SUMMARY
Brodman divided the neocortex into 47 different cortical areas based on histological differences in laminar myeloarchitectonic and cytoarchitectonic defined structure. The ability to do so in vivo with anatomical magnetic resonance (MR) methods in awake subjects would be extremely advantageous for many functional studies. However, due to the limitations of spatial resolution and contrast, this has been difficult to achieve in awake subjects. Here, we report that by using a combination of MR microscopy and novel contrast effects, cortical layers can be delineated in the visual cortex of awake subjects (nonhuman primates) at 4.7 T. We obtained data from 30-min acquisitions at voxel size of 62.5 × 62.5 × 1000 µm3 (4 nl). Both the phase and magnitude components of the T2*-weighted image were used to generate laminar profiles which are believed to reflect variations in myelin and local cell density content across cortical depth. Based on this, we were able to identify six layers characteristic of the striate cortex (V1). These were the stripe of Kaes-Bechterew (in layer II/III), the stripe of Gennari (in layer IV), the inner band of Baillarger (in layer V), as well as three sub-layers within layer IV (IVa, IVb, and IVc). Furthermore, we found that the laminar structure of two extrastriate visual cortex (V2, V4) can also be detected. Following the tradition of Brodman, this significant improvement in cortical laminar visualization should make it possible to discriminate cortical regions in awake subjects corresponding to differences in myeloarchitecture and cytoarchitecture.
Keywords: High resolution MRI, MR microscopy, Non-human primate, Awake, Visual cortex, V1, Extrastriate cortex
1. Introduction
The discovery of the stripe of Gennari (Gennari, 1782) in fresh visual cortical tissue was the first indication of lamination in the cerebral cortex. Over a century later, this lamination was formalized by Brodmann who divided the cortex of non-human primates into six layers (Brodmann, 1905) and, on the basis of distinct cytoarchitectural profiles (cell density, type, and size revealed by Nissl stains), delineated 43 separate areas in human neocortex (Brodmann, 1909). Cortical areas defined in this way have been found to correlate with specific functional specializations as determined by functional imaging in human subjects (Bridge et al., 2005; Geyer et al., 2011; Hinds et al., 2008; Sigalovsky et al., 2006) as well as electrophysiological and imaging in non-human primates.
A second way of differentiating cortical areas and layers is based on the pattern of myelinated fibers or myeloarchitectonic analysis (Braak, 1980). One well known myeloarchitectonic landmark is the stripe of Gennari, a 280 µm thick (von Economo and Koskinas, 1929) myelin-dense band, actually visible to the naked eye, located in the middle of gray matter within the striate cortex (V1) (Valverde, 1985). This myelin dense layer, which varies in thickness across cortical areas, is actually found in all parts of the cortex (Baillarger, 1840) and has been used to identify over thirty areas (Smith, 1907). Cortical layers and boundaries of cortical areas defined by detailed myeloarchitectonic analysis have coincided closely with those of Brodmann’s cytoarchitectonic layers and areas (Nieuwenhuys et al., 2008; Vogt and Vogt, 1919, 1954).
With advances in magnetic resonance (MR) imaging techniques, it is possible to reveal detailed structural features in living subjects based on variations in their intrinsic magnetic resonance properties such as proton density and relaxation times. High resolution MR studies in humans have succeeded at detecting the stripe of Gennari at 1.5T (Clark et al., 1992; Eickhoff et al., 2005; Walters et al., 2003). However, due to the usual relatively low ratio between the in-plane resolution and the width of the stripe of Gennari, partial volume effects lead to a stripe with patchy appearance and fuzzy borders. The spatial resolution in MR imaging is limited by multiple factors including the signal and contrast to noise ratios and the gradient strengths used (Callaghan et al., 1994). The signal per voxel decreases linearly with the reduction of voxel volume but increases with magnetic field strength, and decreases with coil size and signal bandwidth (Mansfield and Morris, 1982). Therefore, MR images can have similar signal to noise ratio (SNR) at higher spatial resolution in higher magnetic fields by using smaller coils. The ability to detect the stripe of Gennari generally improves with improved in-plane resolution (Barbier et al., 2002; Bridge et al., 2005; Carmichael et al., 2006) and by using thinner slices (Trampel et al., 2011; Turner et al., 2008), partly because this reduces the effects of partial volume averaging. Moreover, several groups have suggested that some contrast mechanisms (e.g. magnitude and phase changes in T2*-weighted imaging) are more favorable at high field than conventional MR contrasts (e.g. T1 and T2) and are more able to separate gray matter and white matter (Abduljalil et al., 2003; Duyn et al., 2007; Haacke et al., 2004).
Recent studies show that MR imaging in vivo can achieve spatial resolutions of better than 100 µm and that MR images acquired at such resolution in living animals can differentiate multiple cortical layers in mice (Boretius et al., 2009); moreover, additional myelinated structures outside the stripe of Gennari can apparently be detected within V1 of non-human primates (Goense and Logothetis, 2006; Goense et al., 2007). However, high-resolution MR imaging at 100 × 100 µm2 in-plane resolution (or higher) has thus far been achieved primarily in anesthetized subjects. Compared with scanning in anesthetized subjects, the two main challenges facing improved spatial resolution in awake subjects are head-related motion blurring and the relatively short scan time. A combination of extensive training, specially designed training paradigms, and customized head-fixation may help overcome these problems (Chen et al., 2011). Here, we demonstrate the ability to reveal fine brain microstructure beyond the stripe of Gennari by high spatial resolution MR methods in awake behaving non-human primates at 4.7T, a method we term ‘MR microscopy’ (Benveniste and Blackband, 2002). This will be the basis for addressing the correlation between cortical layers as well as laminar specific functional activation in awake subjects (Geyer et al., 2011; Trampel et al., 2011) and will be a useful approach for distinguishing cortical areas in such studies.
2. Material and methods
2.1 MR imaging data acquisition
MR images were acquired using a Varian 4.7T vertical MR scanner (Varian Inc., Palo Alto, CA) with a 2-cm surface coil. Our results are obtained from two Macaque monkeys (Macaca mulatta) scanned on multiple sessions. All procedures conformed to the guidelines of the National Institute of Health and were approved by the Institutional Animal Care and Use Committee of Vanderbilt University. Before imaging, animals were implanted with MR-compatible headposts under general anesthesia (1~2% isoflurane). Monkeys were then placed in the vertical bore and trained to perform a continuous fixation task with fluid reward every 20 s throughout the scan. T2*-weighted gradient-echo images were acquired (repetition time = 200 to 350 ms, flip angle = 30° to 45°, echo time = 12 to 40 ms, in-plane resolution = 62.5 × 62.5 µm2 to 250 × 250 µm2). Each scanning session consisted of up to five 30-minute high-resolution anatomical runs.
2.2 Data analysis
Images were reconstructed and analyzed using Matlab (Mathworks, Natric, MA) without zero filling and spatial filtering. The real (r) and imaginary (i) components of complex MR signals were used to calculate the magnitude (M) and phase maps (P) as following:
| [1] |
| [2] |
Phase maps were corrected for macroscopic magnetic field variations, which were estimated by an eight-order 2D polynomial function after phase unwrapping (Duyn et al., 2007; Yao et al., 2009).
Relatively flat regions of interest were selected (Supplementary Fig. 1A). The outer (dura/gray matter boundary) and inner borders (white/gray matter boundary) of a cortical area were determined by manual segmentation. The distances of each voxel in the ROI to the outer (Supplementary Fig. 1B) and inner borders (Supplementary Fig. 1C) were calculated using a minimum distance algorithm (Schleicher et al., 2000). The cortical thickness was defined as the sum of distances to outer and inner borders. To compensate for variations in cortical thickness, cortical depth (Supplementary Fig. 1D) is normalized as follows:
| [3] |
Profiles of cell body and myelin density in V1 were interpreted based on the literature (Billings-Gagliardi et al., 1974; Brodmann, 1905; Peters and Sethares, 1996). The histological sections were smoothed mildly by a moving average filter with span of 5% of the cortical depth. All profiles were z-normalized to account for the difference among modalities (Boretius et al., 2009; Eickhoff et al., 2005). Areal borders between visual cortical areas were based on published atlases (Saleem and Logothetis, 2007).
The relative displacement between two structural images was estimated by an in-plane rigid body model. The index of motion Displacement was calculated from the obtained translational movements Tranx and Trany:
| [4] |
The contrast-to-noise ratio between gray matter (GM) and white matter (WM) was calculated as the signal difference between GM and WM divided by the noise in the background.
3. Results
3.1. Effect of imaging parameters on contrast and quality of high-resolution images
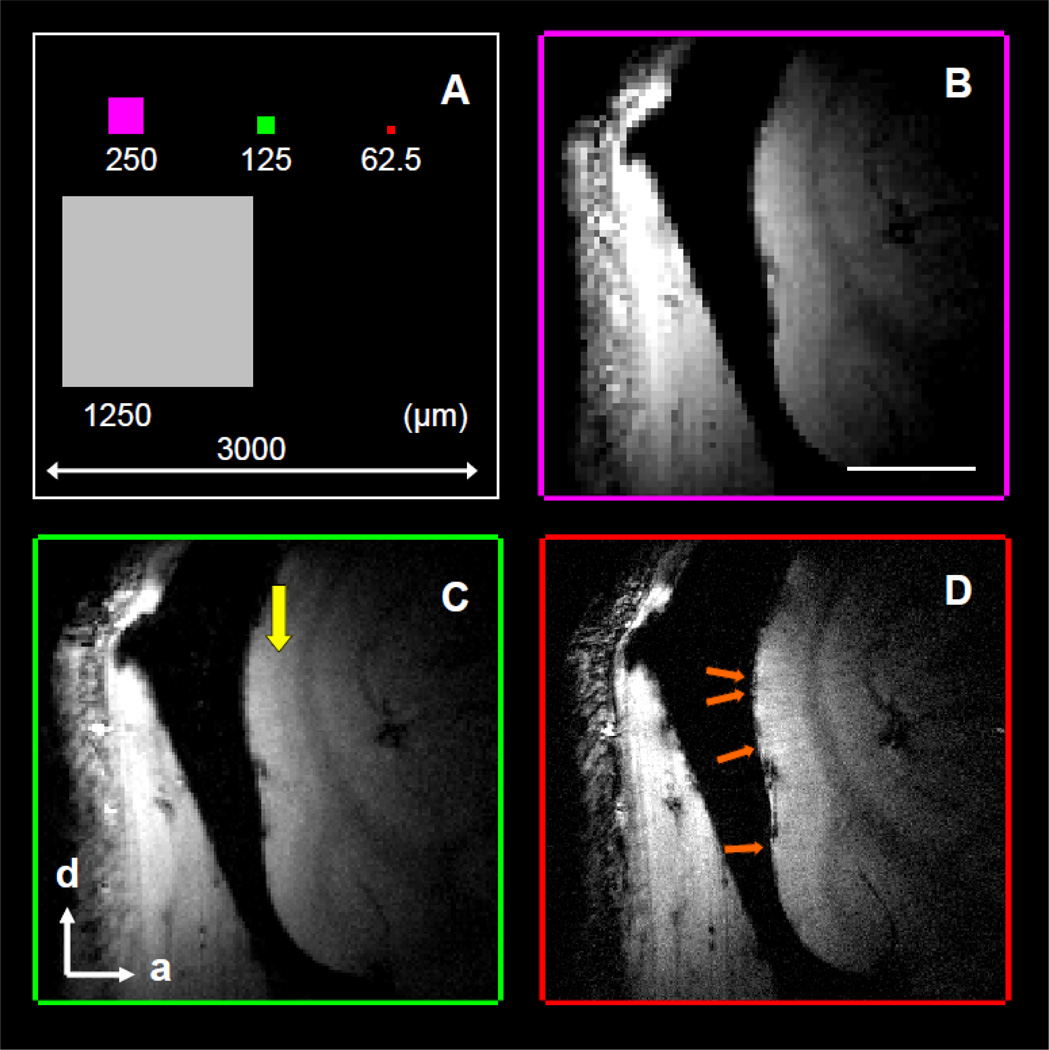
The in-plane sizes of voxels used in this study are summarized in Fig. 1A. The smallest voxel we employed was an in-plane size of 62.5 × 62.5 µm2 (red square), which is 0.04% and 0.25% of the in-plane voxel size used in most human (3000 × 3000 µm2, white square) and awake non-human primate (1250 × 1250 µm2, gray square) functional MR imaging studies. This small size increases our ability to reveal fine anatomical structures. The T2*-weighted magnitude images of a sagittal slice in an awake monkey at different in-plane resolutions with the same slice thickness of 2 mm are shown in Fig. 1B – 1D. These anatomical runs were collected in the same session and lasted up to 15 minutes. At in-plane voxel size of 250 × 250 µm2 (Fig. 1B), the gray matter and white matter can be discriminated. In Fig. 1C, the stripe of Gennari (yellow arrow), which is about 200 µm thick in monkey V1, is detectable in the middle of gray matter at in-plane resolution of 125 × 125 µm2. By further increasing the spatial resolution to 62.5 × 62.5 µm2 (Fig. 1D), small intracortical veins (orange arrows) within the gray matter are distinguishable. Dura and pial veins can be seen at the surface of some cortical areas.
Figure 1. Influence of in-plane resolution on image quality.
(A) The relative in-plane size of voxels used in this and other MR imaging studies. (B–D) The T2*-weighted images (slice thickness 2 mm) from the visual cortex of an awake monkey as a function of in-plane resolution. The in-plane resolutions are 250 × 250 µ2, 125 × 125 µm2, and 62.5 × 62.5 µm2 for (B), (C), and (D), respectively. The stripe of Gennari (yellow arrow in C) and cortical veins (orange arrows in D) are detectable. Scale bar in (B): 5 mm. d, dorsal; a, anterior.
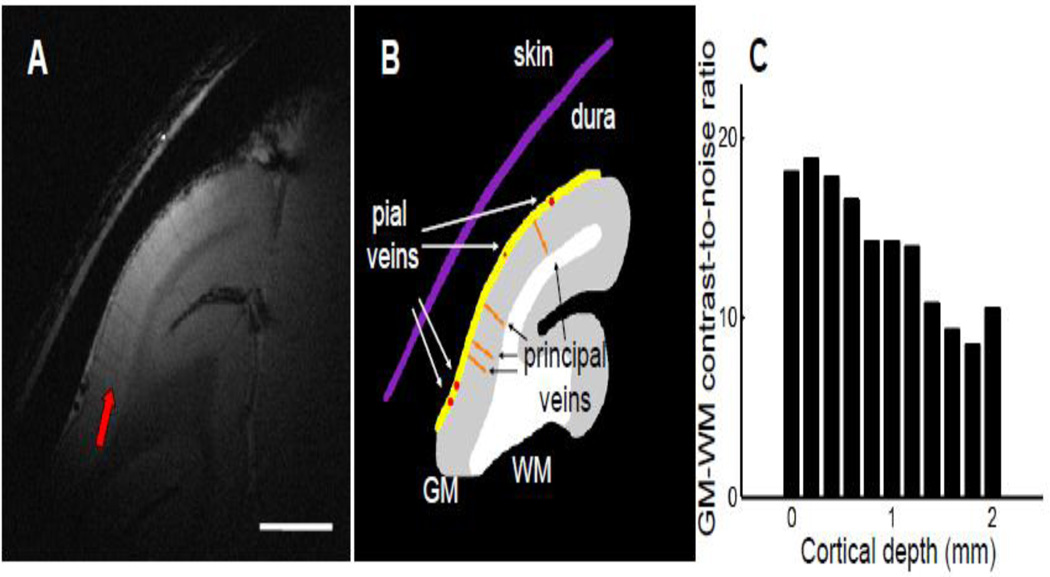
To further improve the quality of the structural image, we oriented the imaging plane perpendicular to the cortical surface and used a slice thickness of 1 mm (Trampel et al., 2011; Turner et al., 2008) to minimize any partial volume effect. Figure 2A shows the averaged results of three 30-minute runs with adjusted slice parameters. The dura (yellow line in Fig. 2B) and pial veins (red dots) can be clearly detected at the cortical surface. Principal veins (orange lines), which run through the gray matter (Duvernoy et al., 1981), had lower signals and higher contrast than those in the thicker slice shown in Fig. 1D. The contrast between gray matter (GM) and white matter (WM) regions was high in most cortical areas. Due to the use of a 2-cm surface coil for both transmission and reception, the sensitivity of MR signal decreases with distance to the coil. In Fig. 2C, we plotted the GM-WM contrast-to-noise ratio (CNR) against the cortical depth. As expected, the CNRs were the greatest at the cortical surface (around 20:1) and gradually decreased with depth. Even in cortical areas located 2 mm from the surface, the CNRs were about 10:1, a ratio still sufficient for clearly discriminating GM from WM.
Figure 2. High-resolution structural image in the awake monkey V1.
(A) A high-resolution structural image with in-plane resolution of 62.5 × 62.5 µm2 and slice thickness of 1 mm shows fine anatomical structures of V1. The slice is oriented perpendicular to the cortical surface. The gray and white matter are clearly separable, and the stripe of Gennari (red arrow) can be seen in the middle of gray matter. (B) Illustration of anatomical structures. Skin and dura are marked as purple and yellow lines, respectively. Pial veins are indicated by red dots, and principal veins running through the gray matter are shown as orange lines. WM, white matter. GM, gray matter. Scale bar in (A): 5 mm. (C) The contrast-to-noise ratio between the gray matter and white matter plotted against the cortical depth.
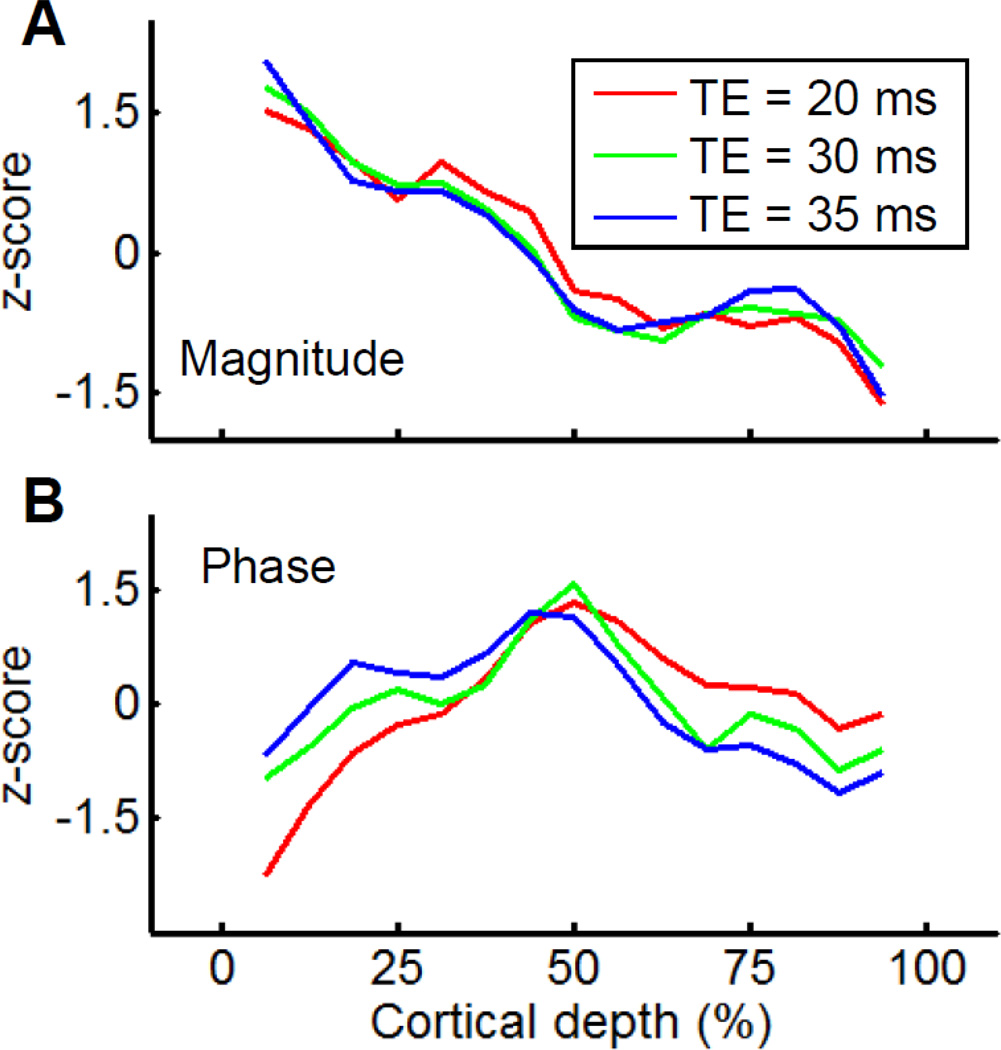
The other important parameter to consider is the echo time (TE), the delay between the RF excitation and data acquisition, used in the gradient-echo sequence. The optimal contrast between GM and WM is usually achieved when a TE between T2* values of GM and WM is used. To determine the optimal TE for best contrast within GM, we tested TE values of 20, 30, and 35 ms. We plot the z-normalized profiles of magnitude (Fig. 3A) and phase (Fig. 3B) acquired at these three TEs against the cortical depth. The magnitude profile obtained with the shortest TE (20 ms, red line) has two dips, at about 25% and 50% cortical depth (Fig. 3A). Dips are also observed at TEs of 30 ms (green line) and 35 ms (blue line). The shapes of these three magnitude profiles are very similar. In contrast, the phase profiles differ as a function of TE (Fig. 3B). At superficial depths (υ0% cortical depth) the phase tends to be greatest for 35 ms TE, smaller for 30 ms TE, and smallest for 20 ms TE, while at greater depths (e.g. >50% cortical depth) the phase is greatest for 20 ms TE, less for 30 ms TE, and least for 35 ms TE. We relate these depth-related differences in TE profile to laminar divisions within the neocortex: the supragranular, granular, and infragranular layers (roughly 0 – 33%, 33 – 66%, and 66 – 100% cortical depth, respectively). These three anatomically defined laminar divisions are defined based on relative location to granular layer IV and each occupies roughly one-third of the cortical depth. All three phase profiles had a peak within the granular layer. Such peak in the middle of the gray matter is the only one for the profile of 20 ms TE (red line). No clear peak could be found within either supragranular or infragranular layers. When the TEs are lengthened to 30 ms (green line) and 35 ms (blue line), the contrasts between the supragranular and the granular layers decreased and the contrasts between the granular and infragranular layers increased. At a TE of 30 ms, we found these two contrasts were almost the same and that, in addition to the one in the granular layer, there were two additional peaks within the supragranular and infragranular layers. Thus, at TE of 30 ms, we find balanced contrasts between the supragranular and infragranular layers and therefore maximal structural contrast within GM; this TE value is close to the T2* value of gray matter previously reported at 4.7T (Pfeuffer et al., 2004).
Figure 3. Cortical depth profiles of T2* weighted images are a function of echo time (TE).
The magnitude (A) and the phase (B) profiles of T2* weighted images. The average profiles at different echo time of 20 ms, 30 ms, and 35 ms are marked as red, green, and blue lines. Profiles are z-normalized and plotted against the distance from the cortical surface in percent of cortical depth for a better comparison.
3.2. Influence of head motion on image quality of MR microscopy in awake subjects
A major challenge in MR microscopy (Benveniste and Blackband, 2002) and high resolution structural imaging in awake subjects (Duyn, 2010) is the problem of subtle head motion. We have previously shown that, with proper training paradigms and customized head fixation, head displacements of awake monkeys can be limited to less than 100 µm (Chen et al., 2011; Lu et al., 2010; Tanigawa et al., 2010). Here, we examined whether the high resolution structural images collected in behaving monkeys with in-plane resolution of 100 × 100 µm2 or higher were contaminated by head motion.
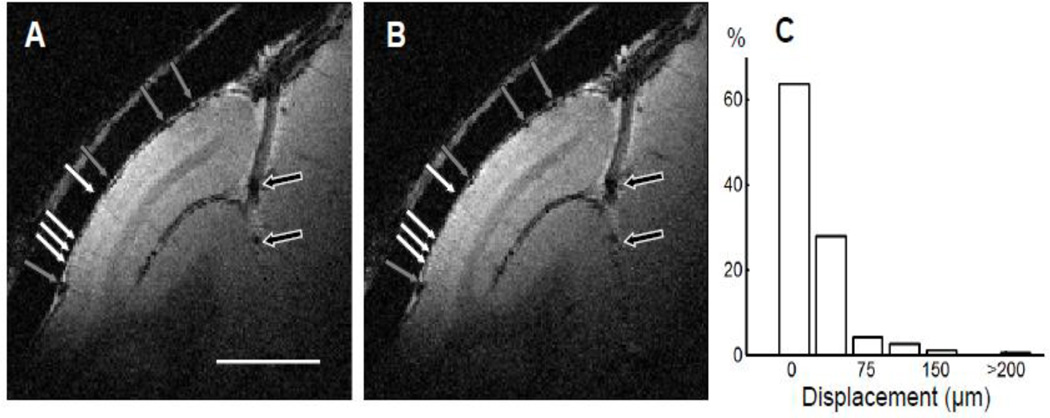
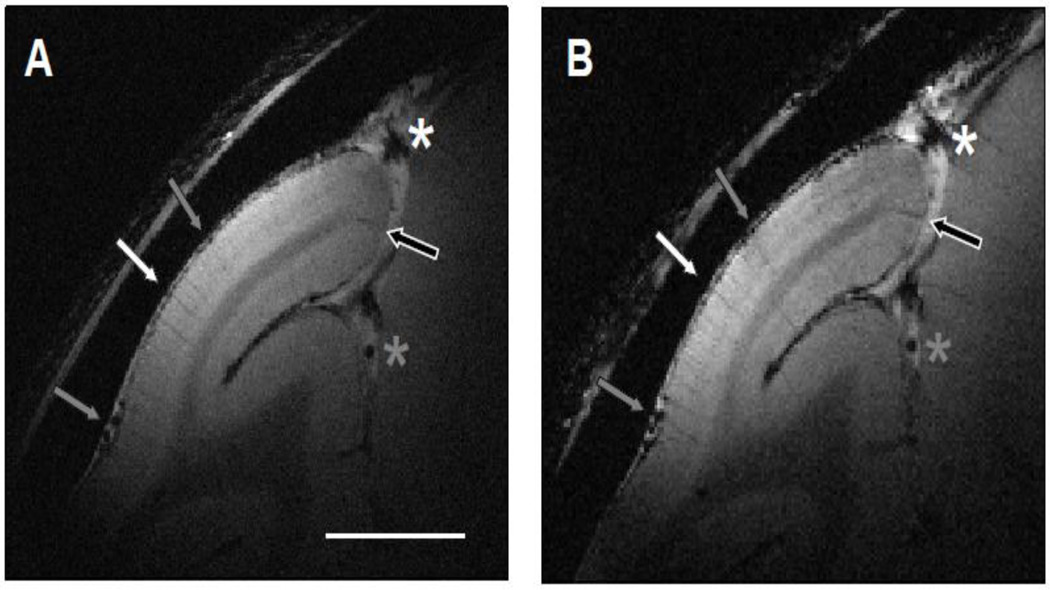
Figure 4A shows the magnitude map from a 30-minute anatomical run, which had an in-plane resolution of 100 × 100 µm2 and thickness of 1 mm from an awake monkey. Although the duration of the scan was one-third of the one presented in Fig. 2A, the GM-WM CNRs were sufficient to discriminate GM from WM. Dura (location of yellow line in Fig. 2B) could be clearly distinguished from the cortex. Draining veins on the cortical surface (gray arrows) and within lunate sulcus (black arrows) contain high concentration of deoxygenated hemoglobin, which are seen as dark spots in the T2*-weighted image. If there were serious motion blur, images of principal veins, which have an average diameter of 80 – 120 µm (Duvernoy et al., 1981), would be wider and harder to detect. This was not the case. Several principal veins (white arrows), whose diameters were about 100 µm, were detected within the gray matter. A second run (Fig. 4B) acquired one hour later had the same duration, in-plane resolution, and thickness. All anatomical structures marked in Fig. 4A can be found, including the pial veins (gray arrows) and deep draining veins (black arrows). In this second run, principal veins (white arrows) were located at the same location and had similar diameter, indicating the average head motion between two runs is effectively well under 100 µm.
Figure 4. Reproducibility of structural images within a session.
(A and B) Structural images collected in two runs (30 minutes each) that were one hour apart (slice thickness, 1 mm; in-plane resolution, 100 × 100 µm2). The locations of the pial veins (black and gray arrows) and principal veins within gray matter (white arrows) are the same. Scale bar: 5 mm. (C) The distribution of relative displacement between runs in the duration of 30 minutes (the length of a typical run).
A rigid body registration between the images shown in Fig. 4A and Fig. 4B confirms this impression. The net displacement along the x-axis and y-axis were estimated to be 16 µm and 40 µm, respectively. Both displacements were less than half of the in-plane voxel size (100 × 100 µm2). As shown in Fig 4C, in well trained animals, we found only subtle head motion across anatomical runs (each run lasting 30 minutes). As seen in the distribution of relative displacement between runs, the average displacement was 24 ± 32 µm (mean ± SD). The majority of displacements (>90%) were smaller than 50 µm. Those larger than 100 µm comprised less than 4%. As the influence of head movement within a session was small compared with the voxel size, runs acquired in the same session can be averaged without significant loss of structural detail.
The small head displacements between runs from the same session (Fig. 4C) permitted temporal averaging of runs and provided improved image quality without reduction in resolution. We obtained good reproducible profiles from multiple runs within a session (Fig. 4A and Fig. 4B) and even from sessions acquired two weeks apart (Fig. 5A and 5B). Main anatomical markers in Fig. 5A, including pial veins (gray arrows), principal veins on the cortical surface (white arrow) and within the lunate sulcus (black arrow), and draining veins (white and gray stars) were repeatedly detected (Fig. 5B).
Figure 5. Reproducibility of structural images between sessions.
Structural images of V1 from the same awake monkey collected in two sessions that were 2 weeks apart are shown. (A) The in-plane voxel size was 62.5 × 62.5 µm2 and the thickness was 1 mm. (B) In-plane spatial resolution of 100 × 100 µm2 and a thickness of 1 mm. The location and shape of the pial veins within the lunate sulcus (white and gray stars) and on the cortical surface (gray arrows) are almost the same. The repeatable detection of principal veins within gray matter (gray and white arrows) further support that the high-resolution structural images from different sessions are highly reproducible. Scale bar: 5 mm.
3.3. Laminar structure in V1 revealed by magnitude and phase maps
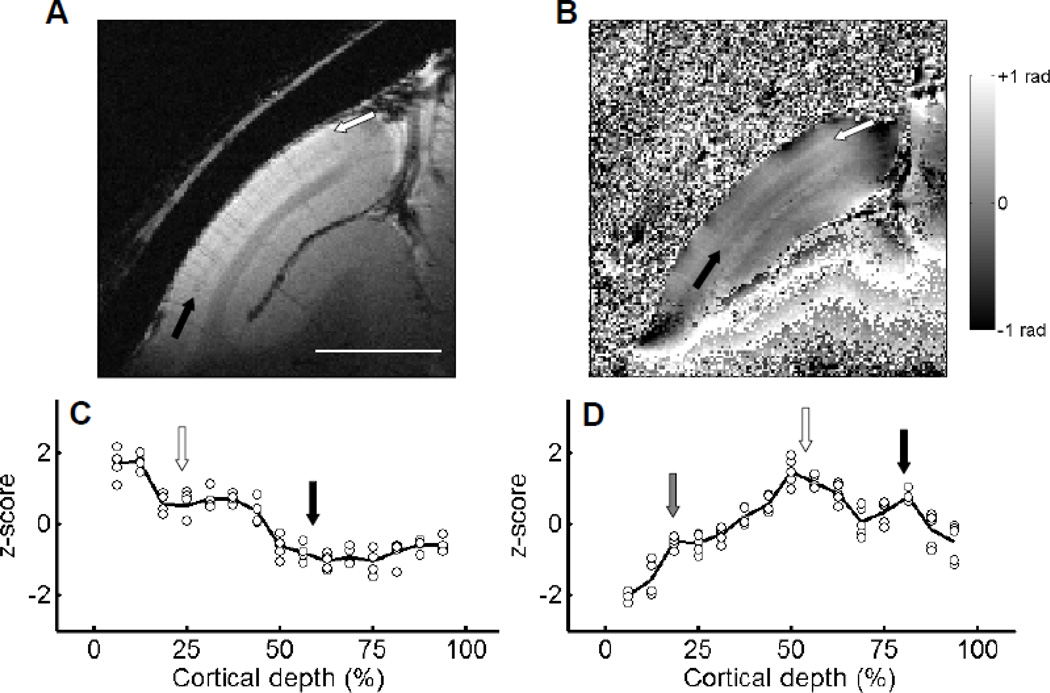
The signal-to-noise ratio of the acquired data increases with the square root of the imaging time, so we attempted to maximize the duration of each run and the number of runs. In a well trained monkey, we are able to collect up to five 30-minute runs from a single session. Data from such a two and a half hour session are shown in Fig. 6: the averaged magnitude (Fig. 6A) and phase (Fig. 6B) maps from a session using the optimal TE of 30 ms, thickness of 1 mm, voxel volume of 10 nl (100 × 100 µm2 in-plane), and flip angle of 30° are shown. Comparison of the map in Fig. 6A (2.5 hour run) with that in Fig. 4A (30 min run) reveals that the SNR of the magnitude map was significantly improved. Not only are GM and WM readily distinguished, but two bands can be detected in the middle of the gray matter: one band which corresponds with the stripe of Gennari (black arrow) and another between the first band and the outer border of the GM (white arrow). Examination of the z-normalized magnitude profile with the cortical depth further supports the existence of additional fine structure within the GM (Fig. 6C, black line: average of five runs, circles: individual runs). There are two dips in this profile located at approximately 20% (white arrow) and 60% cortical depth (black arrow) which correspond to the two dark bands seen in Fig. 6A. Note that variations between different runs were small.
Figure 6. Cortical profiles of magnitude and phase in V1.
The magnitude (A) and phase (B) images of averaged results from five 30-minute runs of a slice over V1 (slice thickness, 1 mm; in-plane resolution, 100 × 100 µm2). The stripe of Gennari (black arrow, layer IV) can be detected in (A). An additional dark layer can be found between the cortical surface and the layer IV (white arrow) from the magnitude map (A). From the phase map (B), a second bright layer (black arrow) exists between the layer IV (white arrow) and the white/gray matter border. (C) and (D) show the z-normalized profiles of magnitude and phase against cortical depth. The black lines indicate the averaged results, and circles represent results of individual runs. Locations of prominent laminar structures are indicated by arrows with the same color used in (A) and (B). A small third peak at 20% cortical depth can be detected in the phase profile (gray arrow). Scale bar in (A): 5 mm.
Variations with laminar depth were also observed in phase maps. Variations in phase in T2*-weighted images has been reported to be related to the density of ferritin, which co-localizes with myelin in V1 (Fukunaga et al., 2010). Figure 6B presents the results based on phase data. Phase information from areas outside the cortex and cortical regions with low SNR exhibit large variations (Duyn et al., 2007). The cortical area within V1 shows relatively smooth variations after the phase unwrapping and polynomial fitting procedures (see Experimental Procedures). In the middle of the gray matter in V1, a bright band (white arrow) 200 to 300 µm in width is seen, likely indicating a band of high myelin density corresponding to layer IVb (the stripe of Gennari). A second bright band (black arrow) can be detected between the first bright band and the GM-WM border with a thickness of 100 to 200 µm. When plotted with respect to laminar depth, the phase profile (Fig. 6D) reveals two peaks (vertical white and black arrows). Again, phase values are fairly consistent across runs (black line: mean, circles: individual runs). A small third peak can also be seen at 20% cortical depth (gray arrow). Given these relative depths, we suggest these three bands correspond, respectively, to the stripe of Kaes-Bechterew (layer II/III) (gray arrow), the stripe of Gennari (white arrow) and the inner band of Baillarger (black arrow).
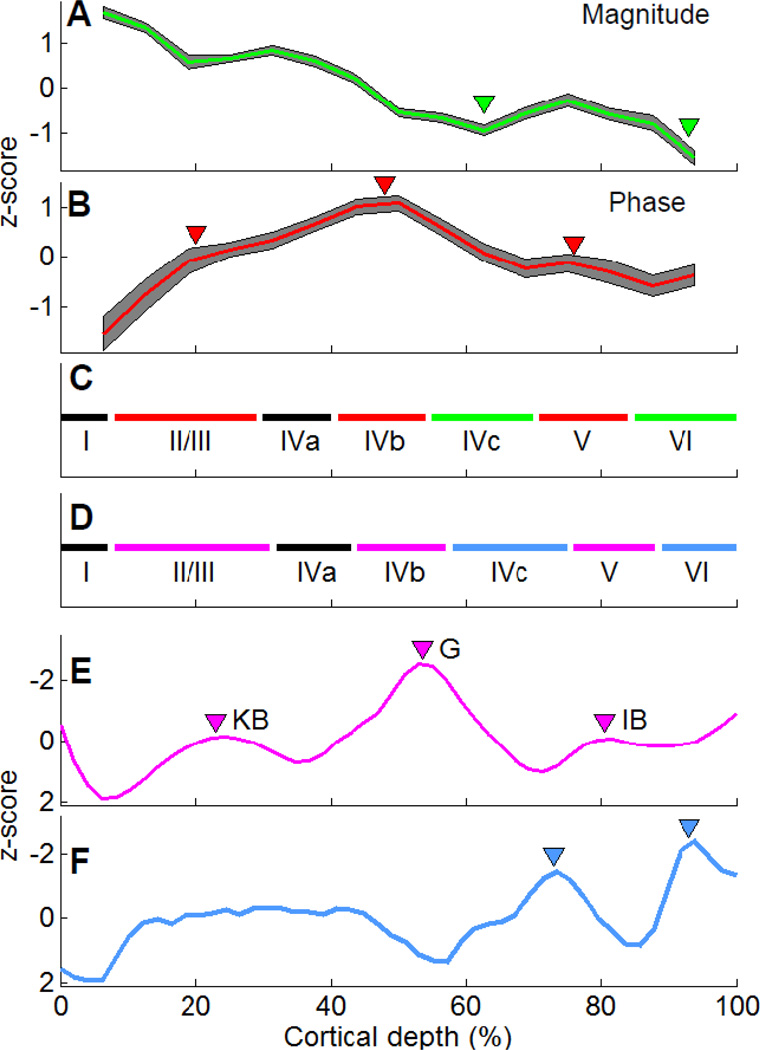
We observed similar laminar profiles within GM of V1 across slices obtained in all experiments. The mean magnitude profile (Fig. 7A) and phase profile (Fig. 7B) from all slices illustrates the consistency of these laminar profiles within gray matter. Previous studies have suggested that both magnitude and phase profiles reflect myelin density (Boretius et al., 2009; Duyn et al., 2007; Fukunaga et al., 2010), while the cell density may contribute mainly to the magnitude profile (Boretius et al., 2009). By combining the fluctuations indicated by these two profiles, we are able to infer laminar positions consistent with histologically determined cellular and myelin profiles. Based on the magnitude profile, the two observed dips at around 60% and 90% cortical depth (Fig. 7A, green arrowheads) are consistent with the location of the two high cellular and myelin density layers, layer IVc and layer VI (Fig. 7F, blue arrowheads, based on (Billings-Gagliardi et al., 1974; Brodmann, 1905), replicated in Supplementary Fig. 2A). Based on the phase profile, the three observed local peaks at around 20%, 50%, and 80% cortical depth (Fig. 7B, red arrowheads) overlap with the location of highly myelinated bands, layers II/III, IVb, and V (Fig. 7E, pink arrowheads, based on (Peters and Sethares, 1996), replicated in Supplementary Fig. 2B). From these combined profiles, layers I and IVa can be inferred (Fig. 7C and 7D). Thus overall, seven laminated structures in V1, including 3 within layer IV, can be identified (I, II/III, IVa, IVb, IVc, V, and VI). As illustrated in Fig. 7C and 7D, lamina distinction from the MR microscopy approach (Fig. 7C) reveals a laminar profile very similar to that obtained from cytoarchitectonic and myeloarchitectonic approaches (Fig. 7D).
Figure 7. Results from high resolution MRI reflect the myeloarchitecture and the cytoarchitecture of V1.
The averaged results of magnitude (A) with dips marked (green triangles) from all runs and slices. The averaged results of phase profiles (B) with peaks marked (red triangles). The gray shadings represent the 95% confidence level in (A) and (B). (C) The laminar structure determined by MRI with peaks in phase profile (red lines) and dips in magnitude profile (green lines) marked. The laminar structure determined by conventional histology is shown in (D). Cell (E) and myelin densities (F) of V1 from literature are z-normalized and plotted against cortical depth with cortical areas of high myelin density marked by pink triangles and regions with high cell density marked by blue triangles. KB: the stripe of Kaes-Bechterew. G: the stripe of Gennari. IB: the inner band of Baillarger. The direction of y-axis of (D) and (E) is reversed for comparison.
3.4. Identification of cortical lamination in extrastriate visual cortex
In addition to the striate cortex (V1), we also examined the laminar structure of two extrastriate visual cortex, V2 and V4, using MR microscopy. Both cortical areas have distinct myeloarchitectonic structure compared with V1. V2 has a relatively homogeneous and broad band of fibers between layer IV to layer IV (Gattass et al., 1981). In contrast, both the inner and outer bands of Baillarger in V4 are recognizable (Gattass et al., 1988). A direct histological comparison of myelin staining from these three cortical areas can be found in (Gattass et al., 1988) and is replicated as the Supplementary Fig. 3.
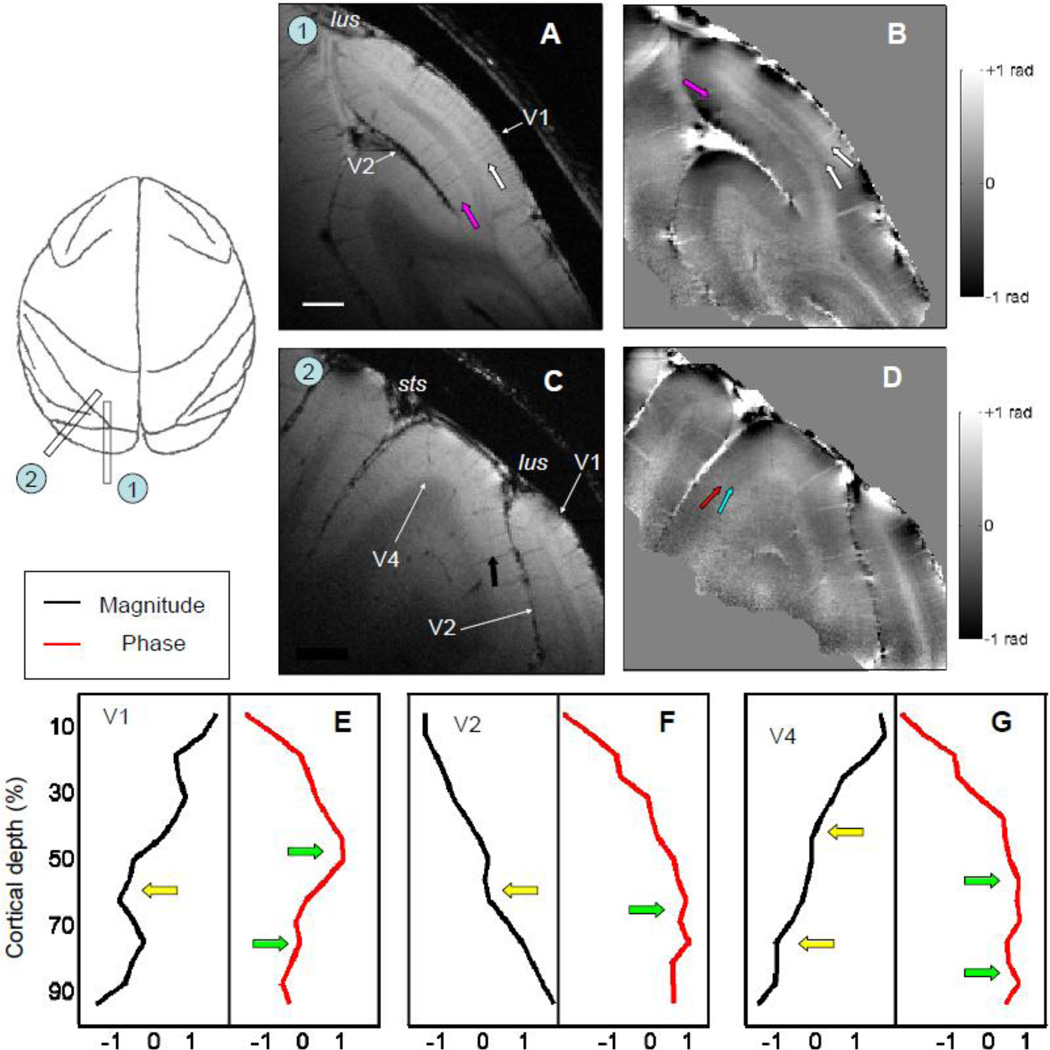
A MR image covering both V1 and V2 is shown in Fig. 8A. The relative position of the surface coil on the head and parameters used in the scanning were similar as those in Fig. 6 except that the flip angle was increased from 30° to 45° to improve the SNR of MR signals from V2. A dark band (pink arrow) can be detected in the middle of V2 from the magnitude map (Fig. 8A). Its relative cortical depth is same as the stripe of Gennari in V1 (white arrow) but with less contrast relative to gray matter. In the phase map (Fig. 8B), one broad bright band (pink arrow) is seen within V2, while there are two bright bands in V1 (white arrows). The difference between the laminar structure of V1 and V2 are more prominent in the averaged profiles (Fig. 8E, V1, and Fig. 8F, V2). In contrast to V1, where there is a prominent peak peak in phase profile near 50% and a smaller peak near 75% (Fig. 8E, green arrows), in V2 there is one broad peak (Fig. 8F, green arrow) in the phase profile (red line) centered around 60% cortical depth (Fig. 8F). This profile matches the description of the myeloarchitecture in V2 (Gattass et al., 1981, see Supplementary Fig. 3).
Figure 8. MR structural images of extrastriate visual cortex.
The magnitude (A) and phase (B) images of a parasagittal slice revealed V2 buried in the lunate sulcus (lus). Besides the stripe of Gennari (white arrow) in V1, a dark layer (pink arrow) can be found in the middle of V2 from the magnitude map (A). From the phase map (B), one bright layer exists in V2 (pink arrow) in additional to the two bright layers in V1 (white arrows). (C) and (D) An oblique slice that includes the portion of V4 that located between superior temporal sulcus (sts) and lus. Laminar structures within V4 (black arrows) can be found both from magnitude map (C) and phase map (D). The averaged results of magnitude (black lines) and phase profiles (red lines) from prestriate visual cortex of V1 (E), V2 (F), and V4 (G) are summarized. The peaks in phase profiles and dips in magnitude profiles are marked by green and yellow arrows, respectively. Both images were acquired with in-plane voxel size of 100 × 100 µm2, thickness of 1 mm, and flip angle of 45°. Scale bar: 2 mm in (A).
With MR microscopy, we found that V4 has a more complicated laminar structure than V2. As shown in Fig. 8D, Two bright bands are seen in the phase map. The broader one is located in the middle of gray matter (red arrow), and a second bright layer (blue arrow) can be detected between the first band and the GM-WM border. Unlike V1 in which the deeper peak of the phase profile is stronger than the shallower one (red line, Fig. 8E), the two peaks in V4 are similar in size (red line, Fig. 8G). In the magnitude map (Fig. 8C), a dark layer (black arrow) in the middle of the gray matter is seen at a depth shallower (closer to the pial layer) than the stripe of Gennari.
In summary, we demonstrate that extrastriate cortical areas V2 and V4 have distinct laminar structure that can be differentiated by MR microscopy in awake monkeys.
4. Discussion
4.1. Laminar architecture in the visual cortex
The neocortex is defined by the presence of six cytoarchitectonic layers (Billings-Gagliardi et al., 1974; Brodmann, 1905). In the striate cortex (V1), these six layers are marked by specific characteristics. The most superficial layer, molecular layer I, contains only few neurons. In contrast, the external granular cell layer (layer II) contains a high density of small pyramidal neurons as well as stellate neurons and the external pyramidal layer (layer III) comprises somewhat larger pyramidal cells similar in cell density to that of layer II. Within V1, the internal granular layer IV can be further divided into a superficial portion (IVa) containing many round cells, a cell-poor middle portion (IVb), and a cell-rich deep portion (IVc). Below layer IVc, the internal pyramidal cell layer (Layer V) is less cell dense and the deepest multiform layer VI contains densely arranged spindle-shaped cells. Based on cell density, layers I, IVb, and V are cell-poor zones while layers IVc, and VI are cell-rich zones (blue lines in Fig. 7D and blue triangles in Fig. 7F). In addition to cell type and density, several bands of horizontal bands of myelination are prominent (pink lines in Fig. 7D and pink triangles in Fig. 7E) providing a basis for myeloarchitecture. The outermost band is the stripe of Kaes-Bechterew (Bechterew, 1891; Kaes, 1907), which is a thin band of myelinated fibers (Fig. 7E, KB) located between layer II and layer III (Braak, 1980). In the middle layers, the stripe of Gennari (Fig. 6E, G) is a band of densely packed fibers, which is coincident with layer IVb (Valverde, 1985). A third band with high myelin density (inner band of Baillarger, IB in Fig. 7E) is located in layer V and is less prominent and thinner than the stripe of Gennari.
Several intrinsic MR parameters, which have differential sensitivity to cellular and myelin content, can be employed to reveal laminar architecture by MR imaging. Most high resolution MR histological studies in awake subjects are based on T1-weighted images (Barbier et al., 2002; Clare and Bridge, 2005; Eickhoff et al., 2005; Walters et al., 2003), an approach which emphasizes the longitudinal relaxation process and produces excellent contrast between gray and white matter. However, the T1 contrast within gray matter is relative low (Pfeuffer et al., 2004). At 7T, the T1 values for the stripe of Gennari were only 60 ms shorter than the remaining gray matter layers within V1, a value which is small compared with the variance of T1 value (150 ms). Therefore, even with long scan times, no T1-contrast based study in awake subjects was able to use in-plane voxel sizes less than 300 × 300 µm2. With a thickness of around 300 µm (von Economo and Koskinas, 1929), the stripe of Gennari was detected as a faint line with single pixel width.
4.2. Phase and magnitude profiles
In the present study, we used MR contrasts from both the magnitude and phase components of T2*-weighted image with in-plane spatial resolution of 100 × 100 µm2 or higher. The contrast in the phase component mainly originates from differences in magnetic susceptibility between tissues arising mainly from differences in blood, iron and myelin density (Duyn et al., 2007). In V1, cortical iron co-localizes mainly with myelin (Fukunaga et al., 2010), so the variation of the phase component in V1 reflects primarily the change of myelin density with cortical depth. In this study, the phase profile revealed three peaks at cortical depths of 20%, 50%, and 80% (Fig. 7B). These depths coincide well with the depths of cortical layers II/III, layer IVb, and layer V and, more specifically, with the stripe of Kaes-Bechterew, stripe of Gennari, and the inner band of Baillarger (compare phase profile (Fig. 7B) with myelin profile (Fig. 7E)).
The second MR parameter we used to reveal laminar structure is based on the magnitude component of the T2*-weighted image. The magnitude reflects variations in proton density (water content) and T2* values, which themselves depend on macromolecular content and variations in susceptibility. In our studies, using a short TR value and surface coil also withdraw a T1 dependent that varies with distance from the coil. When cell density increases the water content likely decreases and the voxel average T1 and T2* values also should be reduced. Additionally, myelin contains populations of protons that are essentially MR-invisible at long echo times used for imaging (Horch et al., 2011). It has been suggested that the contrast in T2-weighted images reflect both myelin content and cell body density (Boretius et al., 2009; Yoshiura et al., 2000), although convincing evidence that directly links neural cell density to MR signal is still lacking. This may explain our finding that cell-rich layers (layers IVc, and VI) appear dark in the magnitude profile (Fig. 7A, green triangles). As shown in Fig. 7A, the heavily myelinated, relatively cell-sparse cortical layer IVb was visibly darker than the myelin-poor, cell-dense layer IVa. Thus, our results are consistent with known laminar structure and with localization of cell density and to myelination.
In principle, the physical origins of MRI contrast and the fine cortical microstructure can be characterized more fully by quantitatively measuring values of T1, T2, and T2*. However, this was not done in this study due to the limited duration of imaging sessions in awake subjects. In the future, direct links between MRI parameter maps and their histological basis can be obtained by using more myelin-specific measurements (Laule et al., 2007; Ou et al., 2009) or in correlative ex-vivo experiments that require sacrificing the animals, scanning the fixed brain at very high resolution and then performing quantitative histological analysis on corresponding slices.
Using improved in-plane resolution of 100 × 100 µm2 or higher, we have successfully delineated for the first time sub-layers within layer IV. Magnitude profiles obtained with in-plane resolution up to 240 × 240 µm2 show a single dip in the middle of the gray matter (Duyn et al., 2007). In this study, by acquiring images with fivefold-smaller volumes (58 nl vs. 10 nl), we found there were in fact two dips in the magnitude profile. The locations of these dips were in the middle of the gray matter, one corresponding to the highly myelinated layer IVb and the second, about 150 µm deeper than the first, corresponding to the high cell dense layer IVc. An alternative explanation to the presence of this dip is that the magnitude profile reflects relatively denser vascular networks within layer IVc (Smirnakis et al., 2007; Zheng et al., 1991), leading to relatively higher concentration of deoxygenated hemoglobin and shortening of T2* (Ogawa and Lee, 1990; Schenck, 1992; Thulborn et al., 1982).
4.3. MR microscopy differentiates visual cortical areas in awake subjects based on distinct laminar structure
In addition to V1 (striate cortex), there are more than 30 cortical areas either dedicated to or associated with visual function in the macaque monkey (Felleman and Van Essen, 1991). Most of them can be differentiated based on their myeloarchitecture. One example is shown in Supplementary Fig. 3 from (Gattass et al., 1981). This myelin stained slice illustrates the distinct laminar structures within striate area V1 and extrastriate areas V2 and V4. Both V1 and V4 have two bands of Baillarger. In V1, the outer one (red arrow, the stripe of Gennari) is more dense and thicker than the inner one (green arrow). On the contrary, the outer band (yellow arrow) has lower density of fiber compared to the inner band in V4 (pink arrow). Furthermore, the outer band of Baillarger in V4 is relatively broader than the stripe of Gennari. In comparison, V2 has only one relatively homogeneous fiber band between the typical two bands of Baillarger.
As we have discussed in section 4.2, the MR contrast of the phase component is heavily influenced by the myelin density. Hence, based on their different myeloarchitectonic profiles, V1, V2, and V4, should be readily distinguished by their phase profiles (red lines in Fig.8E – 8G). Accordingly, we found that both V1 and V4 had two peaks in their phase profiles. The shallow peak is narrower in V1 than V4, and the difference between the amplitude of two peaks is relatively smaller in V4. As shown in Fig. 8F, the phase profile of V2 has only one broad peak covering cortical depths from 40% to 80%. Therefore, the laminar information from the phase component alone provides enough information to differentiate V1, V2, and V4. Interestingly, there are at least two dips in magnitude profiles of V1 and V4 while only one clear dip in V2 (black lines in Fig. 8E – 8G). The locations of dips are also different among three cortical areas. Topics of our future research will include the origins of these differences and whether other extrastriate visual cortical areas (such as V3, MT, and FEF) can be differentiated using MR microscopy.
4.4. Other factors
Other improvements may have contributed to the success of high resolution imaging in awake subjects. First, to minimize partial volume effects, we purposely used imaging planes oriented perpendicular to the cortical surface. The shapes of the cortical surface in adjacent slices were used to verify the extent of orthogonality between the slice and the cortex. Thus, after proper adjustment, the long axis of a 100 × 100 × 1000 µm3 voxel was almost perfectly parallel to cortical lamination. We found thicker slices (2 mm thick) were greatly influenced by partial volume effects (e.g. Fig. 1) as suggested by previous studies (Bridge et al., 2005; Carmichael et al., 2006), whereas thinner slices (< 1 mm thick) had relatively low SNR at the in-plane resolution we used. One main disadvantage of our setup is that, due to the curvature of the brain, the fine anatomical structures of only a limited cortical area perpendicular to the slice can be revealed. The problem is more serious in MR microscopy of extrastriate cortex. For example, the fine structure of the cortex close to the V1/V2 border (Fig. 8B) and part of V4 folded within superior temporal sulcus (Fig. 8C) are relativly indistinct. To reveal the cortical layers over a larger brain area, on gyri, or in sulci, isotropic voxels or thinner slices should be used (Trampel et al., 2011; Turner et al., 2008); this can be achieved by increasing the in-plane resolution. Second, our monkeys which were exceptionally well-trained for the MR environment, performed with less than 100 µm head motion during scans (Chen et al., 2011). This permitted critical temporal averaging of runs and improved image quality without reduction in resolution (Fig. 6A). Third, we optimized MR sequence parameters to improve image quality. The choice of spatial resolution is the most important parameter for visibility of anatomical structure as suggested in Fig. 1 and in previous studies (Boretius et al., 2009). By acquiring MR images with high resolution, we eliminated the need to use interpolation to increase the nominal spatial resolution, a method which often introduces ringing artifacts (Clark et al., 1992; Wald et al., 2006). The other parameter we optimized was the TE. While previous studies showed that TE influences the phase contrast between gray and white matter (Duyn et al., 2007), here we found the TE also influences phase contrast within the gray matter (Fig. 3B). A prolongation of the TE improves the phase contrast between the superficial and granular layers, although at a cost of poorer contrast between the granular and deeper layers (perhaps due to variance of T2* as function of cortical depth in V1, (Fukunaga et al., 2010). Note that, at least in V1, the selection of TE did not have significant influence on the magnitude contrast (Fig. 3A).
In sum, the use of phase and magnitude profiles to infer laminar myelination and cell density patterns improves the ability to detect detailed laminar cytoarchitecture in awake subjects using high resolution MRI. These combined improvements revealed for the first time seven cortical layers (including the three sub-layers within layer IV) in V1 of awake subjects. Laminae separated by as little as 150 µm could be distinguished. In addition to that, we visualized two extrastriate cortical areas with laminar structure distinct from V1. We predict that other cortical regions may be differentiated anatomically in this way and that such laminar signatures may be used to directly identify borders between cortical areas in awake subjects. In combination with multi-coil arrays (de Zwart et al., 2004; Goense et al., 2010; Wiggins et al., 2006), which cover larger areas with even better SNR and accelerated image acquisition, it will hopefully be possible to conduct both high resolution anatomic and functional MR imaging within the same scanning session. Such advances in MR methodology will provide highly needed understanding of high spatial resolution relationships between brain structure and function in awake subjects. Importantly, the application of these methods for evaluation of cortical lamination changes in neurodegenerative disease and brain damage may lead to important breakthroughs in diagnosis and treatment.
Highlights.
-
►
High resolution MR microscopy in awake non-human primates
-
►
T2* phase and magnitude contrast reveals cyto/myeloarchitectonics
-
►
Visualization of 6 cortical layers in striate cortex (V1)
-
►
Visualization of three sub-layers within layer IV (IVa, IVb, and IVc) of V1
-
►
Visualization of laminar structure in two extrastriate cortical areas (V2 and V4)
Supplementary Material
Acknowledgements
This work was supported by NIH NS44375, EY11744, Vanderbilt Vision Research Center, Vanderbilt University Center for Integrative & Cognitive Neuroscience. The authors thank Chaohui Tang, Yanyan Chu, and Mary R. Feutado for animal care; and Bruce Williams, Roger Williams, Sasidha Tadanki, and Ken Wilkens for equipment and technical support. We are also grateful to Malcolm Avison for insightful comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abduljalil AM, Schmalbrock P, Novak V, Chakeres DW. Enhanced gray and white matter contrast of phase susceptibility-weighted images in ultra-high-field magnetic resonance imaging. J Magn Reson Imaging. 2003;18:284–290. doi: 10.1002/jmri.10362. [DOI] [PubMed] [Google Scholar]
- Baillarger JGF. Recherches sur la structure de la couche corticale des circonvolutions du cerveau. Mem. Acad. R. Med. 1840;8:149–183. [Google Scholar]
- Barbier EL, Marrett S, Danek A, Vortmeyer A, van Gelderen P, Duyn J, Bandettini P, Grafman J, Koretsky AP. Imaging cortical anatomy by high-resolution MR at 3.0T: detection of the stripe of Gennari in visual area 17. Magn Reson Med. 2002;48:735–738. doi: 10.1002/mrm.10255. [DOI] [PubMed] [Google Scholar]
- Bechterew W. Zur Frage uber die ausseren Associationsfasern der Hirnrinde. Neurol Zentrbl. 1891;10:682–684. [Google Scholar]
- Benveniste H, Blackband S. MR microscopy and high resolution small animal MRI: applications in neuroscience research. Prog Neurobiol. 2002;67:393–420. doi: 10.1016/s0301-0082(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Billings-Gagliardi S, Chan-Palay V, Palay SL. A review of lamination in area 17 of the visual cortex Macaca mulatta. J Neurocytol. 1974;3:619–629. doi: 10.1007/BF01097627. [DOI] [PubMed] [Google Scholar]
- Boretius S, Kasper L, Tammer R, Michaelis T, Frahm J. MRI of cellular layers in mouse brain in vivo. Neuroimage. 2009;47:1252–1260. doi: 10.1016/j.neuroimage.2009.05.095. [DOI] [PubMed] [Google Scholar]
- Braak H. Architectionics of the Human Telencephalic Cortex. Berlin: Springer; 1980. [Google Scholar]
- Bridge H, Clare S, Jenkinson M, Jezzard P, Parker AJ, Matthews PM. Independent anatomical and functional measures of the V1/V2 boundary in human visual cortex. J Vis. 2005;5:93–102. doi: 10.1167/5.2.1. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Beitrage zur histoloyische lokalization der Grosshirnrinde: Dritte Mitteilung, Die Eindcnfeldern dcr nicdcren Affen. J Psychol Neurol. 1905;4:177–226. [Google Scholar]
- Brodmann K. Vergleichende Lokalisationlehre der Grosshirnrinde. Leipzig, Germany: Johann Ambrosius Barth Verlag; 1909. [Google Scholar]
- Callaghan PT, Clark CJ, Forde LC. Use of static and dynamic NMR microscopy to investigate the origins of contrast in images of biological tissues. Biophys Chem. 1994;50:225–235. doi: 10.1016/0301-4622(94)85034-8. [DOI] [PubMed] [Google Scholar]
- Carmichael DW, Thomas DL, De Vita E, Fernandez-Seara MA, Chhina N, Cooper M, Sunderland C, Randell C, Turner R, Ordidge RJ. Improving whole brain structural MRI at 4.7 Tesla using 4 irregularly shaped receiver coils. Neuroimage. 2006;32:1176–1184. doi: 10.1016/j.neuroimage.2006.04.191. [DOI] [PubMed] [Google Scholar]
- Chen G, Wang F, Dillenburger BC, Friedman R, Chen L, Gore J, Avison MJ, Roe AW. Functional magnetic resonance imaging of awake monkeys: some approaches for improving imaging quality. Magn Reson Imaging. 2011 doi: 10.1016/j.mri.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare S, Bridge H. Methodological issues relating to in vivo cortical myelography using MRI. Hum Brain Mapp. 2005;26:240–250. doi: 10.1002/hbm.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Courchesne E, Grafe M. In vivo myeloarchitectonic analysis of human striate and extrastriate cortex using magnetic resonance imaging. Cereb Cortex. 1992;2:417–424. doi: 10.1093/cercor/2.5.417. [DOI] [PubMed] [Google Scholar]
- de Zwart JA, Ledden PJ, van Gelderen P, Bodurka J, Chu R, Duyn JH. Signal-to-noise ratio and parallel imaging performance of a 16-channel receive-only brain coil array at 3.0 Tesla. Magn Reson Med. 2004;51:22–26. doi: 10.1002/mrm.10678. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull. 1981;7:519–579. doi: 10.1016/0361-9230(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Duyn JH. Study of brain anatomy with high-field MRI: recent progress. Magn Reson Imaging. 2010 doi: 10.1016/j.mri.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A. 2007;104:11796–11801. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S, Walters NB, Schleicher A, Kril J, Egan GF, Zilles K, Watson JD, Amunts K. High-resolution MRI reflects myeloarchitecture and cytoarchitecture of human cerebral cortex. Hum Brain Mapp. 2005;24:206–215. doi: 10.1002/hbm.20082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Li TQ, van Gelderen P, de Zwart JA, Shmueli K, Yao B, Lee J, Maric D, Aronova MA, Zhang G, Leapman RD, Schenck JF, Merkle H, Duyn JH. Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. Proc Natl Acad Sci U S A. 2010;107:3834–3839. doi: 10.1073/pnas.0911177107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, Gross CG, Sandell JH. Visual topography of V2 in the macaque. J Comp Neurol. 1981;201:519–539. doi: 10.1002/cne.902010405. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Gross CG. Visuotopic organization and extent of V3 and V4 of the macaque. J Neurosci. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari F. Observat. Accedunt. Parma, Italy: Regio Typographeo; 1782. Francisci gennari parmensis medicinae doctoris collegiati de peculiari structura cerebri nonnullisque eius morbis - paucae aliae anatom. [Google Scholar]
- Geyer S, Weiss M, Reimann K, Lohmann G, Turner R. Microstructural Parcellation of the Human Cerebral Cortex - From Brodmann's Post-Mortem Map to in vivo Mapping with High-Field Magnetic Resonance Imaging. Front Hum Neurosci. 2011;5:19. doi: 10.3389/fnhum.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goense J, Logothetis NK, Merkle H. Flexible, phase-matched, linear receive arrays for high-field MRI in monkeys. Magn Reson Imaging. 2010;28:1183–1191. doi: 10.1016/j.mri.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Goense JB, Logothetis NK. Laminar specificity in monkey V1 using high-resolution SE-fMRI. Magn Reson Imaging. 2006;24:381–392. doi: 10.1016/j.mri.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Goense JB, Zappe AC, Logothetis NK. High-resolution fMRI of macaque V1. Magn Reson Imaging. 2007;25:740–747. doi: 10.1016/j.mri.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- Hinds OP, Rajendran N, Polimeni JR, Augustinack JC, Wiggins G, Wald LL, Diana Rosas H, Potthast A, Schwartz EL, Fischl B. Accurate prediction of V1 location from cortical folds in a surface coordinate system. Neuroimage. 2008;39:1585–1599. doi: 10.1016/j.neuroimage.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch RA, Gore JC, Does MD. Origins of the ultrashort-T2 1H NMR signals in myelinated nerve: a direct measure of myelin content? Magn Reson Med. 2011;66:24–31. doi: 10.1002/mrm.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaes T. Die Grobhirnrinde des Menschen in ihren Maben und in ihrem Fasergehalt. Jena: Fischer; 1907. [Google Scholar]
- Laule C, Vavasour IM, Kolind SH, Li DK, Traboulsee TL, Moore GR, MacKay AL. Magnetic resonance imaging of myelin. Neurotherapeutics. 2007;4:460–484. doi: 10.1016/j.nurt.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HD, Chen G, Tanigawa H, Roe AW. A motion direction map in macaque V2. Neuron. 2010;68:1002–1013. doi: 10.1016/j.neuron.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield P, Morris PG. NMR imaging in biomedicine. New York: Academic Press; 1982. [Google Scholar]
- Nieuwenhuys R, Voodg J, Huijzen C. The Human Central Nervous System. 4 ed. Berlin: Springer; 2008. [Google Scholar]
- Ogawa S, Lee TM. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magn Reson Med. 1990;16:9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- Ou X, Sun SW, Liang HF, Song SK, Gochberg DF. The MT pool size ratio and the DTI radial diffusivity may reflect the myelination in shiverer and control mice. NMR Biomed. 2009;22:480–487. doi: 10.1002/nbm.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C. Myelinated axons and the pyramidal cell modules in monkey primary visual cortex. J Comp Neurol. 1996;365:232–255. doi: 10.1002/(SICI)1096-9861(19960205)365:2<232::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Merkle H, Beyerlein M, Steudel T, Logothetis NK. Anatomical and functional MR imaging in the macaque monkey using a vertical large-bore 7 Tesla setup. Magn Reson Imaging. 2004;22:1343–1359. doi: 10.1016/j.mri.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; 2007. [Google Scholar]
- Schenck JF. Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Ann N Y Acad Sci. 1992;649:285–301. doi: 10.1111/j.1749-6632.1992.tb49617.x. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Amunts K, Geyer S, Kowalski T, Schormann T, Palomero-Gallagher N, Zilles K. A stereological approach to human cortical architecture: identification and delineation of cortical areas. J Chem Neuroanat. 2000;20:31–47. doi: 10.1016/s0891-0618(00)00076-4. [DOI] [PubMed] [Google Scholar]
- Sigalovsky IS, Fischl B, Melcher JR. Mapping an intrinsic MR property of gray matter in auditory cortex of living humans: a possible marker for primary cortex and hemispheric differences. Neuroimage. 2006;32:1524–1537. doi: 10.1016/j.neuroimage.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnakis SM, Schmid MC, Weber B, Tolias AS, Augath M, Logothetis NK. Spatial specificity of BOLD versus cerebral blood volume fMRI for mapping cortical organization. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600434. [DOI] [PubMed] [Google Scholar]
- Smith GE. New Studies on the Folding of the Visual Cortex and the Significance of the Occipital Sulci in the Human Brain. J Anat Physiol. 1907;41:198–207. [PMC free article] [PubMed] [Google Scholar]
- Tanigawa H, Lu HD, Roe AW. Functional organization for color and orientation in macaque V4. Nat Neurosci. 2010;13:1542–1548. doi: 10.1038/nn.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta. 1982;714:265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- Trampel R, Ott DV, Turner R. Do the Congenitally Blind Have a Stria of Gennari? First Intracortical Insights In Vivo. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq282. [DOI] [PubMed] [Google Scholar]
- Turner R, Oros-Peusquens AM, Romanzetti S, Zilles K, Shah NJ. Optimised in vivo visualisation of cortical structures in the human brain at 3 T using IR-TSE. Magn Reson Imaging. 2008;26:935–942. doi: 10.1016/j.mri.2008.01.043. [DOI] [PubMed] [Google Scholar]
- Valverde F. The organizing principles of the primary visual cortex in the monkey. In: Peters A, Jones EG, editors. Visual cortex. New York: Plenum; 1985. pp. 207–257. [Google Scholar]
- Vogt C, Vogt O. Allgemeinere Ergebnisse unserer Hirnforschung. J Psychol Neurol. 1919;25:279–461. [Google Scholar]
- Vogt C, Vogt O. Gestaltung der topistischen Hirnforschung und ihre Forderung durch den Hirnbau und seine Anomalien. J Hirnforsch. 1954;1:1–46. [Google Scholar]
- von Economo CF, Koskinas GN. The cytoarchitectonics of the human cerebral cortex. London: Oxford University Press; 1929. [Google Scholar]
- Wald LL, Fischl B, Rosen BR. High-Resolution adn Microscopic Imaging at High Field. In: Robitaille PM, Berliner L, editors. Ultra High Field Magnetic Resonance Imaging. New York: Springer; 2006. pp. 343–371. [Google Scholar]
- Walters NB, Egan GF, Kril JJ, Kean M, Waley P, Jenkinson M, Watson JD. In vivo identification of human cortical areas using high-resolution MRI: an approach to cerebral structure-function correlation. Proc Natl Acad Sci U S A. 2003;100:2981–2986. doi: 10.1073/pnas.0437896100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56:216–223. doi: 10.1002/mrm.20925. [DOI] [PubMed] [Google Scholar]
- Yao B, Li TQ, Gelderen P, Shmueli K, de Zwart JA, Duyn JH. Susceptibility contrast in high field MRI of human brain as a function of tissue iron content. Neuroimage. 2009;44:1259–1266. doi: 10.1016/j.neuroimage.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura T, Higano S, Rubio A, Shrier DA, Kwok WE, Iwanaga S, Numaguchi Y. Heschl and superior temporal gyri: low signal intensity of the cortex on T2-weighted MR images of the normal brain. Radiology. 2000;214:217–221. doi: 10.1148/radiology.214.1.r00ja17217. [DOI] [PubMed] [Google Scholar]
- Zheng D, LaMantia AS, Purves D. Specialized vascularization of the primate visual cortex. J Neurosci. 1991;11:2622–2629. doi: 10.1523/JNEUROSCI.11-08-02622.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.