by the addition of a glycosylphosphatidylinositol (GPI) anchor, an apical targeting signal that is acquired in the endoplasmic reticulum 24–25. Protease cleavage releases uromodulin from the GPI anchor to be secreted in the urine 26. Interestingly, as has been demonstrated in independent studies, there is also a lesser yet significant basolateral release of uromodulin 3, 27. For example, using immuno-electron microscopy of the rat kidney, Bachmann et al showed that the ratio of apical to basolateral uromodulin was about 2:1 3. In addition, uromodulin is detected in the serum of healthy individuals at a concentration of 30–540 ng/ml 28–30. The significance and mechanism of this basolateral release are not entirely understood. Additionally, little is known about uromodulin expression and release at the ultra-structural level in stressed conditions. More recent evidence suggests that basolateral release, as opposed to apical secretion, may even be significantly increased during kidney injury 14.
The urinary uromodulin excreted in 24 hours by humans ranges from 9 to 66 mg in most reports 29–33, although several other studies reported as much as 70 mg to 113 mg of uromodulin in 24-hour urine 28, 34. While some degree of variability is to be expected due to the differences in nephron mass between participants, an additional reason for the discrepancies is the use of different methodologies in assaying and reporting urinary uromodulin and the small sample size within each study. Although the rate of uromodulin excretion may not be related to kidney function in healthy individuals 33, the relationship of uromodulin excretion to kidney function becomes more apparent in established kidney diseases. Unfortunately, the lack of large-scale studies that define a standardized normal rate of excretion of uromodulin is a major hindrance to correlating its urinary level to kidney health and disease.
Factors that control uromodulin expression, secretion, and excretion have not been well explored. It is known that the transcription factor hepatic nuclear factor 1 β beta (HNF1B) positively regulates the expression of uromodulin 35. HNF1B binds to the promoter elements of the UMOD gene, and inactivation of HNF1B in vivo is associated with decreased UMOD transcription 35. Interestingly, mutations in HNF1B are associated with features of familial juvenile hyperuricemic nephropathy 36, possibly through a uromodulin link. Mice deficient in COX-2 (prostaglandin-endoperoxide synthase 2) also had profoundly lower uromodulin than wild-type counterpart 37, but it is unclear whether the effect of COX-2 deletion is specific to uromodulin or if it affects the TAL globally. Limited studies in humans and animals have described experimental manipulations or pharmacologic agents that affect uromodulin expression or secretion. Some studies suggested that uromodulin excretion depends on urinary volume 38, although this could not be confirmed by others 39. Factors that could increase uromodulin expression or excretion are dietary salt alone or combined with furosemide 40–41 and high protein diet 39. Factors that could decrease uromodulin expression or excretion are angiotensin-converting enzyme inhibitors 42, possibly colchicine 43–44 and urinary tract obstruction 45. It is important to note that the UMOD mutations causing medulary cystic kidney disease/familial juvenile hyperuricemic nephropathy are associated with a significantly decreased secretion of normal uromodulin 46, which is explained by the fact that these mutations hinder the trafficking of uromodulin to the apical membrane of the TAL 27, 47–48.
Uromodulin and kidney injury: a historical perspective
Initial studies localized uromodulin to renal casts seen in acute kidney injury 49, and subsequently in almost all urinary casts 50–51. This led to the notion that the aggregating properties of uromodulin promote cast formation and obstruction in AKI 18, 52–53. After localization of uromodulin to the thick ascending limb 3–4, predominantly in an apical membrane domain, the findings of “abnormal deposits” of uromodulin in the interstitium during tubulo-interstitial injury suggested a pathologic role of this protein in interstitial inflammation 49, 54–56. The potential deleterious effect of uromodulin was further supported by the fact that intravenous administration of uromodulin in rodents was highly immunogenic 57. Therefore, it was speculated that interstitial uromodulin deposits could potentially cause an immunologic response that promulgates interstitial inflammation. However, these conclusions did not consider the possibility that the presence of uromodulin in a damaged area may be coincidental or reactive. In fact, more conclusive evidence was recently provided with the use of uromodulin knockout mice indicating that this protein in effect may be protective in acute kidney injury 15. However, a cardinal unresolved issue which perpetuates the confusion surrounding the role of uromodulin in kidney injury is the relationship of uromodulin to inflammation.
Uromodulin and inflammation
When uromodulin was rediscovered by Muchmore and Decker, it was described as having immunosuppressive effects on T cells in vitro 5. Uromodulin was also found to bind renal cytokines and lymphokines (interleukin 1 and tumor necrosis factor α) 58, thereby inhibiting inflammation. However, a number of subsequent studies suggested a pro-inflammatory role of uromodulin, specifically in activating neutrophils 59–61, monocytes 62–63 and dendritic cells 64. More recently, Saemann and colleagues demonstrated that uromodulin activates myeloid dendritic cells via Toll-like receptor 4 (TLR4), triggering them to reach a fully mature phenotype 64. Bone marrow-derived macrophages are also activated by uromodulin. Even though intravenous administration of uromodulin is highly immunogenic and produces cytokines 57, 64, several clinical and experimental observations indicate that uromodulin deposits in the kidney do not have a crucial role in the inflammatory process. For example, there was no correlation between uromodulin accumulation in the interstitium and tubular damage on kidney biopsies 65. In addition, an absent or low inflammatory response to extratubular uromodulin deposits has also been reported in kidney allografts with interstitial disease 66. Although in vitro experiments suggest that uromodulin antibody formation may be crucial for engaging an inflammatory response 67, these antibodies do not necessarily form in response to extratubular uromodulin in vivo 68. In fact, it remains unclear whether the uromodulin found in the interstitium is derived from urine extravasation or from basolateral tubular secretion. We recently showed that ischemic injury shifts uromodulin from the apical towards the basolateral domain 14, suggesting that interstitial uromodulin emanates from increased basolateral release. This was associated with down-regulation of inflammatory signaling in contiguous proximal tubules 14.
How can we then reconcile the pro-inflammatory properties of uromodulin on myeloid cells in vitro with the anti-inflammatory properties reported in epithelial cells during kidney injury in vivo? We believe there are two different scenarios. First, the opposing effects of uromodulin on different cell types (epithelial vs. myeloid) may cooperatively be geared towards preventing unchecked and persistent inflammation after AKI. For instance, by stimulating renal dendritic cells 64, 69, uromodulin may promote a prompt inflammatory response to clean up cellular debris immediately after the onset of kidney injury 70. Simultaneously, uromodulin may also down-regulate inflammatory signaling in epithelial cells 14. Both effects could serve to minimize the inflammatory burst after injury and/or signal the beginning of repair. The second scenario could be that basolaterally released uromodulin may not have the same pro-inflammatory properties on immune cells as the urinary uromodulin. For example, basolateral uromodulin may not undergo the same type and/or extent of glycosylation as compared to apical uromodulin 71, which could affect the glycoprotein’s function. This possibility is consistent with the finding that the pro-inflammatory effect of uromodulin on neutrophils may be mediated by a single class of carbohydrate-specific receptors 72, although other receptors for uromodulin on neutrophils could also exist 73. Furthermore, Pfistershammer et al recently demonstrated the presence of additional receptors for uromodulin (scavenger receptors, not typically carbohydrate specific) that do not mediate any pro-inflammatory signaling 74. We hypothesize that alteration of uromodulin glycosylation by basolateral release limits the interaction of uromodulin to specific receptors present on renal epithelial cells, and these receptors mediate a predominant anti-inflammatory effect.
Table 2 summarizes the possible outcome of various interactions of uromodulin with various cell types within the kidney or urinary tract.
Table 2.
Possible outcome of interactions of uromodulin with cell types within the kidney or urinary tract
| Cell Type | Possible Site(s) of Interaction | Outcome | References |
|---|---|---|---|
| Epithelium | Epithelial basolateral surface | Anti-inflammatory | 14–15 |
| T Lymphocyte | Urinary space | Anti-inflammatory | 5 |
| Neutrophil | Urinary space; interstitium | Pro-inflammatory | 59–61 |
| Macrophage | Urinary space; interstitium | Pro-inflammatory | 62–64 |
| Dendritic Cell | Interstitium | Differentiation; migration to lymph node |
64 69 |
Recent Advances
Uromodulin and cast formation in ischemic AKI
Uromodulin has been implicated in the pathogenesis of cast formation during ischemic AKI, but the data supporting this premise is either circumstantial 75 (casts staining positive for uromodulin) or based on in vitro or ex vivo aggregating properties of the glycoprotein 49, 53. More conclusive evidence emerged recently in an ischemia reperfusion model of AKI in uromodulin knockout mice 15. Cast formation was not only unaffected by the absence of uromodulin, but in fact was markedly increased in uromodulin knockout mice, correlating with more severe injury 15. Therefore, it appears that uromodulin is not essential for tubular cast formation in AKI. Nevertheless, this conclusion does not apply to myeloma kidney, for which Sanders and colleagues have elegantly shown that uromodulin binds specifically to Bence Jones proteins to form myeloma casts 44, 76.
Uromodulin expression and secretion after AKI
Only few published studies have investigated the change of uromodulin expression during AKI. Safirstein and colleagues described a decrease in uromodulin messenger RNA in rats after ischemia-reperfusion injury produced by 50 minutes of clamping 77. Using 30 minutes’ clamping, Yoshida et al also observed a decrease in uromodulin gene expression using microarray analysis 78. To our knowledge, there are no conclusive data on the changes in uromodulin protein expression in experimental AKI 15. We recently showed that ischemia partially shifted uromodulin from the default apical pathway to the basolateral side 14. This could theoretically influence its pattern of expression and excretion in AKI.
Several studies have examined uromodulin excretion during clinical AKI in the setting of cardiac surgery 79, intensive care unit 80 and kidney transplant 81. Although these are small studies, they suggest that the excretion of this protein is decreased during AKI. Further proteomic studies are needed to determine whether a decrease in urinary uromodulin would be a suitable biomarker for AKI 82. Could the baseline rate of urinary uromodulin excretion be predictive of susceptibility to AKI? A study by Romero et al suggested that a higher uromodulin level in patients receiving a liver transplant was associated with a lower risk of developing AKI 34. A high excretion rate in patients without pre-existing kidney disease could be suggestive of a larger nephron mass, thereby more renal reserve. Alternatively, if uromodulin does have anti-inflammatory protective properties in kidney injury 14–15, a higher urinary level could be protective.
Role of uromodulin in AKI: a case for a protective tubular cross-talk
Renal ischemia-reperfusion in uromodulin knockout mice is significantly worse than in wild type animals. This is associated with increased inflammatory response and increased neutrophil infiltration in the outer-medulla 15. The phenotype of injury is predominantly that of tubular necrosis affecting the S3 segments but not the uromodulin-deficient TAL 14–15. The proximal S3 segments have an increased expression of the pro-inflammatory protein TLR4 and a neutrophil chemoattractant, the chemokine CXCL2. Neutralization of CXCL2 is protective, and partially rescues the S3 segments, suggesting that a TLR4-CXCL2 pro-inflammatory pathway may be important in the pathophysiology of injury in the uromodulin knockout mouse 14.
That uromodulin produced in TAL affects the susceptibility of neighboring proximal tubules to injury strongly suggests a uromodulin-dependent TAL-S3 tubular crosstalk, a phenomenon previously proposed by Safirstein 77, 83. A shift of uromodulin from the apical to the basolateral surface and the presence of this protein in the interstitium during AKI could suggest that uromodulin itself is the principal mediator of such crosstalk. The presence and up-regulation of a putative uromodulin receptor on proximal tubule also supports this notion 14. However, we cannot rule out a more complex mechanism that involves other intermediary molecules from TAL or uromodulin interactions with other cells in the interstitium. Figure 1 is a conceptual model that summarizes potential mechanisms for this uromodulin-dependent TAL-S3 protective tubular cross-talk during AKI. We also cannot rule out that a pre-existing TAL tubular dysfunction in uromodulin knockout mice may have a contributory role in the increased susceptibility to AKI by affecting auto-regulatory mechanisms within the nephron such as tubulo-glomerular feedback. For example, in a different model of uromodulin knockout mice, Bachmann et al showed increased expression of distal nephron transporters and deceased expression of important juxtaglomerular effectors such as COX-2 and renin 7. However, these mice did not have a difference in any solute excretion compared to wild type 7. More recently, Mutig et al also showed that uromodulin could play a permissive role for the proper functioning sodium/potassium/chloride (Na+/K+/2Cl−) cotransporter 84. Indeed, further characterization of the physiological phenotype of uromodulin knockout mice will clarify the importance of these factors during AKI.
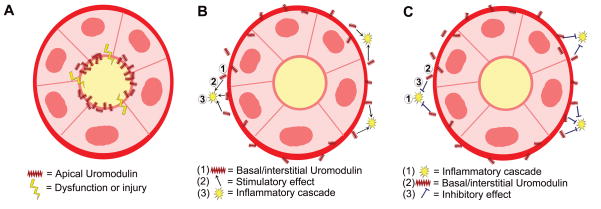
Figure 1. Conceptual model of the uromodulin-dependent protective cross-talk in Acute Kidney Injury (AKI).
This figure represents a cross-section of the outer medulla, where thick ascending limb (TAL), and S3 segments of the proximal tubules are contiguous. During AKI, S3 segments are predominantly injured, and express several pro-inflammatory factors. Path 1 illustrates a direct role of basolateral released uromodulin in down-regulating inflammatory signaling in neighboring S3 segments. Path 2 shows the possibility of a secondary paracrine mediator, released by the TAL in a uromodulin-dependent process with the net effect of protecting neighboring tubule. Path 3 shows a possible “hybrid” mechanism, whereby the protective effect of uromodulin depends on its interaction with an interstitial cell (IC), which in turn regulates or inhibits inflammatory signaling in S3 segments.
The translational relevance of the protective role of uromodulin in AKI remains to be seen. Further investigations are needed to determine whether stimulating uromodulin expression in clinical AKI is protective and to clarify the role of measuring urinary uromodulin in that context.
Uromodulin expression and excretion in chronic kidney disease
The rationale for measuring urinary uromodulin would be to either provide a prognostic tool or a biomarker of disease. There are a multitude of small studies in humans that have measured the rate of urinary uromodulin excretion in chronic disease states (comprehensively reviewed in20). Interpreting these studies is limited by small sample size. Uromodulin excretion could be reflective of at least two factors: the functioning nephron mass present and the regulation of expression imparted by injury 33. Indeed, uromodulin excretion does decline with reduced GFR 30, 33. However, to better correlate excretion with biologic function, uromodulin excretion should be adjusted to the remaining functioning nephron mass by normalizing the 24-hour uromodulin excretion to glomerular filtration rate, similar to what was shown by Thornely et al 33. These investigators demonstrated that the adjusted 24-hour uromodulin excretion (reported as μg/ml of creatinine clearance) had a higher range in patients with CKD 33. Furthermore, they showed in kidney biopsies that intact tubules in patients with CKD are associated with a significantly higher excretion rate. Therefore, the absolute uromodulin excretion declines with the reduction in total nephron mass seen with CKD, but the amount of uromodulin secreted by each single functioning nephron unit is increased. Consequently, in patients with early CKD and a preserved GFR, we expect uromodulin excretion per 24 hours to be increased. Indeed, this has been clearly demonstrated in early diabetes without GFR impairment as discussed below. This could also explain the findings by Kottgen et al that increased levels of uromodulin precede the onset of CKD 85. We advocate the use of 24 hour uromodulin excretion normalized to GFR as the best index for measurement. Potential uses and interpretation of this “uromodulin index” are outlined in figure 2.
Figure 2. Measurement of urinary uromodulin in the form of an index.
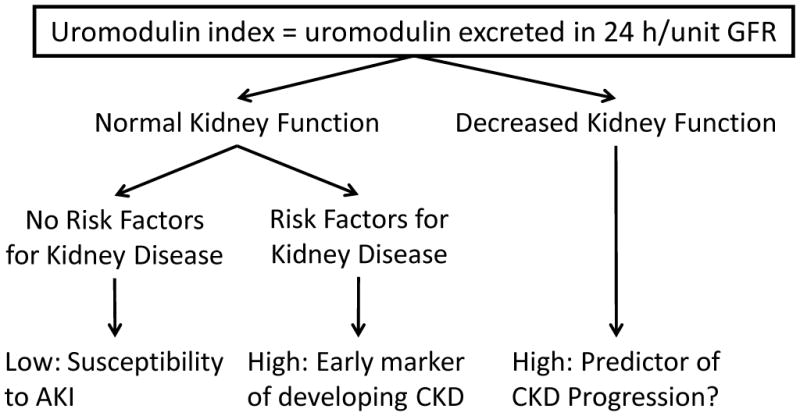
The interpretation of this index depends on the clinical context: no kidney disease, at risk for kidney disease, and established disease. In patients with normal kidney function and no risk factors for kidney disease, a low index could suggest susceptibility to acute kidney injury (AKI) (reference 34). In patients with established risk factors for chronic kidney disease (CKD), such as diabetes mellitus, a high index could be reactive to subtle injury, and thereby predict the development of CKD (reference 85). In patients with established CKD, a high index could signal ongoing injury and further progression. However, in very advanced disease, a low index could imply depletion of tubular reserves and progression to end-stage. Abbreviations: GFR, glomerular filtration rate.
Uromodulin in diabetic kidney disease
The relationship of uromodulin with CKD is probably best studied in diabetic nephropathy. Experiments in rats show that uromodulin excretion is persistently increased after inducing diabetes using streptozotocin 38, 86, and this occurs independently of renal mass 38. Uromodulin excretion in patients with diabetes mirrors these experimental findings. Patients with diabetes and early kidney disease but without a significantly reduced GFR have an increase in 24 hour uromodulin excretion 87–89. This could correlate with the hyper-filtration stage of diabetes 88. However, the effect of urine flow on uromodulin excretion rate remains unclear 38–39, 90 and uromodulin excretion frequently does not correlate with microalbuminuria 87, 89, suggesting an independent effect of diabetes on distal tubular function. With more advanced disease, 24 hour excretion decreases 91–92, which could be a reflection of a decrease in functioning renal mass. Baseline uromodulin measurements could potentially be useful in predicting worse future outcomes 93. However, successive longitudinal measurements which could be more informative have yet to be performed.
Uromodulin function in chronic kidney disease
The recent association of uromodulin variants with chronic kidney disease 16, 85, 94, in addition to the link of UMOD mutations with familial juvenile hyperuricemic nephropathy 9, revitalized the field of uromodulin research 17, but a causative link between uromodulin and CKD is still missing. Uromodulin production per functioning nephron unit increases in chronic kidney disease 33. It appears that this increase in urinary excretion is also associated with an increase in basolateral release of uromodulin evidenced by an increase in the level of serum uromodulin in CKD, as previously shown by Thornelly 33 and more recently by Prajczer et al 95. Interestingly, when serum uromodulin does not increase, it is associated with a worse GFR and a higher tubular atrophy score 95. The significance of increased basolateral/interstitial and serum uromodulin is unclear. Although the increased uromodulin in the interstitium could stimulate a pro-inflammatory response and aggravate injury 95–96, we propose that this increase is in effect reactive and counter-inflammatory14–15. Figure 3 depicts the various proposed pathways whereby uromodulin exerts a modulatory role in chronic kidney disease.
Figure 3. Possible mechanisms whereby uromodulin can modulate chronic injury.
(A) a possible role of apical uromodulin in maintaining the integrity of the thick ascending limb (TAL) by forming a protective layer at the apex of TAL. Uromodulin may also regulate the function of some ion channels and transporters (references 7–8, 84). If injury affects the normal sorting or function of uromodulin at the apical membrane, a breach of TAL integrity and significant electrolyte dysregulation can occur. Panel B illustrates a pro-inflammatory role of uromodulin. Chronic injury can lead to increased basolateral/interstitial uromodulin (1). In this location, uromodulin can stimulate inflammatory signaling (2) through an immunologic response, thereby aggravating injury (3) and promoting subsequent fibrosis. Panel C shows the possibility that uromodulin has a protective role in chronic injury. A persistent inflammatory response during chronic injury (1) can stimulate the release of basolateral/interstitial uromodulin (2). This reactive increase of uromodulin in the interstitium serves to counter the inflammatory response and limit its progression (3). Supportive data for this model are derived from acute injury experiments on uromodulin knockout mice.
The association between single-nucleotide polyporphism (SNP) common variants in the UMOD gene and the risk of CKD using genome-wide association studies (GWAS) has generated significant enthusiasm. Two SNPs (the T allele of reference SNP identification number 12917707 [rs12917707] and the C allele of rs4293393) within the promoter region of the UMOD gene are associated with a decreased risk of CKD and a lower level of urinary uromodulin excretion 16, 85, 97. Do these associations give insight into the role of uromodulin in the pathogenesis of CKD? One explanation could be that if uromodulin has a pathogenic role in CKD, a lower urinary level associated with these variants may be protective. However, a major problem underlying this interpretation is that UMOD SNPs account for an extremely small portion of the variance in kidney function 16, arguing against a major pathogenic role of the above described loci. Unfortunately, this is a common pitfall of GWAS 98, and a few SNPs may unevenly associate with undiscovered rare variants, structural mutations, or other interactions that could be more directly linked to disease pathogenesis 98–99. Therefore, an alternative explanation would be that the “protective” UMOD promoter variants may be associated with an undiscovered interaction (affecting uromodulin or its regulators) that is responsible for the phenotypic “resistance to CKD”. Consequently, uromodulin excretion may be reactive to such phenotype, rather than related directly to the SNP variant itself. More detailed identification of UMOD mutations could help bridge the gap in knowledge between SNP genotype and disease phenotype.
Can we learn about uromodulin function in CKD from the mutations that have been linked to familial juvenile hyperuricemic nephropathy/medulary cystic kidney disease? Indeed, these mutations of uromodulin are associated with progressive kidney disease, and share common features such as accumulation of misfolded uromodulin in the endoplasmic reticulum, and decrease in secretion of the normal protein 10, 46–48. However, a cardinal question that remains unanswered is whether the described progressive injury is related to a gain of abnormal function of the mutant protein, to a loss of function of the normal protein, to the stress in the endoplasmic reticulum from the accumulation of the misfolded mutant protein, or a combination of these factors. Also, is the modulatory effect of uromodulin mediated by its activity on the apical side or on the de novo basolateral-interstitial pathway? Studies of chronic injury models in the uromodulin knockout mice will clarify whether a uromodulin loss of function is a key factor in the progression of kidney disease. Further characterization of mice transgenic for uromodulin mutants 48 will also be crucial to understanding the functions of this protein in chronic kidney disease.
Summary
In returning to the case vignette, this patient has several risk factors for kidney disease. If we assume that uromodulin is the predominant protein excreted in his urine, then his uromodulin index would have increased from 1.1 mg/unit eGFR (105.2 mg/93.5 ml/min/1.73m2) in year 1 to 2.3mg/unit eGFR (238.0 mg/103.1ml/min/1.73m2) in year 12, suggesting a high likelihood of kidney disease progression. This patient should undergo intensive therapy to control his CKD risk factors. This case illustrates how the future incorporation of uromodulin measurements in the care of patients with CKD could potentially influence management and help in providing a better prognosis.
In conclusion, a renewed interest in the understanding of the role of uromodulin in kidney injury has been witnessed over the last few years. This has been enhanced by the availability of knockout and transgenic mice, which provide invaluable tools for in vivo functional studies. It appears that uromodulin has a protective role in acute kidney injury, but the link between uromodulin and chronic kidney disease is still not evident. Indeed, further studies are needed to clarify the role of uromodulin in kidney injury and inflammation. In addition, this review underscores the need for large-scale human studies to validate the use of urinary uromodulin as a tool to investigate prognosis and management options of kidney disease.
Acknowledgments
The authors acknowledge Dr Pierre Dagher from Indiana University for critical review of the manuscript.
Support: Dr El-Achkar was supported by a Carl Gottschalk award from the American Society of Nephrology and by a Veterans Affairs Merit Review award. Dr Wu was supported by grants from the National Institute of Health (DK56903) and Veterans Affairs Merit Review award
Footnotes
Financial Disclosures: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tamm I, Horsfall FL., Jr Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med. 1950 May;74(1):106–108. [PubMed] [Google Scholar]
- 2.Tamm I, Horsfall FL., Jr A mucoprotein derived from human urine which reacts with influenza, mumps, and Newcastle disease viruses. J Exp Med. 1952 Jan;95(1):71–97. doi: 10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann S, Koeppen-Hagemann I, Kriz W. Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry. 1985;83(6):531–538. doi: 10.1007/BF00492456. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer JR, Sisson SP, Vernier RL. Tamm-Horsfall glycoprotein: ultrastructural immunoperoxidase localization in rat kidney. Lab Invest. 1979 Aug;41(2):168–173. [PubMed] [Google Scholar]
- 5.Muchmore AV, Decker JM. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science. 1985 Aug 2;229(4712):479–481. doi: 10.1126/science.2409603. [DOI] [PubMed] [Google Scholar]
- 6.Pennica D, Kohr WJ, Kuang WJ, et al. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science. 1987 Apr 3;236(4797):83–88. doi: 10.1126/science.3453112. [DOI] [PubMed] [Google Scholar]
- 7.Bachmann S, Mutig K, Bates J, et al. Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol. 2005 Mar;288(3):F559–567. doi: 10.1152/ajprenal.00143.2004. [DOI] [PubMed] [Google Scholar]
- 8.Renigunta A, Renigunta V, Saritas T, Decher N, Mutig K, Waldegger S. Tamm-Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function. J Biol Chem. 2011 Jan 21;286(3):2224–2235. doi: 10.1074/jbc.M110.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart TC, Gorry MC, Hart PS, et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002 Dec;39(12):882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rampoldi L, Caridi G, Santon D, et al. Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Hum Mol Genet. 2003 Dec 15;12(24):3369–3384. doi: 10.1093/hmg/ddg353. [DOI] [PubMed] [Google Scholar]
- 11.Bates JM, Raffi HM, Prasadan K, et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 2004 Mar;65(3):791–797. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 12.Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. 2004 Sep;66(3):1159–1166. doi: 10.1111/j.1523-1755.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 13.Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol. 2004 Apr;286(4):F795–802. doi: 10.1152/ajprenal.00357.2003. [DOI] [PubMed] [Google Scholar]
- 14.El-Achkar TM, McCracken R, Rauchman M, et al. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am J Physiol Renal Physiol. 2011 Apr;300(4):F999–F1007. doi: 10.1152/ajprenal.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol. 2008 Aug;295(2):F534–544. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kottgen A, Glazer NL, Dehghan A, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009 May 10;41(6):712–717. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedor JR. Uromodulin and translational medicine: will the SNPs bring zip to clinical practice? J Am Soc Nephrol. 2010 Feb;21(2):204–206. doi: 10.1681/ASN.2009121283. [DOI] [PubMed] [Google Scholar]
- 18.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis. 2003 Oct;42(4):658–676. doi: 10.1016/s0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 19.Scolari F, Caridi G, Rampoldi L, et al. Uromodulin storage diseases: clinical aspects and mechanisms. Am J Kidney Dis. 2004 Dec;44(6):987–999. doi: 10.1053/j.ajkd.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Vyletal P, Bleyer AJ, Kmoch S. Uromodulin biology and pathophysiology - an update. Kidney Blood Press Res. 2010;33(6):456–475. doi: 10.1159/000321013. [DOI] [PubMed] [Google Scholar]
- 21.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011 Jun 8;80(4):338–347. doi: 10.1038/ki.2011.134. [DOI] [PubMed] [Google Scholar]
- 22.Serafini-Cessi F, Malagolini N, Hoops TC, Rindler MJ. Biosynthesis and oligosaccharide processing of human Tamm-Horsfall glycoprotein permanently expressed in HeLa cells. Biochem Biophys Res Commun. 1993 Jul 30;194(2):784–790. doi: 10.1006/bbrc.1993.1890. [DOI] [PubMed] [Google Scholar]
- 23.El-Achkar TM, Plotkin Z, Marcic B, Dagher PC. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol. 2007 Oct;293(4):F1187–1196. doi: 10.1152/ajprenal.00217.2007. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson MA, Williams AF. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- 25.Rindler MJ, Naik SS, Li N, Hoops TC, Peraldi MN. Uromodulin (Tamm-Horsfall glycoprotein/uromucoid) is a phosphatidylinositol-linked membrane protein. J Biol Chem. 1990 Dec 5;265(34):20784–20789. [PubMed] [Google Scholar]
- 26.Santambrogio S, Cattaneo A, Bernascone I, et al. Urinary uromodulin carries an intact ZP domain generated by a conserved C-terminal proteolytic cleavage. Biochem Biophys Res Commun. 2008 Jun 6;370(3):410–413. doi: 10.1016/j.bbrc.2008.03.099. [DOI] [PubMed] [Google Scholar]
- 27.Jennings P, Aydin S, Kotanko P, et al. Membrane targeting and secretion of mutant uromodulin in familial juvenile hyperuricemic nephropathy. J Am Soc Nephrol. 2007 Jan;18(1):264–273. doi: 10.1681/ASN.2006020158. [DOI] [PubMed] [Google Scholar]
- 28.Horton JK, Davies M, Woodhead JS, Weeks I. A new and rapid immunochemiluminometric assay for the measurement of Tamm-Horsfall glycoprotein. Clin Chim Acta. 1988 May 31;174(2):225–237. doi: 10.1016/0009-8981(88)90389-0. [DOI] [PubMed] [Google Scholar]
- 29.Dawnay AB, Thornley C, Cattell WR. An improved radioimmunoassay for urinary Tamm-Horsfall glycoprotein. Investigation and resolution of factors affecting its quantification. Biochem J. 1982 Sep 15;206(3):461–465. doi: 10.1042/bj2060461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynn KL, Marshall RD. Excretion of Tamm-Horsfall glycoprotein in renal disease. Clin Nephrol. 1984 Nov;22(5):253–257. [PubMed] [Google Scholar]
- 31.Bichler KH, Ideler V, Harzmann R. Uromucoid excretion in normal individuals and stone formers. Curr Probl Clin Biochem. 1979;(9):309–324. [PubMed] [Google Scholar]
- 32.Glauser A, Hochreiter W, Jaeger P, Hess B. Determinants of urinary excretion of Tamm-Horsfall protein in non-selected kidney stone formers and healthy subjects. Nephrol Dial Transplant. 2000 Oct;15(10):1580–1587. doi: 10.1093/ndt/15.10.1580. [DOI] [PubMed] [Google Scholar]
- 33.Thornley C, Dawnay A, Cattell WR. Human Tamm-Horsfall glycoprotein: urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci (Lond) 1985 May;68(5):529–535. doi: 10.1042/cs0680529. [DOI] [PubMed] [Google Scholar]
- 34.Romero MC, Zanaro N, Gonzalez L, Trigo P, Imventarza O, Nesse A. Tamm-Horsfall protein excretion to predict the onset of renal insufficiency. Clin Biochem. 2002 Feb;35(1):65–68. doi: 10.1016/s0009-9120(02)00274-6. [DOI] [PubMed] [Google Scholar]
- 35.Gresh L, Fischer E, Reimann A, et al. A transcriptional network in polycystic kidney disease. EMBO J. 2004 Apr 7;23(7):1657–1668. doi: 10.1038/sj.emboj.7600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bingham C, Ellard S, van’t Hoff WG, et al. Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1beta gene mutation. Kidney Int. 2003 May;63(5):1645–1651. doi: 10.1046/j.1523-1755.2003.00903.x. [DOI] [PubMed] [Google Scholar]
- 37.Dou W, Thompson-Jaeger S, Laulederkind SJ, et al. Defective expression of Tamm-Horsfall protein/uromodulin in COX-2-deficient mice increases their susceptibility to urinary tract infections. Am J Physiol Renal Physiol. 2005 Jul;289(1):F49–60. doi: 10.1152/ajprenal.00134.2004. [DOI] [PubMed] [Google Scholar]
- 38.Thulesen J, Jorgensen PE, Torffvit O, Nexo E, Poulsen SS. Urinary excretion of epidermal growth factor and Tamm-Horsfall protein in three rat models with increased renal excretion of urine. Regul Pept. 1997 Oct 31;72(2–3):179–186. doi: 10.1016/s0167-0115(97)01058-6. [DOI] [PubMed] [Google Scholar]
- 39.Bachmann S, Dawnay AB, Bouby N, Bankir L. Tamm-Horsfall protein excretion during chronic alterations in urinary concentration and protein intake in the rat. Ren Physiol Biochem. 1991 Nov-Dec;14(6):236–245. doi: 10.1159/000173411. [DOI] [PubMed] [Google Scholar]
- 40.Ying WZ, Sanders PW. Dietary salt regulates expression of Tamm-Horsfall glycoprotein in rats. Kidney Int. 1998 Oct;54(4):1150–1156. doi: 10.1046/j.1523-1755.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- 41.Torffvit O, Melander O, Hulten UL. Urinary excretion rate of Tamm-Horsfall protein is related to salt intake in humans. Nephron Physiol. 2004;97(1):p31–36. doi: 10.1159/000077600. [DOI] [PubMed] [Google Scholar]
- 42.Guidi E, Giglioni A, Cozzi MG, Minetti EE. Which urinary proteins are decreased after angiotensin converting--enzyme inhibition? Ren Fail. 1998 Mar;20(2):243–248. doi: 10.3109/08860229809045108. [DOI] [PubMed] [Google Scholar]
- 43.Cairns HS, Dawnay A, Woolfson RG, Unwin RJ. Evaluation of therapy for cast nephropathy: failure of colchicine to alter urinary Tamm Horsfall glycoprotein excretion in normal subjects. Exp Nephrol. 1994 Jul-Aug;2(4):257–258. [PubMed] [Google Scholar]
- 44.Sanders PW, Booker BB. Pathobiology of cast nephropathy from human Bence Jones proteins. J Clin Invest. 1992 Feb;89(2):630–639. doi: 10.1172/JCI115629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Storch S, Saggi S, Megyesi J, Price PM, Safirstein R. Ureteral obstruction decreases renal prepro-epidermal growth factor and Tamm-Horsfall expression. Kidney Int. 1992 Jul;42(1):89–94. doi: 10.1038/ki.1992.265. [DOI] [PubMed] [Google Scholar]
- 46.Bleyer AJ, Hart TC, Shihabi Z, Robins V, Hoyer JR. Mutations in the uromodulin gene decrease urinary excretion of Tamm-Horsfall protein. Kidney Int. 2004 Sep;66(3):974–977. doi: 10.1111/j.1523-1755.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 47.Choi SW, Ryu OH, Choi SJ, Song IS, Bleyer AJ, Hart TC. Mutant tamm-horsfall glycoprotein accumulation in endoplasmic reticulum induces apoptosis reversed by colchicine and sodium 4-phenylbutyrate. J Am Soc Nephrol. 2005 Oct;16(10):3006–3014. doi: 10.1681/ASN.2005050461. [DOI] [PubMed] [Google Scholar]
- 48.Bernascone I, Janas S, Ikehata M, et al. A transgenic mouse model for uromodulin-associated kidney diseases shows specific tubulo-interstitial damage, urinary concentrating defect and renal failure. Hum Mol Genet. 2010 Aug 1;19(15):2998–3010. doi: 10.1093/hmg/ddq205. [DOI] [PubMed] [Google Scholar]
- 49.Patel R, McKenzie JK, McQueen EG. Tamm-Horsfall Urinary Mucoprotein and Tubular Obstruction by Casts in Acute Renal Failure. Lancet. 1964 Feb 29;1:457–461. doi: 10.1016/s0140-6736(64)90794-9. [DOI] [PubMed] [Google Scholar]
- 50.Fletcher AP, McLaughlin JE, Ratcliffe WA, Woods DA. The chemical composition and electron microscopic appearance of a protein derived from urinary casts. Biochim Biophys Acta. 1970 Aug 21;214(2):299–308. doi: 10.1016/0005-2795(70)90007-3. [DOI] [PubMed] [Google Scholar]
- 51.Fairley JK, Owen JE, Birch DF. Protein composition of urinary casts from healthy subjects and patients with glomerulonephritis. Br Med J (Clin Res Ed) 1983 Dec 17;287(6408):1838–1840. doi: 10.1136/bmj.287.6408.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brenner BM, Rector FC. Brenner & Rector’s the kidney. 8. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 53.Wangsiripaisan A, Gengaro PE, Edelstein CL, Schrier RW. Role of polymeric Tamm-Horsfall protein in cast formation: oligosaccharide and tubular fluid ions. Kidney Int. 2001 Mar;59(3):932–940. doi: 10.1046/j.1523-1755.2001.059003932.x. [DOI] [PubMed] [Google Scholar]
- 54.Resnick JS, Sisson S, Vernier RL. Tamm-Horsfall protein. Abnormal localization in renal disease. Lab Invest. 1978 May;38(5):550–555. [PubMed] [Google Scholar]
- 55.Zager RA, Cotran RS, Hoyer JR. Pathologic localization of Tamm-Horsfall protein in interstitial deposits in renal disease. Lab Invest. 1978 Jan;38(1):52–57. [PubMed] [Google Scholar]
- 56.Cotran RS, Galvanek E. Immunopathology of human tubulo-interstitial diseases: localization of immunoglobulins complement and Tamm-Horsfall protein. Contrib Nephrol. 1979;16:126–131. doi: 10.1159/000402886. [DOI] [PubMed] [Google Scholar]
- 57.Hoyer JR. Tubulointerstitial immune complex nephritis in rats immunized with Tamm-Horsfall protein. Kidney Int. 1980 Mar;17(3):284–292. doi: 10.1038/ki.1980.34. [DOI] [PubMed] [Google Scholar]
- 58.Hession C, Decker JM, Sherblom AP, et al. Uromodulin (Tamm-Horsfall glycoprotein): a renal ligand for lymphokines. Science. 1987 Sep 18;237(4821):1479–1484. doi: 10.1126/science.3498215. [DOI] [PubMed] [Google Scholar]
- 59.Kreft B, Jabs WJ, Laskay T, et al. Polarized expression of Tamm-Horsfall protein by renal tubular epithelial cells activates human granulocytes. Infect Immun. 2002 May;70(5):2650–2656. doi: 10.1128/IAI.70.5.2650-2656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wimmer T, Cohen G, Saemann MD, Horl WH. Effects of Tamm-Horsfall protein on polymorphonuclear leukocyte function. Nephrol Dial Transplant. 2004 Sep;19(9):2192–2197. doi: 10.1093/ndt/gfh206. [DOI] [PubMed] [Google Scholar]
- 61.Horton JK, Davies M, Topley N, Thomas D, Williams JD. Activation of the inflammatory response of neutrophils by Tamm-Horsfall glycoprotein. Kidney Int. 1990 Feb;37(2):717–726. doi: 10.1038/ki.1990.38. [DOI] [PubMed] [Google Scholar]
- 62.Su SJ, Chang KL, Lin TM, Huang YH, Yeh TM. Uromodulin and Tamm-Horsfall protein induce human monocytes to secrete TNF and express tissue factor. J Immunol. 1997 Apr 1;158(7):3449–3456. [PubMed] [Google Scholar]
- 63.Yu CL, Tsai CY, Lin WM, et al. Tamm-Horsfall urinary glycoprotein enhances monokine release and augments lymphocyte proliferation. Immunopharmacology. 1993 Nov-Dec;26(3):249–258. doi: 10.1016/0162-3109(93)90041-n. [DOI] [PubMed] [Google Scholar]
- 64.Saemann MD, Weichhart T, Zeyda M, et al. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest. 2005 Feb;115(2):468–475. doi: 10.1172/JCI22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chambers R, Groufsky A, Hunt JS, Lynn KL, McGiven AR. Relationship of abnormal Tamm-Horsfall glycoprotein localization to renal morphology and function. Clin Nephrol. 1986 Jul;26(1):21–26. [PubMed] [Google Scholar]
- 66.Howie AJ, Brewer DB. Extra-tubular deposits of Tamm-Horsfall protein in renal allografts. J Pathol. 1983 Feb;139(2):193–206. [PubMed] [Google Scholar]
- 67.Cavallone D, Malagolini N, Serafini-Cessi F. Binding of human neutrophils to cell-surface anchored Tamm-Horsfall glycoprotein in tubulointerstitial nephritis. Kidney Int. 1999 May;55(5):1787–1799. doi: 10.1046/j.1523-1755.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- 68.Fasth AL, Hoyer JR, Seiler MW. Extratubular Tamm-Horsfall protein deposits induced by ureteral obstruction in mice. Clin Immunol Immunopathol. 1988 Apr;47(1):47–61. doi: 10.1016/0090-1229(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 69.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int. 2005 Sep;68(3):1096–1108. doi: 10.1111/j.1523-1755.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 70.Duffield JS. Macrophages in kidney repair and regeneration. J Am Soc Nephrol. 2011 Feb;22(2):199–201. doi: 10.1681/ASN.2010121301. [DOI] [PubMed] [Google Scholar]
- 71.Tveit H, Dick G, Skibeli V, Prydz K. A proteoglycan undergoes different modifications en route to the apical and basolateral surfaces of Madin-Darby canine kidney cells. J Biol Chem. 2005 Aug 19;280(33):29596–29603. doi: 10.1074/jbc.M503691200. [DOI] [PubMed] [Google Scholar]
- 72.Thomas DB, Davies M, Peters JR, Williams JD. Tamm Horsfall protein binds to a single class of carbohydrate specific receptors on human neutrophils. Kidney Int. 1993 Aug;44(2):423–429. doi: 10.1038/ki.1993.260. [DOI] [PubMed] [Google Scholar]
- 73.Toma G, Bates JM, Jr, Kumar S. Uromodulin (Tamm-Horsfall protein) is a leukocyte adhesion molecule. Biochem Biophys Res Commun. 1994 Apr 15;200(1):275–282. doi: 10.1006/bbrc.1994.1445. [DOI] [PubMed] [Google Scholar]
- 74.Pfistershammer K, Klauser C, Leitner J, et al. Identification of the scavenger receptors SREC-I, Cla-1 (SR-BI), and SR-AI as cellular receptors for Tamm-Horsfall protein. J Leukoc Biol. 2008 Jan;83(1):131–138. doi: 10.1189/jlb.0407231. [DOI] [PubMed] [Google Scholar]
- 75.McQueen EG. Composition of urinary casts. Lancet. 1966 Feb 19;1(7434):397–398. doi: 10.1016/s0140-6736(66)91392-4. [DOI] [PubMed] [Google Scholar]
- 76.Huang ZQ, Kirk KA, Connelly KG, Sanders PW. Bence Jones proteins bind to a common peptide segment of Tamm-Horsfall glycoprotein to promote heterotypic aggregation. J Clin Invest. 1993 Dec;92(6):2975–2983. doi: 10.1172/JCI116920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Safirstein R, Megyesi J, Saggi SJ, et al. Expression of cytokine-like genes JE and KC is increased during renal ischemia. Am J Physiol. 1991 Dec;261(6 Pt 2):F1095–1101. doi: 10.1152/ajprenal.1991.261.6.F1095. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida T, Kurella M, Beato F, et al. Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney Int. 2002 May;61(5):1646–1654. doi: 10.1046/j.1523-1755.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 79.Dehne MG, Boldt J, Heise D, Sablotzki A, Hempelmann G. Tamm-Horsfall protein, alpha-1- and beta-2-microglobulin as kidney function markers in heart surgery. Anaesthesist. 1995 Aug;44(8):545–551. doi: 10.1007/s001010050187. [DOI] [PubMed] [Google Scholar]
- 80.Dehne MG, Sablotzki A, Muhling J, Papke G, Kuntzsch U, Hempelmann G. Acute kidney failure. Non-invasive diagnosis of acute kidney failure in operative intensive care patients. Anaesthesist. 1998 Mar;47(3):193–201. doi: 10.1007/s001010050547. [DOI] [PubMed] [Google Scholar]
- 81.McLaughlin PJ, Aikawa A, Davies HM, et al. Uromodulin levels are decreased in urine during acute tubular necrosis but not during immune rejection after renal transplantation. Clin Sci (Lond) 1993 Feb;84(2):243–246. doi: 10.1042/cs0840243. [DOI] [PubMed] [Google Scholar]
- 82.Aregger F, Pilop C, Uehlinger DE, et al. Urinary proteomics before and after extracorporeal circulation in patients with and without acute kidney injury. J Thorac Cardiovasc Surg. 2010 Mar;139(3):692–700. doi: 10.1016/j.jtcvs.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Safirstein R. Gene expression in nephrotoxic and ischemic acute renal failure. J Am Soc Nephrol. 1994 Jan;4(7):1387–1395. doi: 10.1681/ASN.V471387. [DOI] [PubMed] [Google Scholar]
- 84.Mutig K, Kahl T, Saritas T, et al. Activation of the Bumetanide-sensitive Na+,K+,2Cl− Cotransporter (NKCC2) Is Facilitated by Tamm-Horsfall Protein in a Chloride-sensitive Manner. J Biol Chem. 2011 Aug 26;286(34):30200–30210. doi: 10.1074/jbc.M111.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kottgen A, Hwang SJ, Larson MG, et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J Am Soc Nephrol. 2010 Feb;21(2):337–344. doi: 10.1681/ASN.2009070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rasch R, Torffvit O, Bachmann S, Jensen PK, Jacobsen NO. Tamm-Horsfall glycoprotein in streptozotocin diabetic rats: a study of kidney in situ hybridization, immunohistochemistry, and urinary excretion. Diabetologia. 1995 May;38(5):525–535. doi: 10.1007/BF00400720. [DOI] [PubMed] [Google Scholar]
- 87.Pfleiderer S, Zimmerhackl LB, Kinne R, Manz F, Schuler G, Brandis M. Renal proximal and distal tubular function is attenuated in diabetes mellitus type 1 as determined by the renal excretion of alpha 1-microglobulin and Tamm-Horsfall protein. Clin Investig. 1993 Dec;71(12):972–977. doi: 10.1007/BF00180026. [DOI] [PubMed] [Google Scholar]
- 88.Torffvit O, Agardh CD. Urinary excretion rate of NC1 and Tamm-Horsfall protein in the microalbuminuric type I diabetic patient. J Diabetes Complications. 1994 Apr-Jun;8(2):77–83. doi: 10.1016/1056-8727(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 89.Zimmerhackl LB, Pfleiderer S, Kinne R, Manz F, Schuler G, Brandis M. Tamm-Horsfall-Protein excretion as a marker of ascending limb transport indicates early renal tubular damage in diabetes mellitus type I. J Diabet Complications. 1991 Apr-Sep;5(2–3):112–114. doi: 10.1016/0891-6632(91)90037-p. [DOI] [PubMed] [Google Scholar]
- 90.Catalano C, Torffvit O. Urinary excretion of Tamm-Horsfall protein in normotensive, normo-albuminuric type 1 diabetic patients. Nephron. 1996;72(3):436–441. doi: 10.1159/000188909. [DOI] [PubMed] [Google Scholar]
- 91.Torffvit O, Agardh CD. Tubular secretion of Tamm-Horsfall protein is decreased in type 1 (insulin-dependent) diabetic patients with diabetic nephropathy. Nephron. 1993;65(2):227–231. doi: 10.1159/000187479. [DOI] [PubMed] [Google Scholar]
- 92.Torffvit O, Agardh CD, Thulin T. A study of Tamm-Horsfall protein excretion in hypertensive patients and type 1 diabetic patients. Scand J Urol Nephrol. 1999 Jun;33(3):187–191. doi: 10.1080/003655999750015970. [DOI] [PubMed] [Google Scholar]
- 93.Sejdiu I, Torffvit O. Decreased urinary concentration of Tamm-Horsfall protein is associated with development of renal failure and cardiovascular death within 20 years in type 1 but not in type 2 diabetic patients. Scand J Urol Nephrol. 2007 Sep 28;42(2):168–174. doi: 10.1080/00365590701644691. [DOI] [PubMed] [Google Scholar]
- 94.Gudbjartsson DF, Holm H, Indridason OS, et al. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet. 2010 Jul;6(7):e1001039. doi: 10.1371/journal.pgen.1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prajczer S, Heidenreich U, Pfaller W, Kotanko P, Lhotta K, Jennings P. Evidence for a role of uromodulin in chronic kidney disease progression. Nephrol Dial Transplant. 2010 Jun;25(6):1896–1903. doi: 10.1093/ndt/gfp748. [DOI] [PubMed] [Google Scholar]
- 96.Lhotta K. Uromodulin and chronic kidney disease. Kidney Blood Press Res. 2010;33(5):393–398. doi: 10.1159/000320681. [DOI] [PubMed] [Google Scholar]
- 97.Shlipak MG, Li Y, Fox C, Coresh J, Grunfeld C, Whooley M. Uromodulin concentrations are not associated with incident CKD among persons with coronary artery disease. BMC Nephrol. 2011;12:2. doi: 10.1186/1471-2369-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010 Jul 8;363(2):166–176. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 99.Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009 Apr 23;360(17):1696–1698. doi: 10.1056/NEJMp0806284. [DOI] [PubMed] [Google Scholar]