Abstract
In several models of aging, microglia become more inflammatory and reactive to immune challenges. For example, peripheral LPS injection causes exaggerated microglial activation associated with prolonged sickness and depressive-like behavior in aged BALB/c mice. Therefore, the purpose of this study was to determine the extent to which age-related amplified microglial activation was associated with reduced sensitivity to the anti-inflammatory and M2 promoting cytokines interleukin (IL)-10 and IL-4. In initial studies with adult mice, LPS induced a time-dependent increase in M1 and M2 mRNA profiles in microglia. Furthermore, peripheral LPS injection markedly increased surface expression of IL-4 receptor-alpha (IL-4Rα), but not IL-10 receptor-1 (IL-10R1) on microglia. In BV-2 cells, IL-4, but not IL-10, re-directed LPS-activated microglia towards an M2 phenotype. Based on these findings, comparisons of M1 and M2 activation profiles, induction of IL-4Rα, and sensitivity to IL-4 were determined in microglia from adult (3–4 mo) and aged (18–22 mo) mice. In aged microglia, LPS promoted an exaggerated and prolonged M1 and M2 profile compared to adults. Moreover, IL-4Rα protein was not increased on aged microglia following LPS injection. To determine the consequence of impaired IL-4Rα upregulation, adult and aged mice were injected with LPS and activated microglia were then isolated and treated ex vivo with IL-4. While ex vivo IL-4 induced an M2 profile in activated microglia from adult mice, activated microglia from aged mice retained a prominent M1 profile. These data indicate that activated microglia from aged mice are less sensitive to the anti-inflammatory and M2-promoting effects of IL-4.
Keywords: Microglia, Aging, IL-4, IL-10, M1 and M2 activation profiles
1. Introduction
Within the central nervous system (CNS) microglia are responsible for the induction of an innate immune response by receiving and propagating inflammatory signals (Nguyen et al., 2002). Even in the absence of inflammatory stimuli, microglia are actively surveying their local microenvironment (Nimmerjahn et al., 2005). Once activated by an immune stimulus, microglia perform several macrophage-like functions including phagocytosis, inflammatory and anti-inflammatory cytokine production, and antigen presentation (Garden and Moller, 2006). This classical activation (M1) profile is transient with microglia returning to a surveying state as the immune stimulus is resolved. Key to this transition is regulation by several anti-inflammatory mediators (Biber et al., 2007) including neuronal factors, hormones, and cytokines that attenuate microglial activation and promote anti-inflammatory or repair (M2) profiles in microglia (Mantovani et al., 2004; Mosser and Edwards, 2008).
In rodent models of normal and non-neurodegenerative aging, there is an increase in “primed or reactive” microglia that have increased expression of a number of M1 and inflammatory markers including CD86 (Downer et al., 2010), CD68 (Wong et al., 2005), MHC II (Frank et al., 2006; Godbout et al., 2005b; Henry et al., 2009), and toll-like receptors (Letiembre et al., 2007). A consequence of a more inflammatory microglial profile is an exaggerated inflammatory response following peripheral immune activation (Godbout et al., 2005b; Perry et al., 2003). In support of this notion, central (Abraham et al., 2008; Huang et al., 2008) or peripheral innate immune challenges (Chen et al., 2008; Godbout et al., 2005a; Henry et al., 2009; Wynne et al., 2010) lead to amplified and prolonged neuroinflammation (oxidative stress and cytokines) mediated, in part, by a hyperactive MHC II+ microglial population (Henry et al., 2009). An exaggerated M1 microglial response in aged mice is relevant because it is coupled with a myriad of complications including cognitive impairment (Barrientos et al., 2009; Barrientos et al., 2006; Chen et al., 2008), exaggerated and prolonged sickness behavior (Abraham et al., 2008; Barrientos et al., 2009; Godbout et al., 2005a; Huang et al., 2008), and protracted depressive-like behavior (Godbout et al., 2008) following an innate immune challenge (for reviews see (Dantzer et al., 2008; Godbout and Johnson, 2009; Jurgens and Johnson, 2010)). This is paralleled in clinical studies in which elderly patients with peripheral infections or other illnesses have an increased frequency of concomitant neurobehavioral complications including delirium (Lipowski, 1983; Mulsant et al., 1999) and depression (Alexopoulos, 2005; Godbout and Johnson, 2009; Koenig et al., 1988; Yirmiya et al., 2000) compared to younger adults with the same peripheral insults.
The reason aged mice have a reduced capacity to resolve amplified microglial activation after an immune challenge is unknown, but may be related to a reduced sensitivity to anti-inflammatory feedback by neuronal regulators (CD200, CX3CL1) (Lyons et al., 2007a; Wynne et al., 2010) and anti-inflammatory cytokines. Previous studies demonstrate that IL-10 and IL-4, two key anti-inflammatory and M2-promoting cytokines (Mantovani et al., 2004; Strle et al., 2001) are reduced within the brain of older rodents (Nolan et al., 2005; Szczepanik et al., 2001; Ye and Johnson, 2001). In the context of M2 activation profiles, IL-10 promotes an M2c (classical deactivation) profile and IL-4 promotes an M2a (alternative activation) profile in macrophages. Both M2a and M2c phenotypes reduce M1 cytokines and other inflammatory mediators (Mantovani et al., 2004; Mosser and Edwards, 2008). An M2b activation profile has also been shown with expression of both M1 and M2c markers. The degree to which these activation profiles are conserved in microglia is less understood. In our work, peripheral injection of LPS amplified mRNA and intracellular protein expression of both IL-1β and IL-10 in microglia from aged mice (Henry et al., 2009). Despite exaggerated IL-10 production by microglia from aged mice, neuroinflammation persisted and corresponded with prolonged sickness and depressive-like behaviors (Godbout et al., 2005b; Godbout et al., 2008). In addition, other reports indicate that decreased IL-4 in the brain of aged rats was associated with reduced long term potentiation (LTP) (Maher et al., 2005), impaired neurogenesis in the hippocampus (Ziv et al., 2006), and increased brain inflammation (Maher et al., 2005; Nolan et al., 2005).
The purpose of this study was to determine the degree to which activated microglia from adult and aged mice were sensitive to anti-inflammatory effects of IL-10 and IL-4. Initial studies were completed using adult mice, BV-2, and primary microglia to determine microglial M1 and M2 profiles, expression of the receptors for IL-4 (IL-4Rα) and IL-10 (IL-10R1), and sensitivity to IL-10 and IL-4 following LPS. Collectively, these initial experiments demonstrated that LPS- activated microglia shifted towards an M2b phenotype, markedly upregulated IL-4Rα protein expression, and were redirected towards a less inflammatory M2a profile following IL-4 post- treatment. These studies also indicated that IL-10R1 was not induced following LPS-associated activation and IL-10 had little effect in redirecting activated microglia towards a less inflammatory phenotype. In age comparisons, LPS injection induced an exaggerated M2b phenotype in microglia from aged mice compared to adults, but IL-4Rα surface expression was not increased. Moreover, when LPS-activated microglia were isolated and treated ex vivo with IL-4, only microglia from adult mice successfully transitioned from an M1 towards an M2 profile. Thus, failure to increase IL-4Rα surface expression on aged microglia was associated with a reduced sensitivity to the M2 promoting effects of IL-4.
2. Methods
2.1. Animals
Adult (3–4 month-old) male BALB/c mice were obtained from a breeding colony kept in barrier-reared conditions in a specific-pathogen-free facility at the Ohio State University. Mice were individually housed in polypropylene cages and maintained at 25° C under a 12 h light/12 h dark cycle with ad libitum access to water and rodent chow. For age comparisons, male BALB/c mice (18–22 mo) were purchased from the National Institute on Aging specific-pathogen-free colony (maintained at Charles River Laboratories, Inc., MA). The median lifespan for BALB/c mice is approximately 26 months (Morley and Trainor, 2001). Aged mice were acclimated to the facilities for one week prior to experimentation. To investigate changes that occur from adulthood to what is considered aged, 3–4 month-old (adult) and 18–22 month-old (aged) male BALB/c mice were used. Upon arrival, mice were individually housed as described above. All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2 Experimental Protocols
In the first experiment adult (3–4 mo) male BALB/c mice were injected intraperitoneally (ip) with saline or Escherichia coli lipopolysaccharide (LPS) (0.33 mg/kg; serotype 0127:B8, Sigma, St. Louis, MO) and euthanized 4 h or 24 h later by CO2 asphyxiation (n=5). This LPS dosage was selected because it elicits a pro-inflammatory cytokine response in the brain resulting in a transient sickness response in adult mice (Berg et al., 2004; Godbout et al., 2005a; Henry et al., 2008). In a related study, adult (3–4 mo) male BALB/c mice were injected ip with vehicle or minocycline (50 mg/kg, Sigma, St. Louis, MO) for 3 consecutive days (Henry et al., 2008). Twelve hours following the last injection mice were injected ip with saline or LPS and were euthanized 4 h later (n=9). In both sets of experiments, brains were homogenized and microglia were isolated using a discontinuous Percoll density gradient. RNA isolation from enriched microglia was performed and M1, M2a, and M2c gene expression was determined.
In the second experiment adult (3–4 mo) male BALB/c mice were injected ip with saline or LPS (0.33 mg/kg). Brains were homogenized and microglia were isolated using a discontinuous Percoll density gradient either 4 h or 24 h later. CD11b, CD45, IL-10R1 and IL-4Rα were determined on enriched microglia by flow cytometry (n=6).
In the third experiment the BV-2 and primary microglia were used. Microglia were treated with either saline or LPS (10 ng/mL) for 1 h. Next, cells were incubated for an additional 3 h with the appropriate vehicle or recombinant cytokine: 10 ng/ml of IL-10 (n=9), or 20 ng/ml of IL-4 (n=6) (R&D Systems. MN). Concentrations of these cytokines were selected based on previous reports using IL-10 (Frei et al., 1994; Sheng et al., 1995) and IL-4 (Chao et al., 1993; Haque et al., 1998) in macrophage and microglial cultures. After 3 h cell lysates were collected. RNA was isolated and M1, M2a, and M2c gene expression was determined by real-time PCR (RT-PCR). Results represent two independent experiments.
In the fourth experiment, adult (3–4 mo) or aged (18–22 mo) male BALB/c mice were injected ip with saline or LPS (0.33 mg/kg) and euthanized 4 h or 24 h later by CO2 asphyxiation. The brain was collected and a 1 mm coronal brain section (+0.38 mm from Bregma) (Paxinos and Franklin, 2004) was taken using a rodent brain matrix (ASI instruments, Warren, MI). Brain sections were used for analysis of mRNA levels of IL-4 (n=5). The remainder of the brain was homogenized and microglia were isolated using a discontinuous Percoll density gradient. Half of the microglia from each sample were used for RNA isolation and the other half for flow cytometry analysis. RNA was isolated and M1, M2a, and M2c gene expression was determined by RT-PCR (n=6). CD11b, CD45, and IL-4Rα surface expression was determined by flow cytometry (n=4).
In the fifth experiment, adult (3–4 mo) and aged (18–22 mo) male BALB/c mice were injected ip with saline or LPS (0.33 mg/kg). After 4 h brains were homogenized and microglia were isolated using a discontinuous Percoll density gradient and plated on poly-L-lysine coated plates for 1 h. After 1 h, enriched microglia were treated with vehicle or IL-4 (20 ng/ml) for an additional 3 h. After 3 h, RNA was isolated and M1 (IL-1β, iNOS) and M2a (Arg) gene expression was determined by RT-PCR.
2.3. BV-2 cell culture
BV-2 cells were cultured in growth medium (DMEM (Bio-Whittaker, Walkersville, MD) supplemented with 10% FBS (Hyclone, Logan, UT), 3.7 g/L sodium bicarbonate, 200 mM glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin, 0.25 μg/ml fungizone) as previously described (Wynne et al., 2010). Cultures were maintained and incubated at 37°C with 95% humidity and 5% CO2 and growth medium was replenished every third day until confluence. Prior to experimentation cells were plated at 1×105 cells per well in 24-well plates and allowed to adhere for 20 h. Immediately before treatment, cells were washed twice with serum-free DMEM medium and supplemented with warm serum-free DMEM medium containing experimental treatments. Following experimental treatments cells were homogenized and RNA or protein was isolated.
2.4. Primary microglia culture
Microglia cultures were established from neonatal mice as previously described (Godbout, 2004). In brief, whole brains were aseptically removed and mechanically dissociated after a 15 min trypsinization (0.25% trypsin) and passed through a 100 mm nylon mesh, washed twice in D-HBSS, and plated on poly-L-lysine coated 162 cm2 culture flasks in growth medium (DMEM supplemented with 20% FBS, 3.7 g/L sodium bicarbonate, 200 mM glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin, 0.25 μg/ml fungizone, 50 μg/ml gentamicin). Mixed glia cultures were maintained at 37° C with 95% humidity and 5% CO2 and growth medium was replenished every third day until confluence. Mixed glia cultures were shaken at 120 rev/min and 37° C for 3.5 h to harvest microglia from the confluent cell layer. Cells were collected, counted by trypan blue staining, and plated at a density of 1 × 105 cells per 500 μl on poly-L-lysine coated 24-well plates. After 48 h, microglia were washed twice with serum free DMEM medium (growth medium without FBS) and supplemented with warm serum free DMEM medium containing experimental treatments.
2.5. Microglial isolation
Microglia were isolated from whole brain homogenates as described previously (Henry et al., 2009; Wynne et al., 2010). In brief, brains were homogenized in 1x phosphate buffered saline (PBS, pH 7.4) by passing through a 70 μm nylon cell strainer. Resulting homogenates were centrifuged at 600 × g for 6 min. Supernatants were removed and cell pellets were re-suspended in 70% isotonic Percoll at room temperature. A discontinuous Percoll density gradient was layered as follows: 70%, 50%, 35%, and 0% isotonic Percoll. The gradient was centrifuged for 20 minutes at 2000 × g and microglia were collected from the interface between the 70% and 50% Percoll layers. Microglia were washed and re-suspended in sterile filtered PBS or FACS buffer. Each brain extraction yielded approximately 3 × 105 viable cells. We have previously characterized these cells as approximately 85% CD11b+/CD45low microglia (Henry et al., 2009). Based on this previous characterization, cells isolated by Percoll density separation will be referred to as “enriched microglia”.
2.6. Ex vivo microglia cultures
Enriched microglia were washed with PBS and counted by hemocytometer. Cells from each animal were then plated at 1×105 cells/well into two wells of a poly-L-lysine coated 24-well plate in serum-free DMEM medium. Cells were incubated at 37°C with 95% humidity and 5% CO2 and allowed to adhere for 1 h. After 1 h cells were treated with vehicle or IL-4 (20 ng/mL) and incubated at 37°C with 95% humidity and 5% CO2 for 3 h. After 3 h cells were homogenized and RNA was isolated.
2.7. RNA isolation and RT-PCR
RNA was isolated from a coronal brain slice, BV-2 cells, primary microglia, or enriched microglia. For the coronal brain slice, BV-2 cells, and primary microglia total RNA was isolated using the Tri-Reagent protocol (Sigma, MO) and subjected to the DNA-free™ RNA clean up procedure (Ambion, TX). For enriched microglia, RNA was isolated using the RNeasy plus mini kit (Qiagen, CA) or the PrepEase kit (USB, OH). In all RNA isolation procedures, RNA concentration was determined by spectrophotometry (Eppendorf, NY) and RNA was reverse transcribed to cDNA.
Real time PCR (RT-PCR) was performed using the Applied Biosystems (Foster, CA) Taqman® Gene Expression assay as previously described (Godbout et al., 2005b). In brief, cDNA was amplified by RT-PCR where a target cDNA and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems, CA). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold difference from saline controls (Henry et al., 2009). In the microglia ex vivo cultures established from the brains of adult or aged mice, fold change results are expressed as fold change from the age-matched saline controls.
2.8. Flow cytometry
Enriched microglia were assayed for surface antigens by flow cytometry as previously described (Henry et al., 2008; Henry et al., 2009). In brief, Fc receptors were blocked with anti-CD16/CD32 antibody. Next, enriched microglia were split for staining using two separate panels of antibodies. Cells for Panel 1 were incubated with rat anti-mouse antibodies (eBioscience, CA): CD11b-APC, CD45-PerCP-Cy5.5, and IL-10R1-PE. Cells for Panel 2 were incubated with rat anti-mouse antibodies (eBioscience, CA): CD11b-APC, CD45-PerCP-Cy5.5, and IL-4Rα-PE. Expression of these surface receptors was determined using a Becton-Dickinson FACSCaliber four color Cytometer. Twenty thousand events were recorded and microglia were identified by CD11b+/CD45low expression (Wohleb et al., 2011). For each antibody, gating was determined based on appropriate negative isotype stained controls. In age comparisons of IL-4Rα, separate isotypes were used for adult and aged mice to control for the increased non-specific staining detected in microglia from aged mice. Flow data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
2.9. Statistical Analysis
To ensure a normal distribution, data were subjected to the Shapiro-Wilk test using Statistical Analysis Systems (SAS) software (Cary, NC). To determine significant main effects and interactions between main factors, data were analyzed using one- (i.e., Age, Pretreatment, Treatment), or two-way (i.e., Age × Pretreatment, Age × Treatment, Pretreatment × Treatment) ANOVA using the General Linear Model procedures of SAS. When appropriate, differences between treatment means were evaluated by an F-protected t-test using the Least-Significant Difference procedure of SAS or individual student’s t-tests. All data are expressed as treatment means ± standard error of the mean (SEM). Values were considered significant at p-values < 0.05 and a tendency at p-values ≤ 0.1.
3. Results
3.1. Peripheral LPS injection promoted an M1 and M2c mRNA profile in enriched microglia
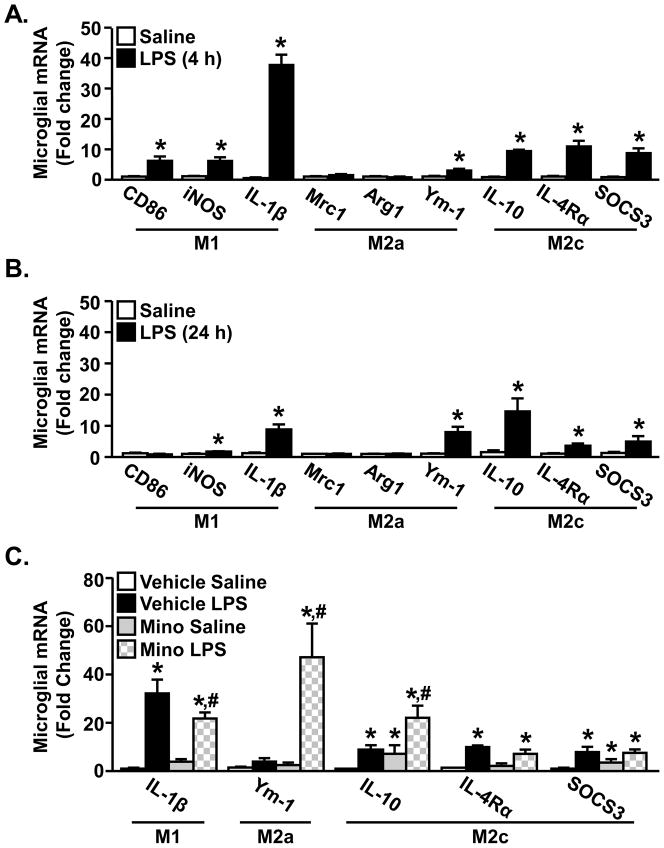
We have previously reported that peripheral LPS injection causes amplified and prolonged microglial activation (Henry et al., 2009; Wynne et al., 2010) in aged BALB/c mice that corresponds with protracted sickness and depressive-like behaviors (Godbout et al., 2005b; Godbout et al., 2008). We hypothesize that exaggerated microglial activation in aged mice is related to a reduced sensitivity to anti-inflammatory cytokines that impair the transition from an M1 phenotype to an M2 phenotype. To begin to address this hypothesis, adult mice were used in an initial set of studies to determine the expression of microglial genes associated with classical activation (M1), alternative activation (M2a), and classic deactivation (M2c) (Mantovani et al., 2004) at two time-points following peripheral LPS injection. These time-points correspond to the peak of neuroinflammation (i.e., 4 h) (Wynne et al., 2010) and the resolution of the sickness response (i.e., 24 h) (Godbout et al., 2005b). Fig. 1A shows the relative microglial mRNA expression of M1 genes: CD86, inducible nitric oxide synthase (iNOS), and IL-1β, M2a genes: mannose receptor (Mrc), arginase (Arg) and chitinase3-like 3 (Ym-1) and M2c genes IL-10, IL-4 receptor-alpha (IL-4Rα) and suppressor of cytokine signaling (SOCS3) in adult mice 4 h after ip injection of LPS. As expected, LPS increased the mRNA levels of all M1 genes (CD86, iNOS, IL-1β) in microglia from adult mice (main effect of LPS, p<0.05 for each). Moreover, M2c genes (IL-10, IL-4Rα, SOCS3) were also increased in enriched microglia 4 h after LPS injection (main effect of LPS, p<0.001 for each). LPS injection did not increase the expression of alternatively activated (M2a) genes Mrc-1 and Arg, but did increase Ym-1 mRNA expression (main effect of LPS, p<0.005). These results indicate that M1 and M2c related genes are both increased in microglia within 4 h after peripheral injection of LPS. Because markers of both classical activation and classic deactivation are simultaneously increased, this activation profile is consistent with the proposed M2b monocyte phenotype (Mantovani et al., 2004; Mosser and Edwards, 2008).
Figure 1. Peripheral LPS injection promoted an M1 and M2c mRNA profile in microglia.
Adult (3–4 mo) BALB/c mice were injected ip with saline or LPS and M1, M2a and M2c related genes were determined from enriched microglia isolated A) 4 h or B) 24 h later (n=6). Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from Saline controls. C) Adult (3–4 mo) BALB/c mice were injected with vehicle or minocycline for 3 consecutive days. On the third day mice were injected ip with saline or LPS and M1-, M2a-, and M2c-related genes were determined from enriched microglia isolated 4 h later (n=9). Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from Vehicle Saline and means with # are significantly different (p<0.05) from Vehicle LPS.
At the 24 h time-point, higher levels of IL-1β and iNOS were still evident in the microglia from LPS treated mice (Fig. 1B, main effect of LPS, p<0.01 for each). These levels, however, were reduced compared to levels detected 4 h after LPS injection (Fig. 1A). Similar to the results at 4 h, Ym-1 was the only M2a gene that was elevated 24 h following LPS treatment (main effect of LPS, p<0.001). All M2c-related genes including, IL-10, IL-4Rα, and SOCS3, remained elevated 24 h after LPS (Fig. 1B, main effect of LPS, p<0.05 for each).. These data indicate that M1-related genes were decreased by 24 h after LPS and that M2c-related genes were maintained.
Because minocycline pretreatment attenuates microglial activation and sickness behavior associated with LPS injection (Henry et al., 2008), the degree to which minocycline alters the M1 and M2 profile of microglia after LPS challenge was determined in adult mice (Fig. 1C). As anticipated, LPS injection markedly increased IL-1β mRNA in enriched microglia (main effect of LPS, p<0.0001) and this induction was attenuated by minocycline (minocycline × LPS interaction, F(1,33)=4.24, p<0.05). In addition, there was a significant interaction of minocycline and LPS on mRNA expression of Ym-1 (F(1,30)=8.15, p<0.009). The LPS-induced increase of IL-10 in microglia was also enhanced by minocycline pretreatment (p<0.02). Other M2c genes including IL-4Rα and SOCS3 were induced by LPS (main effect of LPS, p<0.008 for each), but were not affected by minocycline. These data indicate that minocycline attenuates the induction of an M1 activation profile after LPS and enhances an M2 profile with increased expression of Ym-1 and IL-10.
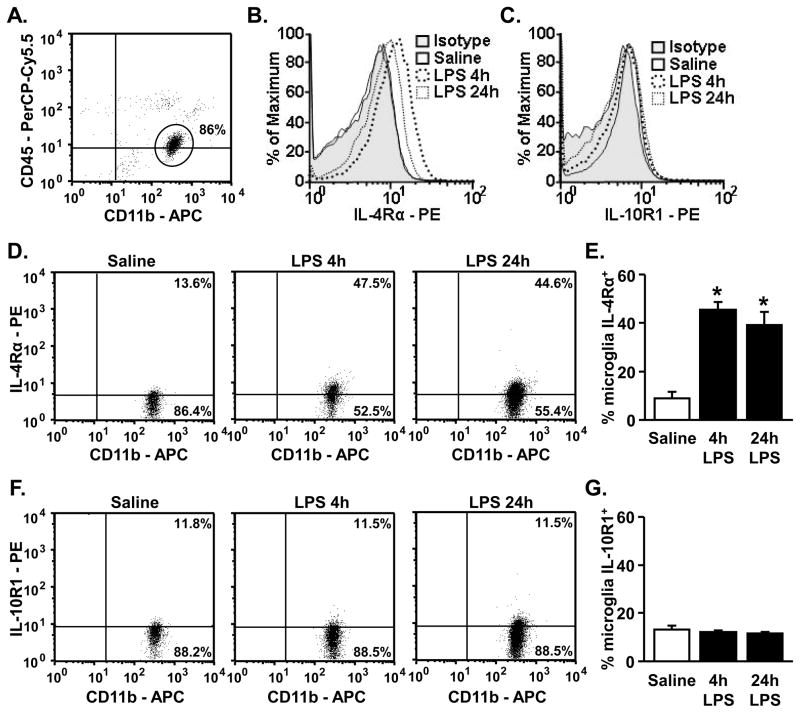
3.2. Peripheral LPS injection increased microglial surface expression of IL-4Rα, but not IL-10R1
Because LPS injection increased several M2 genes in enriched microglia, including IL-10 and IL-4Rα, surface expression of IL-4Rα and IL-10R1 were determined on microglia of adult mice 4 or 24 h after LPS injection. Fig. 2A shows a representative bivariate dot plot of CD11b and CD45 staining of Percoll isolated cells. Microglia were gated based on CD11b+/CD45low staining and IL-4Rα and IL-10R1 protein expression was determined. Representative histograms of the mean fluorescence intensity (MFI) for IL-4Rα-PE or IL-10R1-PE are shown in Figs. 2B&C. There was a robust increase in MFI for IL-4Rα on microglia after LPS injection, but the MFI for IL-10R1 was not increased after LPS. To further support these data, representative bivariate dot plots of IL-4Rα and IL-10R1 staining on microglia (CD11b+/CD45low), are shown in Fig. 2D and Fig. 2E, respectively. These dot plots confirm that IL-4Rα, but not IL-10R1, protein expression on the surface of microglia was markedly increased 4 h after LPS injection (main effect of LPS, p<0.0001). Moreover, the increase in IL-4Rα surface expression was maintained 24 h after LPS (main effect of LPS, p<0.003).
Figure 2. Peripheral LPS injection increased microglial surface expression of IL-4Rα, but not IL-10R1.
Adult (3–4 mo) BALB/c mice were injected ip with saline or LPS and CD11b, CD45, IL-4Rα and IL-10R1 were determined from enriched microglia isolated 4 h or 24 h later. A) A representative bivariate dot plot of Percoll isolated cells gated on CD11b+/CD45low expression for microglia. All analyses were completed using the CD11b+/CD45low microglia. Representative histograms of mean fluorescent intensity (MFI) for B) IL-4Rα and C) IL-10R1. Representative bivariate dot plots of microglia stained for D) IL-4Rα (n=4–7) and F) IL-10R1 (n=4). Average percentage of microglia that were E) IL-4Rα+ and G) IL-10R1+. Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from Saline controls.
3.3 IL-4 re-directs active BV-2 and primary microglia towards an M2 profile
Next, a series of experiments were completed using the BV-2 microglial cell line to determine the ability of IL-4 or IL-10 to re-direct active, M1 microglia towards a repair or anti-inflammatory M2 profile. In the first experiment, BV-2 cells were stimulated with LPS to induce a M1 phenotype and then treated with recombinant IL-4 (20 ng/mL). Table 1 shows that LPS stimulation (LPS Vehicle) increased mRNA levels of M1 genes iNOS and IL-1β (main effect of LPS, p<0.0001 for each) and increased M2 genes IL-10 and SOCS1 (main effect of LPS, p<0.0003 for each). Arg mRNA levels were reduced following LPS (main effect of LPS, p<0.009). These data are consistent with microglia expression of M1 and M2 related genes after peripheral injection of LPS (Fig. 1A). Moreover, IL-4 treatment alone decreased iNOS mRNA and increased mRNA levels of Arg, IL-10, and SOCS1 (main effect of IL-4, p<0.0001 for each). In this experiment, SOCS1 was selected as a target gene over SOCS3 because it is an M2c gene that is strongly and more specifically induced by IL-4 (Losman et al., 1999). IL-4 reduced the LPS dependent increase in iNOS mRNA (IL-4 × LPS interaction, F(1,23)=338.91, p<0.0001) and IL-1β mRNA (IL-4 × LPS interaction, F(1,23)=28.91, p<0.0001). Furthermore, pretreatment with LPS enhanced the IL-4-induced increase in SOCS1 mRNA (IL-4 × LPS interaction, F(1,11)=10.06, p<0.02). IL-4 induced Arg and IL-10 mRNA expression were unaffected by LPS (Table 1).
Table 1. IL-4 re-directed active BV-2 microglia towards an M2 profile.
BV-2 cells were plated and pre-treated with saline or LPS (10 ng/mL) for 1 h. After 1 h cells were treated with vehicle or IL-4 (20 ng/mL) for an additional 3 h (n=6). RNA was isolated and M1, M2a, and M2c gene expression was determined. These results represent 2 independent experiments.
| mRNA | Saline Vehicle | Saline IL-4 | LPS Vehicle | LPS IL-4 | |
|---|---|---|---|---|---|
| M1 | iNOS | 1.00 ± 0.4 (a) | 0.28 ± 0.02 (b) | 9.37 ± 0.22 (c) | 3.88 ± 0.14 (d) |
| IL-1β | 1.00 ± 0.02 (a) | 0.80 ± 0.04 (a) | 21.76 ± 0.64 (b) | 16.54 ± 0.68 (c) | |
| M2 | Arg | 1.02 ± 0.09 (a) | 29.38 ± 2.37 (b) | 0.68 ± 0.05 (c) | 28.80 ± 1.92 (b) |
| IL-10 | 1.01 ± 0.07 (a) | 3.22 ± 0.22 (b) | 1.84 ± 0.07 (c) | 3.11 ± 0.22 (b) | |
| SOCS1 | 1.00 ± 0.03 (a) | 58.56 ± 6.42 (c) | 3.06 ± 0.17 (b) | 85.63 ± 4.57 (d) | |
Table represents the mean ± SEM. Means with different letters (a,b,c,d) are significantly different (p<0.05) from each other.
In the second experiment, BV-2 cells were activated with LPS (as above) and then treated with recombinant IL-10 (10 ng/mL). Table 2 shows a similar pattern of LPS-induced gene expression (LPS Vehicle) consistent with the data provided in Table 1. IL-10 alone increased IL-1β, IL-10 and SOCS3 (main effect of IL-10, p<0.02). IL-10, however, was ineffective in redirecting active microglia towards an M2 profile. For instance, co-treatment of IL-10 and LPS induced the highest level of iNOS gene expression (p<0.04) and did not reduce LPS-associated IL-1β. Although, IL-10 did enhance LPS-induced SOCS3 expression (F(1,35)=7.21, p<0.02). This is relevant because SOCS3, much like SOCS1 for IL-4, is strongly induced by IL-10 (Ito et al., 1999). Thus, an increase in SOCS3 following treatment with IL-10 and further enhancement with LPS and IL-10 co-treatment provides evidence that BV-2 cells have the capacity to respond to IL-10 and promote certain aspects of an M2 profile, but not to the same extent as IL-4. Taken together, these data indicate that following classical activation (M1) with LPS, IL-4, but not IL-10, was effective in reducing M1 genes and enhancing M2 genes in BV-2 cells.
Table 2. IL-10 did not redirect active BV-2 microglia towards an M2 profile.
BV-2 cells were plated and pre-treated with saline or LPS (10 ng/mL) for 1 h. After 1 h cells were treated with vehicle or IL-10 (10 ng/mL) for an additional 3 h (n=9). RNA was isolated and M1, M2a, and M2c gene expression was determined. These results represent 3 independent experiments.
| mRNA | Saline Vehicle | Saline IL-10 | LPS Vehicle | LPS IL-10 | |
|---|---|---|---|---|---|
| M1 | iNOS | 1.09 ± 0.16 (a) | 1.65 ± 0.26 (a) | 3.60 ± 0.56 (b) | 6.88 ± 1.87 (c) |
| IL-1β | 1.08 ± 0.13 (a) | 1.73 ± 0.26 (b) | 9.79 ± 2.42 (c) | 12.95 ± 3.72 (c) | |
| M2 | Arg | 1.06 ± 0.13 | 1.16 ± 0.04 | 0.97 ± 0.16 | 0.78 ± 0.09 |
| IL-10 | 1.15 ± 0.23 (a) | 1.93 ± 0.36 (a,b) | 1.31 ± 0.18 (a) | 2.50 ± 0.40 (b) | |
| SOCS3 | 1.07 ± 0.15 (a) | 3.09 ± 0.55 (b) | 2.62 ± 0.31 (b) | 8.25 ± 1.18 (c) | |
Table represents the mean ± SEM. Means with different letters (a,b,c) are significantly different (p<0.05) from each other.
Next, primary microglia were established from the brains of neonatal mice to confirm that IL-4 promotes an M2 phenotype in LPS activated microglia. Similar to the above studies, microglia were activated by LPS for 1 h and then treated with recombinant IL-4 (20 ng/mL) for an additional 3 h. Table 3 shows that LPS increased M1 markers iNOS and IL-1β (main effect of LPS, p<0.0001 for each) and M2 markers IL-10 and SOCS1 (main effect of LPS, p<0.0001 for each). IL-4 alone increased M2 markers Arg, SOCS1 (main effect of IL-4, p<0.02), and IL-10 (tendency for IL-4, p=0.08) and decreased basal expression of M1 marker iNOS (tendency for IL-4, p=0.1). IL-4 reduced the LPS-dependent increase in iNOS mRNA (tendency for IL-4 × LPS, F(1,20)=2.58, p=0.1), but had no effect on LPS-induced IL-1β. IL-4 promoted Arg in the presence of LPS and enhanced SOCS1 induction compared to IL-4 and LPS treatments alone (IL-4 × LPS interaction, F(1,21)=4.98, p<0.04). Overall, these results support that LPS-activated microglia are successfully re-directed towards an anti-inflammatory and M2 phenotype following treatment with IL-4.
Table 3. IL-4 re-directed active primary microglia towards an M2 profile.
Primary microglia were collected from neonatal mice, plated, and pre-treated with saline or LPS (10 ng/mL) for 1 h. After 1 h cells were treated with vehicle or IL-4 (20 ng/mL) for an additional 3 h (n=5). RNA was isolated and M1, M2a, and M2c gene expression was determined. These results represent 2 independent experiments.
| mRNA | Saline Vehicle | Saline IL-4 | LPS Vehicle | LPS IL-4 | |
|---|---|---|---|---|---|
| M1 | iNOS | 1.00 ± 0.05 (a) | 0.76 ± 0.08 (b) | 270.85 ± 43.97 (c) | 187.95 ± 31.65 (c) |
| IL-1β | 1.03 ± 0.13 (a) | 0.64 ± 0.07 (b) | 114.57 ± 10.99 (c) | 102.87 ± 10.7 (c) | |
| M2 | Arg | 1.19 ± 0.38 (a) | 123.89 ± 44.13 (b) | 1.06 ± 0.43 (a) | 14.35 ± 4.16 (c) |
| IL-10 | 1.03 ± 0.11 (a) | 2.71 ± 0.39 (b) | 5.65 ± 0.65 (c) | 7.18 ± 1.41 (c) | |
| SOCS1 | 1.00 ± 0.03 (a) | 13.36 ± 0.73 (b) | 23.15 ± 3.97 (c) | 52.25 ± 4.99 (d) | |
Table represents the mean ± SEM. Means with different letters (a,b,c) are different (p<0.1) from each other. Underlined letters trend towards a difference (p<0.1) from the same letter (c, c).
3.4 M1, M2a, and M2c profile in adult and aged microglia after peripheral LPS injection
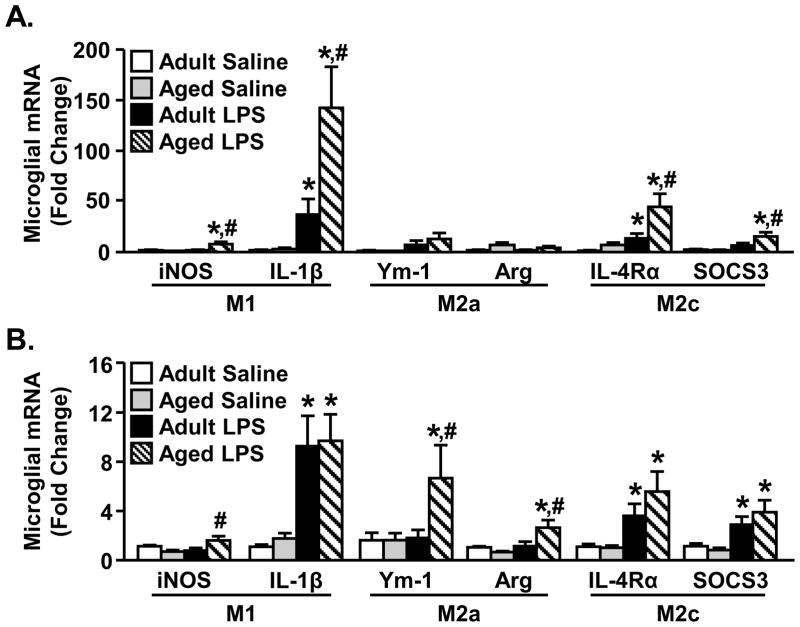
Based on M1 and M2 microglial profiling (Fig. 1–2) and the effects of IL-4 on activated BV-2 and primary microglia (Table 1–3), we next sought to determine differences in M1 and M2 microglial profiles in adult (3–4 mo) mice compared to aged (18–22 mo) mice. In these experiments, mice were ip injected with saline or LPS and enriched microglia were collected 4 h and 24 h later. Fig. 3A shows the relative expression of M1 (iNOS, IL-1β), M2a (Ym-1, Arg) and M2c (IL-4Rα, SOCS3) genes 4 h after LPS. Consistent with our previous work in aged mice, LPS caused an amplified increase in IL-1β (age × LPS interaction, F(1,26)=4.13, p<0.05) and iNOS (age × LPS interaction, F(1,26)=4.58, p<0.05) gene expression. There were no significant differences in LPS associated M2a induction between age groups 4 h after LPS. LPS injection did, however, increase M2c (IL-4Rα, SOCS3) gene expression (main effect of LPS, p<0.01). The highest levels of microglial mRNA expression for both IL-4Rα and SOCS3 were detected in microglia from LPS treated aged mice (age × LPS interaction, IL-4Rα (F(1,25)=4.43, p<0.05; SOCS3 (F(1,25)=2.46, P=0.10) (Fig. 2A). These data indicate that LPS caused an exaggerated induction in both M1 and M2c genes in aged mice compared to adult controls (Fig. 3A).
Figure 3. M1, M2a, and M2c profile in adult and aged microglia after peripheral LPS injection.
Adult (3–4 mo) and aged (18–22 mo) BALB/c mice were injected ip with saline or LPS and M1-, M2a-, and M2c-related genes were determined from enriched microglia isolated A) 4 h or B) 24 h later (n=6). Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from Adult Saline and means with # are significantly different (p<0.05) from Adult LPS.
Twenty-four hours following LPS injections microglia from aged mice maintained elevated expression of iNOS compared to adults treated with LPS (age × LPS interaction (F(1,31)=8.37, p<0.008) (Fig. 3B). IL-1β was also increased 24 h following LPS compared to saline controls, but this difference was independent of age (main effect of LPS, p<0.0001). Levels of IL-1β gene expression in activated microglia from adult mice, however, were higher than we have reported previously. Our previous studies support prolonged IL-1β expression in microglia of aged mice compared to adults (Wynne et al., 2010). At the 24 h time-point, aged mice treated with LPS had a significant increase in M2a gene expression of Arg (age × LPS interaction, F(1,31)=6.40, p<0.02) and Ym-1 (age × LPS interaction, Ym-1, F(1,31)=2.32, P=0.10) that was not detected in microglia from adult mice injected with LPS. Furthermore, 24 h after LPS, increased M2c gene expression was maintained, but this was independent of age (Fig. 3B, main effect of LPS, p<0.01). These results indicate that microglia from aged mice have more profound increases in M1 and M2c gene expression 4 h after LPS compared to adults. In addition, both adult and aged mice maintain elevated M2c gene expression 24 h following LPS injection, but only microglia from aged mice also have prolonged induction of the M1-related gene, iNOS, and M2a-related genes.
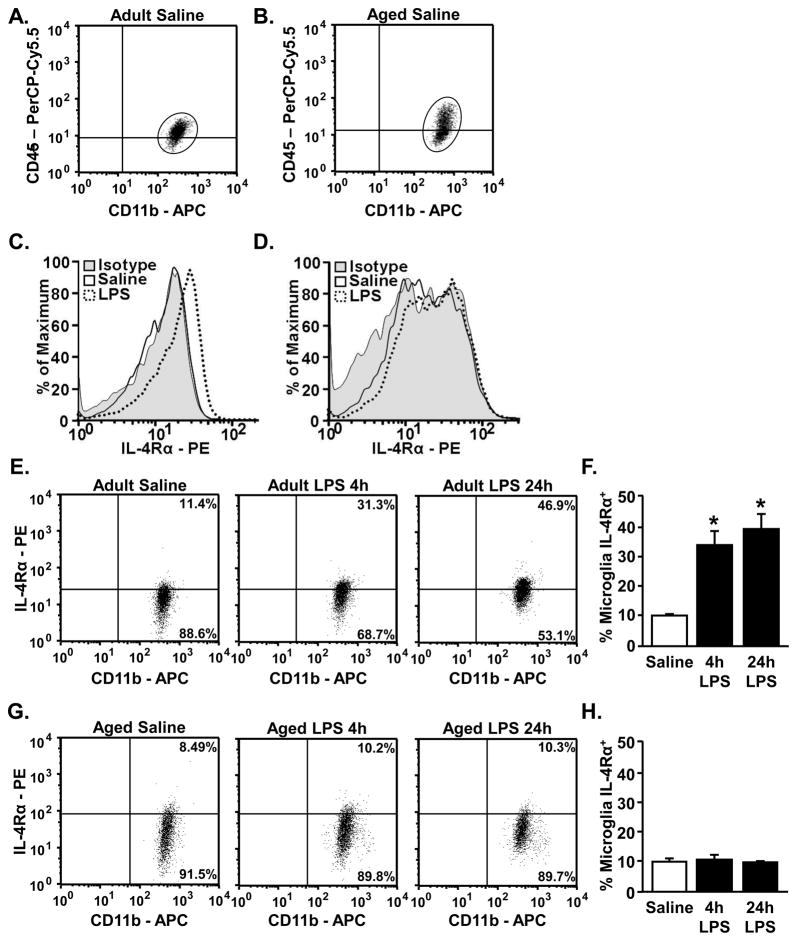
3.5 LPS-induced surface expression of IL-4Rα on microglia is impaired in aged mice
The mRNA data from the microglia profiling between adult and aged microglia indicates an increase in the M2 response in aged microglia compared to adults. We have previously reported a similar pattern for IL-10 mRNA and protein expression in active microglia from aged mice (Henry et al., 2009). Despite the enhanced M2 profile, however, inflammatory responses persist in the brain of aged mice. Therefore, exaggerated and prolonged activation in microglia from aged mice may involve insensitivity to anti-inflammatory feedback. In these age comparison studies we focused our attention on IL-4. This is because IL-4Rα was markedly upregulated on the surface of microglia from adult mice following activation with LPS and IL-4 had more profound M2 promoting capabilities in activated BV-2 cells compared to IL-10. Adult and aged mice were injected ip with saline or LPS and enriched microglia were collected 4 h and 24 h later for analysis of IL-4Rα protein expression. Fig. 4A&B are representative bivariate dot plots for CD11b and CD45 staining in enriched microglia from adult and aged mice used in the analysis for IL-4Rα expression. Fig. 4C&D shows representative histograms of mean fluorescent intensity (MFI) for isotype control, saline, and LPS in these same enriched microglia. Because there was higher nonspecific staining of the isotype for IL4Rα in aged mice, separate isotype controls were used for adult and aged mice. After normalizing to these separate isotypes, saline treated adult and aged mice had the same percentage of IL-4Rα+ microglia (10.0% ± 1.0% and 10.2% ± 0.6%, respectively). Representative dot plots for IL-4Rα protein expression on microglia (CD11b+/CD45low) of adult and aged mice are shown in Fig. 4E and Fig. 4G, respectively. Consistent with the data provided in Fig. 2, LPS injection markedly increased IL-4Rα surface expression on CD11b+/CD45low microglia from adult mice (main effect of LPS, p<0.0001) (Fig. 4E&F). In aged mice, however, LPS injection did not increase the percentage of IL-4Rα+ microglia at either the 4 h or 24 h time-point (Fig. 4G&H). MFI for IL-4Rα in adult and aged microglia confirms that only microglia from adult mice had increased expression of IL-4Rα after LPS injection (Fig. 4C&D). Taken together, these results indicate that IL-4Rα protein expression is not induced on microglia from aged mice after peripheral LPS injection.
Figure 4. Microglia from aged mice do not have increased surface expression of IL-4Rα following LPS.
Adult (3–4 mo) and aged (18–22 mo) BALB/c mice were injected ip with saline or LPS and CD11b, CD45, and IL-4Rα were determined from enriched microglia isolated 4 h and 24 h later (n=4). Representative bivariate dot plots fro microglia from A) adult and B) aged mice showing the gated microglia (CD11b+/CD45low) that were used for IL-4Rα analysis. Representative histograms of the MFI for IL-4Rα in saline and LPS treated C) adult and D) aged mice as well as the age-matched isotype control. Representative bivariate dot plots for microglial IL-4Rα expression from E) adult and G) aged mice. Average percentage of microglia from F) adult and H) aged mice that were IL-4Rα+. Bars represent the mean ± SEM. Means with * are significantly different (p<0.0001) from Saline controls.
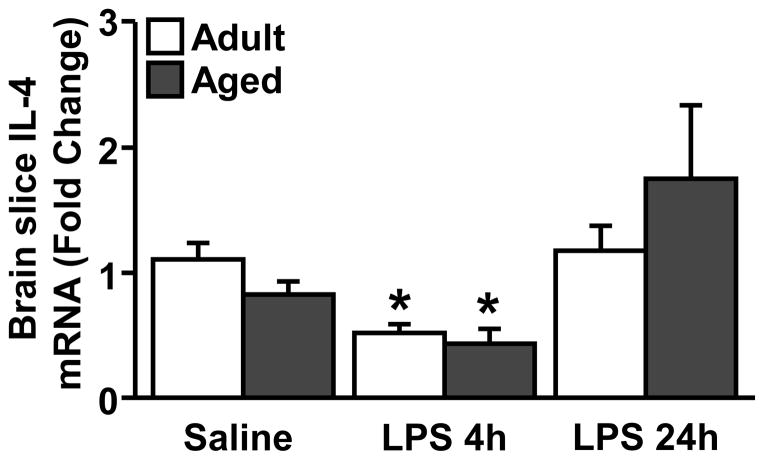
3.6 IL-4 mRNA levels decrease in the brain of adult and age mice after LPS
Next, we sought to determine the extent to which IL-4 mRNA levels change within the brain with age and LPS injection. In this experiment, adult and aged mice were injected ip with saline or LPS and IL-4 mRNA expression was determined from a 1 mm coronal brain section through the prefrontal cortex (+0.38 mm from Bregma) collected either 4 or 24 h later. These brain slices were collected from the same mice that were used in the RT-PCR and flow cytometry experiments. Fig. 5 shows that IL-4 mRNA levels were decreased in both adult and aged mice 4 h after LPS injection (main effect of LPS, p<0.001). IL-4 mRNA expression returned to baseline levels within 24 h after the injection of LPS independent of age. Overall, these data indicate that LPS decreased IL-4 mRNA expression in a time-dependent but age-independent manner.
Figure 5. IL-4 mRNA is decreased in the brain of adult and aged mice after LPS.
Adult (3–4 mo) and aged (18–22 mo) BALB/c mice were injected ip with saline or LPS and IL-4 mRNA was determined from a coronal brain slice 4 h and 24 h later (n=5). Means with * are significantly different (p<0.05) from Adult Saline.
3.7 Ex vivo treatment with IL-4 re-directed active microglia from adult, but not aged, mice towards an M2 profile
To determine the degree to which reduced IL-4Rα on the surface of microglia from aged mice corresponded with impaired sensitivity to IL-4, a series of ex vivo experiments were conducted. In these experiments, adult and aged mice were injected ip with saline or LPS to activate microglia in vivo. After 4 h, enriched microglia were isolated and plated on poly-L-lysine coated 24-well plates. Cells were treated ex vivo with vehicle or IL-4 (20 ng/mL) and RNA was collected 3 h later. Based on the results of the BV-2 and primary microglia experiments with LPS and IL-4, the M1 markers IL-1β and iNOS and the M2 marker Arg were selected for analyses.
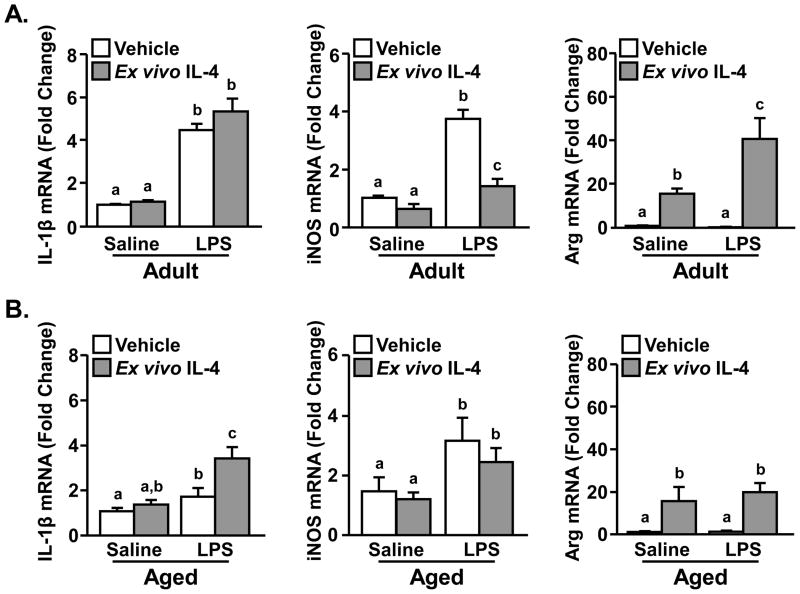
In microglia from adult mice, mRNA levels of IL-1β and iNOS (M1 genes) were increased in mice injected with LPS compared to saline (main effect of LPS, p<0.0001). Thus, the microglia cultured ex vivo retained a degree of activation associated with the ip injection of LPS. Following treatment with ex vivo IL-4, microglia from adult mice had a significant reduction in LPS-induced iNOS mRNA expression (LPS × ex vivo IL-4 interaction, F(1,30)=18.6, p<0.0002), but no change in IL-1β levels (Fig. 6A). The effect of IL-4 on active microglia are similar to the results obtained in primary microglial cultures (Table 3). Moreover, ex vivo IL-4 alone strongly promoted Arg mRNA expression (main effect of IL-4, p<0.0001). This Arg induction was potentiated when microglia from LPS injected mice were stimulated with IL-4 (Fig. 6A, LPS × IL-4 interaction, F(1,36)=6.74, p<0.01). Because adult and aged microglia are inherently different, the results for age × LPS × IL-4 interactions were not directly compared. Nonetheless, IL-4-mediated effects in the aged mice are significantly different than results obtained from the adult experiment.
Figure 6. Ex vivo treatment with IL-4 re-directed active microglia from adult, but not aged, mice towards an M2 profile.
Adult (3–4 mo) and aged (18–22 mo) BALB/c mice were injected ip with saline or LPS. Four hours later microglia were isolated, plated for 1 h, and treated ex vivo with IL-4 (20 ng/mL). RNA was isolated 3 h and expression of IL-1β, iNOS and Arg were determined from A) adult and B) aged mice (n=9). Bars represent the mean ± SEM. Means with different letters (a,b,c) are different (p<0.07) from each other.
Ex vivo cultures of microglia from LPS injected aged mice also had increased mRNA levels of IL-1β and iNOS (M1 genes) compared to saline-injected aged controls (tendency for main effect of LPS, p<0.07). Furthermore, in contrast to adult microglial cultures, ex vivo IL-4 further enhanced LPS-associated IL-1β mRNA expression (LPS × IL-4 interaction, F(1,34)=4.21, p<0.05) and did not reduce LPS-associated iNOS mRNA induction in aged microglia (Fig. 6B). Arg mRNA expression was increased by IL-4 alone in aged ex vivo microglia (main effect of IL-4, p<0.003), but this Arg mRNA induction was not amplified in microglia isolated from the LPS injected aged mice. Taken together, a lack of IL-4Rα upregulation of microglia from aged mice (Fig. 4) is associated with reduced sensitivity to ex vivo IL-4 stimulation.
4. Discussion
Previous studies from our lab and others indicate that aged but otherwise healthy rodents have prolonged sickness behavior (Godbout et al., 2005b; Huang et al., 2008), cognitive impairment (Abraham and Johnson, 2009; Barrientos et al., 2006; Chen et al., 2008), and depressive-like behavior (Godbout et al., 2008) following peripheral or central immune challenge. These behavioral deficits correspond to exaggerated and protracted microglial activation in aged mice (Henry et al., 2009; Wynne et al., 2010). Here we demonstrate that microglia from adult and aged mice express an M2b phenotype following an LPS injection, but only adult mice showed a clear shift towards an M2c phenotype within 24 h. Furthermore, IL-4Rα, but not IL-10R1, was increased on the surface of microglia from adult mice after activation with LPS. In accordance, IL-4, but not IL-10, successfully re-directed activated microglia in culture towards an M2a/M2c profile. In age comparisons, microglia from aged mice did not upregulate surface expression of IL-4Rα following peripheral LPS injection. Furthermore, when activated microglia from adult and aged mice were isolated and treated ex vivo with IL-4, only microglia from adult mice were successfully re-directed towards an anti-inflammatory and M2 profile with reduced iNOS and increased Arg mRNA expression.
One key component of this study was that following a peripheral injection of LPS, microglia simultaneously induced both M1 (IL-1β, iNOS) and M2c (IL-10, IL-4Rα) markers consistent with an M2b phenotype (Mantovani et al., 2004). In microglia from aged mice, there was an exaggerated increase in both the M1 and M2c markers. These data are in line with previous studies showing elevated IL-1β and IL-10 mRNA and intracellular protein expression in microglia 4 and 8 h after LPS injection, and further enhancement of both IL-1β and IL-10 in aged mice (Henry et al., 2009; Sierra et al., 2007). By 24 h after LPS, microglia from adult mice shifted to a predominately M2c phenotype whereas microglia from aged mice maintained an M2b profile. In our earlier studies enhanced IL-1β expression was also retained in LPS-treated aged mice 24 h after LPS. In the current study, however, IL-1β mRNA levels were higher in LPS-treated adult mice than we have previously reported (Godbout et al., 2005b; Henry et al., 2009; Wynne et al., 2010). A prolonged M2b phenotype in aged microglia is important because the shift towards an M2c phenotype 24 h after LPS corresponded with the resolution of the sickness response in adult mice in previous studies (Godbout et al., 2005b; Huang et al., 2008). Consistent with this notion, the present study indicates that pre-treatment with minocycline reduced IL-1β, maintained IL-4Rα and SOCS3, and significantly enhanced IL-10 and Ym-1 mRNA 4 h after LPS. This accelerated shift towards an anti-inflammatory, M2a/M2c phenotype corresponded with previous reports of minocycline-mediated ameliorated sickness response in adult mice (Henry et al., 2008). Based on a previous study (Lyons et al., 2007b) it is plausible that minocycline directly increased IL-4 expression to help promote an anti-inflammatory and M2 phenotype. Taken together these data indicate the time dependent transition of an M2b towards an M2c profile in microglia coincides with the resolution of LPS-induced sickness response.
Another difference between the M1 and M2 profile of microglia was that 24 h after LPS M2a markers were enhanced only in LPS-treated aged mice. Therefore, an increase in the M2a phenotype in microglia from aged mice may represent a greater degree of tissue injury associated with an exaggerated inflammatory response to LPS. In support of this notion, Arg was the most upregulated gene in the spinal cord immediately following induction of experimental autoimmune encephalomyelitis (EAE) (Xu et al., 2003) and was also promoted in mononuclear cells following spinal cord injury (Kigerl et al., 2009; Ochoa et al., 2001). Consequently, a promotion of the M2a phenotype within the aged CNS may be in response to overt CNS damage rather than promotion of an anti-inflammatory state.
Higher induction of mRNA for M2a-related genes may also be misleading because the increase in mRNA for IL-4Rα in microglia from aged mice did not correspond with increased IL-4Rα protein expression. The discrepancy between LPS-induced increased IL-4Rα mRNA levels with a corresponding failure to increase IL-4Rα surface expression in aged microglia may be related to problem with translation, post-translational modifications, or shuttling the receptor subunit to the cell membrane. For example microRNAs block the translation of genes into proteins (Bell, 2007) and multiple sequences and signaling cascades are involved in shuttling a translated protein from the endoplasmic reticulum to the Golgi and then to the cell membrane (Cho and Stahelin, 2005). Thus, it is plausible that a failure to increase IL-4Rα protein expression can occur independent of gene transcription.
Another important finding of this study is that an ip injection of LPS markedly increased protein expression of IL-4Rα, but not IL-10R1, on the surface of microglia from adult mice. IL-4Rα may be upregulated on microglia in preparation for increased levels of IL-4 to promote a microglial phenotype permissive to tissue repair and the resolution of inflammation (Mantovani et al., 2004; Mosser and Edwards, 2008). This may be particularly important in brain injury, EAE, and neurodegenerative diseases where there are tissue damage and repair processes associated with invading immune cells (Popovich and Jones, 2003; Schwartz and Kipnis, 2005). In addition, IL-4Rα is also a co-receptor for IL-13 (Nelms et al., 1999) and therefore microglia may be preparing to encounter IL-13. In the context of the induction of sickness behavior, microglia may enhance IL-4Rα surface expression to respond to a transient reduction in IL-4 mRNA levels after an LPS injection. Alternatively, IL-4Rα may be upregulated to maintain neurotrophic factor expression and support hippocampal neurogenesis in an effort to prevent inflammatory-induced deficits in learning and memory. For example, IL-4 production by T-cells is purportedly involved in increasing brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) during memory tasks (Derecki et al., 2010). Furthermore, IL-4 deficient mice had reduced learning and memory that was reversed following reconstitution with wild type bone marrow (Derecki et al., 2010).
Consistent with a main effect of LPS on microglial induction of anti-inflammatory cytokine receptors, IL-4, but not IL-10, was sufficient to redirect LPS-activated BV-2 and primary microglia towards an M2 phenotype. It is important to note that IL-4 did not reduce LPS-induced IL-1β expression in primary or ex vivo cultures. Several studies have reported no effect of IL-4 on IL-1β mRNA (Allen et al., 1993; Ledeboer et al., 2000), but rather reduced IL-1β bioactivity by enhancing IL-1 receptor antagonist (IL-1RA) through a PI3-kinase dependent mechanism (O’Connor et al., 2007). Nonetheless, these data are consistent with a previous study showing that LPS increased IL-4Rα mRNA on microglia and that both pre- and post-treatment of IL-4 reduced LPS-associated nitric oxide production in microglial and motoneuron co-cultures (Zhao et al., 2006). In contrast to IL-4, IL-10 had little effect in reducing LPS-associated M1 activation in BV-2 and ex vivo microglial cultures (data not shown). The current study was focused on how to re-direct the inflammatory profile of microglia following activation. Other studies have used pre-treatment with IL-10 (Qian et al., 2006; Sawada et al., 1999) or used IL-10 in mixed glial cultures (Ledeboer et al., 2000; Lodge and Sriram, 1996). Nonetheless, IL-10 is an important inflammatory cytokine in the brain. For instance, icv injection of IL-10 reversed LPS-induced sickness behavior (Bluthé et al., 1999) and IL-10 deficient mice had a prolonged LPS-induced sickness response (Richwine et al., 2009). Our results indicate that IL-10R1 expression remained low on microglia and that activated microglia in culture did not respond to the anti-inflammatory promoting effects of IL-10. Thus, it is possible that IL-10 has indirect anti-inflammatory effects on microglia through astrocytes. In support of this notion, cultured astrocytes expressed higher levels of IL-10R1 protein compared to microglia (Ledeboer et al., 2002) and IL-10 treatment of mixed glial cultures reduced LPS-induced inflammatory cytokine production (Ledeboer et al., 2000).
A novel finding of this study was that LPS did not increase IL-4Rα protein expression on the surface of microglia from aged mice and that this was associated with a reduced sensitivity to the M2 promoting-effects of IL-4. Reduced microglial sensitivity to IL-4 may have significant implications in neurological disease, aging, and CNS injury. For example, IL-4 production within the brain during EAE (Begolka et al., 1998) and multiple sclerosis (Benveniste, 1997) was positively correlated with remission of the disease. In addition, increased IL-4 concentrations in the brains of APP+PS1 mice improved NMDA-receptor function and reversed deficits in spatial learning and memory (Kiyota et al., 2010). In aging, reduced IL-4 levels in the brain of aged rats corresponded with increased neuroinflammation and reduced long-term potentiation (LTP) (Maher et al., 2005; Nolan et al., 2005). In addition, induction of hippocampal IL-4 successfully restored LTP in these aged rats (Clarke et al., 2008). We did not detect age-associated reductions in IL-4 mRNA expression in the current study, but this was likely because of species differences in immunity (BALB/c mice versus Wistar rats) (Haley, 2003). Nonetheless, a deficit in IL-4Rα expression and IL-4 sensitivity in aged microglia following an inflammatory stimulus support the idea that IL-4 regulation of microglia is impaired in the aged. Thus, a functional consequence of reduced IL-4-mediated microglial regulation may be reduced cognitive ability and worsened outcome in CNS injury. For example, working memory and long-term memory were reduced in aged mice (Abraham and Johnson, 2009; Chen et al., 2008) and rats (Barrientos et al., 2006) following a peripheral immune challange. In addition, IL-4 and IL-4Rα were strongly upregulated within 24 h following spinal cord (Lee et al., 2010) and brain injury (Xiong et al., 2011). In these traumatic CNS injuries IL-4 deficient mice or mice treated with anti-IL-4 had exaggerated monocyte activation, CNS damage, and had worsened neurological scores. Similar to these mice, cortical impact injury in aged C57BL/6 mice caused exaggerated glial activation (i.e., astrocytes and microglia) and CNS damage compared to injured adults (Onyszchuk et al., 2008; Sandhir et al., 2008). Therefore, it is plausible that worsened injury outcome in aged mice was associated with an impaired microglial response to the M2 and repair promoting effects of IL-4.
In conclusion the present study provides novel evidence that impaired expression of IL-4Rα on microglia from aged mice was associated with reduced sensitivity to the M2-promoting cytokine IL-4. In addition, we propose an important interaction between age-related neuroinflammation and microglial activity where an exaggerated M1 response causes these cells to be refractory to anti-inflammatory feedback by IL-4. These findings support previous work by our lab and others that impaired regulation of microglia in aged mice leads to amplified and prolonged microglial activation. Furthermore, these IL-4 data add to the growing body of literature the glial regulatory systems including CD200-CD200R (Lyons et al., 2007a) and CX3CL1-CX3CR1 (Bachstetter et al., 2011; Lyons et al., 2009; Wynne et al., 2010) are impaired in the aged brain.
Research Highlight.
Microglia from aged mice injected with LPS failed to increase surface expression of IL-4Rα corresponding with a reduced sensitivity to the M2 promoting effects of IL-4.
Acknowledgments
This work is supported by NIH grant R01-AG-033028 to JPG. A.M.F is supported by a Howard Hughes Medical Institute (HHMI) Med into Grad scholarship. A.D. is supported by a Federal Work-Study Grant. The authors thank Dr. John Sheridan (OSU, Dept. of Oral Biology) for the use of a Becton-Dickinson FACSCaliber four color Cytometer and Dr. Ronald Glaser (OSU, Dept. of MVIMG) for the use of an Applied Biosystems PRISM 7300 sequence detection system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuv Res. 2009;12:445. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- Allen JB, Wong HL, Costa GL, Bienkowski MJ, Wahl SM. Suppression of monocyte function and differential regulation of IL-1 and IL-1ra by IL-4 contribute to resolution of experimental arthritis. J Immunol. 1993;151:4344. [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX3CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Begolka WS, Vanderlugt CL, Rahbe SM, Miller SD. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of Multiple Sclerosis. J Immunol. 1998;161:4437. [PubMed] [Google Scholar]
- Bell E. MicroRNAs and the immune response. Nat Rev Immunol. 2007;7:418. [Google Scholar]
- Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- Berg BM, Godbout JP, Kelley KW, Johnson RW. Alpha-tocopherol attenuates lipopolysaccharide-induced sickness behavior in mice. Brain Behav Immun. 2004;18:149–157. doi: 10.1016/S0889-1591(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HWGM. Neuronal ‘on’ and ‘off’ signals control microglia. Trends Neurosci. 2007;30:596. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinol. 1999;24:301. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Chao CC, Molitor TW, Hu S. Neuroprotective role of IL-4 against activated microglia. J Immunol. 1993;151:1473. [PubMed] [Google Scholar]
- Chen J, Buchanan J, Sparkman N, Godbout J, Freund G, Johnson R. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W, Stahelin RV. Membrane-protein interactions in cell signaling and membrane trafficking. Annu Rev Bioph Biom. 2005;34:119–151. doi: 10.1146/annurev.biophys.33.110502.133337. [DOI] [PubMed] [Google Scholar]
- Clarke RM, Lyons A, O’Connell F, Deighan BF, Barry CE, Anyakoha NG, Nicolaou A, Lynch MA. A pivotal role for interleukin-4 in atorvastatin-associated neuroprotection in rat brain. J Biol Chem. 2008;283:1808. doi: 10.1074/jbc.M707442200. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor J, Freund G, Johnson R, Kelley K. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer EJ, Cowley TR, Lyons A, Mills KHG, Berezin V, Bock E, Lynch MA. A novel anti-inflammatory role of NCAM-derived mimetic peptide, FGL. Neurobiol Aging. 2010;31:118. doi: 10.1016/j.neurobiolaging.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Frank M, Barrientos R, Biedenkapp J, Rudy J, Watkins L, Maier S. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frei K, Lins H, Schwerdel C, Fontana A. Antigen presentation in the central nervous system. The inhibitory effect of IL-10 on MHC class II expression and production of cytokines depends on the inducing signals and the type of cell analyzed. J Immunol. 1994;152:2720–2728. [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Godbout J, Berg B, Krzyszton C, Johnson R. [alpha]-Tocopherol attenuates NF[kappa]B activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol. 2005a;169:97. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Godbout J, Chen J, Abraham J, Richwine A, Berg B, Kelley K, Johnson R. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005b;10:1329. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout J, Moreau M, Lestage J, Chen J, Sparkman N, O’Connor J, Castanon N, Kelley K, Dantzer R, Johnson R. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacol. 2008;33:2341. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunol Allergy Clin. 2009;29:321. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003;188:49. doi: 10.1016/s0300-483x(03)00043-x. [DOI] [PubMed] [Google Scholar]
- Haque SJ, Harbor P, Tabrizi M, Yi T, Williams BRG. Protein-tyrosine phosphatase Shp-1 Is a negative regulator of IL-4- and IL-13-dependent signal transduction. J Biol Chem. 1998;273:33893–33896. doi: 10.1074/jbc.273.51.33893. [DOI] [PubMed] [Google Scholar]
- Henry C, Huang Y, Wynne A, Hanke M, Himler J, Bailey M, Sheridan J, Godbout J. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflamm. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1[beta] and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Henry C, Dantzer R, Johnson R, Godbout J. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP, Larner AC, Finbloom DS. Interleukin-10 inhibits expression of both interferon-alpha and interferon-gamma induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–1463. [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Experimental Neurology. 2010 doi: 10.1016/j.expneurol.2010.11.014. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl Kristina A, Gensel John C, Ankeny Daniel P, Alexander Jessica K, Donnelly Dustin J, Popovich Phillip G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:10. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease- like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010;24:3093. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig HG, Meador KG, Cohen HJ, Blazer DG. Depression in elderly hospitalized patients with medical illness. Arch Intern Med. 1988;148:1929. [PubMed] [Google Scholar]
- Ledeboer A, Brevé JJP, Poole S, Tilders FJH, Van Dam AM. Interleukin-10, interleukin-4, and transforming growth factor-β differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia. 2000;30:134. doi: 10.1002/(sici)1098-1136(200004)30:2<134::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Brevé JJP, Wierinckx A, Van Der Jagt S, Bristow AF, Leysen JE, Tilders FJH, Van Dam AM. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur J Neurosci. 2002;16:1175. doi: 10.1046/j.1460-9568.2002.02200.x. [DOI] [PubMed] [Google Scholar]
- Lee SI, Jeong SR, Kang YM, Han DH, Jin BK, Namgung U, Kim BG. Endogenous expression of interleukin-4 regulates macrophage activation and confines cavity formation after traumatic spinal cord injury. J Neurosci Res. 2010;88:2409. doi: 10.1002/jnr.22411. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K. Innate immune receptor expression in normal brain aging. Neuroscience. 2007;146:248. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lipowski ZJ. Transient cognitive disorders (delirium, acute confusional states) in the elderly. Am J Psychiatry. 1983;140:1426. doi: 10.1176/ajp.140.11.1426. [DOI] [PubMed] [Google Scholar]
- Lodge PA, Sriram S. Regulation of microglial activation by TGF-beta, IL-10, and CSF-1. Journal of Leukocyte Biology. 1996;60:502–508. doi: 10.1002/jlb.60.4.502. [DOI] [PubMed] [Google Scholar]
- Losman JA, Chen XP, Hilton D, Rothman P. Cutting edge: SOCS-1 is a potent inhibitor of IL-4 signal transduction. J Immunol. 1999;162:3770–3774. [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Downer E, Crotty S, Nolan Y, Mills K, Lynch M. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007a;27:8309. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Griffin RJ, Costelloe CE, Clarke RM, Lynch MA. IL-4 attenuates the neuroinflammation induced by amyloid-β in vivo and in vitro. J Neurochem. 2007b;101:771. doi: 10.1111/j.1471-4159.2006.04370.x. [DOI] [PubMed] [Google Scholar]
- Lyons A, Lynch AM, Downer EJ, Hanley R, O’Sullivan JB, Smith A, Lynch MA. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem. 2009;110:1547. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- Maher FO, Nolan, Yvonne, Lynch, Marina A. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:12. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Morley AA, Trainor KJ. Lack of an effect of vitamin E on lifespan of mice. Biogerontology. 2001;2:109. doi: 10.1023/a:1011589218219. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulsant BH, Ganguli M. Epidemiology and diagnosis of depression in late life. J Clin Psychiatry. 1999;60:9. [PubMed] [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, Mills KHG, Lynch MA. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J Biol Chem. 2005;280:9354. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Sherry CL, Guest CB, Freund GG. Type 2 Diabetes impairs insulin receptor substrate-2-mediated phosphatidylinositol 3-kinase activity in primary macrophages to induce a state of cytokine resistance to IL-4 in association with overexpression of suppressor of cytokine signaling-3. J Immunol. 2007;178:6886–6893. doi: 10.4049/jimmunol.178.11.6886. [DOI] [PubMed] [Google Scholar]
- Ochoa JB, Bernard AC, O’Brien WE, Griffen MM, Maley ME, Rockich AK, Tsuei BJ, Boulanger BR, Kearney PA, Morris SM., Jr Arginase I expression and activity in human mononuclear cells after injury. Ann Surg. 2001;233:393. doi: 10.1097/00000658-200103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NEJ, Brooks WM. Detrimental effects of aging on outcome from traumatic brain Injury: a behavioral, magnetic resonance imaging, and histological study in mice. J Neurotraum. 2008;25:153. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2 2004. [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Jones TB. Manipulating neuroinflammatory reactions in the injured spinal cord: back to basics. Trends Pharmacol Sci. 2003;24:13. doi: 10.1016/s0165-6147(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Qian L, Block ML, Wei SJ, Lin C-f, Reece J, Pang H, Wilson B, Hong JS, Flood PM. Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. J Pharmacol Exp Ther. 2006;319:44. doi: 10.1124/jpet.106.106351. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Sparkman NL, Dilger RN, Buchanan JB, Johnson RW. Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide. Brain Behav Immun. 2009;23:794. doi: 10.1016/j.bbi.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NEJ. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Experimental Neurology. 2008;213:372. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Suzumura A, Hosoya H, Marunouchi T, Nagatsu T. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J Neurochem. 1999;72:1466. doi: 10.1046/j.1471-4159.1999.721466.x. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J. Protective autoimmunity and neuroprotection in inflammatory and noninflammatory neurodegenerative diseases. J Neurol Sci. 2005;233:163. doi: 10.1016/j.jns.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Kravitz FH, Peterson PK, Chao CC. Tumor necrosis factor alpha upregulates human microglial cell production of interleukin-10 in vitro. Clin Diagn Lab Immunol. 1995;2:604. doi: 10.1128/cdli.2.5.604-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Strle Klemen, Zhou Jian H, Shen Wen H, Broussard Suzanne R, Johnson Rodney W, Freund Gregory G, Dantzer Robert, Kelley Keith W. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427. [PubMed] [Google Scholar]
- Szczepanik AM, Funes S, Petko W, Ringheim GE. IL-4, IL-10 and IL-13 modulate A[beta](1-42)-induced cytokine and chemokine production in primary murine microglia and a human monocyte cell line. J Neuroimmunol. 2001;113:49. doi: 10.1016/s0165-5728(00)00404-5. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LTM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. [Beta]-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AM, Patel NV, Patel NK, Wei M, Morgan TE, de Beer MC, de Villiers WJS, Finch CE. Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neurosci Lett. 2005;390:76. doi: 10.1016/j.neulet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Wynne A, Henry C, Huang Y, Cleland A, Godbout J. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Barreto GE, Xu L, Ouyang YB, Xie X, Giffard RG. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke. 2011;42:2026. doi: 10.1161/STROKEAHA.110.593772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Hilliard B, Carmody RJ, Tsabary G, Shin H, Christianson DW, Chen YH. Arginase and autoimmune inflammation in the central nervous system. Immunology. 2003;110:141. doi: 10.1046/j.1365-2567.2003.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. An Age-Related Decline in Interleukin-10 May Contribute to the Increased Expression of Interleukin-6 in Brain of Aged Mice. Neuroimmunomodulat. 2001;9:183. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, PollmÄCher T. Illness, cytokines, and depression. Ann NY Acad Sci. 2000;917:478. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- Zhao W, Xie W, Xiao Q, Beers DR, Appel SH. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J Neurochem. 2006;99:1176. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]