SUMMARY
Background
Epithelial tissues undergo extensive collective movements during morphogenesis, repair and renewal. Collective epithelial cell migration requires the intercellular coordination of cell-cell adhesions and the establishment of anterior-posterior polarity, while maintaining apical-basal polarity, but how this is achieved at the molecular level is not well understood.
Results
Using an RNAi-based screen to identify Rho family GTPase regulators required for the collective migration of human bronchial epithelial cells, we identified myosin-IXA (gene name: Myo9a). Depletion of myosin-IXA, a RhoGAP and actin motor protein, in collectively migrating cells led to altered organization of the actin cytoskeleton and tension-dependent disruption of cell-cell adhesions, followed by an inability to form new adhesions resulting in cell scattering. Closer examination revealed myosin-IXA is required during the formation of junction-associated actin bundles soon after cell-cell contact. Structure-function analysis of myosin-IXA revealed that the motor domain is necessary and sufficient for binding to actin filaments, while expression of the RhoGAP domain partially rescued the cell scattering phenotype induced by myosin-IXA depletion. Finally, a FRET biosensor revealed a significant increase in Rho activity at nascent cell-cell contacts in myosin-IXA depleted cells compared to controls.
Conclusion
We propose that myosin-IXA locally regulates Rho and the assembly of thin actin bundles associated with nascent cell-cell adhesions and that this is required to sustain the collective migration of epithelial cells.
INTRODUCTION
Collective cell migration is an important process in tissue morphogenesis, regeneration and in tumor dissemination [1]. The collective migration of epithelia is particularly interesting, since cells maintain cell-cell adhesion and apical-basal polarity, while establishing anterior-posterior polarity to promote directed migration. Collective epithelial migrations can be seen throughout development, for example in the elongation of mammary ducts [2], or the formation of kidney nephrons [3]. During zebrafish gastrulation and in the migration of Drosophila border cells, cell-cell adhesion is essential for directional migration [4, 5]. A highly cooperative behavior has been seen during the migration of cancer cells in in vitro assays, while in vivo imaging of primary breast tumors in mice has revealed collective migration of cancer cells towards and into lymph nodes [6–9].
The mechanisms that regulate cooperativity between cells during collective migration are not well characterized. The assembly and disassembly of actin filaments is known to be the major driving force for cell migration, and actin-driven protrusive activity in the basal plane has been observed at the front of leading edge cells and follower cells, during epithelial cell migration [10]. At the apical surface of epithelial cells, the actin cytoskeleton interacts intimately with cell-cell junction proteins, such as ZO-1 and vinculin. One suggestion is that communication between cells is transmitted by mechanical tension induced by leader cells acting through cell-cell contacts and affecting the actin cytoskeleton [11–13]. In combination with actin-based, myosin motor proteins, this could facilitate the transmission of tensile forces across adjacent cells [14–16] Alternatively, biochemical signals could mediate coordinated changes in migrating cells, as during planar cell polarity (PCP), where molecular changes can be induced across a tissue, even in the absence of migration [17, 18].
Rho GTPases are important regulators of the actin cytoskeleton in eukaryotic cells [19]. They control many aspects of cell behavior, but in particular are required for cell migration and for the formation of cell-cell junctions. FRET studies have revealed that Rac1 is recruited by E-cadherin during the initiation of cell-cell contact formation in epithelial cells and is required to promote actin polymerization [20–22]. RhoA and Cdc42 are activated at these early stages, as well as during cell-cell contact expansion [21–23]. Several guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) that regulate Rho proteins, and several downstream effectors have been implicated in the assembly of cell-cell junctions [24–27]. Whether they are required to facilitate collective migration, however, is not known.
We have used an siRNA screen to identify regulators of the Rho family that are required for junctional integrity in a human bronchial epithelial cell line (16HBE) undergoing collective cell migration, and identified myosin-IXA, a RhoGAP and actin-binding motor protein [28]. In the absence of myosin-IXA, 16HBE cells have an altered actin cytoskeleton and are unable to maintain their adherens junctions during migration leading to cell scattering. Mutational studies of myosin-IXA revealed that the motor domain is required for its association with actin bundles, while the RhoGAP activity can partially rescue cell scattering induced by myosin-IXA depletion. Finally, a FRET biosensor revealed a significant increase in RhoA activity at nascent cell-cell contacts in myosin-IXA-depleted cells compared to control. We conclude that myosin-IXA is an important negative regulator of Rho at nascent cell-cell junctions and is required for collective migration of cells in the bronchial epithelium.
RESULTS
Collective cell migration in bronchial epithelial cells
Time-lapse analysis of cultured 16HBE cells revealed important features indicative of collective cell behavior: (i) complex cell flows within a monolayer (Figure 1A, arrows 2-3; Movie S1) and (ii) coordinated movement of individual islands (Figure 1B). Within the monolayer, cell groups stream (Figure 1A, arrows), with similar directional trajectories, while in sparse cultures, cell islands move directionally as a single unit over period of a few hours. Migration rates of cells within the monolayer and in an island are similar (0.6–0.9 μm/min). These observations indicate a highly cooperative mode of collective migration in 16HBE cultures.
Figure 1. Identification of myosin-IXA as a regulator of collective epithelial migration.
(A) Migration of 16HBE cells in a monolayer is highly coordinated (140 min timecourse). Regions 1, 2 and 3 (black arrows) are streams distinguished by parallel tracks of cell groups (Movie S1). (B) Cell islands migrate in a highly coordinated manner (white arrow), with almost parallel cell tracks (140 min timecourse). Bar = 100 μm. (C) Scattering of island cells after smartpool siRNA knockdown of RhoA, RhoC or Cdc42. Bar = 50 μm. (D) Schematic of 96-well migration assay used in siRNA screen (insert diameter = 1.5 mm). (E) Myosin-IXA depletion with siRNA inhibits forward migration. (F) Myosin-IXA depletion with siRNA causes cell scattering of migrating 16HBE islands (Movie S2). The initial (green) and last (red) position of the island is outlined. Bar = 30 μm. (G) Quantification of cell scattering. 4 independent experiments, ± SEM. ***, P = 0.0003, unpaired t test.
Identification of myosin-IXA in a screen for collective migration
To determine whether Rho GTPases are required for collective migration, RhoA, RhoC and Cdc42 were depleted from 16HBE cells using siRNA, and found to disrupt cell-cell contacts leading to cell scattering (Figure 1C). This indicates that Rho and Cdc42 are not required for migration per se, but are required to maintain collective migration. To identify regulators of Rho GTPases important for collective migration, a library of siRNA SMARTpools (each pool = 4 distinct siRNAs) targeting the 67 known Rho family GAPs was screened using a 96-well plate migration assay (Platypus Technologies) (Figure 1D). Myosin-IXA depletion caused a severe inhibition of forward cell migration (Figure 1E) (area of free space = 1.9 mm2 ± 0.5) similar to Cdc42 depletion (2.2 mm2 ± 0.2), when compared to parental cells (0.02 mm2 ± 0.01), or Lamin A/C depleted cells (0.05 mm2 ± 0.03) (3 independent experiments).
Time-lapse microscopy revealed that myosin-IXA depletion induced cell scattering in migrating islands (Figure 1F–G, 4h timecourse; Movie S2) and in scratched monolayers (Figure S1A; Movie S3). Wound edge advancement was inhibited by 90% when compared to control cells (Figure S1B). Myosin-IXA depletion led to uncoordinated migration in monolayers and islands without significant changes in cell migration rates (Figure S1C–D).
Validation of myosin-IXA phenotype
The cell scattering phenotype was induced with siRNA duplex-1 and -2, while duplex-3 and -4 gave a partial effect (Figure 2A and data not shown), in good agreement with the extent of mRNA depletion, as judged by qRT-PCR (Figure 2B), and protein levels, as judged by depletion of the expected band of 300 kDa on western blots (Figure 2C). To validate the phenotype, rescue experiments were performed. Stable pools of cells expressing EYFP-actin (control), or EGFP-rat-myosin-IXA were isolated. The level of EGFP-rat-myosin-IXA expression detected by live cell imaging (Figure 2D) was variable between individual cells, but nevertheless cell scattering induced by siRNA duplex-1 was significantly inhibited (Figure 2E–G). qRT-PCR analysis confirmed the efficiency of endogenous myosin-IXA depletion in this experiment (Figure 2F).
Figure 2. Validation of myosin-IXA phenotype.
(A) Two siRNA duplexes and the SMARTpool cause cell scattering. Bar = 50 μm. (B) mRNA quantification by qRT-PCR after siRNA treatment. 3 independent experiments, ± SEM. (C) Western blot analysis of myosin-IXA siRNA depletion. (D) 16HBE cells stably expressing EGFP-rat-myosin-IXA. Bar = 30 μm. (E) Migration of EGFP-rat-myosin-IXA-expressing cells before and after transfection with myosin-IXA siRNA. 16HBE stable pools expressing EYFP-actin were used as control. siRNA targeting Lamin A/C (control) or myosin-IXA duplex-1 were used. Bar = 100 μm. (F) Quantification of endogenous human and exogenous rat myosin-IXA mRNA by qRT-PCR. Depletion of endogenous myosin-IXA by duplex-1 siRNA is seen in control cells and EGFP-rat-myosin-IXA-expressing cells. ± SD, triplicate. (G) Quantification of cell scattering in control and rat myosin-IXA expressing cells, after depletion of endogenous myosin-IXA. 2 independent experiments, ± SEM, *, P = 0.0176, unpaired t test.
Myosin-IXA accumulates at nascent cell-cell contacts
To explore the function of the protein at cell-cell contacts, immunostaining revealed the endogenous myosin-IXA and ectopic expressed EGFP-Myosin-IXA localize at cell-cell contacts in 16HBE monolayers (Figure S2A–C) [29]. Careful linescan analysis revealed that myosin-IXA co-localizes with ZO-1, but not with E-cadherin. After myosin-IXA depletion, cells that still remained associated with neighbors showed a loss of ZO-1 staining, while E-cadherin adhesions became irregularly shaped (radial and punctate), in contrast to the tangential linear contacts observed in control cells (data not shown). Radial cell-cell contact geometry is characteristic of high tension between cells and time-lapse microscopy confirmed myosin-IXA-depleted cells pull away from adjacent cells (Movie S2). These results suggest myosin-IXA is involved in stabilizing junctional complexes. In an analysis of cell-cell collisions, EGFP-myosin-IXA (Figure S2D,F), but not EGFP (Figure S2E), accumulates in regions of overlapping lamellipodia and filopodia, where nascent junctions form. The accumulation of myosin-IXA at nascent junctions can also be seen in calcium switch experiments (Figure S2G).
Myosin-IXA is required for the assembly of radial actin bundles at cell-cell contacts
Cell scattering induced by myosin-IXA depletion could be a consequence of at least two events: (i) loss of mature cell-cell contacts and (ii) inability to reform new adhesions as cells move relative to each other (Movie S2). To examine the role of myosin-IXA in contact formation, stable pools of cell expressing EYFP-actin, or GFP-E-cadherin were isolated and the assembly of cell junctions monitored after cell-cell collisions. In control cells, an overlapping zone between two lamellipodia formed within the first 3 min of contact (Figure 3A, arrows, Movie S4). During a period of 2–4 minutes post-collision, 16HBE cells established cell-cell contact expansion (Figure 3B, upper graph). In contrast, in colliding cells depleted of myosin-IXA, cell-cell contact expansion was inhibited and cells were unable to form an overlapping zone (Figure 3A, arrowheads, Figure 3B; Movie S4). The average area of lamellipodial overlap in myosin-IXA-depleted cells is about 4 times less than in control (Figure 3B, lower graph). These data indicate that myosin-IXA is important during lamellipodial overlapping and early stabilization of cell-cell contacts.
Figure 3. Myosin-IXA is required for junctional radial actin bundle formation.
(A) Time-lapse images of colliding 16HBE cells show lamellipodial overlaps (arrows) inhibited in myosin-IXA-depleted cells (arrowheads) (siMyosin-IXA) (Movie S4). Min:sec; bar = 10 μm. (B) Cell-cell contact stability was quantified. ± SEM, 3 independent experiments, 13 collisions analyzed. The inability of siRNA smartpool myosin-IXA-depleted cells to establish stable contacts can also been seen in the area of lamellipodial overlap. 3 independent experiments, ± SEM. **, P=0.004, unpaired t test. (C) Epifluorescence microscopy of cells expressing EYFP-actin. Collision between control lamellipodia leads to actin enrichment (upper panel arrows) followed by the formation of radial actin bundles bridging two cells together (arrowheads). Smartpool siRNA myosin-IXA depletion does not abolish actin enrichment (lower panel arrows), but inhibits radial actin bundle formation (Movie S5). Min:sec, bar = 5 μm. (D) Schematic summary of time-lapse sequences in (C). Actin is shown in red. (E) Epifluorescence microscopy of cells expressing EYFP-actin and GFP-E-cadherin. Development of actin radial fingers (arrows) during collision between a fluorescent and a non-florescent cell (outlined with blue line) in control (Movie S6) and myosin-IXA-depleted cells (Movie S7). In smartpool myosin-IXA-depleted cells, E-cadherin clustering (arrowheads) was observed between colliding cells, but without significant lamellipodial overlap. Min:sec; bar = 5 μm. (F) Quantification of actin fingers size. 3 independent experiments, 24 cell-cell contact zones analyzed. *, P=0.036, unpaired t test. Top and bottom of a box indicates min and max values, line is the mean.
Time-lapse sequences of EYFP-actin expressing cells revealed further details of cell-cell interactions in 16HBE cells upon collision. In control cells, junctional actin accumulated at contact sites within the first 2 min of collision (Figure 3C, upper panels, arrows) and over time, this transformed into radial actin bundles, or actin fingers, bridging the two colliding cells (Figure 3C, arrowheads, Movie S5). After myosin-IXA depletion, junctional actin initially accumulated similar to control (Figure 3C, lower panels, arrows), but although some minor bundles could be seen, these bundles did not bridge two colliding cells and cells retracted from each other (shown schematically in Figure 3D).
The formation of radial bundles or actin fingers can best be observed when imaging a collision between a fluorescent and a non-fluorescent cell (Figure 3E, arrows, blue line outlines non-fluorescent cell). In control cells (Movie S6), actin fingers emerged from an overlapping lamellipodium formed on top of its neighbor, supporting a recently proposed asymmetric push-pull mechanism of contact formation [12]. These filopodia-like structures contained GFP-E-cadherin signal (Figure 3E, arrowheads). In myosin-IXA-depleted cells (Movie S7), actin fingers either did not form or if they did, they were very small (Figure 3E, arrow in EYFP-actin panel, Figure 3F). GFP-E-cadherin clustering did occur (Figure 3E), but clusters were unstable and disappeared as cells moved apart. These results suggest that myosin-IXA regulates the formation of cell-cell contacts via remodeling of the actin cytoskeleton.
Myosin-IXA localizes with actin filaments at the leading edge
To examine further the relationship between myosin-IXA and actin, we examined individual 16HBE cells. EGFP-myosin-IXA localized in puncta associated with the prominent marginal bundle (MB) of actin filaments at the periphery (a pattern similar to non-muscle myosin II, but different from myosin IXB, which accumulated more diffusely) (Figure S3A) [30]. Myosin-IXA puncta in lamellipodia underwent retrograde flow (Figure S3B), while live imaging of cells co-expressing EGFP-myosin-IXA and mDsRed-actin, revealed co-localization of myosin-IXA puncta with actin bundles (Figure S3C; top panels), which were disrupted with latrunculin (Figure S3C, lower panels), or the Rho kinase inhibitor Y-27632 (not shown).
To investigate the relationship between myosin-IXA and actin filament organization, myosin-IXA was depleted in EYFP-actin/16HBE cells. Whereas lamellipodia in control migrating cells have a smooth contour, in myosin-IXA-depleted cells, lamellipodia have irregular curvature and are rich in ruffles (Figure S3D). In addition, myosin-IXA depletion promoted reorganization of the marginal bundle into multiple actin bundles oriented perpendicular to the leading edge (Figure S3E, arrowheads). We conclude that myosin-IXA interacts with contractile acto-myosin filaments and is involved in organizing actin filaments at the leading edge.
Myosin-IXA is recruited to actin bundles through its motor domain
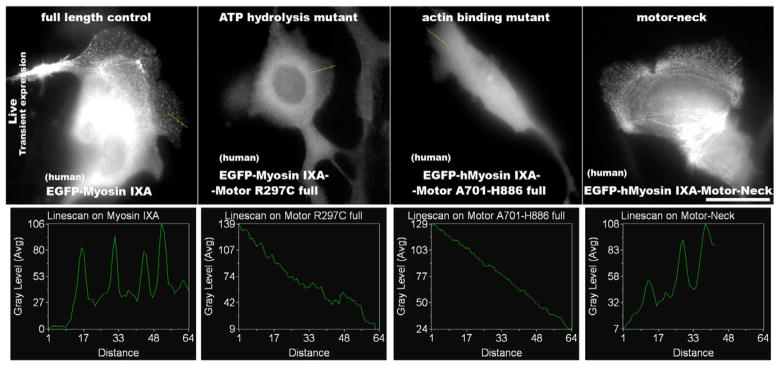
To characterize myosin-IXA function further, mutants of EGFP-human-myosin-IXA were generated (Figure S4) and expressed in 16HBE cells. The role of myosin-IXA motor function was evaluated using the ATP hydrolysis mutant, R297C, which is analogous to a myosin-IXB motor mutant, R295C [30]. Unlike wild type EGFP-myosin-IXA, EGFP-myosin-IXA-R297C showed almost no localization to actin bundles, with most cells showing a cytoplasmic diffuse distribution (Figure 4, panels 1–2) suggesting that a functional motor domain is necessary to target myosin-IXA to actin. The punctate distribution of the wild type, but not the motor mutant protein, is clearly seen in the linescan analysis (Figure 4, lower panels).
Figure 4. Motor domain is necessary and sufficient for targeting myosin-IXA to actin bundles.
16HBE cells were microinjected with full-length EGFP-myosin-IXA, EGFP-myosin-IXA-motor R297C, EGFP-myosin-IXA-motor (deletion aa701-886), or EGFP-myosin-IXA-motor-neck plasmid expression constructs. Fluorescent cells were imaged live. Bar = 30 μm. Linescans through lamellipodia (yellow line, top panels) were used to analyze presence of EGFP puncta shown as peaks on graphs in the lower panels. Note, clear peaks found in full-length EGFP-myosin-IXA and EGFP-myosin-IXA-motor-neck expressing cells. Distance is in pixels.
Class-IX myosins are single-headed plus-end directed motors and have unique two-domain-motor separated by a large loop-2 insertion important for regulation of myosin-IX affinity to actin (Figure S4A) [30]. Deletion of this insertion (A701-H886 full) abolished binding to actin bundles (Figure 4, 3rd panel). In contrast, expression of the N-terminus of myosin-IXA (Motor-Neck) led to a cellular distribution similar to full-length myosin-IXA (Figure 4, 4th panels). This indicates that a functional motor domain is necessary and sufficient for localization of myosin-IXA to peripheral actin bundles.
Functional analysis of RhoGAP domain
Individual cells depleted of myosin-IX showed blebbing and abnormal protrusive activity (Figure 5A, arrows), consistent with the loss of RhoGAP activity leading to Rho hyperactivation at the cell periphery. To confirm that increased Rho activity contributes to the myosin-IXA depletion phenotype, Y-27632 or blebbistatin were used to inhibit Rho-kinase, or myosin-II, respectively. Inhibition of acto-myosin contractility in myosin-IXA-depleted cells led to cell flattening (Figure 6A, arrows) and disappearance of the blebs and protrusive swellings seen in the absence of the inhibitors (Figure 6A, arrowheads). Furthermore, upon collision and in the presence of inhibitor, cells maintained some contact and did not retract from each other (Figure 6A,B, Movie S8).
Figure 5. Functional analysis of myosin-IXA RhoGAP domain.
(A) Epifluorescence or DIC microscopy of cells expressing EYFP-actin. Myosin-IXA depletion by siRNA causes blebbing and lamellipodia swellings (arrows). (B) Confocal and DIC images of a cell expressing full-length, EGFP-rat myosin-IXA showing the formation of long protrusions (arrows). Expression of EGFP-myosin-IXA-deltaGAP has little effect on normal cell morphology. Bar = 30 μm. (C) Diffuse cytoplasmic localization of EGFP-myosin-IXA-neck-tail leads to dramatic morphological changes, with long tails (arrow) characteristic of strong Rho inhibition. This phenotype is not induced by a GAP-defective mutant (R2098M) of EGFP-myosin-IXA-neck-tail. Live cell imaging by epifluorescence microscopy. Linescans (right panels) through lamellipodia were used to quantify presence of EGFP puncta shown as peaks on graphs. Note, absence of clear peaks found in cells expressing either EGFP-myosin-IXA-neck-tail or its GAP-defective mutant (R2098M). Distance is in pixels.
Figure 6. Rho-dependent activities of myosin-IXA.
(A) Partial rescue of cell-cell contact stability in myosin-IXA-depleted cells (smartpool siRNA) after inhibiting Rho-dependent acto-myosin contractility, with Rho-kinase inhibitor Y-27632 (5μM). Cells with swollen lamellipodia (arrowheads) and rounded cell body in myosin-IXA-depleted cells become flattened (arrows) after treatment with Y-27632 (Movie S8). Time is in min:sec. Bar = 30 μm. (B) Quantification of rescue of cell-cell contact stability by Y-27632. 2 independent experiments, ± SEM. (C) Partial rescue of cell-cell contact stability by RhoGAP expression (EGFP-tail) (lower panels), but not by motor-neck expression (EGFP-Motor-Neck) (upper panels) in myosin-IXA-depleted cells. Depletion of endogenous myosin-IXA in EGFP-Motor-Neck expressing line (duplex-1) and in EGFP-tail expressing line (duplex-2). Upon cell-cell collision, cells remain attached for up to 1 h (arrow) (Movie S9). Min:sec; bar = 30 μm. (D) Quantification of rescue of cell-cell contact stability. 2 independent experiments, ± SEM. (E) Partial rescue of cell scattering phenotype in myosin-IXA-depleted cells after expression of EGFP-tail, but not EGFP-motor-neck, constructs. 2 independent rescue experiments, ± SEM. *, P = 0.03, unpaired t test.
The overexpression of full-length myosin-IXA, on the other hand, resulted in the formation of long processes consistent with downregulation of Rho activity, whereas expression of myosin-IXA lacking RhoGAP domain (myosin-IXA-deltaGAP, Figure S4A) had little effect of cell morphology (Figure 5B) [31]. Expression of a construct lacking the motor domain (Neck-Tail, Figure S4A) induced a complete loss of actin bundles and dramatic morphological changes (Figure 5C, arrow), while a similar construct but with an inactivating point mutation in the GAP domain (Neck-Tail-R2098M, Figure S4A) had no effect (Figure 5C). We conclude that the N-terminal motor-neck region attenuates RhoGAP activity.
Finally, we attempted to rescue the myosin-IXA-depletion phenotype by stable expression of different myosin-IXA constructs in 16HBE cells followed by treatment with myosin-IXA siRNA. Low-level expression of the tail domain or the motor-neck domain did not disrupt cell-cell contacts and had only modest effects on cell morphology (Figure S5A). Treatment of these lines with myosin-IXA siRNA led to endogenous myosin-IXA depletion as expected (Figure S5B). EGFP-motor-neck did not significantly rescue junction formation during cell-cell collision, as observed by time-lapse microscopy (Figure 6C, top panels, Figure 6D, and Movie S9) and did not prevent cell scattering (Figure 6E). Expression of EGFP-tail, however, partially rescued myosin-IXA-depleted cells from cell-cell retraction after collision, leading to increased stability of contacts (Figure 6D). Time-lapse observations of cells (Figure 6C, lower panels) revealed extensive contact between adjacent cells, often lasting for up to 1 h (Movie S9). The extent of cell scattering was significantly reduced in these cells (Figure 6E). Cell tracking of migrating islands revealed that random cell migrations induced by myosin-IXA depletion were partially rescued (Figure S5C). These results suggest that RhoGAP activity is required to prevent the cell scattering phenotype induced by myosin IX depletion in migrating cells.
Rho activity at nascent cell-cell contacts
To investigate further the effect of myosin-IXA depletion on Rho activity, we analyzed levels of phospho-myosin-light-chain (P-MLC), a downstream target of Rho-dependent contractility signaling. Western blot analysis revealed a 10% increase in MLC phosphorylation in myosin-IXA-depleted cells compared to control cells (Figure 7A). We also detected a 25% increase in total cellular Rho.GTP levels after myosin-IXA depletion, using G-linked immunosorbent assay (G-LISA), which likely reflects myosin-IXA regulating only a subset of endogenous Rho. As expected, expression of the GAP-containing EGFP-tail reduced Rho activity (by 37%) in comparison to control cells expressing EGFP-motor-neck (Figure 7B).
Figure 7. Quantification of Rho activity after myosin-IXA depletion.
(A) Western blot analysis of myosin-light-chain phosphorylation in myosin-IXA-depleted 16HBE cells. The ratio of phosphorylated over total levels of MLC is shown below. (B) Levels of active RhoA were measured in control, smartpool myosin-IXA-depleted cells, and in cells expressing myosin-IXA mutants. 2 independent experiments, ± SD. *, P < 0.004, unpaired t test. (C) Confocal time-lapse ratio imaging of RhoA FRET biosensor in control and smartpool myosin-IXA-depleted cells. Colliding cells are labeled with asterisks. Newly-formed cell-cell contacts are indicated by arrows. Note, the high FRET/CFP ratio signal at nascent cell-cell contacts after myosin-IXA depletion. Bar = 10 μm. (D) Quantification of normalized FRET/CFP intensity ratio signal. Nascent cell-cell contacts were outlined using the MetaMorph Region tool (2-4 square microns) and similar-sized, contact-free edge regions were selected as controls. Normalized FRET was calculated as ratio between average pixel gray levels over cell-cell contact region relative to average pixel gray levels in control contact-free regions. A total of 14 cell-cell contacts were analyzed. 3 independent experiments, ± SEM. **, P = 0.008, unpaired t test.
To explore the spatio-temporal activity of RhoA, specifically during cell-cell contact formation, we used a single-chain, FRET biosensor as a tool to detect RhoA activity in live cells [32]. In the activated GTP-bound form, the RhoA biosensor shows increased FRET between cyan and yellow fluorescent proteins, and the FRET/CFP emission ratio reflects the level of RhoA activation [32]. Collisions between control or myosin-IXA-depleted cells were imaged over 5–30 minutes. After calculating FRET/CFP ratios, elevated signals were observed at nascent cell-cell contacts in myosin-IXA-depleted cells compared to control cells (Figure 7C, arrows). Normalized FRET efficiency at these cell-cell contact sites was increased about 2-fold after myosin-IXA depletion (Figure 7D). These results suggest that myosin-IXA negatively regulates RhoA activity specifically at nascent cell-cell junctions in 16HBE cells.
DISCUSSION
To study the molecular mechanisms underlying collective epithelial migration, we used the human bronchial epithelial cell line, 16HBE [33]. Scratching a 16HBE monolayer induces collective migration of cells into the space created. Cell streams, or flows can be seen within the migrating epithelium, resulting in cells changing their position with respect to each other, a common characteristic of epithelial collective migration [34, 35].
Rho GTPases, particularly Rho, Rac and Cdc42, regulate the actin cytoskeleton and cell polarity in epithelial tissues. Through effector proteins such a Rho-kinase, WASP and mDia, they promote actin polymerization and actin-myosin filament contraction, and through effectors such Par6 and IQGAP, they mediate the establishment of apical-basal polarity and cell-cell junction formation [19]. They are, therefore, important regulators of epithelial morphogenesis and migration. A key feature of these signaling pathways lies in the ability of upstream activators (GEFs) and inactivators (GAPs) to regulate Rho GTPases in a spatially restricted way. To explore their contribution to collective cell migration, we screened an siRNA library targeting the 67 known Rho family GAPs, in 16HBE cells and identified myosin-IXA, an actin binding, RhoGAP domain containing protein, as an important player in collective migration.
Depletion of myosin-IXA by siRNA resulted in uncoordinated, random migration in scratched monolayers and in islands, through the inability of cells to maintain cell-cell contacts. A large number of proteins are recruited to adherens junctions and tight junctions at cell-cell contact sites to control their assembly, organization, maintenance and association with actin filaments, including numerous actin-based motors, such as myosin-II, -VII, -VI and -X. Myosins regulate cell-cell contacts by remodeling the actin cytoskeleton with their actin cross-linking activity, or by motor function creating tension between actin filaments, or by delivering cargo components. A myosin-IXA knockout mouse revealed a crucial role for this protein in the differentiation of ependymal cells, where it is highly expressed, with defects seen in morphology, gene expression and junction formation, supporting our results on the importance of myosin-IXA function at cell-cell contacts [29].
Myosins are a large family of actin binding motors, structurally diverse but sharing a conserved motor domain that binds to actin filaments and hydrolyzes ATP leading to translocation along the filament [36]. Myosins bind selectively to actin filaments with different composition and spatial arrangement [37]. Endogenous myosin-IXA and EGFP-myosin-IXA localize in puncta at cell-cell contact sites, along the circumferential, contractile actin bundle [29]. The circumferential localization suggests that myosin-IXA harbors recognition motifs for contractile actin filaments, perhaps in the neck-tail region, similar to myosin-IIA and -IIB [38].
Rho signaling is important during cell-cell contact formation and we recently described a role for the Rho target PRK2 in cell-cell junction maturation in 16HBE cells [39]. Localized activation of RhoA at the cell-cell contact zone, visualized by FRET, has a distinct spatiotemporal pattern upon cell-cell interactions suggesting tight regulation by both GEFs and GAPs [21, 22]. Here we present evidence that the RhoGAP activity of myosin-IXA facilitates the assembly of cell-cell contacts and that this is particularly important during collective cell migration. First, we found that the contractile phenotype induced by depletion of myosin-IXA can be partially reversed by inhibiting Rho-kinase. Second, the phenotype observed after overexpression of myosin-IXA is abolished by an inactivating mutation in the RhoGAP catalytic site. Third, low level expression of the constitutively active RhoGAP domain partially rescues the cell scattering phenotype induced by myosin-IXA depletion. Fourth, RhoA activity at nascent cell-cell contacts, detected by FRET ratio imaging, is significantly increased in myosin-IXA-depleted cells. These data suggested that the GAP domain of myosin-IXA acts as a localized, negative regulator of Rho to control contractile forces during the early stages of cell-cell contact formation.
The interaction of lamellipodia from two colliding cells stimulates local clustering of E-cadherin and the recruitment of actin filaments, referred to as junctional actin. Junctional actin can be seen at very early stages of cell-cell interactions in both control and myosin-IXA-depleted colliding 16HBE cells, but in the absence of myosin-IXA this did not reorganize into radial actin filaments. As a consequence, junctions were not stabilized and cells moved away from each other. These observations indicate that myosin-IXA is essential during the assembly of radial actin filaments (Figure 3D).
We conclude that myosin-IXA is required during the assembly of radial actin filaments, seen within a few minutes after cell-cell contact. In migrating 16HBE cells, these appear to be essential for subsequent stabilization of contact sites and depletion of myosin-IXA leads to cell scattering. Maturation of cell-cell junctions is accompanied by Rho-dependent conversion of radial actin filaments into tangential acto-myosin filaments. We speculate that myosin-IXA is required locally to downregulate Rho activity during early stages of junction formation, as well as during the dynamic reorganization of cell-cell contacts seen during collective migration.
EXPERIMENTAL PROCEDURES
Cell culture and transfections
Human bronchial epithelial cells 16HBE14o- (16HBE) [33] were maintained as described [24]. 60–120ng siRNA (Dharmacon) were used to transfect 0.5–1x105 16HBE cells. Duplex sequences were: duplex-1 GAAAGAAGCUUAGCCCUUAUU; duplex-2 GAUAAUACCUGCAUAAUU; duplex-3 GAACAUACAUUACGGAUAUUU; duplex-4 GAACAAAGGCUAAGAGAAAUU. For transient expression, DNA (4 μg) was nucleofected into 0.5x106 16HBE using kitT (Lonza), with program A-23, according to manufacturer’s protocol. All stable lines were obtained by selection in G418.
RNAi-based screen
16HBE cells were plated at 1×104 cells/well in a 96-well plate each containing a silicon insert (Platypus Technologies). The next day, the medium was replaced with OptiMEM reduced serum and antibiotic-free medium (Invitrogen) and cells were transfected using Oligofectamine with SMARTpool siRNAs (Dharmacon/ThermoFisher Scientific). 72h post-treatment, silicon inserts were removed to allow migration for 24h. Cells were fixed in 3.7% formaldehyde, stained with Calcein AM (Invitrogen) and images taken using Olympus dissection fluorescence microscope.
Antibodies, Immunofluorescence, western blot analysis
Primary antibodies: anti-ZO-1 (1:100) (Zymed Laboratories, San Francisco, CA); anti-E-cadherin (1:100) (Zymed); anti-GFP (1:100) (Invitrogen, Eugene, OR); anti-myosin-IXA (1:1000–1:10,000) [31]; rabbit polyclonal anti-myosin-light-chain-2 (Cell Signaling) (1:500); anti-phospho-myosin-II-Ser19 (Cell Signaling) (1:1000); mouse monoclonal anti-β-actin IgG2a isotype (clone AC-74) (Sigma). Secondary antibodies: Alexa-488, Alexa-568 and Alexa-350 conjugated donkey, anti-rabbit and goat anti-mouse (Molecular Probes/Invitrogen); horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse IgG (1:5000) (Jackson Immunoresearch Laboratories, West Grove, PA). For staining, cells were fixed in −20° C methanol or 3.7% formaldehyde and treated with blocking/permeabilization solution (0.1% saponin, 50 mM NH4Cl in PBS) for 25 min prior to antibody application. For western blots, cells were lysed with sample buffer, sonicated, boiled and separated by 3–8% Tris-Acetate SDS-PAGE as described [24].
Image analysis
Image analysis was performed using MetaMorph. Cell tracking analysis was performed by thresholding phase contrast images for dark regions (cytoplasm is dark, cell-cell contacts are bright), determining region centroids and automatic tracking. Alternatively, manual tracking was performed using cell centroids (Track points function). Extent of cell scattering was calculated as the ratio of scattered cells over total number of cells. Scattered cells are defined as cells with no contacts. Cell-cell contact stability was calculated by plotting the length of the cell-cell contact (determined by the straight line, Line region tool) over time. Actin fingers were defined in time-lapse sequences as actin-rich filopodia-like structures protruding after lamellipodia collisions.
FRET ratio analysis was performed using MetaMorph. FRET images were background corrected using the MetaMorph FRET application and the FRET/CFP ratio was calculated using an Arithmetic MetaMorph function. Normalized FRET efficiency was calculated from the FRET/CFP images: average pixel gray intensity of cell-cell contact region was divided by average gray intensity of protruding cell free edge region. The size of the region, the same for each image, was 2–4 square microns depending on the size of the cell-cell contact. Only images with cell-cell contacts formed within 5 min after collision were analyzed.
RhoA activity assay
16HBE cells were transfected with siRNAs or nucleofected with pEGFP-myosin-IXA mutants in 6-well plates and at day 3 (myosin-IXA depletion), or day 2 (post-nucleofection), cell lysates were collected and analyzed for levels of active RhoA using the G-linked immunosorbent assay (G-LISA) kits (Cytoskeleton). Equal amounts of protein were used for the different conditions.
Supplementary Material
HIGHLIGHTS.
Myosin-IXA, an actin-motor- and RhoGAP-protein is required for collective migration
Myosin-IXA is required for radial actin bundle assembly at nascent cell-cell contacts
The localization of myosin-IXA to actin bundles is dependent on its motor domain
Myosin-IXA RhoGAP domain locally inhibits Rho stabilizing nascent cell-cell contacts
Acknowledgments
We thank Dr. D. Gruenert for providing 16HBE14o- cells, Dr. Martin Bähler for a generous gift of Tü78 anti-myosin-IXA antibody, pEGFP-rat-myosin-IXA and pEGFP-rat-myosin-IXB, Dr. Tatiana Svitkina for pmCherry-actin and Dr. Michael Sheetz for pRFP-MLC. We thank Dr. Aurelia Lahoz for technical help, Dr. Michael Overholtzer for the E-cadherin-GFP and use of the spinning disk confocal microscope, Dr. Klaus Hahn for pBABE-RhoA-biosensor, Dr. Songhai Shi for use of the Olympus dissection microscope and Dr. Fanny Jaulin for discussions and support. We thank MSKCC Molecular Cytology Core Facilities staff for the use of the spinning disk confocal used in FRET imaging. We thank Dr. Isabelle Migeotte for critical reading of the manuscript and members of the Hall, Overholtzer and Shi laboratories for helpful discussions. The work was supported by a National Institutes of Health (NIH) grant GM081435 to A.H. and a National Cancer Institute core center grant (P30-CA 08748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 2.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasilyev A, Liu Y, Mudumana S, Mangos S, Lam PY, Majumdar A, Zhao J, Poon KL, Kondrychyn I, Korzh V, et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 2009;7:e9. doi: 10.1371/journal.pbio.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arboleda-Estudillo Y, Krieg M, Stuhmer J, Licata NA, Muller DJ, Heisenberg CP. Movement directionality in collective migration of germ layer progenitors. Curr Biol. 2010;20:161–169. doi: 10.1016/j.cub.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Melani M, Simpson KJ, Brugge JS, Montell D. Regulation of cell adhesion and collective cell migration by hindsight and its human homolog RREB1. Curr Biol. 2008;18:532–537. doi: 10.1016/j.cub.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Kolega J. The movement of cell clusters in vitro: morphology and directionality. J Cell Sci. 1981;49:15–32. doi: 10.1242/jcs.49.1.15. [DOI] [PubMed] [Google Scholar]
- 7.Hegerfeldt Y, Tusch M, Brocker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–2130. [PubMed] [Google Scholar]
- 8.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 10.Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- 11.Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr Opin Cell Biol. 2006;18:472–481. doi: 10.1016/j.ceb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Brevier J, Montero D, Svitkina T, Riveline D. The asymmetric self-assembly mechanism of adherens junctions: a cellular push-pull unit. Phys Biol. 2008;5:16005. doi: 10.1088/1478-3975/5/1/016005. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krendel M, Gloushankova NA, Bonder EM, Feder HH, Vasiliev JM, Gelfand IM. Myosin-dependent contractile activity of the actin cytoskeleton modulates the spatial organization of cell-cell contacts in cultured epitheliocytes. Proc Natl Acad Sci U S A. 1999;96:9666–9670. doi: 10.1073/pnas.96.17.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannone G, Mege RM, Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009;19:475–486. doi: 10.1016/j.tcb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci. 2008;13:6662–6681. doi: 10.2741/3180. [DOI] [PubMed] [Google Scholar]
- 17.Weijer CJ. Collective cell migration in development. J Cell Sci. 2009;122:3215–3223. doi: 10.1242/jcs.036517. [DOI] [PubMed] [Google Scholar]
- 18.Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 19.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 20.Perez TD, Tamada M, Sheetz MP, Nelson WJ. Immediate-early signaling induced by E-cadherin engagement and adhesion. J Biol Chem. 2008;283:5014–5022. doi: 10.1074/jbc.M705209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamazaki Y, Umeda K, Wada M, Nada S, Okada M, Tsukita S. ZO-1- and ZO-2-dependent integration of myosin-2 to epithelial zonula adherens. Mol Biol Cell. 2008;19:3801–3811. doi: 10.1091/mbc.E08-04-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuhara T, Shimizu K, Kawakatsu T, Fukuyama T, Minami Y, Honda T, Hoshino T, Yamada T, Ogita H, Okada M, et al. Activation of Cdc42 by trans interactions of the cell adhesion molecules nectins through c-Src and Cdc42-GEF FRG. J Cell Biol. 2004;166:393–405. doi: 10.1083/jcb.200401093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace SW, Durgan J, Jin D, Hall A. Cdc42 regulates apical junction formation in human bronchial epithelial cells through PAK4 and Par6B. Mol Biol Cell. 2010;21:2996–3006. doi: 10.1091/mbc.E10-05-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Braga VM, Yap AS. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr Opin Cell Biol. 2005;17:466–474. doi: 10.1016/j.ceb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–166. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coluccio LM, BÄhler M. Myosins. Vol. 7. Springer; Netherlands: 2008. Class Ix Myosins; pp. 391–401. [Google Scholar]
- 29.Abouhamed M, Grobe K, San IV, Thelen S, Honnert U, Balda MS, Matter K, Bahler M. Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus. Mol Biol Cell. 2009;20:5074–5085. doi: 10.1091/mbc.E09-04-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Boom F, Dussmann H, Uhlenbrock K, Abouhamed M, Bahler M. The Myosin IXb motor activity targets the myosin IXb RhoGAP domain as cargo to sites of actin polymerization. Mol Biol Cell. 2007;18:1507–1518. doi: 10.1091/mbc.E06-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chieregatti E, Gartner A, Stoffler HE, Bahler M. Myr 7 is a novel myosin IX-RhoGAP expressed in rat brain. J Cell Sci. 1998;111(Pt 24):3597–3608. doi: 10.1242/jcs.111.24.3597. [DOI] [PubMed] [Google Scholar]
- 32.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 33.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 34.Zallen JA, Blankenship JT. Multicellular dynamics during epithelial elongation. Semin Cell Dev Biol. 2008;19:263–270. doi: 10.1016/j.semcdb.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- 36.Krendel M, Mooseker MS. Myosins: tails (and heads) of functional diversity. Physiology (Bethesda) 2005;20:239–251. doi: 10.1152/physiol.00014.2005. [DOI] [PubMed] [Google Scholar]
- 37.Brawley CM, Rock RS. Unconventional myosin traffic in cells reveals a selective actin cytoskeleton. Proc Natl Acad Sci U S A. 2009;106:9685–9690. doi: 10.1073/pnas.0810451106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J Cell Biol. 2008;183:543–554. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace SW, Magalhaes A, Hall A. The Rho target PRK2 regulates apical junction formation in human bronchial epithelial cells. Mol Cell Biol. 2011;31:81–91. doi: 10.1128/MCB.01001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.