Abstract
Cancer immunoediting is a process by which immune cells, particularly lymphocytes of the adaptive immune system, protect the host from the development of cancer and alter tumour progression by driving the outgrowth of tumour cells with decreased sensitivity to immune attack1,2. Carcinogen-induced mouse models of cancer have shown that primary tumour susceptibility is enhanced in immune-compromised mice, while conversely, the capacity for such tumours to grow after transplantation into wild-type mice is reduced2,3. However, many questions about the process of cancer immunoediting remain unanswered due, in part, to the known antigenic complexity and heterogeneity of carcinogen-induced tumours4. Here we have adapted a genetically engineered, autochthonous mouse model of sarcomagenesis to investigate the process of cancer immunoediting. This system allowed us to monitor the onset and growth of immunogenic and non-immunogenic tumours induced in situ that harbor identical genetic and histopathological characteristics. By comparing the development of such tumours in immune-competent mice to mice with broad immunodeficiency or specific antigenic tolerance, we show that recognition of tumour-specific antigens (TSAs) by lymphocytes is critical for immunoediting against sarcomas. Furthermore, primary sarcomas were edited to become less immunogenic through the selective outgrowth of cells that were able to escape T lymphocyte attack. Loss of tumour antigen expression or MHCI presentation was necessary and sufficient for this immunoediting process to occur. These results highlight the importance of TSA expression in immune surveillance, and potentially, immunotherapy.
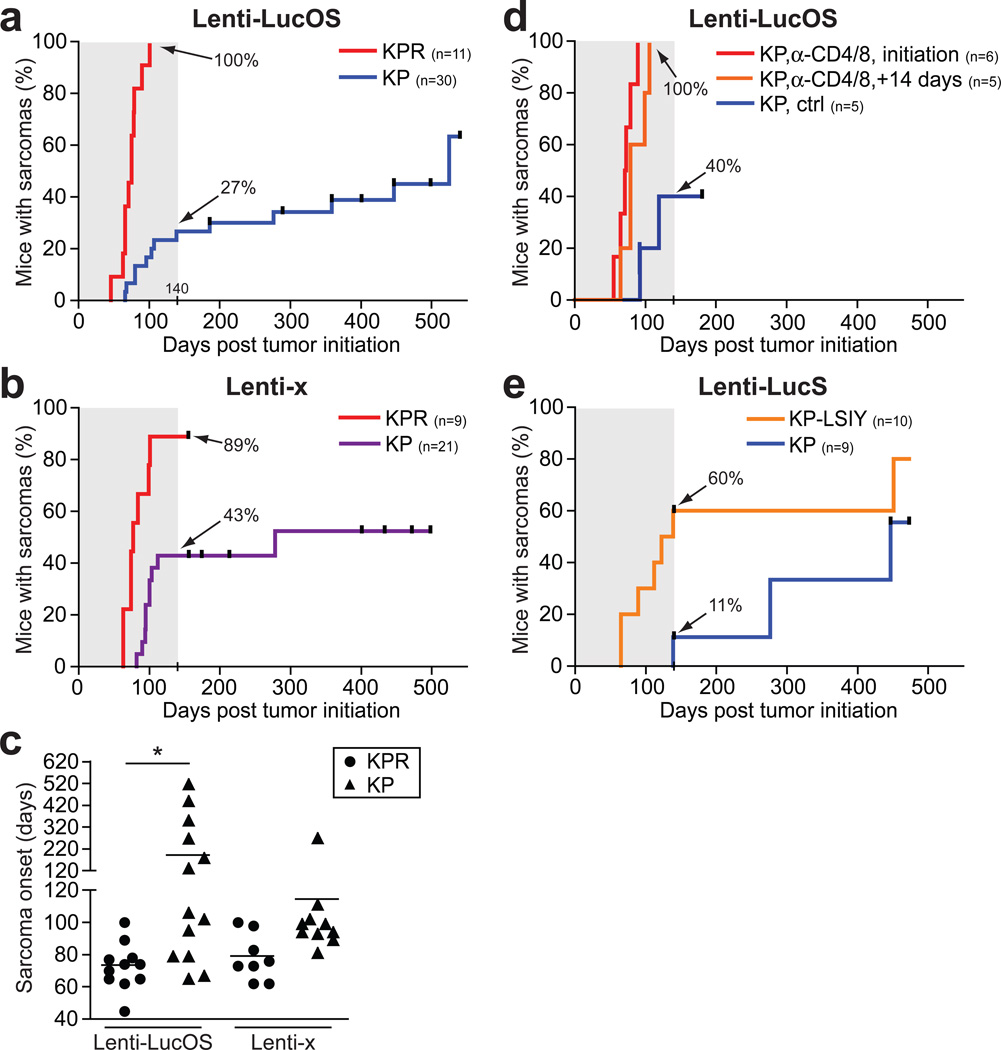
To determine whether T lymphocytes influence tumour development, we adapted a mouse model of human soft tissue sarcomagenesis driven by Cre/LoxP-regulated expression of oncogenic K-rasG12D and deletion of p53 to allow for the control of tumour immunogenicity5. Sarcomas were induced in either immune-competent K-rasLSL-G12D/+; p53fl/fl;Rag-2+/− (KP) or lymphocyte-deficient K-rasLSL-G12D/+;p53fl/fl;Rag-2−/− (KPR) mice by intramuscular injection of lentiviral vectors that expressed Cre recombinase alone (Lenti-x) or, to induce sarcomas with potentially immunogenic antigens, we used vectors that also expressed the T cell antigens SIYRYYGL (SIY) and two antigens from ovalbumin (SIINFEKL (SIN, OVA257–264) and OVA323–339) fused to the C-terminus of luciferase (Lenti-LucOS). Intramuscular injection of Lenti-LucOS led to tumour formation in 100% of KPR mice but only 27% of KP mice by 140 days (Fig. 1a, p< 0.0001). Additional sarcomas ultimately developed in KP mice but with dramatically delayed kinetics (latency of 194.8 ± 43.4 days) compared with KPR mice (73.6 ± 4.3 days) (Fig. 1c, p< 0.02). We also observed a difference in the penetrance of sarcoma development in KPR versus KP mice by 140 days with Lenti-x (89% versus 43%, respectively), although the difference was less dramatic than observed with Lenti-LucOS (Fig. 1b, p< 0.0005). This suggests that in this model, tumour immunosurveillance may not necessitate the introduction of highly immunogenic tumour-specific antigens (TSAs). The observed immunosurveillance against Lenti-x tumours could result from the lentiviral infection required to induce tumours, the acquisition of TSAs during tumour development, or the immunogenicity of Cre itself. However, in a previous study, we found that Cre was not highly immunogenic when expressed in developing lung adenocarcinomas6. Although Lenti-x-induced sarcoma development was slightly delayed in immune-competent (KP) mice (114.9 days in KP versus 79.5 days in KPR mice), it was not significant (Fig. 1c, p= 0.11). The increased latency that is specific to Lenti-LucOS tumours may be the result of an equilibrium between replicating tumour cells and T cells that recognize antigens expressed from the LucOS vector and restrain tumour progression1,7.
Figure 1. Sarcoma formation in immunodeficient mice occurs with increased penetrance and reduced latency.
a and b, KPR or KP mice were injected intramuscularly with Lenti-LucOS (a) or Lenti-x (b) and the onset of palpable sarcomas was monitored. c, Time for palpable tumour formation with Lenti-LucOS or Lenti-x in KPR (circles) or KP (triangles) mice. d, Sarcoma formation in KP mice either untreated or treated with anti-CD4 and anti-CD8 antibodies beginning coincident with or 14 days after Lenti-LucOS injection. e, Sarcoma onset after injection of KP-LSIY or KP littermates with Lenti-LucS. The percentage of total mice (n) with sarcomas by 140 days (grey boxes) is indicated.
Rag-2 deficiency prevents both T and B lymphocyte development and, therefore, could have pleiotropic effects on the immune response to tumour antigens. To specifically test the significance of T cell responses, we treated mice with antibodies against CD4 and CD8 to deplete T cells concurrent with, or subsequent to, intramuscular injection of Lenti-LucOS. T cell depletion at tumour initiation, or even 14 days after tumour initiation, led to sarcoma development with complete penetrance and early onset similar to KPR mice (Fig. 1d, p= 0.001 and p= .013 compared to untreated, respectively). To specifically test the importance of CD8+ T cells that recognize the model TSAs, we made use of a regulatable luciferase-SIY fusion gene engineered into the murine Rosa26 locus (R26LSL-LSIY)8. These mice develop specific tolerance to luciferase and SIY due to weak thymic expression and deletion of reactive T cells (Supplementary Fig. 1)8. K-rasLSL-G12D/+;p53fl/fl;R26LSL-LSIY/+ (KP-LSIY) mice injected with Lenti-LucS, a lenti-vector that expresses Cre and SIY fused to luciferase, were more susceptible to sarcoma formation and developed tumours earlier than KP littermates (Fig. 1e, p= 0.058). Thus, lymphocyte-mediated protection from sarcoma formation requires CD8+ T cells that respond to non-self antigens expressed in tumours.
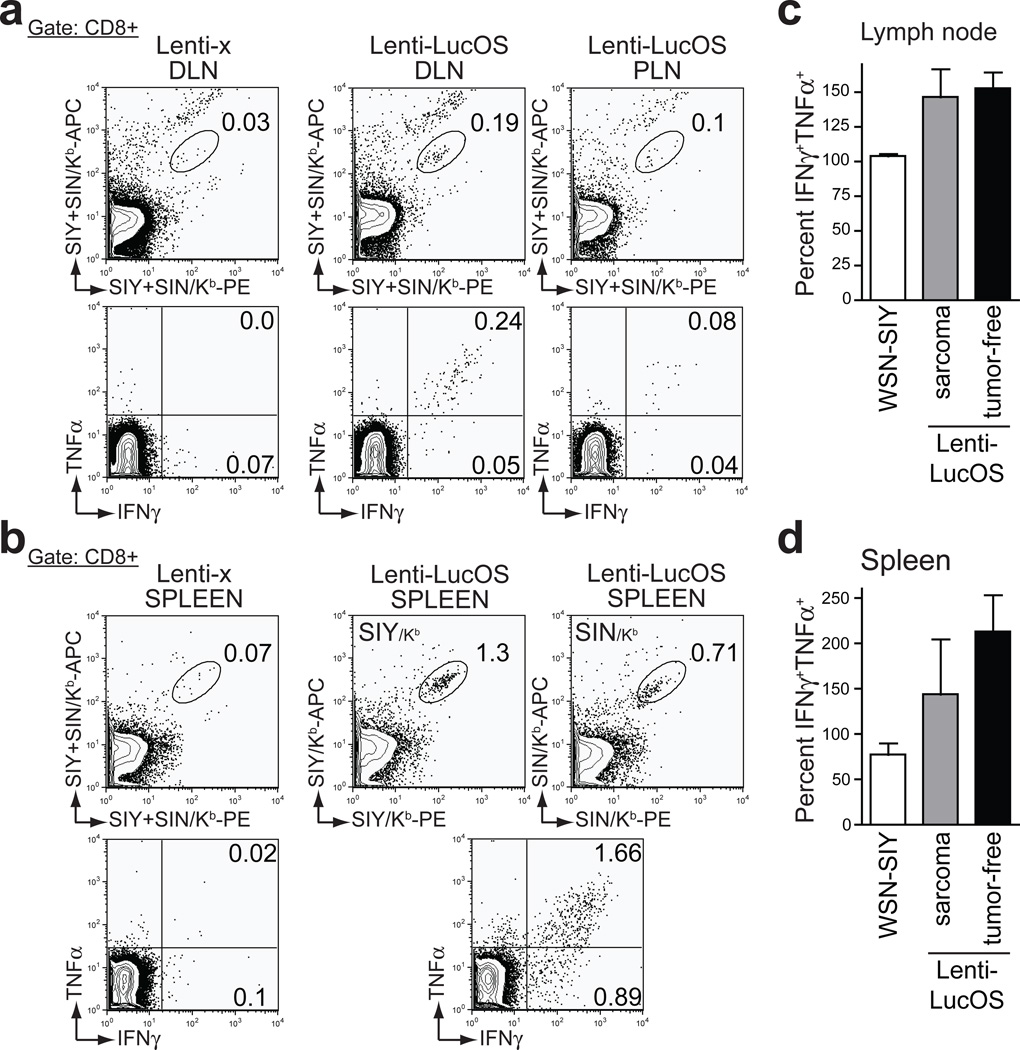
A key advantage of this conditional, genetically engineered cancer model over carcinogen-induced models is the capacity to track endogenous T cells specific for tumour antigens during primary tumour development. We used SIY and SIN loaded MHCI/Kb reagents to track tumour-reactive CD8+ T cells by flow cytometry. Only mice with Lenti-LucOS sarcomas harbored CD8+ T cells specific to SIY and SIN in the lymph nodes nearest the tumour site as well as in the spleen (Fig. 2a, b). These CD8+ T cells appeared to be completely functional because they produced both IFN-γ and TNF-α upon stimulation (Fig. 2a–d). Interestingly, this contrasts sharply with results from an analogous model of lung adenocarcinoma in which the activity of T cells responding to the same tumour antigens was very weak, suggesting that different tumour types may use different mechanisms to escape immune attack6. We also investigated whether KP mice that did not develop sarcomas after injection with Lenti-LucOS harbored antigen-specific T cells, since such T cells could have protected these mice from sarcoma development. Indeed, we detected fully functional antigen-specific T cells in these mice (Fig. 2c, d and Supplementary Fig. 1), demonstrating that T cells specific to these model TSAs are functional and likely provide significant protection against the development of Lenti-LucOS sarcomas.
Figure 2. Functional T cell responses are generated against antigens expressed in sarcomas.
a, Top: Percent of CD8+ cells specific for SIY- and SIN in the inguinal lymph nodes either draining (DLN) or peripheral to (PLN) Lenti-x or Lenti-LucOS tumours. Bottom: IFN-γ and TNF-α cytokine production in SIY+SIN-stimulated CD8+ T cells from mice analyzed above. b, Analysis of splenocytes as in (a). c and d, Cumulative data depicting the percentage of SIY and SIN-specific T cells that were IFN-γ+ TNF-α+ from lymph nodes (c) or spleens (d) of KP mice infected with Lenti-LucOS that developed a “sarcoma” or were “tumour-free” at 170 days. T cells reactive to SIY were analyzed four months after challenge with WSN-SIY (influenza strain expressing SIY). Data represents analysis of 3–4 mice per group, mean ± s.e.m.
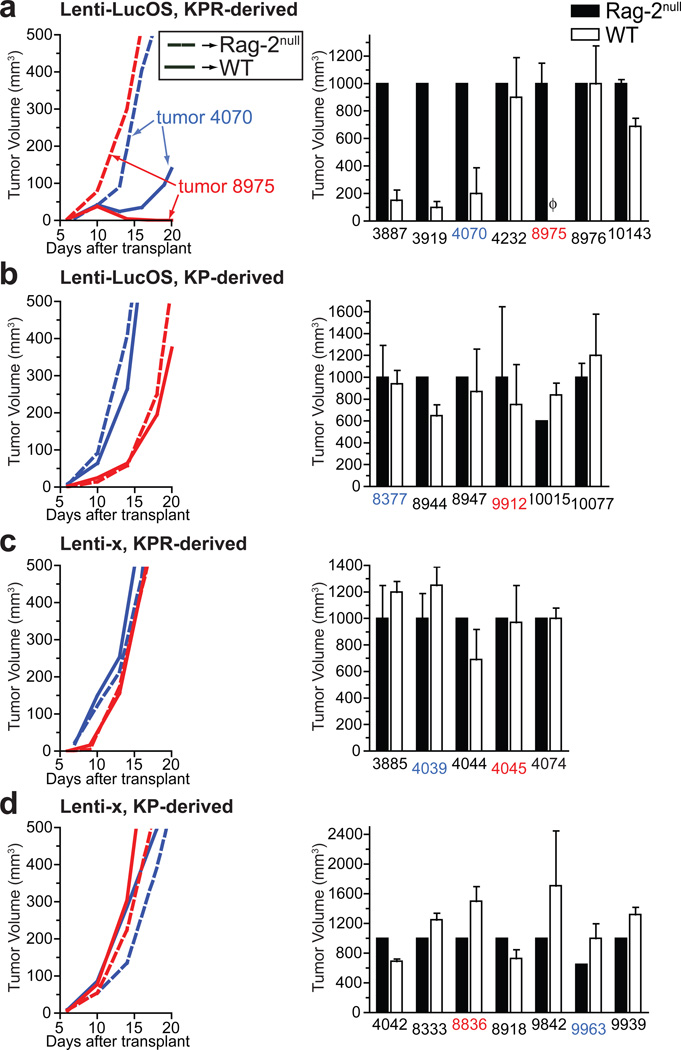
Pivotal experiments using MCA-induced sarcomas revealed that tumours derived in immune-compromised mice, and thus not immunoedited, are more susceptible to rejection upon transplantation into immune-competent mice2. To assay whether autochthonous sarcomas driven by targeted genetic mutations would also display an unedited phenotype, we transplanted independently derived sarcomas from KPR or KP mice into either wild-type or Rag-2−/− mice. While freshly isolated Lenti-LucOS-induced tumours (or cell lines) generated in KP mice grew similarly upon transplantation into either wild-type or Rag-2−/− mice, most Lenti-LucOS tumours generated in KPR mice were rejected (1/7) or had significantly delayed growth (4/7) (Fig. 3a, b and Supplementary Fig. 2). These results recapitulate the original findings from carcinogen-induced sarcomas in a genetically engineered mouse model of sarcomagenesis.
Figure 3. Cancer immunoediting phenotypes require the presence of potent T cell antigens.
Transplanted tumour growth of Lenti-LucOS-induced sarcomas generated in KPR (a) or KP (b) mice and Lenti-x-induced sarcomas generated in KPR (c) or KP (d) mice. At left, representative tumour growth curves from two different primary tumours (colored red or blue) after transplantation into Rag-2null (dashed lines) or wild-type (WT, solid lines) mice. At right, comparison of the mean tumour volume (mm3) ± s.e.m. for all tumours transplanted. Φ indicates no detectable mass. See Supplementary Fig. 2 for growth curves of tumour lines.
Next we wanted to determine whether Lenti-x-induced sarcomas, which lack the strong T cell antigens from LucOS, would yield similar results. Interestingly, Lenti-x tumours generated in KPR or KP mice grew equally well when transplanted into wild-type or Rag-2−/− mice (Fig. 3c, d). It is noteworthy that while autochthonous tumours initiated by Lenti-x appeared partially inhibited by an adaptive immune response (Fig. 1b), in the context of transplantation, we found no evidence of immunoediting (Fig. 3c). This difference may be due to Rag-dependent innate immune cells (NKT and γδ T cells) that recognize stress or inflammatory ligands. These cells may be sufficient to eliminate a limited number of nascent tumour cells in the context of transformation by lentiviral infection, but not in response to the transplantation of fully developed tumours1,9. Nevertheless, we hypothesize that Lenti-x sarcomas from KPR mice grew unabated after transplantation into KP mice because immunoediting by T lymphocytes requires potent TSAs, which these tumours lack. The observed immunogenicity of carcinogen-induced sarcomas derived in immune-compromised mice may be due to the de novo generation of potent tumour neoantigens during transformation with mutagens9–12. Importantly, in a complimentary study reported in this issue, somatically mutated spectrin-β2 in a MCA-induced sarcoma was found to act as a potent neoantigen that drove the immunoediting process (ref*). In an attempt to introduce immunogenic mutations in Lenti-x tumours, we treated cell lines from these tumours with MCA in vitro. Interestingly, such treatment rarely yielded clones with increased immunogenicity (Supplementary Fig. 3). This may indicate that while carcinogens can produce mutations that are immunogenic, it may be a rare event.
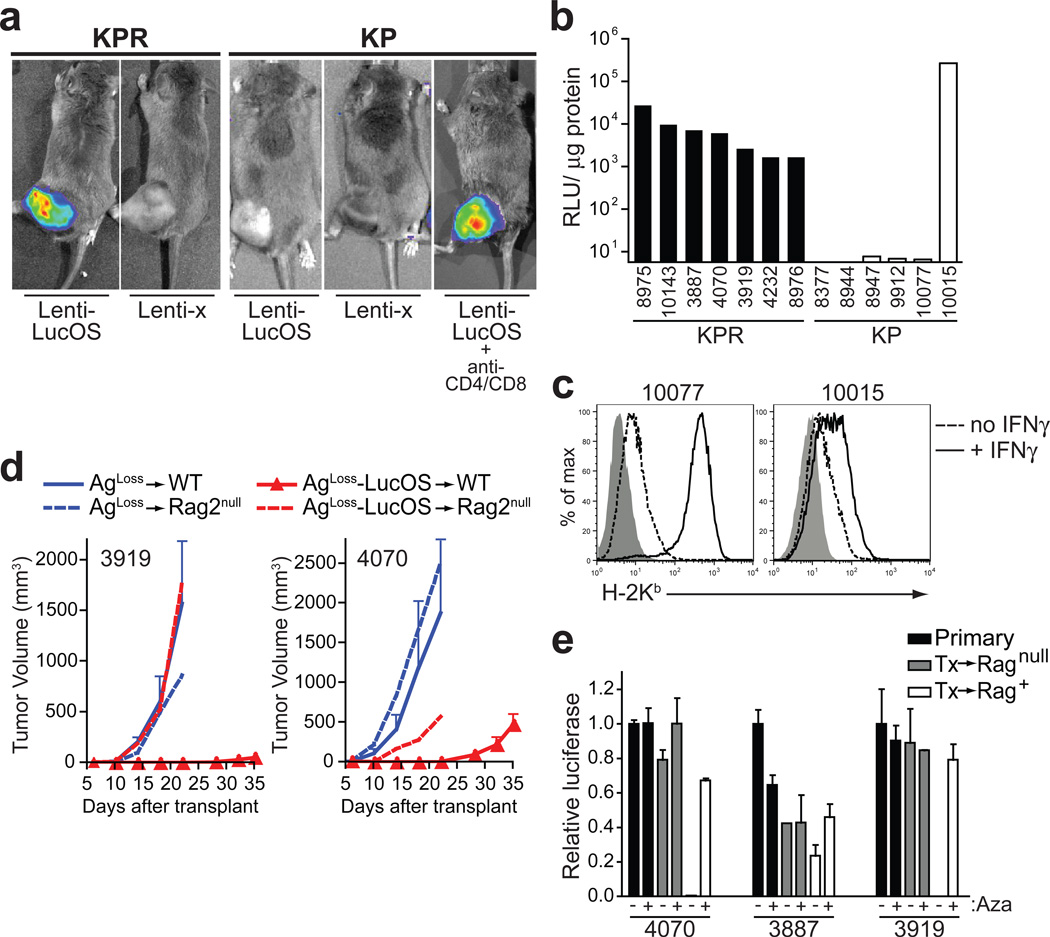
If cancer immunoediting by lymphocytes requires potent TSAs, then Lenti-LucOS-induced tumours that appear edited after forming in KP mice may have evaded an immune response by the selective outgrowth of cells lacking these potent antigens13–15. To assess antigen expression, we measured luciferase activity in tumours. Whereas tumours from KPR mice were universally luciferase positive, tumours from KP mice had drastically reduced luciferase activity in all but one of six sarcomas (Fig. 4a, b). Interestingly, this sarcoma had significantly reduced H-2Kb expression, the MHC class I allele responsible for presenting the SIY and SIN antigens (Fig. 4c). Sarcomas from KP mice treated with anti-CD4 and anti-CD8 antibodies at tumour initiation also retained luciferase activity (5/6 sarcomas luc+, Fig. 4a). However, fewer sarcomas retained luciferase expression when mice were treated with anti-CD4 and anti-CD8 antibodies beginning 14 days after tumour initiation (1/5 sarcomas luc+), suggesting that immunoediting can occur very early during sarcoma development. Thus, by selectively eliminating cells that express potent TSAs, T lymphocytes drive the escape of tumour cells that either do not express potent antigens or cannot present the antigens to reactive T cells.
Figure 4. Immunoediting occurs by selecting for tumour cells that do not express targeted antigens.
a, Representative luciferase activity of Lenti-LucOS and Lenti-x-induced sarcomas in KPR, KP or anti-CD4/CD8 treated KP mice. b, Luciferase expression in Lenti-LucOS-induced sarcoma cell lines derived in KPR or KP mice. c, Freshly harvested sarcomas cultured with IFN-γ (solid line) or untreated (dashed line) were analyzed for H-2Kb surface expression (shaded, control antibody). d, Growth of two independent tumours (3919 and 4070) that had lost antigen expression (AgLoss, blue lines) or the same tumour lines after reintroduction of LucOS (AgLoss-LucOS, red lines). Mean tumour volume ± s.e.m. after transplantation into three wild-type mice (solid lines) or one Rag-2null mouse (dashed lines). e, Relative luciferase activity (compared to the primary sarcoma) ± 5-aza-2’-deoxycytidine (Aza) of Lenti-LucOS sarcomas from KPR mice (Primary, black columns) that were passaged through Rag-2−/− (Tx->Ragnull, grey columns) or wild-type mice (Tx->Rag+). Mean ± s.e.m. from two experiments.
Similar to the antigen loss observed in autochthonous sarcomas, Lenti-LucOS-induced sarcomas from KPR mice lost antigen expression when transplanted into wild-type mice (Supplementary Fig. 4). Importantly, tumours that lost antigen expression after being passaged through wild-type mice grew comparably upon secondary transplantation into wild-type and Rag-2−/− mice, whereas tumours passaged through Rag-2−/− mice did not (Supplementary Fig. 4). To test whether antigen loss was sufficient to provide a means of escape for Lenti-LucOS sarcomas derived in KP mice, we reintroduced the LucOS antigens into sarcomas that had lost expression of the antigens after passage through wild-type mice (referred to as AgLoss tumours). Indeed, re-expression of LucOS led to severely reduced tumour growth (Fig. 4d), indicating that loss of antigen expression was the primary means of tumour escape in this setting.
Epigenetic silencing of tumour antigen expression via DNA methylation could be responsible for antigen loss and tumour escape16,17. To test this hypothesis, we treated cell lines that had lost luciferase expression after transplantation into immune-competent mice with 5-aza-2’-deoxycytidine (Aza), which reverses epigenetic silencing by inhibiting DNA methylation. In several lines tested, luciferase activity was restored with Aza treatment (Fig. 4e). Therefore, epigenetic silencing of tumour antigens may represent an important mechanism by which tumours can be edited in response to immune surveillance.
Here we have overcome many of the obstacles of carcinogen-induced models of cancer by using an autochthonous, genetically engineered model of sarcomagenesis to show that T lymphocyte-driven tumour antigen loss is a critical means by which cancer immunoediting occurs in a primary tumour setting. While this study was limited to investigating the role of anti-tumour immunity by T cells, this model could be adapted to investigate the role of other critical immune cells in cancer immunoediting, such as B cells or NK cells, by either introducing surface-expressed or stress-related antigens into tumours, respectively18–20. This study resulted in two key discoveries. First, oncogene-driven, endogenous tumours can undergo immunoediting in a manner similar to carcinogen-driven tumours if engineered to express model TSAs. The immunogenicity of MCA-induced sarcomas is well-documented, and may be a direct consequence of TSAs that arise from carcinogen-induced mutations of normal genes during tumour development9,11,12(ref*). In contrast, cancers that arise spontaneously or by targeted genetic mutations in mice have been reported to be weakly immunogenic21–24. However, the mutational requirements for tumourigenesis in humans may be greater than in mice,25 and thus it is possible that spontaneous or genetically engineered mouse models of cancer might underestimate the mutational and antigenic load of most human cancers. This idea is supported by the second critical finding of this study that tumour immunogenicity is not a universal characteristic of cancer development. By obviating the need for carcinogens, we could induce sarcomas that potentially lacked potent TSAs. These tumours had significantly reduced immunogenicity despite no previous engagement with the adaptive immune system and hence no opportunity for immunoediting. These results provide the first experimental system to unify the heretofore apparently conflicting results obtained using either carcinogen-induced or genetically targeted mouse models of cancer by identifying TSAs as the critical determinants that invoke adaptive immunosurveillance and immunoediting2,21. We propose that identifying and characterizing TSAs in human cancers may be critical for the generation of more effective anti-cancer immunotherapies in patients suffering with this disease.
METHODS SUMMARY
Experiments used mice of the 129S4/SvJae strain. All animal studies and procedures were approved by the Massachusetts Institute of Technology’s Committee for Animal Care. Sarcomas were induced in KP and KPR mice by intramuscular injection of the hind limb with replication-incompetent lentiviruses expressing Cre recombinase as reported previously5,6. To deplete T cells, anti-CD4 (GK1.5) and anti-CD8 (YTS169.4) antibodies were administered at a dose of 250 µg/mouse by i.p. injection once weekly for the duration of the experiment. Flow cytometry was performed as described6. For transplantation experiments, 2×105 freshly isolated tumour cells or cultured tumour cells were transplanted subcutaneously into immune-competent or Rag-2−/− mice of the 129S4/SvJae background. Tumour volumes were calculated by multiplying the length × width × height of each tumour. To detect luciferase activity, freshly explanted tumours or cell lines were lysed, mixed with Luciferin reagent (Promega), and relative light units (RLU) were detected with a luminometer (MGM Instruments). Aza treatment used 1 µM 5-aza-2’-deoxycytidine for three days. In vivo bioluminescence images were acquired with the NightOWLII LB983 (Berthold Technologies) or the IVIS Spectrum (Xenogen Corp.) after intraperitoneal injection of 1.5 mg Beetle Luciferin (Promega). Statistical analyses utilized unpaired two-tailed Fisher exact probability tests or Student’s t tests.
METHODS
Mice and tumour induction
129S4/SvJae strains backcrossed 8 generations were used for all experiments. Trp53fl mice were provided by A. Berns, K-rasLSL-G12D were generated in our laboratory, and Rag-2−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Sarcomas were induced in KP and KPR mice by intramuscular injection of the left hind limb with replication-incompetent lentiviruses expressing Cre recombinase as reported previously5,6. Mice were monitored twice weekly for palpable sarcoma formation beginning 50 days after intramuscular injection. All animal studies and procedures were approved by the Massachusetts Institute of Technology’s Committee for Animal Care.
Lentiviral production
Lentivirus was produced by transfection of 293T cells with Δ8.2 (gag/pol), CMV-VSV-G, and the various transfer vectors expressing Cre as described26.
Antibody depletion
Anti-CD4 (GK1.5) and anti-CD8 (YTS169.4) antibodies were administered at a dose of 250 µg/mouse by i.p. injection once weekly for the duration of the experiment.
Preparation, culture, and transplantation of primary sarcomas
Primary sarcomas were explanted and single cell suspensions were generated by mincing and digesting the tissues for ~1 hour at 37°C in 125 U/ml Collagenase Type I (Gibco), 60 U/ml Hyaluronidase (Sigma), and 2 mg/ml Collagenase/Dispase (Roche), followed by passage through a 70 µm filter. Subcutaneous transplantation utilized 2×105 cells from freshly isolated tumour cells or cell lines from primary autochthonous tumours that were trypsinized and washed three times in plain DME medium. Transplant recipients were immune-competent or Rag-2−/− mice on the 129S4/SvJae background from the same mouse colony used to generate the autochthonous tumours. Subcutaneously transplanted tumour volumes were calculated by multiplying the length × width × height of each tumour. In Figure 3, the mean volume ± s.e.m. of each tumour line is depicted after transplantation into wild-type mice (WT, open columns) at the time point when the same tumour line reached a volume of 1000 mm3 in the Rag-2null transplanted mice (Rag-2null, filled columns).
Flow cytometry
Cell suspensions from lymphoid organs were prepared by mechanical disruption between frosted slides. Cells were then stained with antibodies for 20–30 min after treatment with FcBlock (BD Pharmingen). α-CD8α (53-6.7), α-IFNγ (XMG1.2), α-TNFα (MP6-XT22), and DimerX I (Dimeric Mouse H-2Kb:Ig) were from BD Pharmingen. All antibodies were used at 1:200 dilution. Peptide-loaded DimerX reagents were prepared as directed and used at 1:75 dilution. To improve the sensitivity of the DimerX reagent, we utilized both PE and APC labeled dimers to co-stain CD8+ T cells. Propidium iodide was used to exclude dead cells. Cells were read on a FACSCalibur and analyzed using Flowjo software (Tree Star). In Figure 2c–d, data was determined by comparing the fraction of CD8+ cells in duplicate samples stained with Kb dimers or for cytokine production and exceeds 100% due to the incomplete sensitivity of the Kb dimers to detect antigen specific cells. In Figure 4c, freshly harvested sarcomas were cultured for 24 hours in the presence of 10 U IFN-γ (solid line) or untreated (dashed line) and analyzed for H-2Kb surface expression (shaded, control antibody)
Cytokine production
Cells were resuspended in the presence or absence of SIYRYYGL and SIINFEKL peptides in OPTI-MEM I (Gibco) supplemented with GolgiPlug (BD Pharmingen) for ~4 hours at 37°C, 5% CO2. Cells were then fixed and stained for intracellular cytokines using the Cytofix/Cytoperm kit (BD Biosciences).
Luciferase detection
Freshly explanted tumours or cell lines were lysed in Cell Culture Lysis Reagent, mixed with Luciferase Assay Reagent according to the manufacturer’s instructions (Promega), and relative light units (RLU) were detected using the Optocomp I luminometer (MGM Instruments). RLUs were standardized by the total amount of protein (Bio-Rad Protein Asssay) in each sample. In vivo bioluminescence images were acquired with the NightOWLII LB983 (Berthold Technologies) or the IVIS Spectrum (Xenogen Corp.) after intraperitoneal injection of 1.5 mg Beetle Luciferin (Promega).
5-aza-2’-deoxycytidine treatment
Tumour cell lines were plated at low confluency (2×105 cells/ well of 6-well plate), and treated with 1 µM 5-aza-2’-deoxycytidine replaced daily for three consecutive days and then analyzed for luciferase activity.
Influenza
WSN-SIY (20 pfu/ mouse) provided by J. Chen. FACs analysis performed four months after intratracheal infection.
Statistical analyses
P-values were generated using unpaired two-tailed Fisher exact probability tests or Student’s t tests.
Supplementary Material
ACKNOWLEDGEMENTS
We thank M.M. Winslow and A.G. DuPage for critical reading of this manuscript. This work was supported by Grant 1 U54 CA126515-01 from the NIH and partially by Cancer Center Support (core) grant P30-CA14051 from the National Cancer Institute and the Margaret A. Cunningham Immune Mechanisms in Cancer Research Fellowship Award (M.D.) from the John D. Proctor Foundation. T.J. is a Howard Hughes Investigator and a Daniel K. Ludwig Scholar.
Footnotes
AUTHOR CONTRIBUTIONS
M.D. and T.J. designed the study. M.D. performed all experiments with assistance from C.M. and L.M.S. A.F.C. provided reagents and conceptual advice. M.D. and T.J. wrote the manuscript.
REFERENCES
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 3.Swann JB, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin Z, Blankenstein T. A cancer immunosurveillance controversy. Nat Immunol. 2004;5:3–4. doi: 10.1038/ni0104-3. author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 5.Kirsch DG, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13:992–997. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]
- 6.DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson RT, Schmidt LM, Crowley D, Chen J, Jacks T. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell. 2011;19:72–85. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 8.Cheung AF, Dupage MJ, Dong HK, Chen J, Jacks T. Regulated expression of a tumor-associated antigen reveals multiple levels of T-cell tolerance in a mouse model of lung cancer. Cancer Res. 2008;68:9459–9468. doi: 10.1158/0008-5472.CAN-08-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of "tumor escape" phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–778. [PubMed] [Google Scholar]
- 11.Dubey P, et al. The immunodominant antigen of an ultraviolet-induced regressor tumor is generated by a somatic point mutation in the DEAD box helicase p68. J Exp Med. 1997;185:695–705. doi: 10.1084/jem.185.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monach PA, Meredith SC, Siegel CT, Schreiber H. A unique tumor antigen produced by a single amino acid substitution. Immunity. 1995;2:45–59. doi: 10.1016/1074-7613(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 13.Uyttenhove C, Maryanski J, Boon T. Escape of mouse mastocytoma P815 after nearly complete rejection is due to antigen-loss variants rather than immunosuppression. J Exp Med. 1983;157:1040–1052. doi: 10.1084/jem.157.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou G, Lu Z, McCadden JD, Levitsky HI, Marson AL. Reciprocal changes in tumor antigenicity and antigen-specific T cell function during tumor progression. J Exp Med. 2004;200:1581–1592. doi: 10.1084/jem.20041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stauss HJ, Van Waes C, Fink MA, Starr B, Schreiber H. Identification of a unique tumor antigen as rejection antigen by molecular cloning and gene transfer. J Exp Med. 1986;164:1516–1530. doi: 10.1084/jem.164.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo ZS, et al. De novo induction of a cancer/testis antigen by 5-aza-2'-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res. 2006;66:1105–1113. doi: 10.1158/0008-5472.CAN-05-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gure AO, Wei IJ, Old LJ, Chen YT. The SSX gene family: characterization of 9 complete genes. Int J Cancer. 2002;101:448–453. doi: 10.1002/ijc.10634. [DOI] [PubMed] [Google Scholar]
- 18.Guerra N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schietinger A, et al. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science. 2006;314:304–308. doi: 10.1126/science.1129200. [DOI] [PubMed] [Google Scholar]
- 20.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 21.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 22.Embleton MJ, Heidelberger C. Antigenicity of clones of mouse prostate cells transformed in vitro. Int J Cancer. 1972;9:8–18. doi: 10.1002/ijc.2910090103. [DOI] [PubMed] [Google Scholar]
- 23.Hewitt HB, Blake ER, Walder AS. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. Br J Cancer. 1976;33:241–259. doi: 10.1038/bjc.1976.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott OC. Tumor transplantation and tumor immunity: a personal view. Cancer Res. 1991;51:757–763. [PubMed] [Google Scholar]
- 25.Rangarajan A, Weinberg RA. Opinion: Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer. 2003;3:952–959. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- 26.DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.