Abstract
Extrasynaptic glutamate has been shown to regulate dopamine function in the mesocorticolimbic pathway, which plays an important role in the behavioral pharmacology of psychostimulants. Basal levels of glutamate are primarily regulated by the cystine-glutamate transporter and provide glutamatergic tone on extrasynaptic glutamate receptors. The present study examined the effects of a cystine-glutamate transporter enhancer on the neurochemical and behavioral effects of cocaine and amphetamine in nonhuman primates. It was hypothesized that augmenting extrasynaptic glutamate release with N-acetyl-L-cysteine (NAC), a cystine prodrug, would attenuate cocaine- or amphetamine-induced increases in extracellular dopamine and their corresponding behavioral-stimulant and reinforcing effects. In vivo microdialysis was used to evaluate cocaine-induced changes in extracellular dopamine (DA) in the caudate nucleus (n=3). NAC significantly attenuated cocaine-induced increases in dopamine but had inconsistent effects on amphetamine-induced increases in dopamine (n=4). Separate groups of subjects were either trained on a fixed-interval schedule of stimulus termination (n=6) or on a second-order schedule of self-administration (n=5) to characterize the behavioral-stimulant and reinforcing effects of psychostimulants, respectively. Systemic administration of NAC did not alter the behavioral-stimulant effects of either cocaine or amphetamine. Furthermore, cocaine self-administration and reinstatement of previously extinguished cocaine self-administration were not altered by pretreatment with NAC. Hence, drug interactions on caudate neurochemistry in vivo were not reflected in behavioral measures in squirrel monkeys. The present results in nonhuman primates do not support the use of NAC as a pharmacotherapy for cocaine abuse, although rodent and clinical studies suggest otherwise.
Keywords: cocaine, dopamine, glutamate, nonhuman primate, self-administration, in vivo microdialysis
1. INTRODUCTION
Interactions between dopamine and glutamate in brain are a major focus in the areas of drug addiction and mental health. Anatomical substrates for these interactions have been established in rodents (Sesack et al., 2003). Though dopamine signaling has functions independent of glutamate, glutamate regulates some of the critical modulatory functions of dopamine. Glutamatergic afferents from the prefrontal cortex and the laterodorsal/pedunculopontine tegmentum terminate onto dopamine neurons in both rodents and nonhuman primates. Glutamatergic regulation by these afferents is critical for regulating phasic firing of dopamine to signal behavioral events (Sesack et al., 2003). Midbrain dopamine neurons also project to the cerebral cortex and synapse onto both glutamatergic pyramidal cells and GABAergic medium spiny neurons and interneurons. Understanding this reciprocal signaling may be important for developing pharmacotherapies to treat cocaine addiction.
Changes in the glutamatergic system have been linked to changes in locomotor activity, conditioned place preference, and drug self-administration (Carlezon and Nestler, 2002, Cornish and Kalivas, 2000, 2001, Pierce et al., 1996), three behavioral measures often used to model the effects of drugs commonly abused by humans. Acute systemic administration of cocaine increased synaptic release of both dopamine and glutamate in the nucleus accumbens (McFarland et al., 2003). In contrast, repeated systemic cocaine administration decreased basal extrasynaptic glutamate (Baker et al., 2002a). Although vesicular release of glutamate contributes to the basal tone of glutamate, extracellular glutamate seems to originate primarily from cystine/glutamate exchange via transporters located primarily on glial cells (Baker et al., 2002b, Pow, 2001). This extrasynaptic glutamate provides tonic regulation on dopamine function in the mesocorticolimbic pathway via group II metabotropic receptors (mGluR2/3), which act as autoreceptors to regulate pre-synaptic neurotransmitter release (Baker et al., 2003a, Baker et al., 2002b). Repeated cocaine administration decreased basal glutamate in rodents (McFarland et al., 2003), resulting in decreased activation of mGlu2/3 receptors, which in turn facilitates synaptic release of glutamate and dopamine. In rodents, restoring basal glutamate levels by enhancing cystine-glutamate exchange blocked reinstatement of previously extinguished cocaine self-administration (Baker et al., 2003a).
The purpose of the present study was to extend these findings in rodents to nonhuman primates, examining the effects of manipulating the glutametergic system on the neurochemical and behavioral effects of cocaine. To do this, we used the cystine prodrug, N-acetyl-L-cysteine (NAC) to manipulate the cystine-glutamate transporter. Oxidation of L-cysteine to cystine (Puka-Sundvall et al., 1995) followed by its intracellular reduction to cysteine, results in an extracellular cystine concentration that is 100-fold greater than the intracellular concentration (Murphy et al., 1989). In contrast, the intracellular concentration of glutamate is 10,000-fold greater than the extracellular compartment. Therefore, uptake of NAC increases extracelluar levels of glutamate. Understanding the mechanisms by which this increase in extracellular glutamate modulates dopamine in the context of cocaine neuropharmacology may uncover new targets in developing pharmacotherapeutic medications for cocaine addiction.
2. MATERIALS AND METHODS
2.1. Subjects
Twenty male adult squirrel monkeys (Saimiri sciureus) (5-15 years) weighing between 800-1200g served as subjects. Between experimental sessions, subjects were individually housed and allowed access to food twice daily (Harlan Teklad monkey chow; Harlan Teklad, Madison, WI; fresh fruit and vegetables) and had access to water ad libitum. All subjects had served in previous experiments involving acute administration of cocaine and monoaminergic drugs. These studies were conducted in strict accordance with the NIH “Guidelines for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee of Emory University.
2.2. Apparatus
Experimental sessions were conducted daily in a ventilated, sound-attenuated chamber in which each monkey was seated comfortably in a commercially available primate chair (Modular Primate Chair, Med Associates Inc, St. Albans, VT). The front wall of the chair facing the monkey was equipped with a response lever and red and white lights. The response lever registered responses monitored by computers and integrated circuitry interfaced with the chambers. Typically, operant behavioral sessions lasted 1 h and were conducted five days a week. Drug time course determinations lasted 1.5 h. In vivo microdialysis sessions were limited to 5 h and were conducted no more frequently than once per week in individual animals.
2.3. Surgical Procedures
For in vivo microdialysis experiments, subjects (s147, s152, s155, s159, s168, s175, s180, s181, s188) were implanted with bilateral guide cannulae (CMA/11, CMA/Microdialysis, North Chelmsford, MA) using stereotaxic procedures to target the caudate nuclei utilizing sterile surgical procedures (Czoty et al., 2000). Coordinates for the stereotaxic procedures were obtained from a standard squirrel monkey atlas (Gergen et al., 1962) (from the earbar: A/P±15.0, L/M±3.). Subjects were initially sedated using a cocktail of 0.1 mg/kg atropine, 20 mg/kg ketamine, and 2 mg/kg Telazol to prepare the animal for surgery and position the subject’s head in the stereotaxic frame. Anesthesia was maintained with isoflurane (1-2%) during the surgery. Six nylon screws (2.4 mm, Plastics One, Inc., Roanoke, VA) were implanted surrounding the guide cannulae and the preparation was secured in place with dental cement (Lang Dental Manufacturing Co, Inc., Wheeling, IL). After the surgery, a stainless steel stylet was placed in each guide cannulae to protect the site when not in use. Subjects were allowed two to three weeks to recover before the experiments were initiated.
Subjects involved in behavioral protocols in which drugs were administered i.v. were surgically implanted with an indwelling chronic polyvinyl chloride catheter (0.38 mm ID; 0.76 mm OD). The procedure for the surgery was done according to a previously published protocol (Howell and Wilcox, 2001). Subjects were anesthetized with 0.1 mg/kg atropine, 20 mg/kg ketamine, and 2 mg/kg Telazol, and anesthesia was maintained by ketamine supplements during the surgery. The catheter was implanted in either a right or left femoral or external jugular vein to the level of the right atrium using sterile surgical techniques. The catheter was routed subcutaneously to the interscapular region and exited the skin under a protective nylon-mesh jacket. The catheter was filled with heparinized saline and sealed with a stainless-steel obturator when not in use. Subjects were allowed to recover for a week before beginning any experiments. If the catheter became occluded or damaged, the catheter was removed and another catheter was implanted in the same vein if possible, or in another vein.
2.4. In vivo microdialysis
Teflon tubing passed through the ceiling of the chamber to connect the probe inserted into the guide cannula to the perfusion pump. In order to prevent the subject from disturbing the probe or tubing, a Lexan plate was positioned perpendicular to the medial plane of the body just above the shoulders. Prior to surgery, the animal was acclimated to the chair and Lexan plate over several months. Commercially available probes (CMA/11) with a shaft length of 14 mm and active dialysis membrane measuring 4 × 0.24 mm were inserted into the guide cannulae. A Harvard PicoPlus microinfusion pump continuously flushed artificial cerebrospinal fluid (1.0 mM Na2HPO4, 150 mM NaCl, 3 mM KCl, 1.0 mM CaCl2, 1.0 mM MgSO4 and 0.15 mM ascorbic acid) through the probes via FEP Teflon tubing to the probe at a flow rate of 2.0 μL/min for the duration of the experiment.
For systemic NAC in vivo studies, subjects were administered NAC i.m. as a pretreatment in the home cage 3 hr prior to collecting the baseline samples. This pretreatment time point was selected based on previously published studies in rodents (Baker et al., 2003a, Baker et al., 2003b). As a 30-min pretreatment with NAC did not result in any behavioral changes, we decided to focus on the longer pretreatment interval. Probes were implanted into the cannulae 1 hr prior to collecting baseline samples to allow for equilibration. Four consecutive 10-min samples were collected for determination of baseline dopamine concentration. Following collection of baseline samples, a single dose of saline, cocaine or amphetamine was administered i.m. After saline or drug administration, samples were collected every 10 min for 2 hr. All samples were collected in microcentrifuge tubes and immediately refrigerated. Before and after each experiment, probes were tested in vitro to determine the suitability of probe efficiency and performance. Subjects were tested no more frequently than once per week and each site was accessed no more frequently than once every 2 weeks. Under the conditions described, consistent responses to drug treatments have been observed following repeated access to the caudate without significant gliosis (Czoty et al., 2000).
Small-bore, high-performance liquid chromatography (HPLC) and electrochemical detection quantified levels of dopamine according to previously established protocols (Kimmel et al., 2005, Kimmel et al., 2007). The HPLC system consisted of a small-bore (3 mm i.d. × 100 mm) column (MD-150 Analytical, 3 mm × 15 cm, ESA, Inc.,) with a commercially available mobile phase (ESA, Inc., Chelmsford, MA) delivered by an ESA 582 solvent delivery pump at a flow rate of 0.6 ml/min. After loading onto the refrigerated sample tray, samples (20 μl) were automatically mixed with 3 μl of ascorbate oxidase, and 5 μl of the mixture was injected into the HPLC system by an ESA model 542 autosampler. Samples were analyzed within 12 h of collection, remaining either in a refrigerator or in the refrigerated autosampler tray during this time. Electrochemical analyses were performed using an ESA dual-channel analytical cell (model 5040) and guard cell (model 5020, potential=350 mV) and an ESA Coulochem II detector. The potential of channel 1 was set to -150 mV for oxidation, while the potential of channel 2 was set to 275 mV for reduction. A full range of dopamine standards (0.5 - 25 nM) was analyzed before and after each set of samples to evaluate possible degradation of dopamine. Levels of dopamine below 0.5 nM were considered below the limit of detection. A desktop computer collected data and chromatograms were generated by EZChrom Elite software (version 3.1, Scientific Software, Pleasanton, CA). The chromatograms were analyzed using the EZChrom software, comparing the experimental samples with the standards.
2.5. Fixed-interval stimulus termination
Each subject (s117, s170, s171, s177, s181, s185) was trained on a 300s fixed-interval (FI) schedule of stimulus termination with a 3s limited hold (LH). During the 300s FI, the chamber was illuminated with a red light. After the interval elapsed, the animal had 3s to press a response lever and extinguish the red light associated with an impending electric stimulus. When the animal pressed the lever during the 3s LH and extinguished the red light, a white light was illuminated for 15s followed by a 60s time-out. In the absence of a response in the 3s LH, the animal received a brief (200ms) 4-mA electrical stimulus to a shaved area near the tip of the tail. Each session consisted of 13 consecutive FI 300s components, each followed by a 60-s timeout. This schedule has been used to reliably characterize the behavioral-stimulant effects of drugs (Ginsburg et al., 2005). Pretreatment doses of NAC were given via i.m. injection (volume of 0.4-0.8 mL in the thigh) in the home cage 30 min or 3 hr prior to the injection of saline or drug and the initiation of the session. In one group of subjects (s170, s171, s181, s185) with implanted catheters, cocaine was administered i.v. In a separate group of subjects (s117, s170, s171, s177), cocaine or amphetamine was administered i.m. in the thigh.
2.6. Cocaine self-administration
In order to extend the life of the catheter, subjects (s172, s174, s179, s184) were initially trained under a second-order FI 600-s schedule of stimulus termination with a fixed-ratio (FR) 20 components and a 20-s limited hold (LH) as described previously (Ginsburg et al., 2005). When rates and patterns of responding were stable, venous catheters were implanted as previously described. Subsequently, the method of reinforcement was changed such that termination of the red light resulted in injection of 0.1 mg/kg of cocaine and the LH was increased to 200 s. This technique has been effective at maintaining high rates of responding during drug self-administration (Kimmel et al., 2005, Kimmel et al., 2007). Drug experiments began once rates and patterns of responding were stable. Initially, a range of doses of cocaine (0.03–1.0 mg/injection) was substituted for the training dose to establish a full dose-effect curve for cocaine self-administration and to determine the dose that maintained peak rates of responding. Each dose was tested for 5 consecutive sessions, and the order of drug dose was randomized. Subsequently, subjects were maintained on the dose that maintained peak rates of responding (0.1 mg/kg/infusion: s172, s174, s184; 0.3 mg/kg/infusion: s179). During drug interaction studies, saline was administered as a control at either 30 min or 3 hr pre-session for 3 consecutive days (typically Tuesday, Wednesday, and Thursday). Subsequently, 1 dose of NAC was administered 30-min or 3 hr pre-session for 3 consecutive days of the following week. Mean rate of responding during self-administration sessions was determined for individual monkeys by averaging response rate in the presence of the red light during all components of a session. Mean response rate for a given pretreatment dose was determined across the three drug sessions and expressed as a percentage of the mean rate during the 3 days which saline was administered on the previous week.
2.7. Reinstatement
Subjects (s174, s183, s184, s186). were initially trained on a second-order schedule of stimulus termination as described above [FI 10min (FR20: S)]. Once rates of responding were stable, subjects were implanted with an indwelling catheter as described above. The schedule parameters for the reinstatement procedures were the same as those for the self-administration procedures. A full dose-effect curve for cocaine self-administration was established to determine the dose that maintained peak rates of responding before reinstatement studies began (0.1 mg/kg/infusion: s174, s183, s184; 0.3 mg/kg/infusion: s186). Once rates of responding were stable at the maintenance dose for 4-5 days, subjects were then extinguished for at least two days. During extinction, the schedule parameters remained the same, except that the white stimulus light was not illuminated after completion of each FR and the red light remained illuminated, and cocaine was replaced with vehicle (saline) for self-administration. Reinstatement experiments were initiated once the extinction rates were less than 30% of the cocaine-maintained response rates. During the reinstatement experiments, vehicle was available for self-administration and the cocaine-paired stimulus light was restored. Previous studies demonstrated that reinstatement of previously extinguished cocaine self-administration was greatest when cocaine priming was accompanied by presentations of the cocaine-paired stimulus (Spealman et al., 2004). Reinstatement sessions were conducted on three consecutive days utilizing the same pretreatment dose. NAC was administered 30 min or 3 hr prior to administering either an i.v. saline prime or cocaine prime (0.3 or 1.0 mg/kg) in a pseudo-randomized order. The prime was administered 5 minutes prior to the start of the session. Before testing a new pretreatment dose, subjects were allowed to self-administer cocaine until baseline cocaine self-administration rates were re-established, then this behavior was extinguished again to rates of 30% baseline. Baseline rates of responding between reinstatement probes did not vary by more than 20% and were reached within 4-5 days of cocaine self-administration. All subjects readily extinguished within 2 days of implementing extinction conditions. S174 lost his viable catheter sites before completing the 30 min pretreatment experiments.
2.8. Drugs
Cocaine HCl (National Institute on Drug Abuse, Bethesda, MD), d-amphetamine-H2SO4 (Sigma Aldrich Corporation, St. Louis, MO) and NAC (Sigma Aldrich Corporation, St. Louis, MO) were dissolved in 0.9% saline. Drug doses were determined as salts. All i.m. drug injects were administered into the thigh muscle at a volume of 0.4-0.8 mL.
2.9. Statistical analyse
The time-course data from the neurochemical studies and the stimulus termination studies and the overall rate data for the self-administration and reinstatement studies were each analyzed using repeated-measures ANOVAs. When a significant main effect was observed, Tukey’s post hoc multiple comparisons tests were used to determine statistical significance, defined at the 95% level of confidence (p < 0.05.
3. RESULTS
3.1. In vivo microdialysis
Basal levels of dopamine ranged from 2.0 to 8.0 nM, with a mean (±S.E.M.) of 4.3 ± 2.6 nM dopamine, as reported previously (Bauzo et al., 2009b, Czoty et al., 2002, Ginsburg et al., 2005). Two-way ANOVA revealed that cocaine administration during in vivo microdialysis produced a significant interaction between dose and time on extracellular dopamine (F[30,60]=6.156, p<0.001). There was a significant effect of treatment (F[2,4]=9.587, p=0.03 Figure 1A). Thus, cocaine administration (0.3 and 1.0 mg/kg, i.m.) produced a dose-dependent increase in extracellular dopamine to 150% and 300% basal levels, respectively, as measured by in vivo microdialysis. In contrast, saline did not alter extracellular dopamine. Two-way ANOVA revealed that saline administration with or without a pretreatment of NAC did not produce a significant interaction between dose and time on extracellular dopamine (F[30,90]=1.084, p=0.374; Figure 1B). Thus, NAC given as a 3h pretreatment to saline also did not produce a significant change in extracellular dopamine. Conversely, two-way ANOVA revealed a significant interaction between dose and time when a 3h pretreatment of NAC (3.0 or 10 mg/kg, i.m.) was administered prior to cocaine administration (F[45,90]=3.159, p=<0.001; Figure 1C). Furthermore, there was a significant effect of treatment (F[3,6]=7.057, p=0.022). Thus, a 3h pretreatment of NAC (3.0 or 10 mg/kg, i.m.) significantly attenuated cocaine-induced increases in extracellular dopamine. However, posthoc analysis revealed that there was not a significant difference between the two pretreatment doses (p=0.641). Therefore, this effect was not dose-dependent.
Figure 1.
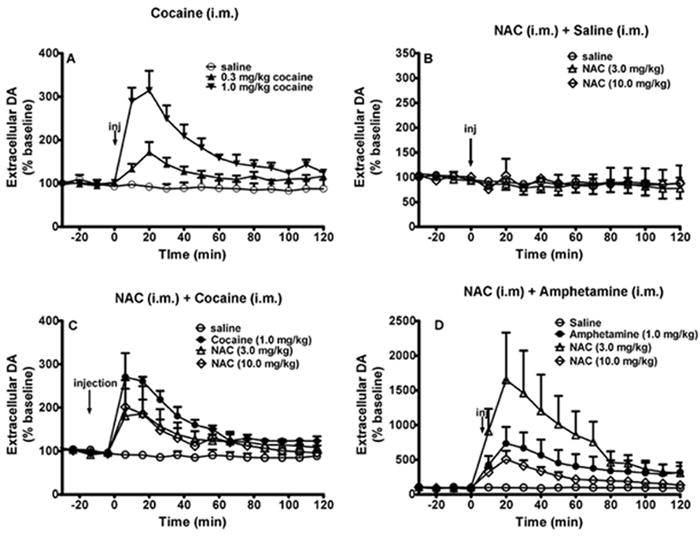
Cocaine administration (0.3 and 1.0 mg/kg, i.m.) dose-dependently increased extracellular dopamine in the caudate (A) of three squirrel monkeys. Neither administration of saline (i.m.) nor of NAC (i.m.) significantly altered extracellular dopamine when administered as a pretreatment to saline (B). However, NAC pretreatment significantly attenuated cocaine-induced increases in extracellular dopamine (C). The lower dose of NAC (3.0 mg/kg) tended to enhance amphetamine-induced increases in extracellular dopamine, while a higher dose of NAC (10 mg/kg) slightly attenuated amphetamine-induced increases in extracellular dopamine (D). Arrows indicate the point at which saline or drug was administered. Data points represent mean ± SEM dopamine levels as a percent of values obtained prior to drug administration. Abscissae: time in minutes. Ordinates: extracellular concentrations of dopamine expressed as a percent of baseline control levels.
Interestingly, NAC did not have similar effects when administered as a pretreatment to amphetamine administration. A two-way ANOVA revealed a significant interaction between dose and time when a 3-hr pretreatment of NAC was administered prior to amphetamine administration (F[30,90]=1.679, p=0.032). Amphetamine (1.0 mg/kg, i.m.) increased extracellular dopamine to about 750% of basal levels which peaked at about 20 minutes post amphetamine administration and returned to baseline at approximately 70 min post amphetamine administration (F[15,45]=1.539, p<0.001, Figure 1D). There was not a significant main effect of treatment (F[2,6]=1.539, 9=0.289). Though not significant, pretreatment with the lower dose of NAC (3.0 mg/kg, i.m.) tended to enhance amphetamine-induced increases in dopamine, whereas a higher dose of NAC (10 mg/kg, i.m.) tended to attenuate amphetamine-induced increases in dopamine (Figure 1D).
3.2. Fixed-interval stimulus termination
In the first set of behavioral experiments, NAC (3.0 - 17 mg/kg, i.m.) was given as a 30-min or 3h pretreatment in subjects trained to respond under a fixed-interval schedule of stimulus termination. Baseline response rates for individual subjects ranged from 0.46 to 1.49 responses/s with a mean (±S.E.M.) of 0.95±0.46 responses/s. Stable rates of responding were maintained throughout the 90-min session following i.v. administration of saline. Two-way ANOVA revealed no significant interaction effect when NAC was administered as a pretreatment to saline administration (30-min: F[42,126]=1.332, p=0.114; 3-hr: F[42,126]=1.110, p=0.324.; Figure 2A,C). Thus, NAC did not altar rates of responding. Though two-way ANOVA also revealed no significant interaction of dose and time when NAC was administered as a pretreatment to cocaine administration (30-min ptx: F[42,126]=1.394, p=0.082; F[42,126]=1.081, p=0.363), there was a significant main effect of time (30-min ptx: F[14,42]=9.207, p<0.001; 3-hr ptx: F[14,42]=9.355, p=<0.001; Figure 2B,D). Rates of responding significantly increased within the first 20 minutes after i.v. administration of cocaine and returned to baseline levels by 60 minutes. However, there was not a significant effect of treatment. Thus, pretreatment with NAC at any dose did not significantly alter cocaine-induced changes in response rates at either pretreatment time point. Thus, attenuation of cocaine-induced increases in extracellular dopamine by NAC did not result in changes in the behavioral-stimulant effects of cocaine. It is important to note that in the current experiment, subjects were given the NAC pretreatments via an i.m. injection and cocaine was administered via an i.v. infusion. While in the microdialysis experiment, all drugs were administered via an i.m. injection.
Figure 2.
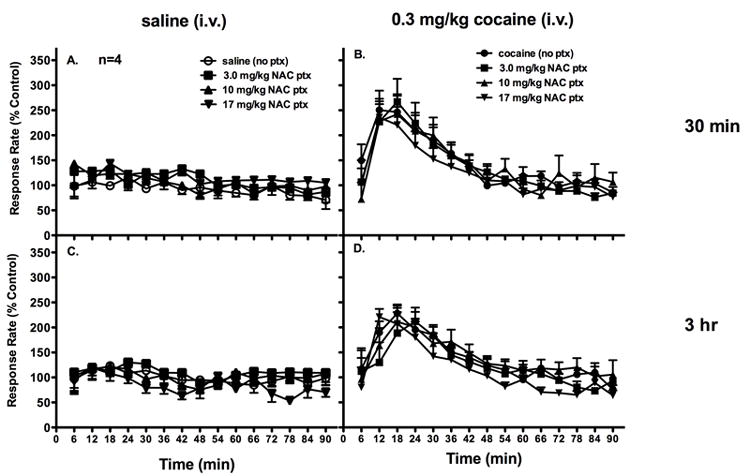
Pretreatment with NAC (i.m.) 30 min or 3 hr prior to i.v. administration of saline did not significantly alter rates of responding under a fixed-interval avoidance schedule (A, C). Pretreatment with NAC (i.m.) 30 min or 3 h prior to cocaine administration did not significantly alter cocaine-induced increases in rates of responding (B, D). Abscissae: time in minutes. Ordinates: rates of responding expressed as a percent of baseline control rates.
In a separate group of subjects, the NAC pretreatments as well as cocaine or saline were administered i.m.. Two-way ANOVA revealed no significant interaction effect when NAC was administered as a 3-hr pretreatment to i.m. saline administration (3-hr: F[28,84]=0.900, p=0.631; Figure 3C). Though there was a significant interaction between dose and time when NAC was administered as a 30-min pretreatment before saline (F[28,179]= 2.237, p=0.003), there was no significant main effect of time (F[14,42]=1.658, p=0.103) or treatment (F[2,6]=0.0591, p=0.943; Figure 3A). Thus, saline administered i.m. did not alter response rates throughout the behavioral session, and NAC pretreatments to saline also did not change response rates (Figure 3A,C). Two-way ANOVA revealed no significant interaction between dose and time when NAC was administered as a 30-min or 3-hr pretreatment to i.m. cocaine administration (30-min: F[28,84]=0.900, p=0.631; 3-hr: F[28,84]=1.155, p=0.301). However, there was a significant effect of time F[14,42]=5.589, p<0.001; 3-hr: F[14,42]=9.337, p<0.001). Cocaine administered i.m. significantly increased rates of responding within the first 20 minutes of the session, then these rates returned to baseline levels by 60 minutes (Figure 3B,D). There was no significant effect of treatment (F[2,6]=1.507, p=0.295; 3-hr: F[2,6]=0.954, p=0.437). Neither 30 min nor 3 hr NAC pretreatments significantly altered cocaine-induced increases in response rates (Figure 3B,D). Therefore, attenuation of cocaine-induced increases in extracellular dopamine by NAC did not result in changes in the behavioral-stimulant effects of cocaine, regardless of the route of administration of cocaine.
Figure 3.
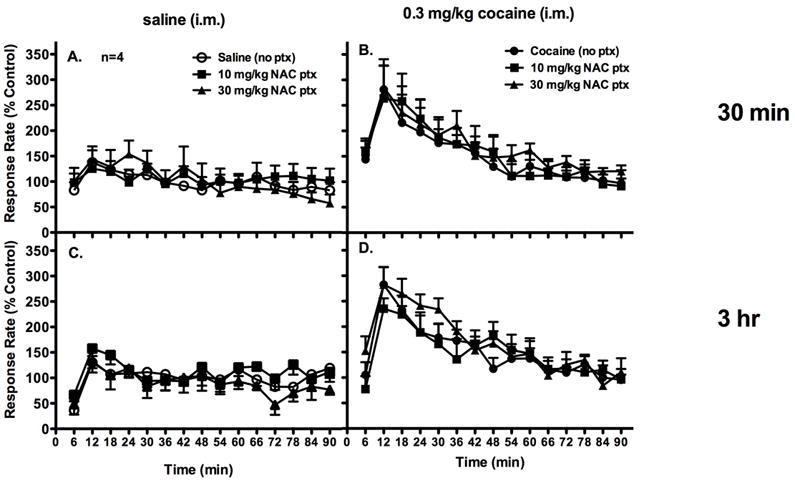
NAC pretreatments (i.m.) did not significantly alter rates of responding under a fixed-interval avoidance schedule when saline was administered i.m. 5 sec prior to the start of the session (A, 30 min ptx; C, 3h ptx). Further, NAC pretreatments did not significantly alter cocaine-induced increases in rates of responding when cocaine (0.3 mg/kg) was administered i.m. 5 sec prior to the start of the session (B, 30 min ptx; D, 3h ptx). Abscissae: time in minutes. Ordinates: rates of responding expressed as a percent of baseline control rates.
Because NAC pretreatments had different effects on the neurochemistry of cocaine and amphetamine in our earlier studies, the same four subjects repeated these experiments and were administered saline or amphetamine (0.1 mg/kg) i.m. Two-way ANOVA revealed no significant interaction effect when NAC was administered as a 30-min or 3-hr pretreatment to i.m. saline administration (30-min: F[28,84]= 0.489, p=0.982; 3-hr: F[28,84]=1.003, p=0.475; Figure 4A,C). Thus, pretreatment with NAC at either time point did not alter rates of responding after saline administration. Two-way ANOVA revealed a significant interaction of dose and time when NAC was administered as a 30-min pretreatment prior to amphetamine (30-min: F[28,84]= 1.761, p=0.025). Though there was no significant interaction when NAC was administered as a 3-hr pretreatment (3-hr: F[28,84]=1.065, p=0.399), there was a significant effect of time (F[14,42]= 5.304, p<0.001; 3-hr: F[14,42]=4.767, p=<0.001). Amphetamine administration increased rates of responding within the first 20 minutes of the session, then these rates returned to baseline levels within 60 min. Two-way ANOVA revealed no significant effect of treatment when NAC was administered as a 3-hr pretreatment to i.m. amphetamine administration (30-min: F[2,6]=0.868, p=0.467; 3-hr: F[2,6]=0.546, p=0.605; Figure 4B,D). Thus, NAC pretreatments did not alter amphetamine-induced increases in rates of responding at either pretreatment time.
Figure 4.
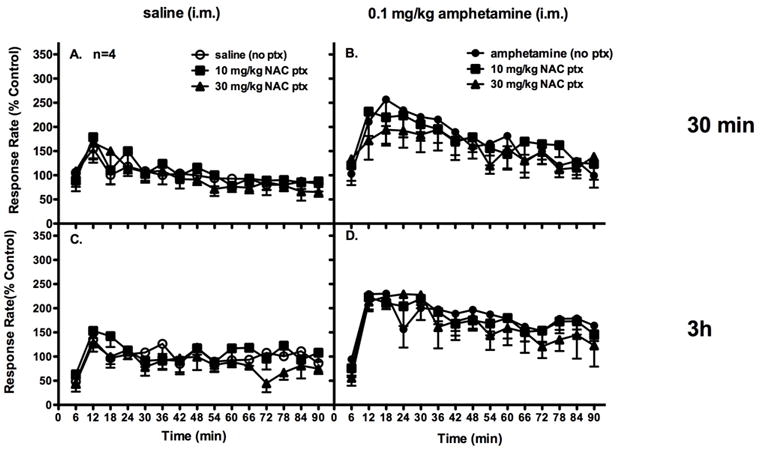
NAC pretreatments (i.m.) did not significantly alter rates of responding under a fixed-interval avoidance schedule when saline was administered i.m. 5 sec prior to the session (A, 30 min ptx; C, 3h ptx). Furthermore, NAC pretreatments did not significantly alter amphetamine-induced increases in rates of responding when amphetamine (0.1 mg/kg) was administered i.m. 5 sec prior to the session (B, 30 min ptx; D, 3h ptx). Abscissae: time in minutes. Ordinates: rates of responding expressed as a percent of baseline control rates.
3.3. Self-administration
To determine if the attenuation of cocaine-induced increases in extracellular dopamine by NAC would result in changes in the reinforcing properties of cocaine, four squirrel monkeys trained on a second-order schedule of cocaine self-administration were pretreated with NAC 30 min or 3 hr prior to the behavioral sessions. During drug interaction studies, baseline responding was determined by administering saline as a pretreatment to the self-administration session for 3 consecutive days and mean rate of responding was averaged across the sessions. The response rates for individual subjects ranged from 0.86 to 1.19 responses/s with a mean (±S.E.M.) of 1.01±0.14 responses/s. One way-ANOVA did not reveal a significant effect of pretreatment dose when NAC was given as a 3-hr pretreatment before cocaine self-administration (3-hr: F[3,15]=1.948, p=0.192; Figure 5). Though one-way ANOVA did reveal a significant effect of dose when NAC was administered as a 30-min pretreatment before cocaine self-administration (30-min: F[3,15]=4.136, p=0.042), posthoc analysis revealed no significant differences between the pretreatment doses and a pretreatment of saline (3.0 mg/kg NAC: p=0.220, 10 mg/kg NAC: p=0.591, 17.0 mg/kg NAC: p=0.801). Thus, pretreatment with NAC did not significantly alter rates of responding during cocaine self-administration.
Figure 5.
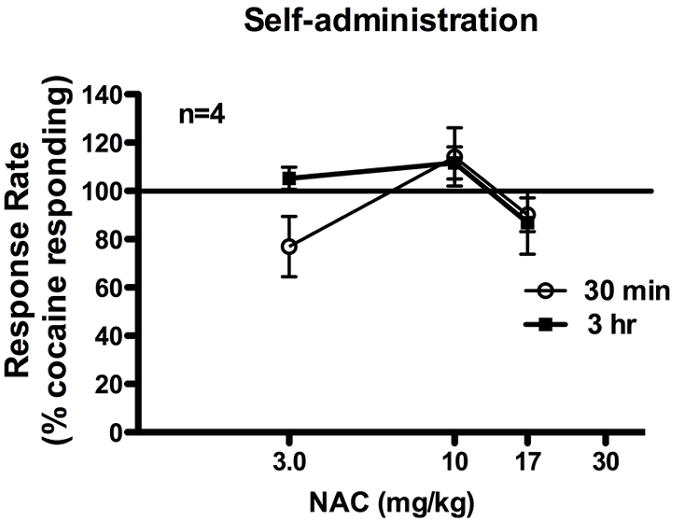
Administration of NAC did not significantly alter rates of responding maintained by a second-order schedule of cocaine self-administration. Each dose of NAC was administered 30 min or 3 hr prior to the start of the self-administration session for three consecutive days. The solid line at 100% represents baseline responding during cocaine self-administration. Abscissae: dose of NAC pretreatment. Ordinates: rates of responding expressed as a percent of baseline control rates.
3.4. Reinstatement
In the reinstatement studies, animals were initially allowed to self-administer their maintenance dose of cocaine until their responding was stable. Response rates for individual subjects at their maintenance dose of cocaine ranged from 0.84 to 3.04 responses/s with a mean (±S.E.M.) of 1.88 ± 0.91 responses/s. When saline was substituted for cocaine during the self-administration session, subjects extinguished responding within two behavioral testing sessions. One-way ANOVA revealed a significant effect of treatment when non-contingent priming injections of cocaine were administered (30-min: F[3,6]=57.841, p=0.001; 3-hr: F[3,6]=23.747, p=0.001; Figure 6). Cocaine (0.3 or 1.0 mg/kg, i.v.) produced a dose-dependent reinstatement of previously extinguished self-administration (Figure 6). However, two-way ANOVA revealed no significant interaction between pretreatment dose of NAC and non-contingent priming dose of cocaine (30-min: F[6,12]=1.185, p=0.377, 3-hr: F[6, 18]=0.971, p=0.472). Thus, NAC pretreatments at either time point did not significantly alter the reinstatement effects of either dose of cocaine.
Figure 6.
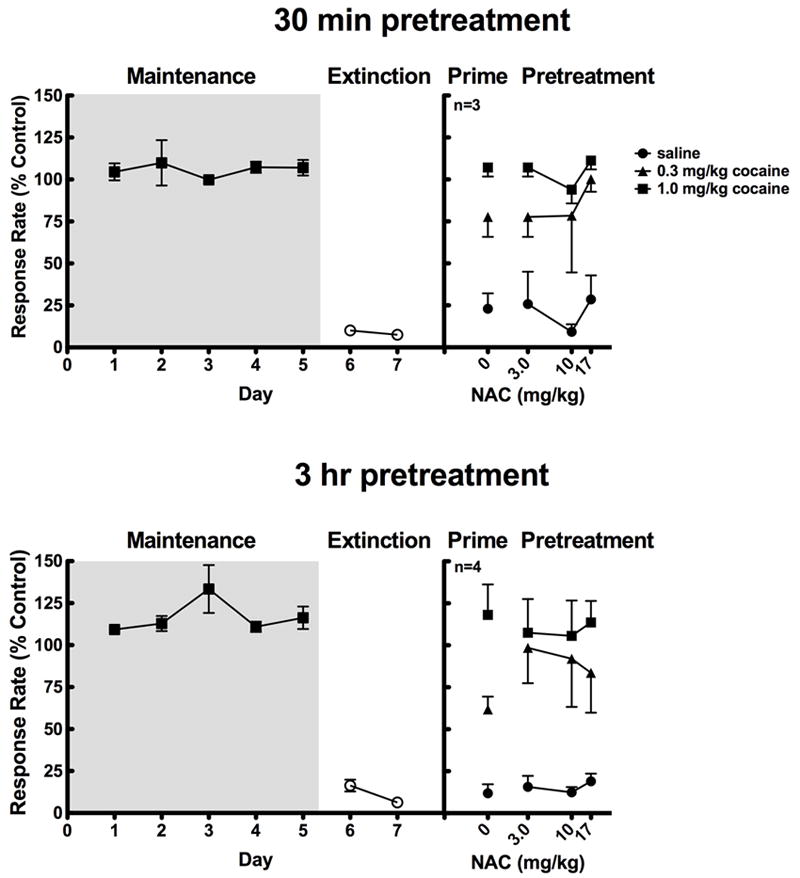
Pretreatment with NAC did not significantly alter reinstatement of previously extinguished cocaine self-administration.Abscissae: day of treatment and pretreatment dose of NAC prior to reinstatement tests. Ordinates: rates of responding expressed as a percent of baseline control rates.
4. DISCUSSION
The purpose of the current study was to determine the effects of the cystine-glutamate transporter enhancer, NAC, on the neurochemical and behavioral effects of cocaine and amphetamine in nonhuman primates. NAC significantly attenuated cocaine-induced increases in extracellular dopamine in the caudate without an apparent alteration of basal extracellular dopamine. Acute systemic administration of NAC pretreatment did not completely abolish cocaine-induced increases in dopamine in this region, but decreased the effectiveness of cocaine such that the effects of 1.0 mg/kg cocaine following NAC pretreatment were equivalent to the effects of 0.3 mg/kg cocaine alone previously reported in squirrel monkeys (Bauzo et al., 2009a, Ginsburg et al., 2005). This lower dose of cocaine (0.3 mg/kg) is one that has induced behavioral-stimulant effects in nonhuman primates.
However, this attenuation of cocaine-induced changes in neurochemistry did not translate to changes in the behavioral effects of cocaine. Thus, a more significant attenuation of cocaine-induced increases in extracellular dopamine may be necessary for changes in behavior to be expressed. Higher doses of cocaine and amphetamine were used in the present microdialysis studies than in the behavioral studies because we wanted to be able to measure the neurochemical changes following these drugs. Lower doses would result in smaller changes in neurochemistry, which might not be measurable. In addition, higher doses of these stimulants fall on the descending limb of the dose-effect curve, with lower rates of responding, which would mask the effects of NAC pretreatment.
The lack of effect of NAC pretreatment on the behavioral changes induced by cocaine suggests that the endogenous glutamatergic tone on mGluR2/3 receptors may not be critical to the cocaine-induced behaviors observed in the current studies in nonhuman primates, which contrasts the results observed in rodents. Rodent studies have shown that NAC administration prevents reinstatement of extinguished cocaine self-administration (Baker et al., 2003b, Kau et al., 2008, Moran et al., 2005, Zhou and Kalivas, 2008) and that this attenuation can be blocked by selective mGluR2/3 antagonists (Moran et al., 2005). Earlier studies in our laboratory indicated that direct activation of mGluR2/3 receptors with the selective agonist, LY379268, did not attenuate the abuse-related effects of cocaine in nonhuman primates (Bauzo et al., 2009a). Thus, the present data provide further evidence for the importance of evaluating drug interactions in nonhuman primate models.
The effects of NAC on amphetamine-induced increases in dopamine were inconsistent and not statistically significant. The lower dose tended to enhance the effects of amphetamine, whereas the higher dose of NAC tended to attenuate the effects of amphetamine, as observed with cocaine. The differential effects of NAC on cocaine and amphetamine-induced increases in extracellular dopamine in the caudate may have been partially due to the differences in the maximal neurochemical effect produced by these two psychostimulants. Since the effects of NAC on amphetamine-induced changes in dopamine were equivocal, we focused on examining the effects of NAC on the behavioral-stimulant and reinforcing effects of cocaine. However, further studies in which the doses of the psychostimulants are matched for effectiveness are needed to determine the mechanism underlying the different effects of NAC observed in the present studies.
NAC ostensibly increases extrasynaptic glutamate via actions at the cystine-glutamate transporter, which results in increased signaling on mGluR2/3 receptors and decreased vesicular release of glutamate. This decreased vesicular release of glutamate would lead to decreased excitation of dopamine neurons. Given that the effects of cocaine on extracellular dopamine are impulse-dependent, reduced presynaptic tone should reduce the neurochemical and subsequent behavioral effects of cocaine. In contrast, the effects of amphetamine on extracellular dopamine are impulse-independent and less likely to be influenced by presynaptic tone. Thus, the indirect effects of NAC on the excitation of dopamine neurons may help to explain the differential effects observed with cocaine and amphetamine.
The current study provides the first evidence that NAC can attenuate cocaine-induced increases in striatal dopamine in vivo in nonhuman primates. In support of these results, two recent clinical reports indicate that NAC is effective in reducing cocaine craving and use in human user (LaRowe et al., 2007, Mardikian et al., 2007). It should be noted that the latter study was open-label, weakening the significance of the results (Karila et al., 2011), and these studies have not been replicated, despite the ready availability of over-the-counter NAC. However, in contrast to previous behavioral studies in rodents, NAC had no significant effect on the behavioral effects of cocaine. The behavioral-stimulant, reinforcing and reinstatement effects of cocaine were not influenced by NAC administration even when dose, route of administration and pretreatment time were varied systematically. The generality of the reported behavioral effects of NAC in rodents do not extend to nonhuman primates under the conditions employed in the current studies. Despite this discrepancy, the current neurochemical data and the recent clinical results provide support for the glutamatergic system as a pharmacotherapeutic target for treating psychostimulant addiction.
Highlights.
-
◆
N-acetyl-L-cysteine (NAC) is a cystine-glutamate transporter enhancer.
-
◆
We hypothesized increasing transporter activity would decrease the neurochemical and behavioral effects of cocaine.
-
◆
NAC reduced cocaine-induced increases in dopamine in squirrel monkey caudate.
-
◆
NAC did not alter self-administration of cocaine or reinstatement of this behavior.
-
◆
Here, drug interactions altered neurochemistry but not behavior.
Acknowledgments
This research was supported by U.S. Public Health Service grants DA00517 (LLH), DA12514 (LLH), DA021476 (RMB) and RR00165 (Division of Research Resources, National Institutes of Health). The authors would like to thank Mi Zhou and Marjorie Sen for their expert technical assistance.
Footnotes
DISCLOSURE STATEMENT None of the authors have any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence our work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rayna M. Bauzo, Email: bauzo@ufl.edu.
Heather L. Kimmel, Email: hlkimme@emory.edu.
Leonard L. Howell, Email: lhowell@emory.edu.
References
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003a;6:743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Annals of the New York Academy of Sciences. 2003b;1003:349–51. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: Modifications by repeated cocaine administration. Amino Acids. 2002a;23:161–2. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002b;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauzo RM, Kimmel HL, Howell LL. Interactions between the mGluR2/3 agonist, LY379268, and cocaine on in vivo neurochemistry and behavior in squirrel monkeys. Pharmacol Biochem Behav. 2009a;94:204–10. doi: 10.1016/j.pbb.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauzo RM, Kimmel HL, Howell LL. Interactions between the mGluR2/3 agonist, LY379268, and cocaine on in vivo neurochemistry and behavior in squirrel monkeys. Pharmacology, biochemistry, and behavior. 2009b;94:204–10. doi: 10.1016/j.pbb.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–5. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Repeated cocaine administration into the rat ventral tegmental area produces behavioral sensitization to a systemic cocaine challenge. Behav Brain Res. 2001;126:205–9. doi: 10.1016/s0166-4328(01)00239-x. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–7. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology (Berl) 2000;148:299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Gergen JA, MacLean PD National Institutes of Health (U.S.) A stereotaxic atlas of the squirrel monkey’s brain (Saimiri sciureus) Bethesda, Md.: U.S. Dept. of Health Education and Welfare Public Health Service National Institutes of Health; 1962. [Google Scholar]
- Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, Howell LL. Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys. Pharmacol Biochem Behav. 2005;80:481–91. doi: 10.1016/j.pbb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. Methods of behavior analysis in neuroscience. Boca Raton: CRC Press; 2001. [Google Scholar]
- Karila L, Reynaud M, Aubin HJ, Rolland B, Guardia D, Cottencin O, et al. Pharmacological treatments for cocaine dependence: is there something new? Curr Pharm Des. 2011;17:1359–68. doi: 10.2174/138161211796150873. [DOI] [PubMed] [Google Scholar]
- Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaineinduced drug seeking. Neuroscience. 2008;155:530–7. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, Ginsburg BC, Howell LL. Changes in extracellular dopamine during cocaine self-administration in squirrel monkeys. Synapse. 2005;56:129–34. doi: 10.1002/syn.20135. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, O’Connor JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86:45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–7. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:389–94. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of nucleus accumbens mediates cocaineinduced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–93. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–58. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–60. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV. Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. Glia. 2001;34:27–38. doi: 10.1002/glia.1037. [DOI] [PubMed] [Google Scholar]
- Puka-Sundvall M, Eriksson P, Nilsson M, Sandberg M, Lehmann A. Neurotoxicity of cysteine: interaction with glutamate. Brain research. 1995;705:65–70. doi: 10.1016/0006-8993(95)01139-0. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Lee B, Tiefenbacher S, Platt DM, Rowlett JK, Khroyan TV. Triggers of relapse: nonhuman primate models of reinstated cocaine seeking. Nebr Symp Motiv. 2004;50:57–84. [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biological psychiatry. 2008;63:338–40. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


