Abstract
Haspin is a serine/threonine kinase that phosphorylates Thr-3 of histone H3 in mitosis that has emerged as a possible cancer therapeutic target. High throughput screening of approximately 140,000 compounds identified the beta-carbolines harmine and harmol as moderately potent haspin kinase inhibitors. Based on information obtained from a structure-activity relationship study previously conducted for an acridine series of haspin inhibitors in conjunction with in silico docking using a recently disclosed crystal structure of the kinase, harmine analogs were designed that resulted in significantly increased haspin kinase inhibitory potency. The harmine derivatives also demonstrated less activity towards DYRK2 compared to the acridine series. In vitro mouse liver microsome stability and kinase profiling of a representative member of the harmine series (42, LDN-211898) are also presented.
The serine/threonine kinase haspin (Haploid Germ Cell-Specific Nuclear Protein Kinase, also known as Germ Cell Specific Gene-2; Gsg2)1 functions in mitosis, where it phosphorylates histone H3 at Thr-3 (H3T3ph).2 During mitosis, this phosphorylation generates a binding site on H3 for Survivin and thereby positions the Chromosome Passenger Complex at centromeres to regulate chromosome segregation,3, 4 and it also displaces proteins such as TFIID that normally bind to H3 through methylated Lys-4.5 Depletion of haspin by RNA interference, or microinjection of H3T3ph antibodies, causes chromosome alignment defects and failure of normal mitosis.2, 3, 6
Human haspin has ATP-binding and catalytic sites structurally similar to other members of the eukaryotic protein kinase (ePK) superfamily with several notable exceptions. For example, the highly conserved DFG motif involved in ATP binding and the APE motif involved in stabilizing the C-terminal lobe among ePKs are altered or absent and the activation loop region is substantially rearranged in haspin compared to other ePKs.7, 8
Haspin kinase inhibitors are expected to be useful probes for elucidating the cellular roles of this protein and may have therapeutic utility in treating cancer. A recently described small molecule, CHR-6494 (1), that inhibits haspin displayed anti-tumor activity in a mouse xenograft model.9 Also, 5-iodotubercidin (2) has been reported as an effective haspin kinase inhibitor.7, 10
We previously utilized a time-resolved fluorescence resonance energy transfer (TR-FRET) high throughput screening (HTS) assay to identify the acridine derivative 3 (LDN-192960) as another potent haspin inhibitor (Figure 1; IC50 = 0.010 µM).11, 12 This assay has now also been used to discover the beta-carbolines harmine, 4, and harmol, 5, as moderately potent haspin inhibitors with IC50 values of 0.59 and 0.77 µM, respectively. Harmine has previously been identified as an inhibitor of DYRK family kinases, with IC50 values between 0.03 and 0.35 µM reported for DYRK1A, and approximately 50-fold lower potency toward DYRK2.13 Herein, we describe the design, synthesis and improved potency of the beta-carboline series for haspin inhibition utilizing the structure-activity relationships previously determined for the acridine series12 combined with in silico docking using a recently disclosed crystal structure of the kinase.7 In addition, in vitro mouse liver microsome stability and kinase profiling of a representative beta-carboline analog are presented.
Figure 1.
Haspin inhibitors identified by radiometric, thermal stability shift and TR-FRET HTS assays.
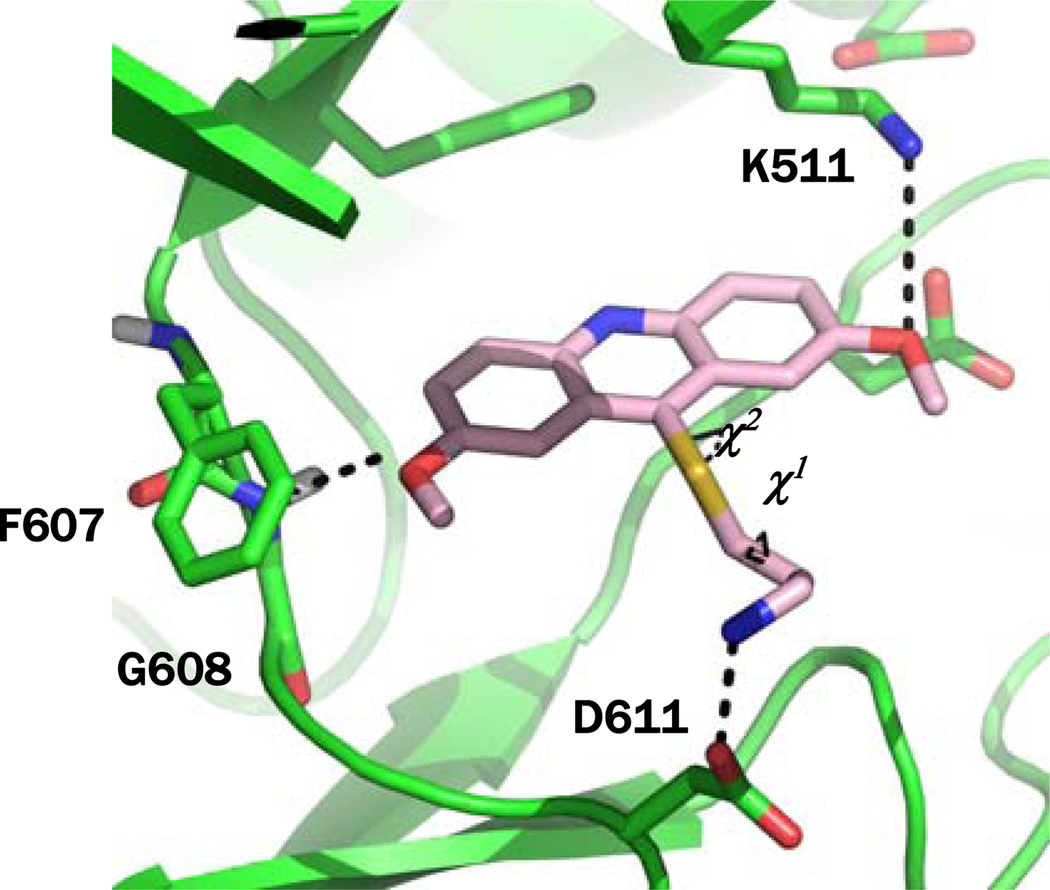
A crystal structure of haspin bound to AMP was used for docking calculation.7, 14 Analysis of this structure revealed key hydrogen bonds between nitrogen atoms of the adenine ring of AMP and protein backbone atoms of residues E606 and G608 (Figure S1).15 A 12Å docking grid was generated using the AMP center of mass as the point of origin with a single hydrogen bond constraint on the backbone amide of G608. Docking calculations were performed on 3, which demonstrated that this inhibitor was well accommodated within the binding site and satisfied the hydrogen bonding constraint on G608 (Figure 2). In addition, the inhibitor also made a hydrogen bond with K511, which likely disrupts a key salt bridge between this residue and E535 that is necessary for closure of the ATP-binding cleft enabling kinase activity. A metadynamic simulation sampling the two torsion angles (Χ1 and Χ2) of the alkylamine as collective variables was also conducted. One low energy conformation was found (Figure S2) that allowed a hydrogen bond between the amine and D611 (Figure 2).
Figure 2.
Molecular docking of 3 at the ATP-binding site of haspin. Χ1 and Χ2 are the torsion angles that were sampled during the metadynamic calculation.
Next, docking calculations were performed on four harmine analogs (7a – b and 9a – b) that incorporate at two different positions alkylamines similar to that present in 3. The two derivatives with the alkylamine on the N9-position (9a and 9b) were well accommodated within the binding site making a hydrogen bond with D687 as well as the hydrogen bonding constraint on G608 (Figure 3B and C). In contrast, compounds 7a and 7b resulted in steric hindrance with the region around F607 and G608 and were not well situated within the ATP-binding site, suggesting that they would unlikely be haspin inhibitors. A similar metadynamic simulation for 9a sampling two torsion angles (Χ1 and Χ2) of the alkylamine as collective variables found two low energy conformations (Figure 3A). One of these allowed a hydrogen bond between the amine and D611 (Figure 3B), similar to 3. However, the second conformation permitted a hydrogen bond between the amine and the backbone carbonyl of I490 in the glycine-rich loop (Figure 3C).
Figure 3.
(A) Free energy surface map from metadynamic calculations sampling two torsion angles (Χ1 and Χ2) of 9a. Blue and red represent low and high energy conformations, respectively. (B and C) Docking of 9a at the ATP-binding site of haspin for the two conformations of the alkylamine side-chain based on metadynamic calculations.
The initial beta-carboline derivatives used in the in silico docking experiments were prepared using the procedure outlined in Scheme 1. Harmol, 5, was O-alkylated with N-Boc-protected alkylamines in the presence of cesium carbonate to give 6a and 6b. The carbamate protecting group was removed under acidic conditions to give 7a and 7b. Harmine, 4, could be N-alkylated. However, phthalimide-protected alkylamines were required and a stronger base (i.e. NaH) was also needed to give 8a and 8b. Removal of the protecting group was accomplished using hydrazine hydrate to yield 9a and 9b.
Scheme 1.
Reagents and conditions: (a) Br(CH2)nNHBoc, Cs2CO3, DMF, rt; (b) HCl, MeOH, rt; (c) Br(CH2)nNPhth, NaH, DMF, rt; (d) NH2NH2•H2O, EtOH, 65 °C. Phth = phthalimide.
Haspin kinase inhibitory activity for the various compounds was assessed using the same assay utilized for the HTS, except in the presence of varying concentrations of test compounds.11, 15 As anticipated 7a and 7b were inactive haspin inhibitors at 10 µM. However, 9a and 9b were both active demonstrating IC50 values of 0.46 and 0.17 µM, respectively (Table 1).
Table 1.
IC50determinations for haspin and DYRK2 inhibition.
 | ||||||
|---|---|---|---|---|---|---|
| Cmpd | R1 | R2 | R3 | n | IC50 (µM) | |
| Haspin | DYRK2 | |||||
| 9a | Me | 7-OMe | NH2 | 2 | 0.46 | > 10 |
| 9b | Me | 7-OMe | NH2 | 3 | 0.17 | > 10 |
| 28 | H | 7-OMe | NH2 | 3 | 0.30 | > 10 |
| 29 | i-Pr | 7-OMe | NH2 | 3 | 0.46 | > 10 |
| 30 | Et | 7-OMe | NH2 | 3 | 0.19 | > 10 |
| 31 | Me | 7-OH | NH2 | 3 | 0.16 | > 10 |
| 32 | Me | 7-F | NH2 | 3 | 0.58 | > 10 |
| 33 | Me | 7-NHSO2Me | NH2 | 3 | 0.69 | > 10 |
| 34 | Me | 7-O-t-Bu | NH2 | 3 | 4.6 | > 10 |
| 35 | Me | 5-OMe | NH2 | 3 | 2.7 | 7.0 |
| 36 | Me | 6-OMe | NH2 | 3 | 2.4 | 4.9 |
| 37 | Me | 8-OMe | NH2 | 3 | 0.22 | > 10 |
| 38 | Me | 6,7-OCH2O | NH2 | 3 | 0.50 | 4.8 |
| 39 | Me | 7-OMe, 8-Cl | NH2 | 3 | 0.83 | 8.0 |
| 40 | Me | 7-OMe | NHMe | 3 | 0.34 | > 10 |
| 41 | Me | 7-OMe | NMe2 | 3 | 0.47 | > 10 |
| 42 | CF3 | 7-OMe | NH2 | 3 | 0.10 | 15 |
| 43 | CF3 | 7-OH | NH2 | 3 | 0.22 | 8.5 |
Based on these results additional beta-carboline analogs were prepared using the general procedure outlined in Scheme 2. Indoles 10 were either directly converted to derivative 11 using Me2NCH=CHNO2 (generated from MeNO2 and Me2NCH(OMe)2 at 85 °C for 0.5 h) or in a two step procedure (i.e. Vilsmeier-Haak followed by Henry reactions). The alkene and nitro groups were both reduced in the presence of lithium aluminum hydride (LAH) to give 12. A Pictet-Spengler reaction between 12 and various aldehydes generated 11. In the case where R1 = CF3, the hemiacetal CF3CHOH(OEt) was used. Oxidation of the tetrahydro-beta-carboline with manganese dioxide gave 14. Finally as previous outlined, alkylation generated 15, which was de-protected to give 16.
Scheme 2.
(a) Me2NCH=CHNO2, TFA, rt, 30 min; (b) POCl3, DMF, 0 °C to rt then MeNO2, NH4OAc, 100 °C, 1 h; (c) LiAlH4, THF, rt; (d) R1CHO or CF3CHOH(OEt), MeOH, cat. HCl, rt; (e) MnO2, 5% Pd/C, DMF, microwave (MW), 150 °C; (f) Br(CH2)nNPhth, NaH, DMF, rt; (g) NH2NH2•H2O, MeOH, DCM.
Several other beta-carboline derivatives were also prepared using the procedures outlined in Scheme 3. Intermediate 17 was de-methylated using HBr in acetic acid to give phenol 18. This material was converted to triflate 17 and then a methylsulfonamide was introduced utilizing a Pd-catalyzed reaction to yield 20. Removal of the protecting group gave amine 21. Likewise, intermediate 16 was converted to the t-butyl ether 22 in the presence of Me2NCH(O-t-Bu)2, which was again de-protected to liberate amine 23. Intermediate 17 was also de-protected to give 24, which was converted to the tertiary amine 25 through reductive amination. Finally, intermediate 24 was converted to the secondary amine 27 via the formamide 26.
Scheme 3.
(a) HBr, AcOH, MW, 130 °C; (b) Tf2O, i-Pr2EtN, DCM, 0 °C to rt; (c) MeSO2NH2, Pd2(dba)3, xPhOS, K3PO4, toluene, 110 °C, 1 h; (d) NH2NH2•H2O, MeOH, DCM; (e) Me2NCH(O-t-Bu)2, DMF, 120 °C, 1 h; (f) CH2O, HCO2H, MW, 150 °C; (g) EtOCHO, MW, 160 °C; (h) LiAlH4, THF, rt.
The additional compounds prepared were used to further explore the structure-activity relationship of 9b for haspin inhibition (Table 1). Removal of the methyl at the 1-position (28) or replacement with an isopropyl (29) were detrimental, where as replacement with an ethyl (30) was equivalent. Replacing the methoxy in the 7-position with a hydroxyl (31) was tolerated, but a fluorine (32), methyl sulfonamide (33) or t-butoxy (34) were detrimental. Transposition of the methoxy to the 5- or 6-positions (35 and 36) resulted in loss of activity, while introduction of the methoxy to the 8-position (37) was more tolerated. The 6,7- and 7,8-disubstituted analogs 38 and 39 demonstrated less activity. The primary amine also appeared to be optimal, where the secondary (40) and tertiary (41) amines were less active. Finally, in order to potentially improve metabolic stability analogs (42 and 43) that replace the methyl group in the 1-position with a trifluoromethyl were evaluated. Gratifyingly, 42 displayed an IC50 value of 100 nM for inhibiting haspin. This compound was also assessed for in vitro metabolic stability in pooled mouse liver microsomes and demonstrated excellent stability with a t1/2 of 341 min and a CLint of 3.8 µL/min/mg protein suggesting that this compound may be useful as an in vivo probe.15, 16
The beta-carboline series was also evaluated for inhibition of human DYRK2 using a previously described assay.12 Harmine and harmol gave IC50 values for DYRK2 inhibition of 0.69 and 1.5 µM, consistent with previous studies.13 Introduction of the tethered amine onto the N9-position substantially reduced the potency for DYRK2 inhibition in all cases examined (Table 1). For example, 42 had an IC50 value for DYRK2 of 15 µM and demonstrated 150-fold selectivity for haspin over DYRK2.
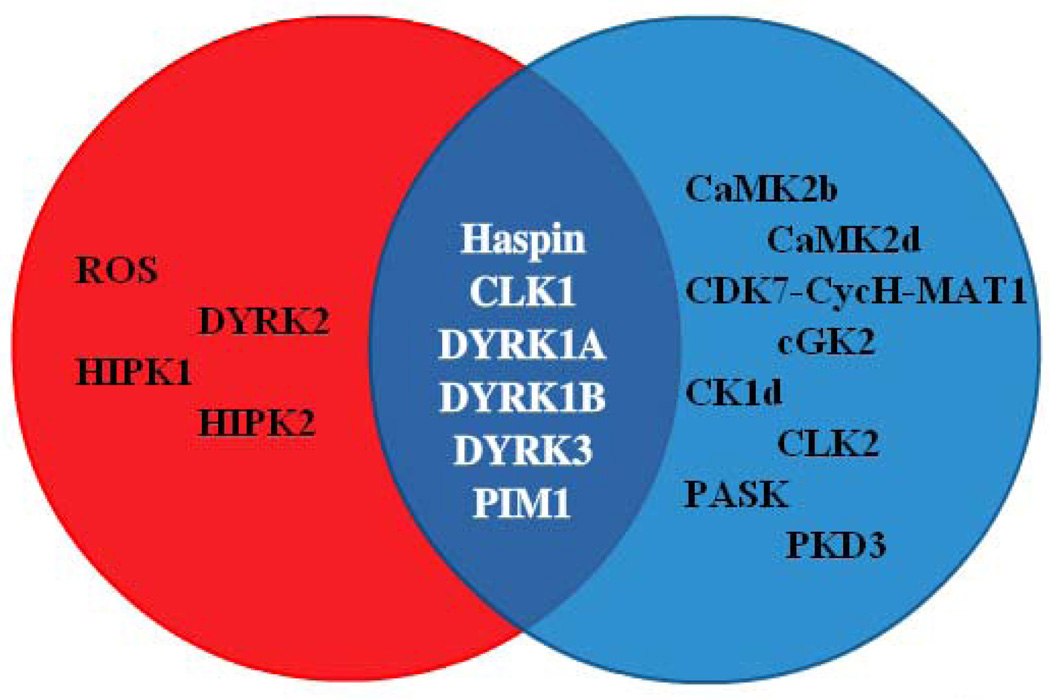
Lastly, haspin inhibitor 42 was assessed against a panel of 292 kinases at 10 µM. At this high concentration, the compound inhibited thirteen kinases, in addition to haspin, ≥ 90%.15, 17 These kinases were CaMK2b, CaMK2d, CDK7-CycH-Mat1, cGK2, CK1d, CLK1, CLK2, DYRK1A, DYRK1B, DYRK3, PASK, PIM1 and PKD3. Interestingly, many of these enzymes belong to the CMGC group of kinases, unlike haspin which is a divergent member of the ePK family.18 In addition, a comparison of the profiles of 3 and 42 suggested that only six kinases, including haspin, were inhibited by both compounds ≥ 90% at 10 µM (Figure 4).12 Profiling of additional haspin kinase inhibitors, such as 1 and 2, may further reduce the number of kinases, besides haspin, which are known to be potently inhibited by all the compounds. In addition, the collective use of 3, 42 and potentially other haspin inhibitors in cell based assays may allow for more concrete conclusions to be reached with regard to haspin’s biological functions.
Figure 4.
A Venn diagram highlights kinases selectively inhibited by 3 (red) or 42 (blue) by ≥ 90% at 10 µM. The overlapping region contains only six kinases inhibited by both compounds.
In conclusion, a structure-activity relationship study of the beta-carbolines 4 and 5, identified utilizing a recently developed HTS assay for haspin kinase inhibitory activity, was performed guided by insights obtained from a previously optimized compound series12 combined with in silico docking and metadynamic calculations. Increased potency was accomplished by introduction of a tethered primary amine onto the N9-position of the beta-carboline. Potency was further increased by replacing the methyl at the 1-position with a trifluoromethyl giving 42. In addition, this analog demonstrated excellent in vitro metabolic stability in pooled mouse liver microsomes. Kinase profiling of 42 suggested that it was fairly selective and inhibited only six kinases (≥ 90% at 10 µM), including haspin, in common with the previously identified acridine inhibitor 3. The beta-carboline haspin inhibitor 42 (LDN-211898) described herein, along with other structurally distinct inhibitors such as 1 – 3 provide valuable molecular probes to study the cellular functions of haspin kinase and may have potential therapeutic utility in treating cancer.
Supplementary Material
Acknowledgments
The authors thank Partners Healthcare for financial support. This work was also supported in part by NIH grant R01CA122608 and a Leukemia and Lymphoma Society Scholar Award to J.M.G.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at ().
References and notes
- 1.(a) Tanaka H, Yoshimura Y, Nishina Y, Nozaki M, Nojima H, Nishimune Y. FEBS Lett. 1994;355:4. doi: 10.1016/0014-5793(94)01155-9. [DOI] [PubMed] [Google Scholar]; (b) Tanaka H, Yoshimura Y, Nozaki M, Yomogida K, Tsuchida J, Tosaka Y, Habu T, Nakanishi T, Okada M, Nojima H, Nishimune Y. J. Biol. Chem. 1999;274:17049. doi: 10.1074/jbc.274.24.17049. [DOI] [PubMed] [Google Scholar]
- 2.(a) Dai J, Sultan S, Taylor SS, Higgins JMG. Genes Dev. 2005;19:472. doi: 10.1101/gad.1267105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Higgins JMG. Chromosoma. 2010;119:137. doi: 10.1007/s00412-009-0250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JMG. Science. 2010;330:231. doi: 10.1126/science.1189435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Science. 2010;330:235. doi: 10.1126/science.1189505. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Science. 2010;330:239. doi: 10.1126/science.1194498. [DOI] [PubMed] [Google Scholar]
- 5.Varier RA, Outchkourov NS, de Graaf P, van Schaik FMA, Ensing HJL, Wang F, Higgins JMG, Kops GJPL, Timmers HThM. EMBO J. 2010;29:3967. doi: 10.1038/emboj.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai J, Kateneva AV, Higgins JMG. J. Cell Sci. 2009;122:4168. doi: 10.1242/jcs.054122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eswaran J, Patnaik D, Filippakopoulos P, Wang F, Stein RL, Murray JW, Higgins JMG, Knapp S. Proc. Natl Acad. Sci. U.S.A. 2009;48:20198. doi: 10.1073/pnas.0901989106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villa F, Capasso P, Tortorici M, Forneris F, de Marco A, Mattevi A, Musacchio A. Proc. Natl Acad. Sci. U.S.A. 2009;48:20204. doi: 10.1073/pnas.0908485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huertas D, Soler M, Moreto J, Villanueva A, Martinez A, Vidal A, Charlton M, Moffat D, Patel S, McDermott J, Owen J, Brotherton D, Krige D, Cuthill S, Esteller M. Oncogene. 2011 doi: 10.1038/onc.2011.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balzano D, Santaguida S, Musacchio A, Villa F. Chem. Biol. 2011;18:966. doi: 10.1016/j.chembiol.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Patnaik D, Xian J, Glicksman MA, Cuny GD, Stein RL, Higgins JMG. J. Biomol. Screen. 2008;13:1025. doi: 10.1177/1087057108326081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuny GD, Robin M, Ulyanova NP, Patnaik D, Pique V, Casano G, Liu J-F, Lin X, Xian J, Glicksman MA, Stein RL, Higgins JMG. Bioorg. Med. Chem. Lett. 2010;20:3491. [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JSC, Alessi DR, Cohen P. Biochem J. 2007;408:297. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Seifert A, Allan LA, Clarke PR. FEBS J. 2008;275:6268. doi: 10.1111/j.1742-4658.2008.06751.x. [DOI] [PubMed] [Google Scholar]; (c) Göckler N, Jofre G, Papadopoulos C, Soppa U, Tejedor FJ, Becker W. FEBS J. 2009;276:6324. doi: 10.1111/j.1742-4658.2009.07346.x. [DOI] [PubMed] [Google Scholar]; (d) Ogawa Y, Nonaka Y, Goto T, Ohnishi E, Hiramatsu T, Kii I, Yoshida M, Ikura T, Onogi H, Shibuya H, Hosoya T, Ito N, Hagiwara M. Nat. Commun. 2010;1:86. doi: 10.1038/ncomms1090. [DOI] [PubMed] [Google Scholar]
- 14.Protein Data Base (PDB) ID: 3DLZ. Docking experiments were conducted using Glide XP v2.5 from Schrödinger Inc.
- 15.See supplementary data for details.
- 16.Baranczewski P, Stańczak A, Sundberg K, Svensson R, Wallin A, Jannson J, Garberg P, Postlind H. Pharmacol. Rep. 2006;58:453. [PubMed] [Google Scholar]
- 17.Although the percent inhibition was conducted as a single point, this should reflect relative potency for each kinase. However, dose-response IC50 value determinations will ultimately be needed.
- 18.(a) Higgins JMG. Protein. Sci. 2001;10:1677. doi: 10.1110/ps.49901. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kannan N, Taylor SS, Zhai Y, Venter JC, Manning G. PLoS Biol. 2007;5:e17. doi: 10.1371/journal.pbio.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.