Abstract
Cardiac surgery presents particular challenges for the anesthesiologist. In addition to standard and advanced monitors typically used during cardiac surgery, anesthesiologists may consider monitoring the brain with raw or processed electroencephalography (EEG). There is strong evidence that a protocol incorporating the processed EEG Bispectral Index (BIS) decreases the incidence intraoperative awareness compared with standard practice. However there is conflicting evidence that incorporating the BIS into cardiac anesthesia practice improves “fast-tracking,” decreases anesthetic drug use, or detects cerebral ischemia. Recent research, including many cardiac surgical patients, shows that a protocol based on BIS monitoring is not superior to a protocol based on end tidal anesthetic concentration monitoring in preventing awareness. There has been a resurgence of interest in the anesthesia literature in limited montage EEG monitoring, including nonproprietary processed indices. This has been accompanied by research showing that with structured training, anesthesiologists can glean useful information from the raw EEG trace. In this review, we discuss both the hypothesized benefits and limitations of BIS and frontal channel EEG monitoring in the cardiac surgical population.
Introduction
Cardiac anesthesiologists frequently care for vulnerable patients with multiple co-morbidities undergoing complex and invasive surgery. In the interest of patient safety and to facilitate appropriate anesthetic and surgical decision making, such patients are monitored extensively and with state-of-the-art technologies, such as transesophageal echocardiography and sophisticated hemodynamic monitors. However, monitoring the brain, the target of general anesthesia and one of the organs susceptible to perioperative injury, is not mandatory during cardiac surgery. Brain monitoring has not been made standard of practice for several reasons, including the lack of compelling evidence of effectiveness and the perception that current monitors are not always reliable or easy to interpret. General anesthesia is associated with neuroelectric changes in the brain, which are partly detected by electroencephalogram (EEG) recordings from scalp electrodes.1 As far back as 1937, Gibbs et al. suggested, “The anesthetist and surgeon could have before them on tape or screen a continuous record of the electric activity of both heart and brain.”2
While the authors of this review endorse the view that in 2012 some type of brain monitoring should potentially be used for all general anesthetics, the unique physiological and procedural challenges make the case for monitoring the brain during cardiac surgery particularly compelling. Practitioners who currently monitor the brain during cardiac surgery do so for three chief reasons: 1) to decrease the incidence of awareness by detection of inadequate anesthesia; 2) to reduce time to awakening and overall anesthetic consumption; and 3) to provide surrogate information on cerebral perfusion. This review will focus on two common methods of monitoring the brain during cardiac surgery: the raw frontal EEG and the Bispectral Index. Currently, the Bispectral Index or BIS® monitor (Covidien, Boulder, CO) is the most frequently used processed EEG (pEEG) monitor in cardiac anesthetic practice. This review does not discuss in detail other pEEG monitoring (e.g., spectral entropy) or evoked brain electrical activity monitoring (e.g., somatosensory evoked potentials, auditory evoked potentials, and motor evoked potentials). We also exclude cerebral oximetry, which is gaining popularity as a candidate surrogate monitor of brain perfusion during cardiac surgery.3 We aim to present some of the evidence for EEG monitoring in cardiac anesthesia, for what is added by use of a pEEG like the BIS, and for the potential drawbacks to EEG and pEEG monitoring.
A Brief Introduction to EEG, the BIS and Anesthesia
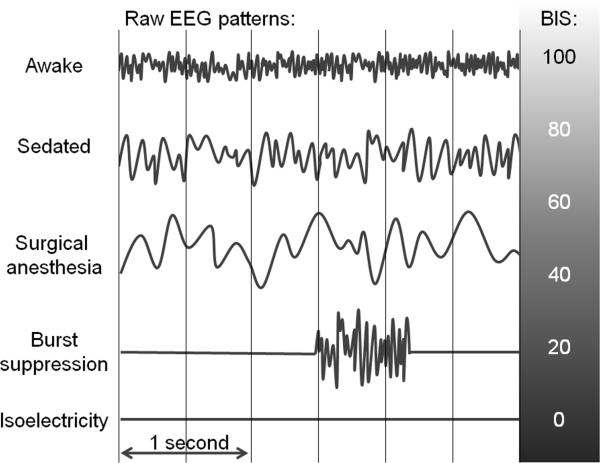
The EEG is a real-time graphical representation of a summation of miniscule (in the microvolt range) spontaneously generated electrical potentials in a small brain area underlying a scalp electrode. It has long been noted that the interindividual variation in EEG patterns during wakefulness tends to diminish with exposure to an anesthetic drug.4 The EEG changes occurring with general anesthesia have been described in classic papers, in which its potential use as a tool to judge depth of anesthesia that is commensurate with impending surgical stimuli is advocated.1,4 The following description of EEG changes induced by progressive anesthetic administration, as related by Martin, et al. in 1959,4 remains instructive: “Early in the variations from normal comes an increase in frequency to 20 to 30 cycles per second. As consciousness is lost, this pattern of small rapid waves is replaced by a large (50 to 300 microvolts) slow wave (1 to 5 cycles per second) that increases in amplitude as it slows. The wave may become irregular in form and repetition time, and it may have secondary faster waves superimposed as the level of anesthesia deepens. The amplitude next begins to decrease, and periods of relative cortical inactivity (the so-called burst suppression) may appear until the depression finally results in the entire loss of cortical activity and a flat or formless tracing.”4 Figure 1 shows stylized versions of raw EEG patterns during different levels of anesthetic depth. Figure 2, taken during cardiopulmonary bypass (CPB) in a cardiac operating room, demonstrates the extent of information available from use of a frontal EEG with nonproprietary processing methods including compressed spectral array and frequency analysis. Bennett et al. have reviewed clinical uses of the raw EEG waveform during anesthesia suggesting that familiarity with it has practical utility;5 they emphasize that uncritical adherence to a pEEG index, which is subject to artifacts and errors, could lead to inappropriate over- or under-dosing of anesthesia.5 In practice, the simplicity of a pEEG index has proved more acceptable than the raw EEG to most practitioners. There are several numerical indices, some nonproprietary (such as the spectral edge frequency6 and permutation entropy7) and some proprietary (such as the BIS and the Narcotrend®8 [MonitorTechnik, Bad Bramstedt, Germany] indices). Because it enjoys the most widespread use, both in practice and as a research tool, we will focus on the BIS; however, many of the strengths and weaknesses discussed here apply also to other pEEG indices. For detailed descriptions on EEG processing9 and the derivation of the BIS,10 we refer readers to comprehensive reviews. The BIS incorporates features of the raw EEG to produce a dimensionless number between 0 and 100, with 0 indicating complete cerebral suppression and 90-100 consistent with the awake state. In brief, the BIS algorithm initially processes the frontal EEG to detect presence of cerebral suppression (i.e., burst suppression or persistent suppression) and performs a fast Fourier transform (FFT) on the waveform. Data from the FFT are used to compute the ratio of higher frequency waves (30 to 47 Hz.) to other waves of lower frequency (11 to 20 Hz.), and to compute the bispectrum, which measures the phase coupling between high frequency (40 to 47 Hz.) and a broader frequency range (0.5 to 47 Hz.) of EEG waves.11 With the exception of the bispectral analysis, these features can be qualitatively assessed from the raw EEG and nonproprietary processed parameters. Based on such parameters and clincal context, a trained observer can estimate the BIS output with reasonable accuracy.12 Whether or not bispectral analysis adds incremental value to raw EEG and non proprietary parameters in improving the accurate detection of awareness or measuring depth of anesthesia has not been established.13,14
Figure 1.
Representative electroencephalogram patterns at different stages of anesthesia as described in the text.
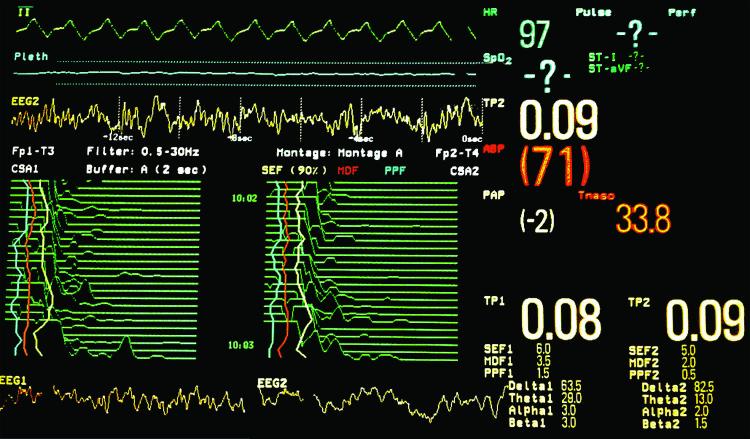
Figure 2.
Photograph of the screen of a Philips IntelliVue MP90 patient monitor®. The MP90 allows several monitors to be used concurrently with different configurations. With the Philips Electroencephalography (EEG) measurement module, the monitor can be configured to display real-time raw waveforms from two EEG channels, compressed spectral array (CSA) for each channel of EEG, total power (TP) percent in each frequency band (delta, theta, alpha, beta), spectral edge frequency (SEF), mean dominant frequency (MDF), and peak power frequency (PPF). This screen shot was taken during cardiopulmonary bypass shortly after aortic clamp removal. The third waveform on the screen is a 16-second epoch from a right-sided frontal EEG channel (EEG2); the sweep speed is 12.5 mm/sec. A slow underlying delta waveform (<1 Hz.) pattern is evident. The two bottom waveforms are each 4-second epochs from a left (EEG1) and right (EEG2) sided frontal EEG channel; the sweep speed is 25 mm/sec. A slow delta pattern (<1 Hz.) is evident as well as predominantly theta (4-8 Hz.) and delta waves (0-4 Hz.). The compressed spectral arrays graphically depict the peaks in different EEG frequencies from 0 to 30 Hz. over time; the display is refreshed every two seconds. Peaks in the lower frequencies are on the left and higher frequencies are on the right. From each of the arrays, 30-second trends can be appreciated. The percent power in each frequency band shows that >90% of the power is in the theta and delta frequencies. This is consistent with both of the spectral edge frequencies (SEF 90%), which are 6 Hz. for EEG1 and 5 Hz. for EEG2. The spectral edge frequency trend for each channel is depicted by the yellow lines on the compressed spectral arrays.
Intraoperative Awareness
One of the most compelling arguments for brain monitoring is that a primary objective of surgical anesthesia is insensibility. The EEG, raw or processed, provides a direct measure of cerebral activity, and with appropriate training, anesthesiologists can be taught to recognize whether a raw EEG is consistent with wakefulness, sedation, or unresponsiveness.15 There are many reasons why cardiac surgical patients might be at higher risk of intraoperative awareness. Patients with impaired cardiac function (e.g., low ejection fraction, pulmonary hypertension) are vulnerable to hemodynamic compromise with anesthetic administration. Practitioners therefore might attempt to minimize anesthetic administration to these patients.16 Titration of anesthetic drugs based simply on heart rate and arterial blood pressure responses is even more unreliable in cardiac anesthesia than in other settings because patients are often receiving medications that mask blood pressure and heart rate responses (e.g., beta blockers) and the intraoperative use of vasoactive drugs is also potentially confounding.
Furthermore, cardiac surgery frequently requires periods of CPB, which presents its own specific monitoring challenges. Blood pressure (determined by CPB pump) and heart rate (often absent) indicate nothing about the patient's depth of anesthesia. The CPB oxygenator exhaust gas port may not be routinely monitored for anesthetic drug concentration, and the common, but arguably unnecessary, practice of muscle relaxant administration during CPB excludes movement as a sign of possible awareness. In addition, total IV anesthesia is a popular technique during cardiac surgery, and markedly altered pharmacokinetics during CPB17 render target-controlled infusions inaccurate. For these reasons, during CPB, anesthesiologists may have no surrogate monitors or indicators that can suggest whether a patient is conscious or unconscious. At least three of the patients who experienced awareness in the BAG RECALL trial provide narratives that are consistent with awareness episodes occurring during CPB.18
Reported rates of intraoperative awareness during cardiac surgery range from 0.2% to 2%, a tenfold increase in risk compared with the general surgical population.18-21 Anesthetic technique is likely to significantly influence the incidence of intraoperative awareness. Patients undergoing balanced anesthesia for cardiac surgery using benzodiazepines, moderate-dose fentanyl (10-15 mcg/kg), isoflurane (end-tidal concentrations 0.5%-1.5%) during anesthetic maintenance and IV propofol infusion (2-6 mg/kg/hr) during and after CPB reportedly have a relatively low incidence of awareness (0.3%; 95% CI, 0.09% to 1.19%)20 compared with patients in the past who underwent cardiac surgery with high-dose opioid anesthesia supplemented by low dose halothane or nitrous oxide (23%; 95% CI, 11.8% to 40.9%).22 An important caveat, apart from the wide confidence interval of the estimate, is that 0.3% was probably an underestimate because patients were only interviewed once at 18 hours postoperatively;20 other studies have found that many patients only remember or report awareness at subsequent postoperative interviews.18,21,23-26 While the incidence of awareness with high-dose opioid anesthesia is likely to be high, 23% is probably an over-estimate because only 30 patients were included in this study and five of the seven patients who recalled intraoperative events required hypnosis to reveal these memories.22 The potential problem with high-dose opioid anesthesia is that maintenance of unconsciousness is unpredictable.27 Studies have shown that during opioid anesthesia perception and cortical processing of auditory information may not be suppressed completely.28
No study has specifically investigated ways to reduce intraoperative awareness in an exclusively cardiac surgical population. However, three randomized clinical trials of the impact of BIS monitoring on awareness included a relatively large number of patients undergoing cardiac surgery (27%, 49%, and 36%) and/or lung transplantation.18,21,23 Post hoc analysis of the incidence of awareness in those undergoing cardiac surgery and/or lung transplantation does not reveal a reduction in either definite or possible awareness associated with BIS monitoring18,21,23 (Table 1).
Table 1.
Summary of studies on BIS-guided anesthesia and intraoperative awareness focusing on cardiac surgical patients
| Characteristics | Myles et al.; 2004 [23] | Avidan et al.; 2008 [21] | Avidan et al., 2011 [18] |
|---|---|---|---|
| Number of patients studied | 2,463 | 1,941 | 5,713 |
| Study period | 2000-2002 | 2005-2006 | 2008-2010 |
| Study design | Randomized clinical trial | Randomized clinical trial | Randomized clinical trial |
| Proportion of cardiac surgery patients, % | 49 | 27 | 36 |
| Proportion of patients receiving TIVA (%) | 43 | 0 | 0 |
| Type of intervention | BIS-guided anesthesia vs. routine care | BIS-guided vs. ETAC-guided anesthesia | BIS-guided vs. ETAC guided anesthesia |
| Incidence of definite awareness among cardiac surgical patients including lung transplants (%) | BIS group: 1/607 (0.16%) Routine care: 5/605 (0.8%) |
BIS group: 0/270 (0%) |
BIS group: 5/1004 0.5% |
| ETAC group: 2/255 (0.78%) |
ETAC group: 1/1037 (0.1%) |
||
| Incidence of definite or possible awareness among cardiac surgical patients including lung transplants (%) | BIS group: 9/607 (1.5%) Routine care: 11/605 (1.8%) |
BIS group: 2/270 (0.74%) |
BIS group: 11/1004 (1.1%) |
| ETAC group: 3/255 (1.18%) |
ETAC group: 4/1037 (0.4%) |
||
| Absolute risk reduction by BIS-guided anesthesia for definite awareness, % (95% CI) | 0.64% (-0.13% to 1.5%) |
0.78% (-0.73% to 2.8%) |
-0.4% (-1.07% to 0.13%) |
| Absolute risk reduction by BIS-guided anesthesia for definite or possible awareness, % (95% CI) | 0.34% (-1.1% to 1.8%) |
0.44% (-1.6% to 2.7%) |
-0.71% (-1.6% to 0.06%) |
| Number needed to treat to prevent one definite awareness event (95% CI) with BIS-guided care* | 151 (NNTH 778 to ∞ to NNTB 69) |
128 (NNTH 137 to ∞ to NNTB 36) |
NNTH 250 (NNTH 93 to ∞ to NNTB 769) |
| Number needed to treat to prevent one definite or possible awareness event (95% CI) with BIS-guided care* | 298 (NNTH 91 to ∞ to NNTB 56) |
227 (NNTH 63 to ∞ to NNTB 37) |
NNTH 141 (NNTH 63 to ∞ to NNTB 1667) |
ASA, American Society of Anesthesiologists, BIS, Bispectral Index; CI, confidence interval; ETAC, end-tidal anesthetic gas concentration; TIVA, total intravenous anesthesia.
Confidence intervals span zero and hence reported as number needed to treat to harm (NNTH) and number needed to treat to benefit (NNTB) as recommended by Altman.105
Although not limited to patients undergoing cardiac surgery, one as yet unpublished trial might provide additional information about the potential of BIS-guided anesthesia to decrease unintended intraoperative awareness in patients undergoing cardiac surgery.29 This trial is the Michigan Awareness Control Study, a prospective, randomized, controlled trial comparing electronic alerts based on BIS monitoring or calculated anesthetic concentration for the prevention of intraoperative awareness in an unselected surgical population.29
Based on the B-Aware trial, it would be reasonable to surmise that routine use of a BIS-guided protocol during cardiac surgery could decrease the incidence of awareness compared with standard anesthetic practice.23 However, alternative approaches, such as protocols based on anesthetic concentration alarms, are probably equally effective, and potentially more cost effective.18,21 There have been no studies evaluating the potential utility of raw EEG or processed EEG indices, apart from BIS, in preventing awareness in cardiac surgery or other surgical settings. The recent evidence18,21,23 reinforces the appropriateness of the stance taken by the American Society of Anesthesiologists’ Practice Advisory on Awareness and Brain Monitoring.30 The opinion of the Task Force is that the decision to use a brain monitor, including a BIS monitor, should be made on a case-by-case basis, and should not be considered standard of care.30
Fast Track Cardiac Surgery
Most of the research suggesting that pEEG monitoring might be helpful in fast-track anesthesia has been conducted in noncardiac surgery settings. The trials showing benefit in this regard have generally been small efficacy trials (i.e., trials that show that pEEG-based protocols can improve postoperative recovery, including time to respond appropriately to commands, time to tracheal extubation, to eye opening, to leaving the operating room and to eligibility for discharge from the postanesthesia care unit).31 Results from larger trials have not reproduced the same impressive successes,23,32,33 and effectiveness data (i.e., trials that demonstrate whether pEEGs such as the BIS actually do improve outcomes such as postoperative recovery outside the context of a protocol-driven trial) are lacking. The B-Aware, B-Unaware and BAG-RECALL trials did not find that BIS monitoring was associated with administration of less anesthesia, decreased hospital stay or decreased mortality.18,21,23,34 In relation to cardiac surgery, there are neither efficacy nor effectiveness data addressing whether pEEG monitoring contributes to the success of fast-tracking. Some studies have shown that using a protocol incorporating BIS monitoring can successfully achieve more rapid tracheal extubation after cardiac surgery.35-38 However, these studies were not randomized clinical trials, so one cannot establish the independent contribution of pEEG monitoring to the success of a fast-track approach. Patients in the B-Aware trial who were randomized to the BIS protocol and went to the intensive care unit (ICU) after their surgery did not have a shorter ICU stay.32 Many of these patients underwent cardiac surgery.
Anesthetic dosing
Patients undergoing cardiac surgery frequently have diminished physiological reserve, and profound hypotension may occur with hypnotic concentrations of general anesthetics. In this setting, whether treating hypotension by reduction in anesthetic administration improves patient outcomes compared with administration of vasoactive medications, such as phenylephrine and norepinephrine, has not been well established. Results from a 60-patient study showed that patients who underwent elective carotid endarterectomy and were randomized to the deep anesthesia with liberal phenylephrine administration (isoflurane, 1.43 minimum alveolar concentration (MAC) or halothane 1.48 MAC, administered respectively) had an almost 3-fold greater incidence of myocardial ischemia at the same blood pressure than did those who were randomized to the light anesthesia groups with no phenylephrine use (isoflurane, 1.04 MAC or halothane 1.01 MAC).39 However, the major limitation of this study is that no patients had a myocardial infarction, so there was no clinically relevant outcome difference between the groups.
In a more recent study, pEEG devices, such as entropy monitoring, have been shown to decrease propofol administration and improve hemodynamic stability during induction of anesthesia in elderly patients. However, these patients were undergoing ophthalmic surgery. Furthermore, the actual decreases in systolic and mean arterial blood pressures after induction were only about 12 and 10 mmHg respectively, and the control patients received a 2 mg/kg bolus dose of propofol, which was a relatively high dose for their age.40
The data supporting the use of pEEG monitoring for total IV anesthesia during cardiac surgery are more compelling than for volatile drug-based anesthesia, because currently there are no clinically approved monitors for tracking propofol concentrations in real time, and estimated concentrations are rendered inaccurate during CPB. Several exploratory studies have shown that the proton transfer reaction mass spectrometer (Ionicon Analytik, GmbH, Innsbruck, Austria)41 or ion-molecule reaction mass spectrometry (Airsense Mass Spectrometry Systems; V&F Medical Development, Absam, Austria)42 may allow reliable, real-time end-tidal propofol concentration monitoring in anesthetized patients. However, issues such as the propofol concentration not returning to the baseline value due to residual propofol in the sampling line41, the inability to account for possible effects of pulmonary and extrahepatic metabolism of propofol, and changes in ventilation/perfusion relations, or diffusion capacity across different disease states or ventilation modes have yet to be resolved.42
Apart from altering pharmacokinetics, CPB is hypothesized to increase the sensitivity of the brain to anesthesia.43 Furthermore, the unbound propofol fraction increases during CPB, which might decrease the blood propofol concentration required to maintain anesthesia during CPB.44 Closed-loop anesthesia using BIS during cardiac surgery has been shown to decrease propofol administration and to increase hemodynamic stability compared to dosing controlled manually by anesthesiologists based upon the BIS reading.45 Three small studies found that BIS monitoring was associated with impressive (>30%) reductions in either propofol46,47,48 or isoflurane administration for patients undergoing both on-pump and off-pump cardiac surgery.48 However, in the much larger B-Aware, B-Unaware and BAG-RECALL trials, this impressive reduction in anesthetic administration with BIS protocols was not demonstrated.18,21,23 (Table 2) Interestingly, decreased sensitivity to anesthesia might persist after CPB because isoflurane requirements assessed by the BIS were found to be less after CPB than before CPB.49 On a cautionary note, changes in the raw EEG (from slow, synchronous, large wave EEG activity to less synchronous EEG activity), which are not reflected in the BIS, have been detected towards the end of CPB.50 This reinforces the concern that current pEEG indices might not always reflect changes in the raw EEG waveform and are not validated metrics of anesthetic depth.
Table 2.
Summary of studies evaluating the effect of a bispectral protocol on anesthetic dosing for patients undergoing cardiac surgery
| Characteristics | Bauer et al.; 2004[46] | Chiu et al.; 2007[47] | Muralidhar et al.; 2008[48] | Myles et al.; 2004[23] | Avidan et al.; 2008[21] | Avidan et al.; 2011[18] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients studied | 40 | 20 | 40 | 2,463 | 1,941 | 5,713 | ||||||
| Study design | RCT | RCT | RCT | RCT | RCT | RCT | ||||||
| Study Aim | Impact of BIS on propofol dose and stress response in cardiac surgery | Impact of BIS on propofol dose during CPB | Impact of BIS on anesthetic dose during OPCAB | Impact of BIS protocol on intraoperative awareness | Impact of BIS protocol on intraoperative awareness | Impact of BIS protocol on intraoperative awareness | ||||||
| Number of cardiac surgery patients (%) | 40 (100%) | 20 (100%) | 40 (100%) | 1,212 (49%) | 525 (27%) | 2,041 (36%) | ||||||
| Proportion of patients receiving TIVA (%) | 100% | 100% | 50% | 43% | 0% | 0% | ||||||
| Interventions | 1.Target controlled infusion protocol 2.BIS guided protocol |
1.Routine practice 2.BIS guided protocol |
1.BIS-guided propofol 2.BIS-guided isoflurane 3.Propofol routine practice 4 Isoflurane routine practice |
1.BIS protocol 2.Routine care |
1.BIS protocol 2.ETAC protocol |
1.BIS protocol 2.ETAC protocol |
||||||
| Result | BIS* | TCI* | BIS* | Routine* | BIS* | Routine* | BIS* | Routine* | BIS* | ETAC* | BIS* | ETAC* |
| BIS value | 45±7 | 43±9 | 43±10 | 41 (38-45) | 41 (38-45) | |||||||
| - Midazolam (mg) | 2 (2-3.5) | 2.5 (2-4.0) | 2 (2-5) | 2 (2-5) | 2 (2-3) | 2 (2-3) | ||||||
| - Propofol infusion (mg/kg/hr) | 4.3 | 6.8 | 2.9 | 6.0 | 5 (4-6) | 5 (3-6) | ||||||
| - Propofol administered (ml; 10 mg/ml)) | 120±6 | 176±9 | 15 (12-20) | 15 (12-20) | 16 (11-20) | 15 (10-20) | ||||||
| - MAC equivalents | 0.57 (0.43-0.72) | 0.61 (0.43-0.78) | 0.81±0.25 | 0.82±0.23 | 0.9 (0.8-1)† | 0.9 (0.8-1)† | ||||||
| - Isoflurane administered (ml) | 24±4 | 37±4 | ||||||||||
| - Fentanyl (mcg) | 500 (100-1000) | 600 (100-1175) | 300 (200-600) | 250 (200-500) | 300 (200-600) | 300 (200-650) | ||||||
| - Morphine (mg) | 10 (7-15) | 10 (7-15) | 10 (6-10) | 10 (6-10) | 10 (5-10) | 10 (5-10) | ||||||
| Summary findings | 37% propofol dose reduction in the BIS group. Higher BIS values in the BIS group. No difference in catecholamines or cytokine | 50% propofol dose reduction in BIS group. Higher BIS values in BIS group | 35% reduction in isoflurane and 32% reduction in propofol administration | No significant difference in median propofol infusion rate, MAC, induction agent dose, or opioid dose between groups. 25% reduction in induction midazolam dose in BIS group. | No significant difference in median MAC, midazolam dose, induction agent dose, opioid dose between groups. | No significant difference in median age-adjusted MAC, midazolam dose, induction agent dose, opioid dose between groups. | ||||||
| Commentary | Comparison between BIS protocol and target controlled infusion. There was minimal correlation between BIS values and propofol concentrations. | Small study. No protocol for control group. Potential for bias (not blinded to group). | Small study. No protocol for control group. Potential for bias (not blinded to group). | Anesthetic dose was a secondary outcome. This might have decreased the potential for bias. Only 49% of patients underwent cardiac surgery. | Anesthetic dose was a secondary outcome. This might have decreased the potential for bias. Only 27% of patients underwent cardiac surgery. | Anesthetic dose was a secondary outcome. This might have decreased the potential for bias. Only 36% of patients underwent cardiac surgery. | ||||||
Abbreviations: BIS, Bispectral Index. CPB, cardiopulmonary bypass. ETAC, end-tidal anesthetic gas concentration. MAC, minimum alveolar concentration. OPCAB, off-pump coronary artery bypass surgery. RCT, randomized controlled trial. TCI, target controlled infusion.
Median (interquartile range) or mean ± standard deviation.
MAC equivalent value is age adjusted.
Weaning patients from CPB is challenging and anesthetic drugs can in a dose-dependent manner decrease myocardial contractility and impair cardiac loading conditions.51 Cardiac anesthesiologists might therefore delay or minimize the administration of both volatile and IV anesthetic drugs until hemodynamic stability has been achieved after separation from CPB. One of the hypothesized benefits of the BIS and other pEEGs is that they provide practitioners with the confidence to decrease anesthesia as long as the index indicates that deep hypnosis is likely. This hypothesis is appealing, but it has not been rigorously tested in clinical trials. Moreover, even with reduction in anesthetic concentration, there is not always a reduction in depth of hypnosis indices; concentration-response relationship curves of pEEG indices versus end-tidal volatile anesthetic concentrations have shown that these indices often plateau over a broad range of clinically relevant anesthetic concentrations.8, 52 (Figure 3) Even when a pEEG index suggests that a patient is sufficiently anesthetized for stimulating surgery, rapid arousal can occur. Furthermore, current pEEG indices have not been shown to have 100% sensitivity in excluding that patients are aware below established threshold values of the index (e.g., BIS <60).53 For example, in the B-Unaware trial, 5 of the 9 patients who experienced definite or possible awareness did not appear to have BIS values higher than 60 during their awareness experiences.21
Figure 3.
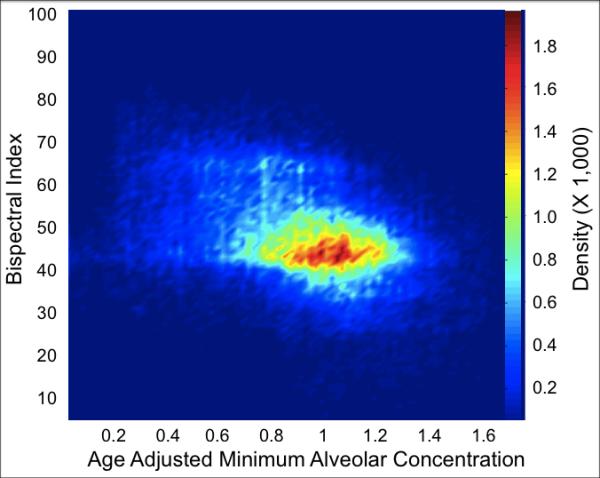
A density contour plot from 700 sequential patients who underwent cardiac surgery and were enrolled in the BAG-RECALL clinical trial.18 Data points are from the maintenance period of anesthesia. This representation, similar to a plot depicted by Whitlock et al.,52 demonstrates that over a clinically relevant range of age-adjusted minimum alveolar concentrations (i.e., 0.5 to 1.5 MAC), the Bispectral Index (the dependent variable) displays its highest densities (mode) in the low forties.
Cerebral ischemia
The EEG, and by extension the BIS, may be a useful tool in detecting cerebral ischemia or injury. In the absence of a recent change in anesthetic concentration, a sudden change in the EEG including increased delta activity, periods of burst suppression, or persistent suppression,54-56 and/or a decrease in the BIS value, may suggest ischemia. It has long been recognized that sudden EEG changes during CPB could be attributable to such problems as superior vena cava obstruction, generalized decrease in cardiac output, and other malfunctions of the CPB apparatus.56 However, the EEG and derived indices are nonspecific in detecting cerebral ischemia. In an editorial Billard indicated that unprocessed EEG monitoring is not absolutely reliable at detecting brain injury.54 It appears reasonably specific when anesthesia is stable but it is not very sensitive, meaning that a stable, unchanged EEG is of limited value in excluding ischemia. Similarly, BIS and raw EEG may be useful for detecting brain ischemia, especially when anesthesia is stable, if the insult is sudden, extended or located in the frontal area, and if the preoperative EEG was normal.57 It is possible that more targeted monitors, such as cerebral oximeters,58,59 transcranial Doppler60 and jugular bulb venous saturation61 might have more utility in this regard.
Whether BIS tracks cerebral perfusion is unknown. Initial studies in patients undergoing awake carotid endarterectomy suggest that BIS decreases may correlate with neurological deficits and/or clinical cerebral ischemia,62,63 although another study showed no relationship.64 Similarly, a study in patients undergoing routine carotid endarterectomy with total IV anesthesia showed that during carotid cross-clamping and under a constant level of IV anesthesia, BIS may increase, decrease or remain unchanged.65 A paradoxical BIS increase was more frequently observed in patients with moderate or poor internal carotid backflow than in those with good internal carotid backflow. However, it was not clear whether the BIS increase was related to ischemia, a change in brain anesthetic drug concentration, or a change in the nociceptive-antinociceptive balance associated with carotid cross-clamping.
The latest iteration of the BIS® monitor incorporates bilateral frontal EEG channels, which might increase the ability of the BIS to detect unilateral frontal ischemia (Figure 4). Whether bilateral BIS will prove to be a valid monitoring technique for regional cerebral hypoperfusion has yet to be determined.66 Neidhart et al. evaluated bilateral BIS monitoring by using two separate BIS monitors simultaneously. They found that for 6% of the time, there were sustained periods of 30 seconds or more where the concurrent readings differed by 10 or more between the two BIS monitors.67 The findings of this 12-patient observational study indicated that, absent cerebral ischemia or pathology, bilateral BIS monitoring might not always provide a similar value. This occasional lack of intrapatient BIS reproducibility could curtail the specificity of bilateral BIS monitoring for the detection of cerebral ischemia.
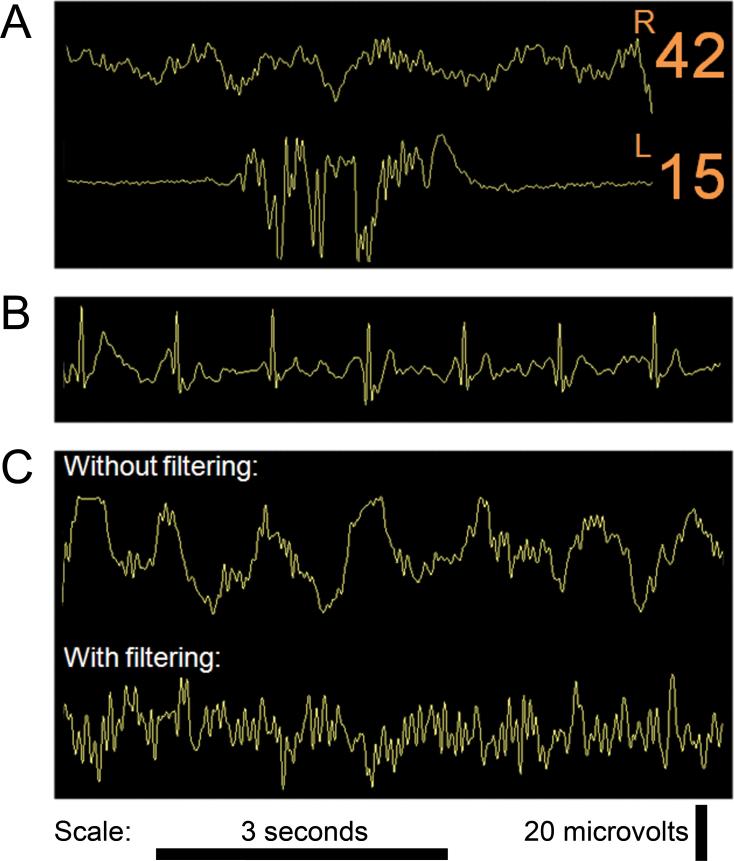
Figure 4.
Notable features on intraoperative electroencephalogram (EEG) or Bispectral Index (BIS) monitoring. A, this shows two EEG traces and corresponding BIS values. These traces reflect a hypothetical situation where bilateral frontal EEG recordings are taken. The right channel shows an underlying slow delta wave pattern with higher frequency waves (e.g., faster delta, theta and alpha waves) also apparent. The left channel shows a burst suppression pattern. Such asymmetry could occur with unilateral (left) frontal hypoperfusion. B, This is an EEG trace obtained from a BIS monitor®. Cardiopulmonary bypass had been initiated and the patient's EEG was persistently suppressed. The raw trace shows marked electrocardiographic (ECG) artifact in the persistently suppressed raw EEG trace. Automated analysis may not recognize the ECG waveform as readily as a trained anesthesiologist. C, the effect of filtering on the EEG. The first trace shows a typical EEG epoch during general anesthesia, with an underlying slow delta pattern. There are also higher frequency waves (e.g., faster delta, theta and alpha waves) apparent. In the second trace, the higher frequency waves are still apparent, but the underlying slow delta pattern has been attenuated by the BIS monitor's® filters. The BIS automatically outputs a filtered EEG trace; filters may obscure useful information. In this case, large delta waves consistent with deep anesthesia were filtered out, potentially preventing the anesthesiologist from appreciating the true anesthetic depth. EEG tracings were obtained using a BIS Quatro Sensor (version XP; Covidien, Boulder, CO).
Prognostic significance: BIS and mortality
Associations between anesthesia-related factors and short- and long-term survival after surgery remain controversial.34,68-71 Monk et al. first showed that, in noncardiac surgical patients, cumulative duration of BIS <45 was associated with increased risk of death within the first postoperative year (relative risk for mortality: 1.24 per hour spent with BIS <45).68 The investigators suggested there may be a link between cumulative anesthesia dose and 1-year mortality. Biological mechanisms advanced for such a relationship include the notion that exposure to increased concentrations of potent inhaled anesthetics may lead to immunosuppression or cerebral hypoxia, or that some individuals may have increased cerebral susceptibility to the effects of anesthetics.68
Only two studies have examined associations between low BIS, BIS monitoring, and intermediate-term mortality in patients who have undergone cardiac surgery. (Table 3)34,68-71 Leslie et al. found that the risk of death in BIS-monitored patients was not significantly different from unmonitored patients, but when a propensity score analysis was performed, patients with BIS values <40 for > 5 minutes had a hazard ratio of 1.41 for mortality compared with those who did not.70 In this study, the dosages of different anesthetic drugs were not recorded or used in the analyses; thus the association between the cumulative dose of anesthetic drugs and BIS in relation to mortality could not be studied.
Table 3.
Summary of studies on BIS and mortality
| Characteristics | Monk et al.; 2005[68] | Lindholm et al.; 2009 [69] | Leslie et al.; 2010 [70] | Kertai et al., 2010 [34] | Kertai et al., 2011[71] |
|---|---|---|---|---|---|
| Number of patients studied | 1,064 | 4,087 | 2,463 | 460 | 1,473 |
| Cardiac surgery patients (%) | 0 | 0 | 49 | 100 | 0 |
| Age (yr) | 51 (37-65) | 50 (36-65) | 61 (46-71) | 64 (55-73) | 59 (48-69) |
| Male sex (%) | 37 | 38 | 62 | 62 | 51 |
| ASA physical status ≥3 (%) | 35 | 6 | 74 | 98 | 63 |
| Duration of anesthesia (h) | 3.1 (2.3-4.3) | 1.8 (1.2-2.5) | 3.1 (1.4-4.4) | 6.1 (5.2-7.1) | 3.4 (2.1-4.9) |
| Volatile maintenance (%) | 91 | 95 | 57 | 100 | 100 |
| BIS monitoring (%) | 100 | 100 | 50 | 100 | 100 |
| Anesthesiologists blind to BIS | Yes | No | No | 50% | 50% |
| Average BIS | 49±9 | 37±7 | 45±7 | 40±7 | 45±8 |
| Follow-up (yr) | 1 | 2 | 4 | 3 | 3 |
| 30-day mortality (%) | 0.7 | 0.7 | 4.3 | 3.5 | 1.5 |
| 1-year mortality (%) | 5.5 | 4.3 | 10.8 | 10.7 | 9.8 |
| 2-year mortality (%) | - | 6.5 | 14.6 | 14.3 | 16.4 |
| Mortality at the end of the follow-up (%) | - | - | 22.2 | 17.8 | 22.8 |
| Relative risk for mortality per hour BIS <45 (95%CI)* | 1.24 (1.06 to 1.44) | 1.08 (0.99 to 1.18) | 1.41 (1.02 to 1.95) | 1.29 (1.12 to 1.49) | 1.03 (0.93 to 1.14) |
Table was modified from Leslie et al.,70; Data are reported as mean±SD, median (interquartile range), count, or percent; ASA, American Society of Anesthesiologists, BIS, Bispectral Index; CI, confidence interval
corrected for clinical risk factors and intraoperative variables
In a predetermined substudy of the B-Unaware trial,21 Kertai et al. attempted to clarify the association between clinical variables, anesthetic drugs, cumulative duration of low BIS and intermediate-term mortality in 460 patients who underwent cardiac surgery.34 The cumulative duration of BIS <45 was independently associated with intermediate-term mortality, with a hazard ratio of 1.29 per hour. Several perioperative risk factors, such as low ejection fraction, were also associated with cumulative duration of BIS <45. However, there was no association between the cumulative duration of BIS <45 and cumulative volatile anesthetic concentration or with the average total dose of IV anesthetic drugs. It therefore seems likely that increased cumulative duration of BIS <45 is a marker of factors such as systemic illness, poor cardiac function, and a complicated intraoperative course.
In summary, there is currently insufficient evidence that limiting depth of anesthesia, either by titration to a specific BIS threshold or by limiting volatile drug concentrations, would decrease intermediate-term mortality after cardiac surgery.
Special Considerations with both BIS and unprocessed EEG
Different clinical conditions have been described that may lead to altered and inaccurate BIS readings unrelated to changes in depth of anesthesia or cerebral perfusion, sometimes through an effect on the raw EEG. BIS values that do not accurately reflect hypnotic depth may arise from underlying brain pathology or may reflect limitations of pEEG algorithms.72,73 (Figures 5 A-D)
Figure 5.
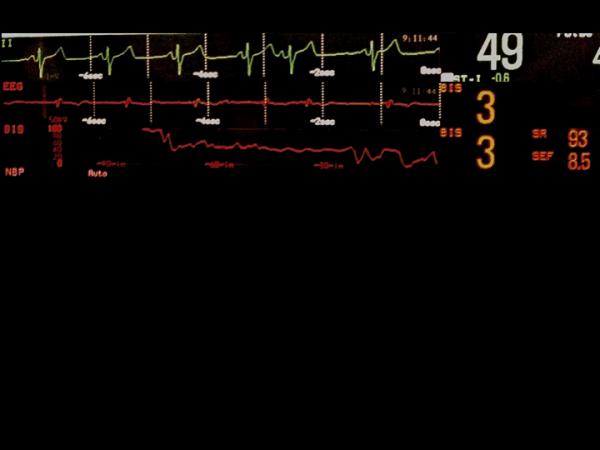
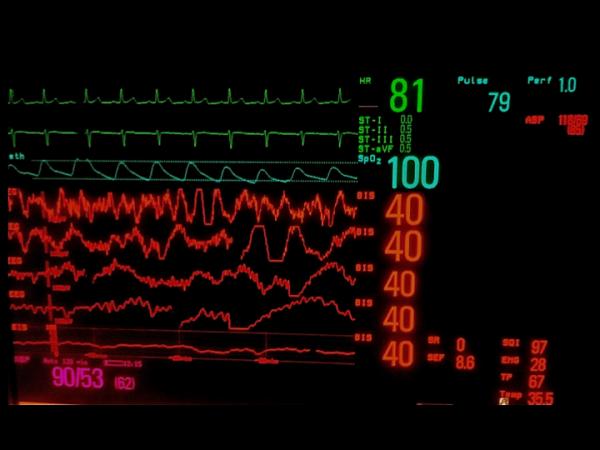
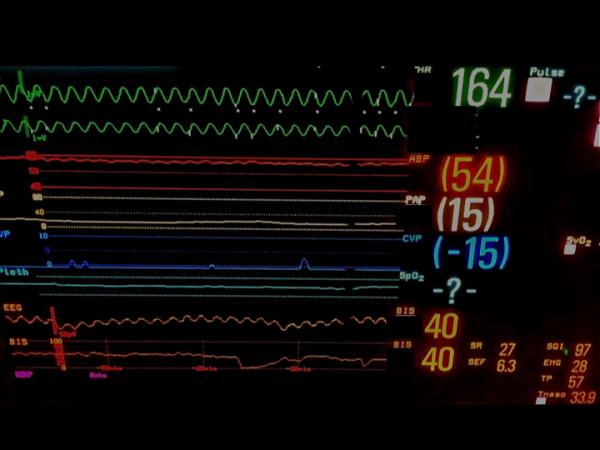
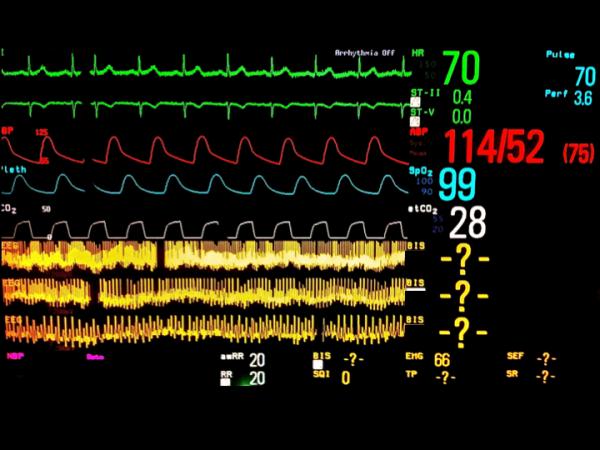
Examples of raw frontal electroencephalographic (EEG) traces with concurrent Bispectral Index (BIS) values. A, electrocardiographic (ECG) artifact is evident with persistently suppressed EEG trace. The BIS value is appropriately very low despite the ECG artifact. B, Slow delta waves and spindles are seen at four different EEG sweep speeds. The BIS value is 40, which is congruent with the raw EEG trace. C, Ventricular fibrillation artifact is present on the EEG trace. The underlying EEG rhythm is persistent suppression. The BIS value should be very low, but instead is showing 40. D, This patient has a deep brain stimulator. EEG traces are shown at three sweep speeds. Spikes from the brain stimulator are evident. No BIS reading is displayed. EEG tracings were obtained using a BIS Quatro Sensor (version XP; Covidien, Boulder, CO).
Neurological disease
Because the BIS algorithm was developed from healthy volunteers with normal EEG, neurological disorders that manifest in abnormal EEG waveforms may significantly affect the behavior of the BIS.5 Neurological disorders such as Alzheimer-type dementia has been shown to be associated with a marked loss of β power across the cortex with largest differences noted in the frontal region.74 Patients with Alzheimer or vascular dementia, both of which may be present and undiagnosed in patients undergoing cardiac surgery, show an increase in slow wave activity of the EEG, associated with a lower mean awake BIS.75 Similarly, caution should be exercised in interpreting the BIS or the EEG in patients undergoing cardiac surgery who have a history of cerebrovascular disease; cerebral ischemia leads to (regional) cortical inactivation, which may be reflected by EEG slowing or a decrease in the BIS.5
Muscle activity
The effects of muscle activity and muscle relaxants on the EEG are controversial. Experimental work has shown that in awake, nonparalyzed volunteers, much of the scalp electrical activity above 20 Hz (high beta and gamma bands) originates from the electromyographic signal (EMG).76 In view of EMG contamination, the EEG in nonparalyzed patients might be inherently unreliable in these high frequency ranges.77 Not surprisingly, EMG activity and the use of neuromuscular blocking drugs reportedly do influence pEEG monitoring. In some instances, the BIS algorithm might not distinguish between EMG signals and higher frequency EEG activity.78 This could be problematic if the BIS algorithm interpreted the abolition of EMG activity after the administration of a neuromuscular blocking drug as deepening of anesthesia. This occurred in an experiment where healthy volunteers received a muscle relaxant without any hypnotic drug, and BIS values decreased to nadirs of 9 to 33.79 It is possible that through advanced statistical techniques such as principal component analysis, high frequency EEG activity can be distinguished from EMG activity.76 Software revisions of the BIS algorithm reportedly now filter out EMG activity.80 However, such filtering might be incomplete;5 a processed EEG monitor could incorrectly indicate increased hypnotic depth based on the loss of high frequency EMG activity.80 This problem reinforces the perspective that the use of pharmacological paralysis should be minimized in cardiac surgery.81 Although it is not the scope of this review, there are different perspectives on the need for muscle relaxants during cardiac surgery. It is becoming common at some centers to administer only a single dose of intermediate-acting muscle relaxant at the start of surgery; patients are sometimes not pharmacologically paralyzed during CPB. Some practitioners might not even administer muscle relaxants at induction for cardiac surgery. A line of reasoning behind such an approach is as follows: purposeful patient movement is currently the only reliable surrogate for intraoperative awareness; muscle relaxants can prevent purposeful patient movement and therefore mask the detection of awareness; there is evidence that muscle relaxants are associated with both an increased incidence and more distressing experience of awareness;25,82 the incidence of awareness is likely to be higher with cardiac surgery than with other kinds of surgery;82 muscle relaxants are typically administered to assist surgical exposure, for example, to relax abdominal skeletal muscles; potassium, not nondepolarizing muscle relaxation, facilitates surgical exposure during cardiac surgery; there is currently no evidence that muscle relaxants improve outcomes for patients undergoing cardiac surgery. Therefore, considering that muscle relaxants increase the risk of traumatic intraoperative awareness without conferring any proven benefits, the almost universal practice of pharmacologically paralyzing cardiac surgery patients should be critically re-appraised.81
Polypharmacy
While a general pattern can be described for the effects of anesthetic drugs on the EEG, each class of anesthetic drugs has a different EEG response.1,4 Some differences are subtle, whereas others are marked. This is important for cardiac surgical patients, who commonly receive multiple drugs, and who historically have received limited doses of GABAergic drugs (e.g., thiopental, propofol, volatile anesthetics), because these are frequently myocardial depressants. Drugs such as benzodiazepines and opioids are regularly administered to cardiac surgery patients. Even when anesthesia is clinically adequate and patients appear to be unconscious in response to these drugs, BIS generally underestimates depth of anesthesia, probably because of the variable effect of benzodiazepines and opioids on the EEG and the pEEG.83 It has been shown that high doses of opioids can cause a paradoxical increase in pEEG.5 Other drugs such as ketamine in low doses can increase high frequency EEG activity and cause misleading increases of pEEG indices, such as the BIS.5
Brain region monitored
Most raw and processed EEG monitoring techniques use a single or paired frontal EEG montage. This might be limited for various reasons. As early as 1953, it was known that anesthetic drugs may not suppress all brain regions equally.84 Areas like the hippocampus and amygdala are directly involved in traumatic memory formation, and monitoring those regions might be higher yield than frontal regions if the purpose of brain monitoring is to detect possible intraoperative awareness. However, these deep brain areas are not easily monitored with surface EEG, and (frontal) cortical changes might provide good surrogacy for unconsciousness with GABAergic anesthesia.85 Recent research suggests that anesthetic-induced unconsciousness might lead to failure of information synthesis in the posterior parietal cortex and in parietal networks. This highlights the potential limitations of an exclusive emphasis on frontal cortical EEG monitoring.86 Another major limitation to single frontal electrode montage is that network relationships between brain regions, which have been shown in recent studies to provide valuable information about anesthesia, cannot be assessed.86,87
Electrical interference
Electric device interference during cardiac surgery can also affect BIS values. Electrocautery produces an impressive artifact on raw EEG, and it is unclear how the BIS software addresses the high-amplitude high-frequency signal artifact. Electrocautery, cardiac pacing and vibrations from a forced air warming blanket during surgery have all been shown to have the potential to disturb the BIS signal.88 The resulting frequencies can interfere with the BIS electrodes simulating the EEG waves of light anesthesia or an awake state.88 Falsely increased BIS values, which were thought to be attributable to forced air flow, were reported in a case series of patients who underwent cardiac surgery.89 When forced air flow devices are used, it might therefore be necessary to pause the warming device in order to distinguish falsely increased BIS values from BIS values indicating insufficient depth of anesthesia.89 Cardiac electrical activity also frequently contaminates EEG recordings. This is often apparent when there is a persistently suppressed EEG with spike deflections concurrent with electrocardiographic QRS complexes. (Figure 4B)
Hypothermia
Hypothermia causes suppression of the EEG.90,91 Several studies have reported the effects of mild to moderate hypothermia on the BIS during cardiac surgery with CPB;92-94 based on these studies it seems that hypothermia reduces the BIS by approximately one BIS unit per degree Celsius. The effect of hypothermia during CPB on the correlation and agreement between the BIS and entropy has also been studied.95 Both BIS and entropy values declined after induction of general anesthesia, and cooling during CPB resulted in an additional decline in BIS, but the decline of state and response entropies was more significant. There were also good correlations and acceptable agreements between BIS and entropy variables under normothermic conditions but during hypothermic conditions both correlations and agreements between BIS and entropy variables were weaker questioning the interchangeability between these two candidate depth of anesthesia indices with hypothermia.
Several studies in patients who underwent cardiac surgery with CPB and deep hypothermic circulatory arrest showed that there was a strong association between BIS values and temperature during deep hypothermia.96,97 The association may be linear,97 or may be biphasic, with faster BIS decline at more extreme degrees of hypothermia.96 It is important to mention that drug regimens in these studies were highly variable and might have influenced BIS changes during hypothermic conditions; the pharmacokinetics of volatile anesthetics98 and propofol99 are also affected by hypothermia. Typically, a persistently suppressed EEG (Figure 1) and a BIS number approaching 0 occur during periods of deep hypothermic arrest.100 Persistent EEG suppression might be desirable during periods of brain ischemia as it might reflect decreased brain metabolic activity. If there is brain activity, such as burst suppression (Figure 1), this could signify that further cooling or anesthetic administration might be beneficial. Seizure activity on the EEG could signify potential for brain injury and could potentially inform early treatment.
Delay in response
Studies have shown that various pEEGs, including the BIS, are slow to respond to sudden state changes, such as arousal or loss of responsiveness.101,102 The time taken for the index to reflect the state change varies from 30 seconds to two minutes. This means that the value displayed by the BIS might reflect the patient's state of arousal a minute in the past, rather than their current state. Given that episodic memory consolidation can occur rapidly, and might be enhanced by aversive or emotionally arousing experiences through amygdala modulation of hippocampus-dependent memory processes,103 such a delay in a depth of anesthesia monitor's responsiveness has important potential clinical implications. There are candidate nonproprietary pEEG indices, such as permutation entropy,7,104 which have much more rapid response times (i.e., <10 seconds). Time delay is an important limitation of currently available pEEGs and points to an area where future commercial pEEG indices should urgently be improved.
Conclusion
The BIS is only one of several candidate depth of anesthesia monitors; the jury is out as to which of the currently available proprietary and nonproprietary monitors is most useful for patients undergoing cardiac surgery. If practitioners do use the BIS for cardiac surgery, there is significant added value to the routine display of the raw EEG trace if the anesthesiologist knows how to interpret the raw EEG.12,15 Specifically, when there are changes in the BIS, the discerning practitioner might be able to determine from the raw trace whether the change is attributable to artifact, such as electrocautery, or to true changes in hypnotic state. One of the important EEG features of surgical anesthesia is a slow delta waveform. Inactivating the filters on the BIS monitor® allows the practitioner to appreciate better the slow delta waves in the BIS monitor's® EEG display during general anesthesia (Figure 4 and Figure 5B). When there is a change in a patient's state (e.g., from unresponsive to awake), changes are apparent in the raw EEG trace about a minute before they are reflected in processed EEG indices like the BIS.102
As with all monitoring techniques, the use of processed or raw EEG during cardiac surgery presents both advantages and disadvantages (Table 4). The effectiveness of BIS monitoring in preventing intraoperative awareness with postoperative explicit recall remains controversial. Similarly, the effectiveness of BIS monitoring in guiding the safe reduction in anesthetic dosing has not been consistently demonstrated. Indeed, minimizing anesthetic dose might have no benefit and may lead to resurgence in the incidence of unintended intraoperative awareness. Despite many years of research and compelling reasons for monitoring the brain during heart surgery, we are not yet at a point when any specific brain monitoring modality can reasonably be mandated for use in patients undergoing cardiac surgery.
Table 4.
Utility and drawbacks of raw and processed electroencephalogram (EEG) in cardiac surgery
| Strengths | Weaknesses | |
|---|---|---|
| Raw EEG | •Relatively available and inexpensive •Should not be affected by muscle relaxant •Artifact recognition •Specific patterns (e.g. isoelectricity) have clinical utility and can be appreciated |
•Requires training to interpret •No well-designed studies evaluating its efficacy at reducing awareness |
| Common to both raw and processed EEG | •Might provide evidence with EEG features or with a numerical index that awareness is unlikely •Continues to provide useful information about the brain during CPB |
•Does not reflect NMDA antagonism (e.g. ketamine, N2O) •Affected by underlying brain pathology |
| Processed EEG | •Reduces risk of awareness compared with standard care •Allows threshold-based alarms to be set •Interpretation under most clinical situations is simple |
•Lag time between current state of EEG and pEEG index •Underlying algorithms may be poorly understood by those using it, hindering interpretation of artifactual readings •Cost |
CPB, cardiopulmonary bypass; NMDA, N-methyl D-aspratate; pEEG, processed EEG
Supplementary Material
Acknowledgments
Funding: The study itself was not funded. Elizabeth Whitlock was supported by grant number UL1 RR024992 and sub-award TL1 RR024995 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
The authors declare no conflicts of interest.
DISCLOSURES:
Name: Miklos D. Kertai, MD, PhD
Contributions: This author helped design the study and write the manuscript.
Attestation: Mikos D. Kertai approved the final manuscript.
Name: Elizabeth L. Whitlock, MD, MSc
Contributions: This author helped design the study and write the manuscript.
Attestation: Elizabeth L. Whitlock approved the final manuscript.
Name: Michael S. Avidan, MBBCh
Contributions: This author helped design the study and write the manuscript.
Attestation: Michael S. Avidan approved the final manuscript.
This manuscript was handled by: Martin J. London, MD
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Miklos D. Kertai, Duke University Medical Center, Durham, NC.
Elizabeth L. Whitlock, Washington University School of Medicine, St. Louis, MO.
Michael S. Avidan, Washington University School of Medicine, St. Louis, MO.
References
- 1.Clark DL, Rosner BS. Neurophysiologic effects of general anesthetics. I. The electroencephalogram and sensory evoked responses in man. Anesthesiology. 1973;38:564–82. [PubMed] [Google Scholar]
- 2.Gibbs FA, Gibbs EL, Lennox WG. Effect on the electro-encephalogram of certain drugs which influence nervous activity. Arch Intern Med. 1937;60:154–66. [Google Scholar]
- 3.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):i3–13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 4.Martin JT, Faulconer A, Jr., Bickford RG. Electroencephalography in anesthesiology. Anesthesiology. 1959;20:359–76. doi: 10.1097/00000542-195905000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Bennett C, Voss LJ, Barnard JP, Sleigh JW. Practical use of the raw electroencephalogram waveform during general anesthesia: the art and science. Anesth Analg. 2009;109:539–50. doi: 10.1213/ane.0b013e3181a9fc38. [DOI] [PubMed] [Google Scholar]
- 6.Rampil IJ, Matteo RS. Changes in EEG spectral edge frequency correlate with the hemodynamic response to laryngoscopy and intubation. Anesthesiology. 1987;67:139–42. doi: 10.1097/00000542-198707000-00033. [DOI] [PubMed] [Google Scholar]
- 7.Olofsen E, Sleigh JW, Dahan A. Permutation entropy of the electroencephalogram: a measure of anaesthetic drug effect. Br J Anaesth. 2008;101:810–21. doi: 10.1093/bja/aen290. [DOI] [PubMed] [Google Scholar]
- 8.Kreuer S, Bruhn J, Ellerkmann R, Ziegeler S, Kubulus D, Wilhelm W. Failure of two commercial indexes and spectral parameters to reflect the pharmacodynamic effect of desflurane on EEG. J Clin Monit Comput. 2008;22:149–58. doi: 10.1007/s10877-008-9116-1. [DOI] [PubMed] [Google Scholar]
- 9.Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002. doi: 10.1097/00000542-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Johansen JW, Sebel PS. Development and clinical application of electroencephalographic bispectrum monitoring. Anesthesiology. 2000;93:1336–44. doi: 10.1097/00000542-200011000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto Y, Hagihira S, Koizumi Y, Ishida K, Matsumoto M, Sakabe T. The relationship between bispectral index and electroencephalographic parameters during isoflurane anesthesia. Anesth Analg. 2004;98:1336–40. doi: 10.1213/01.ane.0000105867.17108.b6. [DOI] [PubMed] [Google Scholar]
- 12.Bottros MM, Palanca BJ, Mashour GA, Patel A, Butler C, Taylor A, Lin N, Avidan MS. Estimation of the bispectral index by anesthesiologists: an inverse turing test. Anesthesiology. 2011;114:1093–101. doi: 10.1097/ALN.0b013e31820e7c5c. [DOI] [PubMed] [Google Scholar]
- 13.Schneider G, Schoniger S, Kochs E. Does bispectral analysis add anything but complexity? BIS sub-components may be superior to BIS for detection of awareness. Br J Anaesth. 2004;93:596–7. doi: 10.1093/bja/aeh612. [DOI] [PubMed] [Google Scholar]
- 14.Miller A, Sleigh JW, Barnard J, Steyn-Ross DA. Does bispectral analysis of the electroencephalogram add anything but complexity? Br J Anaesth. 2004;92:8–13. doi: 10.1093/bja/aeh003. [DOI] [PubMed] [Google Scholar]
- 15.Barnard JP, Bennett C, Voss LJ, Sleigh JW. Can anaesthetists be taught to interpret the effects of general anaesthesia on the electroencephalogram? Comparison of performance with the BIS and spectral entropy. Br J Anaesth. 2007;99:532–7. doi: 10.1093/bja/aem198. [DOI] [PubMed] [Google Scholar]
- 16.Mashour GA, Orser BA, Avidan MS. Intraoperative awareness: from neurobiology to clinical practice. Anesthesiology. 2011;114:1218–33. doi: 10.1097/ALN.0b013e31820fc9b6. [DOI] [PubMed] [Google Scholar]
- 17.Bailey JM, Mora CT, Shafer SL. Pharmacokinetics of propofol in adult patients undergoing coronary revascularization. The Multicenter Study of Perioperative Ischemia Research Group. Anesthesiology. 1996;84:1288–97. doi: 10.1097/00000542-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Avidan MS, Jacobsohn E, Glick D, Burnside BA, Zhang L, Villafranca A, Karl L, Kamal S, Torres B, O'Connor M, Evers AS, Gradwohl S, Lin N, Palanca BJ, Mashour GA. Prevention of intraoperative awareness in a high-risk surgical population. N Engl J Med. 2011;365:591–600. doi: 10.1056/NEJMoa1100403. [DOI] [PubMed] [Google Scholar]
- 19.Serfontein L. Awareness in cardiac anesthesia. Curr Opin Anaesthesiol. 2010;23:103–8. doi: 10.1097/ACO.0b013e328334cb75. [DOI] [PubMed] [Google Scholar]
- 20.Dowd NP, Cheng DC, Karski JM, Wong DT, Munro JA, Sandler AN. Intraoperative awareness in fast-track cardiac anesthesia. Anesthesiology. 1998;89:1068–73. doi: 10.1097/00000542-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Avidan MS, Zhang L, Burnside BA, Finkel KJ, Searleman AC, Selvidge JA, Saager L, Turner MS, Rao S, Bottros M, Hantler C, Jacobsohn E, Evers AS. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097–108. doi: 10.1056/NEJMoa0707361. [DOI] [PubMed] [Google Scholar]
- 22.Goldmann L, Shah MV, Hebden MW. Memory of cardiac anaesthesia. Psychological sequelae in cardiac patients of intra-operative suggestion and operating room conversation. Anaesthesia. 1987;42:596–603. doi: 10.1111/j.1365-2044.1987.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 23.Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B-Aware randomised controlled trial. Lancet. 2004;363:1757–63. doi: 10.1016/S0140-6736(04)16300-9. [DOI] [PubMed] [Google Scholar]
- 24.Nordstrom O, Engstrom AM, Persson S, Sandin R. Incidence of awareness in total i.v. anaesthesia based on propofol, alfentanil and neuromuscular blockade. Acta Anaesthesiol Scand. 1997;41:978–84. doi: 10.1111/j.1399-6576.1997.tb04823.x. [DOI] [PubMed] [Google Scholar]
- 25.Sandin RH, Enlund G, Samuelsson P, Lennmarken C. Awareness during anaesthesia: a prospective case study. Lancet. 2000;355:707–11. doi: 10.1016/S0140-6736(99)11010-9. [DOI] [PubMed] [Google Scholar]
- 26.Ekman A, Lindholm ML, Lennmarken C, Sandin R. Reduction in the incidence of awareness using BIS monitoring. Acta Anaesthesiol Scand. 2004;48:20–6. doi: 10.1111/j.1399-6576.2004.00260.x. [DOI] [PubMed] [Google Scholar]
- 27.Hug CC., Jr. Does opioid “anesthesia” exist? Anesthesiology. 1990;73:1–4. doi: 10.1097/00000542-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Schwender D, Weninger E, Daunderer M, Klasing S, Poppel E, Peter K. Anesthesia with increasing doses of sufentanil and midlatency auditory evoked potentials in humans. Anesth Analg. 1995;80:499–505. doi: 10.1097/00000539-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Mashour GA, Tremper KK, Avidan MS. Protocol for the “Michigan Awareness Control Study”: A prospective, randomized, controlled trial comparing electronic alerts based on bispectral index monitoring or minimum alveolar concentration for the prevention of intraoperative awareness. BMC Anesthesiol. 2009;9:7. doi: 10.1186/1471-2253-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Practice advisory for intraoperative awareness and brain function monitoring: a report by the american society of anesthesiologists task force on intraoperative awareness. Anesthesiology. 2006;104:847–64. doi: 10.1097/00000542-200604000-00031. [DOI] [PubMed] [Google Scholar]
- 31.Punjasawadwong Y, Boonjeungmonkol N, Phongchiewboon A. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2007:CD003843. doi: 10.1002/14651858.CD003843.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Leslie K, Myles PS, Forbes A, Chan MT, Short TG, Swallow SK. Recovery from bispectral index-guided anaesthesia in a large randomized controlled trial of patients at high risk of awareness. Anaesth Intensive Care. 2005;33:443–51. doi: 10.1177/0310057X0503300404. [DOI] [PubMed] [Google Scholar]
- 33.Pavlin JD, Souter KJ, Hong JY, Freund PR, Bowdle TA, Bower JO. Effects of bispectral index monitoring on recovery from surgical anesthesia in 1,580 inpatients from an academic medical center. Anesthesiology. 2005;102:566–73. doi: 10.1097/00000542-200503000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Kertai MD, Pal N, Palanca BJ, Lin N, Searleman SA, Zhang L, Burnside BA, Finkel KJ, Avidan MS. Association of perioperative risk factors and cumulative duration of low bispectral index with intermediate-term mortality after cardiac surgery in the B-Unaware Trial. Anesthesiology. 2010;112:1116–27. doi: 10.1097/ALN.0b013e3181d5e0a3. [DOI] [PubMed] [Google Scholar]
- 35.Gangopadhyay S, Acharjee A, Nayak SK, Dawn S, Piplai G, Gupta K. Immediate extubation versus standard postoperative ventilation: Our experience in on pump open heart surgery. Indian J Anaesth. 2010;54:525–30. doi: 10.4103/0019-5049.72641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemmerling TM, Choiniere JL, Basile F, Le N, Olivier JF, Prieto I. Immediate Extubation after Aortic Valve Surgery Using High Thoracic Epidural Anesthesia. Heart Surg Forum. 2004;7:16–20. [PubMed] [Google Scholar]
- 37.Hemmerling TM, Le N, Olivier JF, Choiniere JL, Basile F, Prieto I. Immediate extubation after aortic valve surgery using high thoracic epidural analgesia or opioid-based analgesia. J Cardiothorac Vasc Anesth. 2005;19:176–81. doi: 10.1053/j.jvca.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Hemmerling TM, Prieto I, Choiniere JL, Basile F, Fortier JD. Ultra-fast-track anesthesia in off-pump coronary artery bypass grafting: a prospective audit comparing opioid-based anesthesia vs thoracic epidural-based anesthesia. Can J Anaesth. 2004;51:163–8. doi: 10.1007/BF03018777. [DOI] [PubMed] [Google Scholar]
- 39.Smith JS, Roizen MF, Cahalan MK, Benefiel DJ, Beaupre PN, Sohn YJ, Byrd BF, Schiller NB, Stoney RJ, Ehrenfeld WK. Does anesthetic technique make a difference? Augmentation of systolic blood pressure during carotid endarterectomy: effects of phenylephrine versus light anesthesia and of isoflurane versus halothane on the incidence of myocardial ischemia. Anesthesiology. 1988;69:846–53. doi: 10.1097/00000542-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Riad W, Schreiber M, Saeed AB. Monitoring with EEG entropy decreases propofol requirement and maintains cardiovascular stability during induction of anaesthesia in elderly patients. Eur J Anaesthesiol. 2007;24:684–8. doi: 10.1017/S026502150700018X. [DOI] [PubMed] [Google Scholar]
- 41.Takita A, Masui K, Kazama T. On-line monitoring of end-tidal propofol concentration in anesthetized patients. Anesthesiology. 2007;106:659–64. doi: 10.1097/01.anes.0000264745.63275.59. [DOI] [PubMed] [Google Scholar]
- 42.Hornuss C, Praun S, Villinger J, Dornauer A, Moehnle P, Dolch M, Weninger E, Chouker A, Feil C, Briegel J, Thiel M, Schelling G. Real-time monitoring of propofol in expired air in humans undergoing total intravenous anesthesia. Anesthesiology. 2007;106:665–74. doi: 10.1097/01.anes.0000264746.01393.e0. [DOI] [PubMed] [Google Scholar]
- 43.Barbosa RA, Santos SR, White PF, Pereira VA, Silva Filho CR, Malbouissson LM, Carmona MJ. Effects of cardiopulmonary bypass on propofol pharmacokinetics and bispectral index during coronary surgery. Clinics (Sao Paulo) 2009;64:215–21. doi: 10.1590/S1807-59322009000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takizawa E, Hiraoka H, Takizawa D, Goto F. Changes in the effect of propofol in response to altered plasma protein binding during normothermic cardiopulmonary bypass. Br J Anaesth. 2006;96:179–85. doi: 10.1093/bja/aei293. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal J, Puri GD, Mathew PJ. Comparison of closed loop vs. manual administration of propofol using the Bispectral index in cardiac surgery. Acta Anaesthesiol Scand. 2009;53:390–7. doi: 10.1111/j.1399-6576.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- 46.Bauer M, Wilhelm W, Kraemer T, Kraemer T, Kreuer S, Brandt A, Adams HA, Hoff G, Larsen R. Impact of bispectral index monitoring on stress response and propofol consumption in patients undergoing coronary artery bypass surgery. Anesthesiology. 2004;101:1096–104. doi: 10.1097/00000542-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Chiu CL, Ong G, Majid AA. Impact of bispectral index monitoring on propofol administration in patients undergoing cardiopulmonary bypass. Anaesth Intensive Care. 2007;35:342–7. doi: 10.1177/0310057X0703500304. [DOI] [PubMed] [Google Scholar]
- 48.Muralidhar K, Banakal S, Murthy K, Garg R, Rani GR, Dinesh R. Bispectral index-guided anaesthesia for off-pump coronary artery bypass grafting. Ann Card Anaesth. 2008;11:105–10. doi: 10.4103/0971-9784.41578. [DOI] [PubMed] [Google Scholar]
- 49.Lundell JC, Scuderi PE, Butterworth JFt. Less isoflurane is required after than before cardiopulmonary bypass to maintain a constant bispectral index value. J Cardiothorac Vasc Anesth. 2001;15:551–4. doi: 10.1053/jcan.2001.26526. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi K, Mita K, Sawa T. Electroencephalographic changes in the late cardiopulmonary bypass period are not reflected in the bispectral index. Clin Neurophysiol. 2010;121:1198–204. doi: 10.1016/j.clinph.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 51.De Hert SG, Van der Linden PJ, ten Broecke PW, Vermeylen KT, Rodrigus IE, Stockman BA. Effects of desflurane and sevoflurane on length-dependent regulation of myocardial function in coronary surgery patients. Anesthesiology. 2001;95:357–63. doi: 10.1097/00000542-200108000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Whitlock EL, Villafranca AJ, Lin N, Palanca BJ, Finkel KJ, Zhang L, Burnside BA, Kaiser HA, Evers AS, Avidan MS. Relationship between bispectral index values and volatile anesthetic concentrations during the maintenance phase of anesthesia in the B-unaware trial. Anesthesiology. 2011;115:1209–1218. doi: 10.1097/ALN.0b013e3182395dcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider G, Gelb AW, Schmeller B, Tschakert R, Kochs E. Detection of awareness in surgical patients with EEG-based indices--bispectral index and patient state index. Br J Anaesth. 2003;91:329–35. doi: 10.1093/bja/aeg188. [DOI] [PubMed] [Google Scholar]
- 54.Billard V. Brain injury under general anesthesia: is monitoring of the EEG helpful? Can J Anaesth. 2001;48:1055–60. doi: 10.1007/BF03020368. [DOI] [PubMed] [Google Scholar]
- 55.Merat S, Levecque JP, Le Gulluche Y, Diraison Y, Brinquin L, Hoffmann JJ. [BIS monitoring may allow the detection of severe cerebral ischemia]. Can J Anaesth. 2001;48:1066–9. doi: 10.1007/BF03020370. [DOI] [PubMed] [Google Scholar]
- 56.Theye RA, Patrick RT, Kirklin JW. The electro-encephalogram in patients undergoing open intracardiac operations with the aid of extracorporeal circulation. J Thorac Surg. 1957;34:709–17. [PubMed] [Google Scholar]
- 57.Morimoto Y, Monden Y, Ohtake K, Sakabe T, Hagihira S. The detection of cerebral hypoperfusion with bispectral index monitoring during general anesthesia. Anesth Analg. 2005;100:158–61. doi: 10.1213/01.ANE.0000139347.64944.95. [DOI] [PubMed] [Google Scholar]
- 58.Tobias JD. Cerebral oxygenation monitoring: near-infrared spectroscopy. Expert Rev Med Devices. 2006;3:235–43. doi: 10.1586/17434440.3.2.235. [DOI] [PubMed] [Google Scholar]
- 59.Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, Hogue CW., Jr. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41:1951–6. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexandrov AV, Sloan MA, Tegeler CH, Newell DN, Lumsden A, Garami Z, Levy CR, Wong LK, Douville C, Kaps M, Tsivgoulis G. Practice Standards for Transcranial Doppler (TCD) Ultrasound. Part II. Clinical Indications and Expected Outcomes. J Neuroimaging. 2010 Oct 26; doi: 10.1111/j.1552-6569.2010.00523.x. doi: 10.1111/j.1552-6569.2010.00523.x. [DOI] [PubMed] [Google Scholar]
- 61.Diephuis JC, Moons KG, Nierich AN, Bruens M, van Dijk D, Kalkman CJ. Jugular bulb desaturation during coronary artery surgery: a comparison of off-pump and on-pump procedures. Br J Anaesth. 2005;94:715–20. doi: 10.1093/bja/aei118. [DOI] [PubMed] [Google Scholar]
- 62.Estruch-Perez MJ, Ausina-Aguilar A, Barbera-Alacreu M, Sanchez-Morillo J, Solaz-Roldan C, Morales-Suarez-Varela MM. Bispectral index changes in carotid surgery. Ann Vasc Surg. 2010;24:393–9. doi: 10.1016/j.avsg.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Estruch-Perez MJ, Barbera-Alacreu M, Ausina-Aguilar A, Soliveres-Ripoll J, Solaz-Roldan C, Morales-Suarez-Varela MM. Bispectral index variations in patients with neurological deficits during awake carotid endarterectomy. Eur J Anaesthesiol. 2010;27:359–63. doi: 10.1097/EJA.0b013e32833618ca. [DOI] [PubMed] [Google Scholar]
- 64.Deogaonkar A, Vivar R, Bullock RE, Price K, Chambers I, Mendelow AD. Bispectral index monitoring may not reliably indicate cerebral ischaemia during awake carotid endarterectomy. Br J Anaesth. 2005;94:800–4. doi: 10.1093/bja/aei115. [DOI] [PubMed] [Google Scholar]
- 65.Bonhomme V, Desiron Q, Lemineur T, Brichant JF, Dewandre PY, Hans P. Bispectral index profile during carotid cross clamping. J Neurosurg Anesthesiol. 2007;19:49–55. doi: 10.1097/01.ana.0000211031.49420.c8. [DOI] [PubMed] [Google Scholar]
- 66.Kodaka M, Nishikawa Y, Suzuki T, Asano K, Maeyama A, Miyao H. Does bilateral bispectral index monitoring (BIS) detect the discrepancy of cerebral reperfusion during carotid endarterectomy? J Clin Anesth. 2009;21:431–4. doi: 10.1016/j.jclinane.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 67.Niedhart DJ, Kaiser HA, Jacobsohn E, Hantler CB, Evers AS, Avidan MS. Intrapatient reproducibility of the BISxp monitor. Anesthesiology. 2006;104:242–8. doi: 10.1097/00000542-200602000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 69.Lindholm ML, Traff S, Granath F, Greenwald SD, Ekbon A, Lennmarken C, Sandin RH. Mortality within 2 years after surgery in relation to low intraoperative bispectral index values and preexisting malignant disease. Anesth Analg. 2009;108:508–12. doi: 10.1213/ane.0b013e31818f603c. [DOI] [PubMed] [Google Scholar]
- 70.Leslie K, Myles PS, Forbes A, Chan MT. The effect of bispectral index monitoring on long-term survival in the B-aware trial. Anesth Analg. 2010;110:816–22. doi: 10.1213/ANE.0b013e3181c3bfb2. [DOI] [PubMed] [Google Scholar]
- 71.Kertai MD, Palanca BJ, Pal N, Burnside BA, Zhang L, Sadiq F, Finkel KJ, Avidan MS. Bispectral index monitoring, duration of bispectral index below 45, patient risk factors, and intermediate-term mortality after noncardiac surgery in the B-Unaware Trial. Anesthesiology. 2011;114:545–56. doi: 10.1097/ALN.0b013e31820c2b57. [DOI] [PubMed] [Google Scholar]
- 72.Duarte LT, Saraiva RA. When the bispectral index (bis) can give false results. Rev Bras Anestesiol. 2009;59:99–109. doi: 10.1590/s0034-70942009000100013. [DOI] [PubMed] [Google Scholar]
- 73.Hagihira S, Takashina M, Mori T, Mashimo T, Yoshiya I. Practical issues in bispectral analysis of electroencephalographic signals. Anesth Analg. 2001;93:966–70. doi: 10.1097/00000539-200110000-00032. [DOI] [PubMed] [Google Scholar]
- 74.Holschneider DP, Leuchter AF, Uijtdehaage SH, Abrams M, Rosenberg-Thompson S. Loss of high-frequency brain electrical response to thiopental administration in Alzheimer's-type dementia. Neuropsychopharmacology. 1997;16:269–75. doi: 10.1016/S0893-133X(96)00220-5. [DOI] [PubMed] [Google Scholar]
- 75.Renna M, Handy J, Shah A. Low baseline Bispectral Index of the electroencephalogram in patients with dementia. Anesth Analg. 2003;96:1380–5. doi: 10.1213/01.ANE.0000059223.78879.0F. [DOI] [PubMed] [Google Scholar]
- 76.Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, Broberg M, Wallace A, DeLosAngeles D, Lillie P, Hardy A, Fronsko R, Pulbrook A, Willoughby JO. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clin Neurophysiol. 2007;118:1877–88. doi: 10.1016/j.clinph.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 77.Whitham EM, Lewis T, Pope KJ, Fitzgibbon SP, Clark CR, Loveless S, DeLosAngeles D, Wallace AK, Broberg M, Willoughby JO. Thinking activates EMG in scalp electrical recordings. Clin Neurophysiol. 2008;119:1166–75. doi: 10.1016/j.clinph.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 78.Baldesi O, Bruder N, Velly L, Gouin F. Spurious bispectral index values due to electromyographic activity. Eur J Anaesthesiol. 2004;21:324–5. doi: 10.1017/s0265021504254126. [DOI] [PubMed] [Google Scholar]
- 79.Messner M, Beese U, Romstock J, Dinkel M, Tschaikowsky K. The bispectral index declines during neuromuscular block in fully awake persons. Anesth Analg. 2003;97:488–91. doi: 10.1213/01.ANE.0000072741.78244.C0. [DOI] [PubMed] [Google Scholar]
- 80.Bonhomme V, Hans P. Muscle relaxation and depth of anaesthesia: where is the missing link? Br J Anaesth. 2007;99:456–60. doi: 10.1093/bja/aem243. [DOI] [PubMed] [Google Scholar]
- 81.Ponte J. Neuromuscular blockers during general anaesthesia. BMJ. 1995;310:1218–9. doi: 10.1136/bmj.310.6989.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesth Analg. 2009;108:527–35. doi: 10.1213/ane.0b013e318193c634. [DOI] [PubMed] [Google Scholar]
- 83.Barr G, Anderson RE, Samuelsson S, Owall A, Jakobsson JG. Fentanyl and midazolam anaesthesia for coronary bypass surgery: a clinical study of bispectral electroencephalogram analysis, drug concentrations and recall. Br J Anaesth. 2000;84:749–52. doi: 10.1093/oxfordjournals.bja.a013587. [DOI] [PubMed] [Google Scholar]
- 84.Bickford RG, Faulconer A, Jr., Sem-Jacobsen CW, Petersen MC, Dodge HW, Jr., Schnugg FJ. Some effects of barbiturate anesthesia on the depth electrogram. Proc Staff Meet Mayo Clin. 1953;28:162–5. [PubMed] [Google Scholar]
- 85.Velly LJ, Rey MF, Bruder NJ, Gouvitsos FA, Witjas T, Regis JM, Peragut JC, Gouin FM. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107:202–12. doi: 10.1097/01.anes.0000270734.99298.b4. [DOI] [PubMed] [Google Scholar]
- 86.Lee U, Muller M, Noh GJ, Choi B, Mashour GA. Dissociable network properties of anesthetic state transitions. Anesthesiology. 2011;114:872–81. doi: 10.1097/ALN.0b013e31821102c9. [DOI] [PubMed] [Google Scholar]
- 87.Lee U, Oh G, Kim S, Noh G, Choi B, Mashour GA. Brain networks maintain a scale-free organization across consciousness, anesthesia, and recovery: evidence for adaptive reconfiguration. Anesthesiology. 2010;113:1081–91. doi: 10.1097/ALN.0b013e3181f229b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dahaba AA. Different conditions that could result in the bispectral index indicating an incorrect hypnotic state. Anesth Analg. 2005;101:765–73. doi: 10.1213/01.ane.0000167269.62966.af. [DOI] [PubMed] [Google Scholar]
- 89.Hemmerling TM, Fortier JD. Falsely increased bispectral index values in a series of patients undergoing cardiac surgery using forced-air-warming therapy of the head. Anesth Analg. 2002;95:322–3. doi: 10.1097/00000539-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 90.Woodhall B, Reynolds DH, Mahaley S, Jr., Sanders AP. The physiologic and pathologic effects of localized cerebral hypothermia. Ann Surg. 1958;147:673–83. doi: 10.1097/00000658-195805000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woodhall B, Sealy WC, Hall KD, Floyd WL. Craniotomy under conditions of quinidine-protected cardioplegia and profound hypothermia. Ann Surg. 1960;152:37–44. doi: 10.1097/00000658-196007000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doi M, Gajraj RJ, Mantzaridis H, Kenny GN. Effects of cardiopulmonary bypass and hypothermia on electroencephalographic variables. Anaesthesia. 1997;52:1048–55. doi: 10.1111/j.1365-2044.1997.229-az0364.x. [DOI] [PubMed] [Google Scholar]
- 93.Mathew JP, Weatherwax KJ, East CJ, White WD, Reves JG. Bispectral analysis during cardiopulmonary bypass: the effect of hypothermia on the hypnotic state. J Clin Anesth. 2001;13:301–5. doi: 10.1016/s0952-8180(01)00275-6. [DOI] [PubMed] [Google Scholar]
- 94.Schmidlin D, Hager P, Schmid ER. Monitoring level of sedation with bispectral EEG analysis: comparison between hypothermic and normothermic cardiopulmonary bypass. Br J Anaesth. 2001;86:769–76. doi: 10.1093/bja/86.6.769. [DOI] [PubMed] [Google Scholar]
- 95.Meybohm P, Gruenewald M, Hocker J, Renner J, Graesner JT, Ilies C, Scholz J, Bein B. Correlation and agreement between the bispectral index vs. state entropy during hypothermic cardio-pulmonary bypass. Acta Anaesthesiol Scand. 2010;54:169–75. doi: 10.1111/j.1399-6576.2009.02138.x. [DOI] [PubMed] [Google Scholar]
- 96.Hayashida M, Sekiyama H, Orii R, Chinzei M, Ogawa M, Arita H, Hanaoka K, Takamoto S. Effects of deep hypothermic circulatory arrest with retrograde cerebral perfusion on electroencephalographic bispectral index and suppression ratio. J Cardiothorac Vasc Anesth. 2007;21:61–7. doi: 10.1053/j.jvca.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 97.Ziegeler S, Buchinger H, Wilhelm W, Larsen R, Kreuer S. Impact of deep hypothermic circulatory arrest on the BIS index. J Clin Anesth. 2010;22:340–5. doi: 10.1016/j.jclinane.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 98.Antognini JF. Hypothermia eliminates isoflurane requirements at 20 degrees C. Anesthesiology. 1993;78:1152–6. doi: 10.1097/00000542-199306000-00020. [DOI] [PubMed] [Google Scholar]
- 99.Russell GN, Wright EL, Fox MA, Douglas EJ, Cockshott ID. Propofol-fentanyl anaesthesia for coronary artery surgery and cardiopulmonary bypass. Anaesthesia. 1989;44:205–8. doi: 10.1111/j.1365-2044.1989.tb11223.x. [DOI] [PubMed] [Google Scholar]
- 100.Stecker MM, Cheung AT, Pochettino A, Kent GP, Patterson T, Weiss SJ, Bavaria JE. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg. 2001;71:14–21. doi: 10.1016/s0003-4975(00)01592-7. [DOI] [PubMed] [Google Scholar]
- 101.Pilge S, Zanner R, Schneider G, Blum J, Kreuzer M, Kochs EF. Time delay of index calculation: analysis of cerebral state, bispectral, and narcotrend indices. Anesthesiology. 2006;104:488–94. doi: 10.1097/00000542-200603000-00016. [DOI] [PubMed] [Google Scholar]
- 102.Zanner R, Pilge S, Kochs EF, Kreuzer M, Schneider G. Time delay of electroencephalogram index calculation: analysis of cerebral state, bispectral, and Narcotrend indices using perioperatively recorded electroencephalographic signals. Br J Anaesth. 2009;103:394–9. doi: 10.1093/bja/aep198. [DOI] [PubMed] [Google Scholar]
- 103.Akirav I, Richter-Levin G. Factors that determine the non-linear amygdala influence on hippocampus-dependent memory. Dose Response. 2006;4:22–37. doi: 10.2203/dose-response.004.01.003.Akirav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jordan D, Stockmanns G, Kochs EF, Pilge S, Schneider G. Electroencephalographic order pattern analysis for the separation of consciousness and unconsciousness: an analysis of approximate entropy, permutation entropy, recurrence rate, and phase coupling of order recurrence plots. Anesthesiology. 2008;109:1014–22. doi: 10.1097/ALN.0b013e31818d6c55. [DOI] [PubMed] [Google Scholar]
- 105.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–12. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.