Abstract
We review recent literature describing protein nucleic acid interactions and nucleic acid organization in viruses. The nature of the viral genome determines its overall organization and its interactions with the capsid protein. Genomes composed of single strand (ss) RNA and DNA are highly flexible and, in some cases, adapt to the symmetry of the particle-forming protein to show repeated, sequence independent, nucleoprotein interactions. Genomes composed of double stranded (ds) DNA do not interact strongly with the container due to their intrinsic stiffness, but form well-organized layers in virions. Assembly of virions with ssDNA and ssRNA genomes usually occurs through a cooperative condensation of the protein and genome, while dsDNA viruses usually pump the genome into a preformed capsid with a strong, virally encoded, molecular motor complex. We present data that suggests the packing density of ss genomes and ds genomes are comparable, but the latter exhibit far higher pressures due to their stiffness.
Introduction
Structural studies of nucleic acid packaging in viruses continues to extend our understanding of the relationship between the capsid and the genome, as well as the organization of RNA and DNA within the confined volumes of the particle. Here we summarize and compare recent structural virology papers that include descriptions of the viral genome structures and functions. We first summarize recent RNA virus structural data, and then recent developments in structural studies of dsDNA bacteriophages and dsDNA animal viruses. Table 1 lists the virus structures that were reported in the last five years where significant information on nucleic acid structure was described. We conclude by comparing factors that affect the packaging densities of ssRNA and ssDNA genomes with packaged dsDNA genomes, focusing on the consequences of the intrinsic persistence length associated with different types of nucleic acid.
Table 1.
Recently published structures of some virus protein-nucleic acid complexes.
| Name | Family | Genome | Morphology | Sample | Resolution (Å) | Access Code | Reference |
|---|---|---|---|---|---|---|---|
| PrV | Tetraviridae | (+)ssRNA | Icos T=4 | Authentic virion | 3.8 | PDB 2QQP | [13**] |
| FHV | Nodaviridae | (+)ssRNA | Icos T=3 | VLP | 3.6 | PDB 3LOB | [14] |
| ϕCb5 | Leviviridae | (+)ssRNA | Icos T=3 | Authentic virion & VLP | 2.9-3.6 | PDB 2W4Z | [8] |
| PDB 2W4Y | |||||||
| PP7 | Leviviridae | (+)ssRNA | Icos T=3 | Mutated subunit | 2.4 | PDB 2QUX | [9*] |
| RSV | Paramyxoviridae | (-)ssRNA | Pleomorphic | Decameric ring of N subunits | 3.3 | PDB 2WJ8 | [4**] |
| VSV | Rhabdoviridae | (-)ssRNA | Bullet shape | Decameric ring of N subunits | 3.0-3.1 | PDB 3PTX | [5*,6] |
| PDB 3PUO | |||||||
| PDB 3PU1 | |||||||
| PDB 3PU4 | |||||||
| STNV | Tombusviridae | (+)ssRNA | Icos T=1 | VLP (E.Coli) | 1.4 | PDB 3S4G | [12] |
| HLSV | Tobamoviridae | (+)ssRNA | Helical rod | Authentic virion | 3.5 | PDB 3PDM | [3] |
|
| |||||||
| P22 | Podoviridae | dsDNA | Icos T=7l | Authentic virion | 5.0-17 | EMD 1220 | [36*,46,47] |
| EMD 1222 | |||||||
| EMD 5232 | |||||||
| ε15 | Podoviridae | dsDNA | Icos T=7l | Authentic virion | 4.5-20 | EMD 1175 | [48,49] |
| EMD 1176 | |||||||
| EMD 5003 | |||||||
| PDB 3C5B | |||||||
| P-SSP7 | Podoviridae | dsDNA | Icos T=7 | Authentic virion | 4.6-9.0 | EMD 1715 | [50] |
| EMD 1713 | |||||||
| PDB 2XD8 | |||||||
| ϕ29 | Podoviridae | dsDNA | Prolate T=3, Q=5 | Authentic virion & tailless VLP | 7.8-20 | EMD 1420 | [51-53] |
| EMD 1419 | |||||||
| EMD 1260 | |||||||
| EMD 1265 | |||||||
| T5 | Siphoviridae | dsDNA | T=13 | Thermal & tailless mutants | 19-20 | N/A | [54] |
RNA Viruses
The ssRNA viruses provide the greatest level of detail for protein-RNA interactions in virus capsids due to the ability of their icosahedral or helical particles to form crystals or fibers for high resolution x-ray diffraction studies [1,2]. While non-enveloped icosahedral virus structures again provide most of the recent examples of packaged, ordered nucleic acid, structures from other capsid morphologies, including two enveloped viruses, offer interesting new insights into how viral RNA is manipulated during assembly and the consequences of its organization on the virus life cycle. This variety complicates attempts to formulate a general mechanism that encompasses how RNA genome packaging is accomplished across the different virus families.
Hibiscus latent Singapore virus (HLSV) is a helical rod virus approximately 300nM in length with the ssRNA genome packaged in a small channel at its center [3]. It is in the Tobamovirus family along with tobacco mosaic virus (TMV) and their symmetry and structure determination by X-ray fiber diffraction set them apart from spherical capsid structures. The LR (left radial) helix of HLSV has the largest bend in the family (46.1°) at its center point. The bend is induced by charge repulsion of His122 and Lys123. In all but two tobamoviruses residue 122 is an arginine that stabilizes guanine 1 during virus assembly, but in HLSV the histidine instead leads to the kinked helix and the adjacent Lys123 makes a unique interaction with phosphate 1. Even with high structural conservation among the rod shaped viruses, unique variations in protein-RNA interactions continue to be discovered, as more structures are determined.
Human respiratory syncytial virus (RSV) [4**] and vesicular stomatitis virus (VSV) [5*,6] are enveloped viruses with pleomorphic and bullet shaped capsids, respectively, that utilize helical nucleoprotein complexes within the capsid to organize negative strand RNA genomes. The complexes sequester the viral RNA and serve as a template for transcription and replication. In both studies a decamer of the nucleoprotein assembled into a nucleocapsid-like particle that bound 7-9 nucleotides of RNA per subunit. The RNA is non-specifically bound in a basic groove centered between the N and C-terminal domains and has alternating buried and exposed bases. Combined with a helical reconstruction from EM studies of intact nucleopcapsids, this suggests how readout by the polymerase is possible without release of the RNA from the complex. Indeed, the most recent VSV study revealed that the RNA can be digested away from the nucleocapsid protein. The helical protein assembly remains intact and creates empty nucleocapsids, showing the RNA is readily accessible. The N protein structure from Rift Valley fever virus (RVFV) has also been determined recently, but in an RNA-free monomeric state. Although folded into two helical lobes resembling the other N proteins, the fold is different, shows no positively charged cleft, and the RNP complexes viewed in EM micrographs show no helical character [7]. Genomes in the Bunyaviridae may instead be packaged as beads on a string, possibly organized into macrocircles.
Two new structures from the T=3 icosahedral Leviviridae bacteriophage family, one of the caulobacter bacteriophage 5 (ϕCb5) virion [8] and one of the Pseudomonas aeruginosa bacteriophage PP7 coat protein-RNA complex [9*], detail different mechanisms for RNA binding compared to MS2 [10,11]. The ϕCb5 shell structure is closely similar to that of MS2 with the largest differences at the N-terminus and two of the loops. Unlike previous structures in this family, a single nucleotide is resolved bound on the periphery of a capsid dimer interface with a third additional subunit from a neighboring dimer. This interaction may help stabilize subunit interactions in ϕCb5 since they have less buried surface area compared to other levivirus particles. The new binding site also suggests an assembly mechanism whereby accelerated oligomerization of subunits occurs via non-specific recognition and binding of the RNA genome at these sites. The high resolution structure of the PP7 complex shows binding of its cognate operator stem loop at the coat protein dimer interface as seen in MS2, but using a considerably different arrangement of adenine binding pockets relative to the dimer axis. Combined with altered roles for conserved residues, this explains the inability of these phages to recognize the other’s hairpin structure and demonstrates the flexibility of the interior β-sheet surface as a scaffold for recognizing a diverse range of specific RNAs.
The capsid protein of satellite tobacco necrosis virus was expressed in E. coli and forms T=1 icosahedral VLPs closely similar to the wild-type virions [12]. While no RNA density was observed in the 1.4Å VLP structure or in wild-type structures, reducing the resolution of the VLP map to 6Å revealed ordered density at the 5-fold axes between clusters of the basic coat protein N-terminal helices, which are positioned in the particle interior. This was interpreted as CP mRNA or degraded cellular RNAs and fitted with a 3-bp duplex. This would account for roughly half of the packaged genome and may represent a native RNA interaction as VLPs of Flock House virus impose a duplex structure on encapsidated cellular RNAs closely similar to that seen in wild-type virions (see below).
The structure of Providence virus (PrV) [13], an icosahedral T=4 tetravirus, and that of a metal free VLP of Flock House virus (FHV) [14], an icosahedral T=3 nodavirus, both showed duplex RNA bound at the particle 2-fold axes. This was anticipated in the case of FHV as both the wild-type capsid [15] and several previous VLP structures have demonstrated that the capsid protein arranges a specific RNA structure at the 2-fold axes as an integral component of the particle structure (Fig. 1A,B) although the protein-RNA contacts themselves are non-specific (unpublished data). Removal of the metal ions destabilizes the capsid and alters the conformation of the lytic peptides near the packaged cellular RNA, which also shows a lower level or order compared to the wild-type virions. In contrast, the duplex RNA was one of many surprises in the structure of PrV, only the second structure of a tetravirus. The structure of NwV [16] did not display any ordered RNA, but showed a similar jelly roll fold, autocatalytic cleavage of lytic peptides (called gamma peptides), and use of coat protein termini to support the capsid architecture as seen in the T=3 nodaviruses. PrV has the same attributes, but the subunit termini have completely different structures and opposite functions compared to those of NwV. The gamma peptides do not support the capsid architecture of the PrV T=4 capsid as they do in the NwV. In PrV the gamma peptides interact with the duplex RNA at the 2-fold axes with a repeating sequence pattern found in other RNA binding proteins. The protein-RNA interaction in PrV may be more genome specific as VLPs do not assemble from coat protein expressed in a baculovirus system [17].
Figure 1.
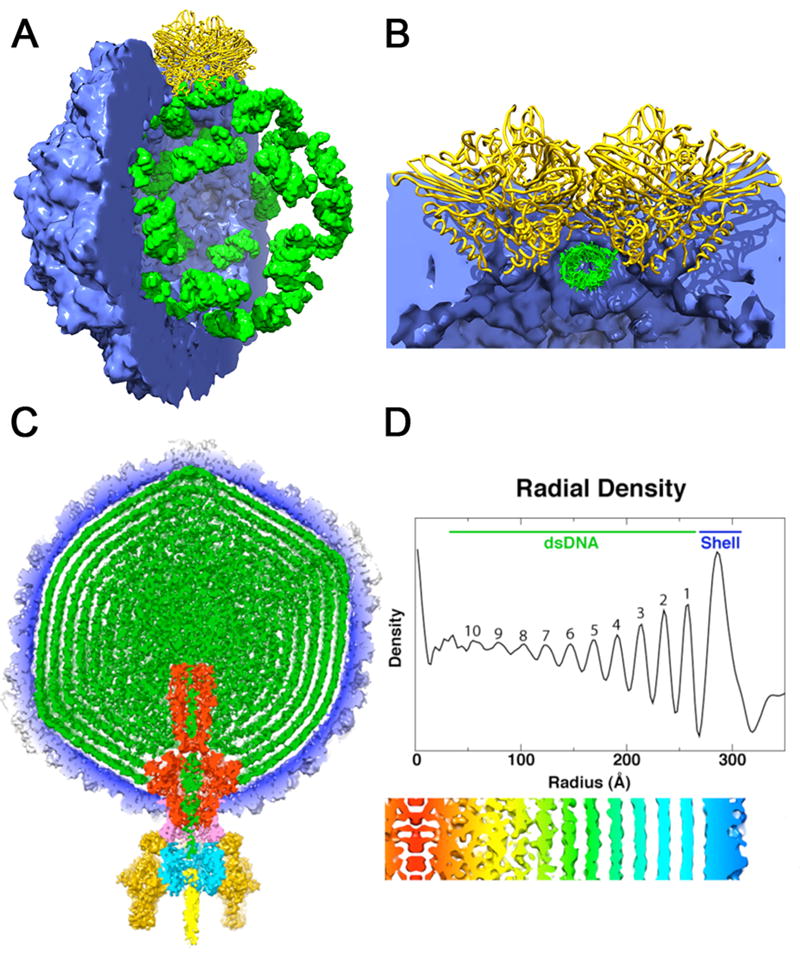
Structures adopted by encapsidated nucleic acid. (a) Packaging of cellular RNA in the metal free FHV virus like particle. The crystal structure coordinates were used to compute density maps for the protein shell (blue) and bound RNA (green). Half of the capsid has been cut away to reveal the dodecahedral arrangement of the ordered duplex RNA segments bound by a coat protein dimer at the icosahedral 2-fold axes (yellow). (b) Close up view of the subunit-RNA interaction looking down the duplex RNA axis and perpendicular to the particle 2-fold. The RNA structure is imposed by the coat protein at the 2-folds to create a molecular switch in the formation of the quasi-equivalent T=3 particle. (c) A 60Å thick central cross-section of the P22 virion showing the concentric layered organization of the packaged dsDNA genome (green). The map is segmented to highlight individual components: coat protein gp5 (blue), gp1 (red), gp4 (pink), gp9 (dark yellow), gp10 (cyan), gp26 (yellow), and pilot proteins (green) [36*]. Here the capsid is a ready made container for the genome and the dsDNA doesn’t play an essential role in particle formation. (d) Radial density plot of the spherically averaged phage reconstruction shows at least ten distinct layers of dsDNA, separated by about 22 Å, exist within the capsid shell. A thin, central section from a map 12-fold averaged about the portal axis and aligned with the radial density plot above it, shows an enlarged view of the DNA layers (red-to-blue, low-to-high radii).
Bacteriophages and dsDNA Viruses
Bacteriophages packaging dsDNA have been extensively studied for decades, but the availability of single molecule methods and high resolution structures of virus capsids in different stages of maturation have rejuvenated these investigations and provided detailed descriptions of the packaging of DNA and its resultant structure. Bustamante and colleagues performed the first single molecule study of dsDNA packaging with the ϕ29 bacteriophage and this demonstrated that the motor could overcome 50 pN of resistance to package the viral genome [18], making it among the most powerful biological motors. The studies were recently extended with bacteriophages lambda [19] and T4 [20**], demonstrating motors of comparable strength for these viruses, but with packaging rates that are roughly proportional to the length of the genome (i.e. for the viruses examined, it takes roughly 10 minutes to package its genome regardless of genome size). Pressures within the capsids were estimated by the force required for dsDNA packaging and confirmed by measuring the osmotic force necessary to prevent release of the dsDNA genome when lambda virions were exposed to the lambda receptor. These measurements were in good agreement with the forces required for packaging [21-23]. There is some consensus that these liquid crystalline densities (and associated pressures) are functionally important for initial genome release [24*], however, the opinion is not universal [25,26]. A hallmark of dsDNA structure in bacteriophages and herpes viruses is the densely packed layers of DNA shown in Figures 1C and 1D [27].
There have been a number of recent computational studies that address dsDNA packing within particles [28-30,31*,32-33]. These studies demonstrated a high degree of sensitivity to persistence length estimates, the presence or absence of a central protein core, the presence of neutralizing cations and the size and shape of the capsid. The available structures and efforts to accurately reproduce them with computation have significantly advanced our knowledge of the various forces contributing to dsDNA organization. The physical chemistry defining dsDNA organization in bacteriophages has recently been reviewed [34].
The magnitude of the DNA packaging problem in bacteriophages is impressive; the organization of the DNA within the particle must be virtually perfect all the time. The particle to plaque forming unit (pfu) for bacteriophage is approximately 1, so every particle delivers its full length of dsDNA without fail. This is compared to values of 200-300 particles to pfu for most ssRNA animal viruses. It is interesting to consider the parameters of the problem and translate them to the macroscopic scale. The persistence length of dsDNA is 5 nM, its width is 2 nM, its length (in P22 phage) is 14600 nM and the container is a sphere that is roughly 60 nM in diameter. This scales to the equivalent of packing 80 meters of stiff, almost wire-like, 1 cm cable into a 30 cm diameter spherical container.
The factors that may affect DNA packaging and release continue to be discovered. Recently it was found that the P22 portal, the dodecameric protein structure through which dsDNA enters and leaves the particle, has a 20 nM helical coiled-coil that extends to the center of the particle. The X-ray crystal structure of ectopically expressed full-length portal protein [35*] revealed the remarkable extension. It was then possible to fit the X-ray model into the 7.8 Å cryoEM structure of the entire virion [36*]. The role of such a remarkable structure is still being established, but it may function to direct the entering dsDNA (possibly like a flexible nozzle) and/or it may clamp the DNA and prevent its loss during the addition of closure proteins to the portal. This coiled-coil is not present in all podoviridae, so it may perform a special function in P22 and its close relatives.
Herpesvirus is clearly phage-like in all of its DNA packaging properties and organization of packaged dsDNA [37]. There is still debate concerning the presence of a preformed capsid and packaging motor in the dsDNA adenovirus. Some data suggest it assembles more like the RNA viruses with capsid proteins assembling on pre-condensed dsDNA [38] while more recent data indicates the presence of a portal and a phage-like packaging mechanism [39]. Adenovirus has a low particle to plaque forming ratio of 10-20 [40]. In vivo studies of the archeal, dsDNA, sulfolobus turreted icosahedral virus (STIV) demonstrate the presence of empty capsids [41**] and there is a candidate gene encoding a packaging ATPase [42], both consistent with a phage like packaging mechanism. Neither adenovirus or STIV display the characteristic layered dsDNA structure in reconstructions of their particles suggesting that the DNA is not as stressed as it is in bacteriophage and herpes viruses. It is possible that additional viral gene products assist in DNA condensation in adenovirus and STIV, reducing the characteristic repulsion observed in phage.
Density and Pressure of Packaged Nucleic Acid in Icosahedral Viruses
Factors affecting dsDNA packaging in viruses are different from those observed in ssRNA and ssDNA viruses. The latter normally assemble in a cooperative condensation of capsid protein and single stranded nucleic acid, while the former package the dsDNA genome into preformed capsids with strong molecular motors driving the dsDNA insertion. The degree of stress or pressure within virus particles is directly related to the persistence length (a measure of polymer stiffness) of the material being packaged [34]. The persistence lengths of ssRNA [43] and ssDNA [44] are estimated to be 15-20Å in solutions of 0.1M ionic strength compared to a persistence length of 50Å for dsDNA [34]. This dramatic difference significantly changes the energetics of nucleic acid packaging in viruses with dsDNA compared to those with single stranded genomes.
Icosahedral viral shells form a rigid container of defined volume for packaging nucleic acid. The packing density of RNA or DNA within these shells provides a quantitative means of comparing packaged genomes within viruses. Crystallographers define a parameter called Vm (Vcontainer (Å3))/Mr(contents) (Daltons)) that describes the volume occupied per Dalton of a biological macromolecule [45]. The crystal unit cell is the “container” and Vm is an indication of the solvent content of the crystal. A small Vm indicates a small solvent content or high packing density. Protein crystals typically contain approximately 50% solvent and RNA crystals slightly more. Here we use the internal capsid volume as the “container” and the molecular weight of the RNA or DNA genome as the macromolecular mass. Table 2 lists values for some representative viruses and the Vm of crystalline duplex RNA, (U((UA)6)A)2. For reference we also compute the Vm for dehydrated RNA. The specific volume for dry RNA is 0.55cm3/g. Conversion to molecular dimension with the conversion factor (1024Å3/cm3)/(6.02×1023 Dalton/gm) gives 1.66gm-Å3/Dalton-cm3 and a Vm value for dehydrated RNA of 0.91Å3/Dalton, the minimum value that RNA can have. It is clear that some viruses packaging single stranded nucleic acid may have a higher packing density than dsDNA viruses, but they are not as energetically stressed because of their smaller persistence lengths. Put another way, the particles appear to have overall similar packing densities; thus, the high pressure measured in the dsDNA bacteriophages is due to the longer persistence length of their genomes.
Table 2.
Genome packing densities for a variety of virus capsids compared to that of dehydrated and crystalline nucleotides.
| Virus1 | Capsid Type | Genome Type | Interior Volume (Å3 × 106)2 | Nucleic Acid Mr (Da × 106)3 | Vm (Å3/Da) |
|---|---|---|---|---|---|
| Dry RNA | -- | -- | -- | -- | 0.9 |
| [U(UA)6A]2 | -- | -- | 0.073 | 0.034 | 2.1 |
|
| |||||
| PCV2 | T=1 | ssDNA | 1.2 | 0.6 | 2.0 |
| AAV6 | T=1 | ssDNA | 2.7 | 1.5 | 1.8 |
| CCMV | T=3 | ssRNA | 4.4 | 1.1 | 4.0 |
| FHV | T=3 | ssRNA | 6.0 | 1.5 | 4.0 |
| ϕCB5 | T=3 | ssRNA | 6.0 | 1.3 | 4.6 |
| HRV14 | pT=3 | ssRNA | 5.2 | 2.5 | 2.1 |
| PrV | T=4 | ssRNA | 8.2 | 3.0 | 2.7 |
| P22 | T=7l | dsDNA | 91 | 29 | 3.1 |
PDB ID’s in order: 1rna, 3r0r, 3oah, 1cwp, 2z2q, 2w4z, 1ncq, 2qqp, 2xyz.
Calculated using the method described in [8].
Calculated by multiplying the genome length by the average weight of an RNA (340 Da) or DNA (330 Da) nucleotide.
Conclusions
Nucleic acid packaging in capsids provides novel insights into the organization of viral genomes and their interactions with the container. It is clear that the structure of ssRNA and ssDNA allow them to adapt to the icosahedral or helical symmetry of the container with structural interactions that obey the intrinsic symmetry of the protein shell occasionally observed. To this end some groups are pursuing apparent links between hairpin loop distributions in the viral genomes relative to the binding sites identified in their capsid structures. Genome organizations in viruses with dsDNA are quite different. There is little affinity with the capsid, which is often negatively charged on its inner surface, and the persistence length creates ordered layers or shells of the dsDNA that is observed in cryoEM reconstructions of many bacteriophages. In the center of the particle this organization breaks down and little is known about how the stiff duplex is accommodated around the portal machinery. The highly orchestrated dsDNA packaging also leads to an inherently ordered and efficient egress. Through all of the adaptations that the dsDNA makes in these structures it is invariably faithfully delivered for a successful infection in bacteriophage. The relation between the genome and capsid is highly evolved and one of the most successful nucleo-protein structures in biology.
Highlights.
ssRNA viruses provide the highest resolution structures of packaged genome segments.
New modes of RNA packaging are seen in enveloped (-)ssRNA nucleoproteins.
Strong motors create ordered layers of dsDNA in bacteriophage genome packaging.
Nucleic acid packaging density is roughly similar in ssRNA, ssDNA, and dsDNA capsids.
The pressure inside dsDNA capsids is related to persistence length rather than packaging density.
Acknowledgments
We apologize to our colleagues if we were not able to adequately cover your contributions due to space constraints. This work was supported in part by grants from the National Institutes of Health (R37-GM34220 & R01-GM54076) to JEJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schneemann A. The structural and functional role of RNA in icosahedral virus assembly. Annu Rev Microbiol. 2006;60:51–67. doi: 10.1146/annurev.micro.60.080805.142304. [DOI] [PubMed] [Google Scholar]
- 2.Speir JA, Johnson JE. Virus Particle Structure: Nonenveloped Viruses. In: Mahy BWJ, van Regenmortel MHV, editors. Encyclopedia of Virology. Third. Elsevier; 2008. pp. 380–393. [Google Scholar]
- 3.Tewary SK, Oda T, Kendall A, Bian W, Stubbs G, Wong SM, Swaminathan K. Structure of hibiscus latent singapore virus by fiber diffraction: a nonconserved his122 contributes to coat protein stability. J Mol Biol. 2011;406:516–526. doi: 10.1016/j.jmb.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 4**.Tawar RG, Duquerroy S, Vonrhein C, Varela PF, Damier-Piolle L, Castagne N, MacLellan K, Bedouelle H, Bricogne G, Bhella D, et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science. 2009;326:1279–1283. doi: 10.1126/science.1177634. The authors combine x-ray and cryoEM structure determination methods to elucidate the complete RSV nucleocapsid helical structure. This provides a model, based on alternating exposed and buried nucleotide bases, for readout of the highly sequestered genome by the viral polymerase. [DOI] [PubMed] [Google Scholar]
- 5*.Green TJ, Rowse M, Tsao J, Kang J, Ge P, Zhou ZH, Luo M. Access to RNA encapsidated in the nucleocapsid of vesicular stomatitis virus. J Virol. 2011;85:2714–2722. doi: 10.1128/JVI.01927-10. Contrary to previous reports, the authors show that (-)ssRNA in the VSV nucleocapsid can be digested away by RNase. The N protein still assembles to form empty capsids, which can bind a variety of polyribonucleotides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green TJ, Zhang X, Wertz GW, Luo M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science. 2006;313:357–360. doi: 10.1126/science.1126953. [DOI] [PubMed] [Google Scholar]
- 7.Raymond DD, Piper ME, Gerrard SR, Smith JL. Structure of the Rift Valley fever virus nucleocapsid protein reveals another architecture for RNA encapsidation. Proc Natl Acad Sci U S A. 2010;107:11769–11774. doi: 10.1073/pnas.1001760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plevka P, Kazaks A, Voronkova T, Kotelovica S, Dishlers A, Liljas L, Tars K. The structure of bacteriophage phiCb5 reveals a role of the RNA genome and metal ions in particle stability and assembly. J Mol Biol. 2009;391:635–647. doi: 10.1016/j.jmb.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 9*.Chao JA, Patskovsky Y, Almo SC, Singer RH. Structural basis for the coevolution of a viral RNA-protein complex. Nat Struct Mol Biol. 2008;15:103–105. doi: 10.1038/nsmb1327. Demonstrates an alternate mode of sequence-specific RNA recognition in PP7 compared to MS2 by using different locations of adenine binding pockets and new roles for conserved residues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valegard K, Liljas L, Fridborg K, Unge T. The three-dimensional structure of the bacterial virus MS2. Nature. 1990;345:36–41. doi: 10.1038/345036a0. [DOI] [PubMed] [Google Scholar]
- 11.Horn WT, Tars K, Grahn E, Helgstrand C, Baron AJ, Lago H, Adams CJ, Peabody DS, Phillips SE, Stonehouse NJ, et al. Structural basis of RNA binding discrimination between bacteriophages Qbeta and MS2. Structure. 2006;14:487–495. doi: 10.1016/j.str.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane SW, Dennis CA, Lane CL, Trinh CH, Rizkallah PJ, Stockley PG, Phillips SE. Construction and Crystal Structure of Recombinant STNV Capsids. J Mol Biol. 2011;413:41–50. doi: 10.1016/j.jmb.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 13**.Speir JA, Taylor DJ, Natarajan P, Pringle FM, Ball LA, Johnson JE. Evolution in action: N and C termini of subunits in related T = 4 viruses exchange roles as molecular switches. Structure. 2010;18:700–709. doi: 10.1016/j.str.2010.03.010. Reports the first structure of ordered RNA in a T=4 capsid, a complete reversal in how the coat protein termini in tetraviruses create a molecular switch, and extensive interactions of a lytic peptide with the RNA. These features place PrV on an evolutionary pathway somewhere between the tetravirus and T=3 nodavirus families. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee M, Speir JA, Kwan MH, Huang R, Aryanpur PP, Bothner B, Johnson JE. Structure and function of a genetically engineered mimic of a nonenveloped virus entry intermediate. J Virol. 2010;84:4737–4746. doi: 10.1128/JVI.02670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher AJ, Johnson JE. Ordered duplex RNA controls capsid architecture in an icosahedral animal virus. Nature. 1993;361:176–179. doi: 10.1038/361176a0. [DOI] [PubMed] [Google Scholar]
- 16.Munshi S, Liljas L, Cavarelli J, Bomu W, McKinney B, Reddy V, Johnson JE. The 2.8 A structure of a T = 4 animal virus and its implications for membrane translocation of RNA. J Mol Biol. 1996;261:1–10. doi: 10.1006/jmbi.1996.0437. [DOI] [PubMed] [Google Scholar]
- 17.Taylor DJ, Speir JA, Reddy V, Cingolani G, Pringle FM, Ball LA, Johnson JE. Preliminary x-ray characterization of authentic providence virus and attempts to express its coat protein gene in recombinant baculovirus. Arch Virol. 2006;151:155–165. doi: 10.1007/s00705-005-0637-3. [DOI] [PubMed] [Google Scholar]
- 18.Smith DE, Tans SJ, Smith SB, Grimes S, Anderson DL, Bustamante C. The bacteriophage phi29 portal motor can package DNA against a large internal force. Nature. 2001;413:748–752. doi: 10.1038/35099581. [DOI] [PubMed] [Google Scholar]
- 19.Fuller DN, Raymer DM, Rickgauer JP, Robertson RM, Catalano CE, Anderson DL, Grimes S, Smith DE. Measurements of single DNA molecule packaging dynamics in bacteriophage lambda reveal high forces, high motor processivity, and capsid transformations. Journal of molecular biology. 2007;373:1113–1122. doi: 10.1016/j.jmb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Fuller DN, Raymer DM, Kottadiel VI, Rao VB, Smith DE. Single phage T4 DNA packaging motors exhibit large force generation, high velocity, and dynamic variability. Proc Natl Acad Sci U S A. 2007;104:16868–16873. doi: 10.1073/pnas.0704008104. Analysis of the dsDNA packaging motor for a bacteriophage with a large genome providing evidence that bacteriophage motors package at a rate proportional to the length of the genome being packaged. Bacteriophage ϕ29, lambda and T4 have different sized genomes, but the DNA packaging time is about 10 minutes for all of them. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evilevitch A, Fang LT, Yoffe AM, Castelnovo M, Rau DC, Parsegian VA, Gelbart WM, Knobler CM. Effects of salt concentrations and bending energy on the extent of ejection of phage genomes. Biophysical journal. 2008;94:1110–1120. doi: 10.1529/biophysj.107.115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evilevitch A, Gober JW, Phillips M, Knobler CM, Gelbart WM. Measurements of DNA lengths remaining in a viral capsid after osmotically suppressed partial ejection. Biophysical journal. 2005;88:751–756. doi: 10.1529/biophysj.104.045088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evilevitch A, Lavelle L, Knobler CM, Raspaud E, Gelbart WM. Osmotic pressure inhibition of DNA ejection from phage. Proc Natl Acad Sci U S A. 2003;100:9292–9295. doi: 10.1073/pnas.1233721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Gelbart WM, Knobler CM. Virology. Pressurized viruses. Science. 2009;323:1682–1683. doi: 10.1126/science.1170645. An excellent summary on the factors that give rise to the high internal pressures of dsDNA observed in bacteriophages. [DOI] [PubMed] [Google Scholar]
- 25.Chang CY, Kemp P, Molineux IJ. Gp15 and gp16 cooperate in translocating bacteriophage T7 DNA into the infected cell. Virology. 2010;398:176–186. doi: 10.1016/j.virol.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panja D, Molineux IJ. Dynamics of bacteriophage genome ejection in vitro and in vivo. Physical biology. 2010;7:045006. doi: 10.1088/1478-3975/7/4/045006. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JE, Chiu W. DNA packaging and delivery machines in tailed bacteriophages. Curr Opin Struct Biol. 2007;17:237–243. doi: 10.1016/j.sbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Petrov AS, Boz MB, Harvey SC. The conformation of double-stranded DNA inside bacteriophages depends on capsid size and shape. J Struct Biol. 2007;160:241–248. doi: 10.1016/j.jsb.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrov AS, Harvey SC. Structural and thermodynamic principles of viral packaging. Structure. 2007;15:21–27. doi: 10.1016/j.str.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Petrov AS, Harvey SC. Packaging double-helical DNA into viral capsids: structures, forces, and energetics. Biophysical journal. 2008;95:497–502. doi: 10.1529/biophysj.108.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Petrov AS, Harvey SC. Role of DNA-DNA interactions on the structure and thermodynamics of bacteriophages Lambda and P4. J Struct Biol. 2011;174:137–146. doi: 10.1016/j.jsb.2010.11.007. A computational study focused on the role of dsDNA-dsDNA interactions during DNA packaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrov AS, Lim-Hing K, Harvey SC. Packaging of DNA by bacteriophage epsilon15: structure, forces, and thermodynamics. Structure. 2007;15:807–812. doi: 10.1016/j.str.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Petrov AS, Locker CR, Harvey SC. Characterization of DNA conformation inside bacterial viruses. Physical review E, Statistical, nonlinear, and soft matter physics. 2009;80:021914. doi: 10.1103/PhysRevE.80.021914. [DOI] [PubMed] [Google Scholar]
- 34.Knobler CM, Gelbart WM. Physical chemistry of DNA viruses. Annual review of physical chemistry. 2009;60:367–383. doi: 10.1146/annurev.physchem.59.032607.093728. [DOI] [PubMed] [Google Scholar]
- 35**.Olia AS, Prevelige PE, Jr, Johnson JE, Cingolani G. Three-dimensional structure of a viral genome-delivery portal vertex. Nat Struct Mol Biol. 2011;18:597–603. doi: 10.1038/nsmb.2023. An X-ray structure of the full length dodecameric portal from the P22 bacteriophage revealed a 20nM coiled-coil at the C-terminus of the subunits. The authors also determined the X-ray structure of the portal without the C-terminal coiled-coil in complex with the dodecameric GP4 protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Tang J, Lander GC, Olia AS, Li R, Casjens S, Prevelige P, Jr, Cingolani G, Baker TS, Johnson JE. Peering down the barrel of a bacteriophage portal: the genome packaging and release valve in p22. Structure. 2011;19:496–502. doi: 10.1016/j.str.2011.02.010. The portal structure observed in Olia et al. [35**] was fitted into the 7.8Å cryoEM recostruction of the P22 virion demonstrating that the coiled-coil extends almost to the center of the particle and has a slightly different conformation in the virion than observed in the crystal structure. The authors suggest that it may transiently hold the dsDNA in the particle while the closure proteins are added. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown JC, Newcomb WW. Herpesvirus Capsid Assembly: Insights from Structural Analysis. Current opinion in virology. 2011;1:142–149. doi: 10.1016/j.coviro.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostapchuk P, Hearing P. Control of adenovirus packaging. Journal of cellular biochemistry. 2005;96:25–35. doi: 10.1002/jcb.20523. [DOI] [PubMed] [Google Scholar]
- 39.Christensen JB, Byrd SA, Walker AK, Strahler JR, Andrews PC, Imperiale MJ. Presence of the adenovirus IVa2 protein at a single vertex of the mature virion. J Virol. 2008;82:9086–9093. doi: 10.1128/JVI.01024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eglon MN, Duffy AM, O’Brien T, Strappe PM. Purification of adenoviral vectors by combined anion exchange and gel filtration chromatography. The journal of gene medicine. 2009;11:978–989. doi: 10.1002/jgm.1383. [DOI] [PubMed] [Google Scholar]
- 41**.Fu CY, Wang K, Gan L, Lanman J, Khayat R, Young MJ, Jensen GJ, Doerschuk PC, Johnson JE. In Vivo Assembly of an Archaeal Virus Studied with Whole-Cell Electron Cryotomography. Structure. 2010;18:1579–1586. doi: 10.1016/j.str.2010.10.005. This study showed partially assembled lipid-protein shells of sulfolobus turreted icosahedral virus that had the curvature observed in fully assembled empty capsids. The appearance of empty capsids indicates that STIV has a phage-like assembly mechanism with dsDNA packaged into preformed particles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice G, Tang L, Stedman K, Roberto F, Johnson JE, Douglas T, Young MJ. The structure of a thermophilic archeal virus shows a dsDNA viral capsid type that spans all domains of life. Proc Natl Acad Sci (U S) 2004;101:7716–7720. doi: 10.1073/pnas.0401773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoffe AM, Prinsen P, Gopal A, Knobler CM, Gelbart WM, Ben-Shaul A. Predicting the sizes of large RNA molecules. Proc Natl Acad Sci U S A. 2008;105:16153–16158. doi: 10.1073/pnas.0808089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tinland B, P A, Sturm J, Weil G. Persistence Length of Single-Stranded DNA. Macromolecules. 1997;30:5763–5765. [Google Scholar]
- 45.Matthews BW. Solvent content of protein crystals. Journal of molecular biology. 1968;33:491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- 46.Chang J, Weigele P, King J, Chiu W, Jiang W. Cryo-EM asymmetric reconstruction of bacteriophage P22 reveals organization of its DNA packaging and infecting machinery. Structure. 2006;14:1073–1082. doi: 10.1016/j.str.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Lander GC, Tang L, Casjens SR, Gilcrease EB, Prevelige P, Poliakov A, Potter CS, Carragher B, Johnson JE. The structure of an infectious P22 virion shows the signal for headful DNA packaging. Science. 2006;312:1791–1795. doi: 10.1126/science.1127981. [DOI] [PubMed] [Google Scholar]
- 48.Jiang W, Baker ML, Jakana J, Weigele PR, King J, Chiu W. Backbone structure of the infectious epsilon15 virus capsid revealed by electron cryomicroscopy. Nature. 2008;451:1130–1134. doi: 10.1038/nature06665. [DOI] [PubMed] [Google Scholar]
- 49.Jiang W, Chang J, Jakana J, Weigele P, King J, Chiu W. Structure of epsilon15 bacteriophage reveals genome organization and DNA packaging/injection apparatus. Nature. 2006;439:612–616. doi: 10.1038/nature04487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, Zhang Q, Murata K, Baker ML, Sullivan MB, Fu C, Dougherty MT, Schmid MF, Osburne MS, Chisholm SW, et al. Structural changes in a marine podovirus associated with release of its genome into Prochlorococcus. Nat Struct Mol Biol. 2010;17:830–836. doi: 10.1038/nsmb.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Comolli LR, Spakowitz AJ, Siegerist CE, Jardine PJ, Grimes S, Anderson DL, Bustamante C, Downing KH. Three-dimensional architecture of the bacteriophage phi29 packaged genome and elucidation of its packaging process. Virology. 2008;371:267–277. doi: 10.1016/j.virol.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 52.Tang J, Olson N, Jardine PJ, Grimes S, Anderson DL, Baker TS. DNA poised for release in bacteriophage phi29. Structure. 2008;16:935–943. doi: 10.1016/j.str.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang Y, Morais MC, Battisti AJ, Grimes S, Jardine PJ, Anderson DL, Rossmann MG. Structural changes of bacteriophage phi29 upon DNA packaging and release. EMBO J. 2006;25:5229–5239. doi: 10.1038/sj.emboj.7601386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Effantin G, Boulanger P, Neumann E, Letellier L, Conway JF. Bacteriophage T5 structure reveals similarities with HK97 and T4 suggesting evolutionary relationships. J Mol Biol. 2006;361:993–1002. doi: 10.1016/j.jmb.2006.06.081. [DOI] [PubMed] [Google Scholar]


