Abstract
Synthetic compounds for controlling or creating human immunity have the potential to revolutionize disease treatment. Motivated by challenges in this arena, we report herein a strategy to target metastatic cancer cells for immune-mediated destruction by targeting the urokinase-type plasminogen activator receptor (uPAR). Urokinase-type plasminogen activator (uPA) and uPAR are overexpressed on the surfaces of a wide range of invasive cancer cells and are believed to contribute substantially to the migratory propensities of these cells. The key component of our approach is an antibody-recruiting molecule that targets the urokinase receptor (ARM-U). This bifunctional construct is formed by selectively, covalently attaching an antibody-binding small molecule to the active site of the urokinase enzyme. We demonstrate that ARM-U is capable of directing antibodies to the surfaces of target cancer cells and mediating both antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC) against multiple human cancer cell lines. We believe that the reported strategy has the potential to inform novel treatment options for a variety of deadly, invasive cancers.
Cancer is currently the second leading cause of death in the United States, having claimed over half a million American lives in 2010.(1) In general, metastatic cancers are particularly difficult to treat and are associated with higher levels of morbidity and mortality compared to localized tumors.(2,3) For example, while the five-year survival rate of patients with localized melanoma is >95%, this survival rate drops to 15–30% for patients whose disease has metastasized to distant locations.(1) Since American men and women have a 38–44% chance, respectively, of developing invasive cancers during their lifetimes,(1) novel strategies for treating advanced-stage invasive cancers have the potential to provide profound therapeutic impact.
Tumor metastasis begins with cancer cells invading surrounding tissues. This process is frequently accelerated by cell-surface proteases, including uPA,(4,5,6) which are capable of breaking down extracellular matrix proteins and activating migration-inducing signal transduction cascades.(7,8) uPA binds uPAR on the extracellular surface of many cancer cells, including those of the breast, colon, stomach, and bladder.(9,10) Extensive evidence suggests that the levels of uPA and uPAR expression are substantially higher on invasive, malignant cancer cells than on either healthy tissues or benign tumors.(5,9,11,12,13,14) Indeed, in clinical settings, high levels of uPA and uPAR are used as diagnostic markers for metastatic potential and poor clinical outcome in numerous malignancies.(4,5,10,15,16,17,18,19,20) For these reasons uPA and uPAR have emerged as promising therapeutic targets.(9,21) Data has shown that inhibitors and cytotoxic fusion proteins that target the uPA–uPAR system can both reduce the invasive potential of cancer cells(22,23) and reduce tumor volumes in animal models(24,25,26) without significantly damaging healthy tissue.(26)
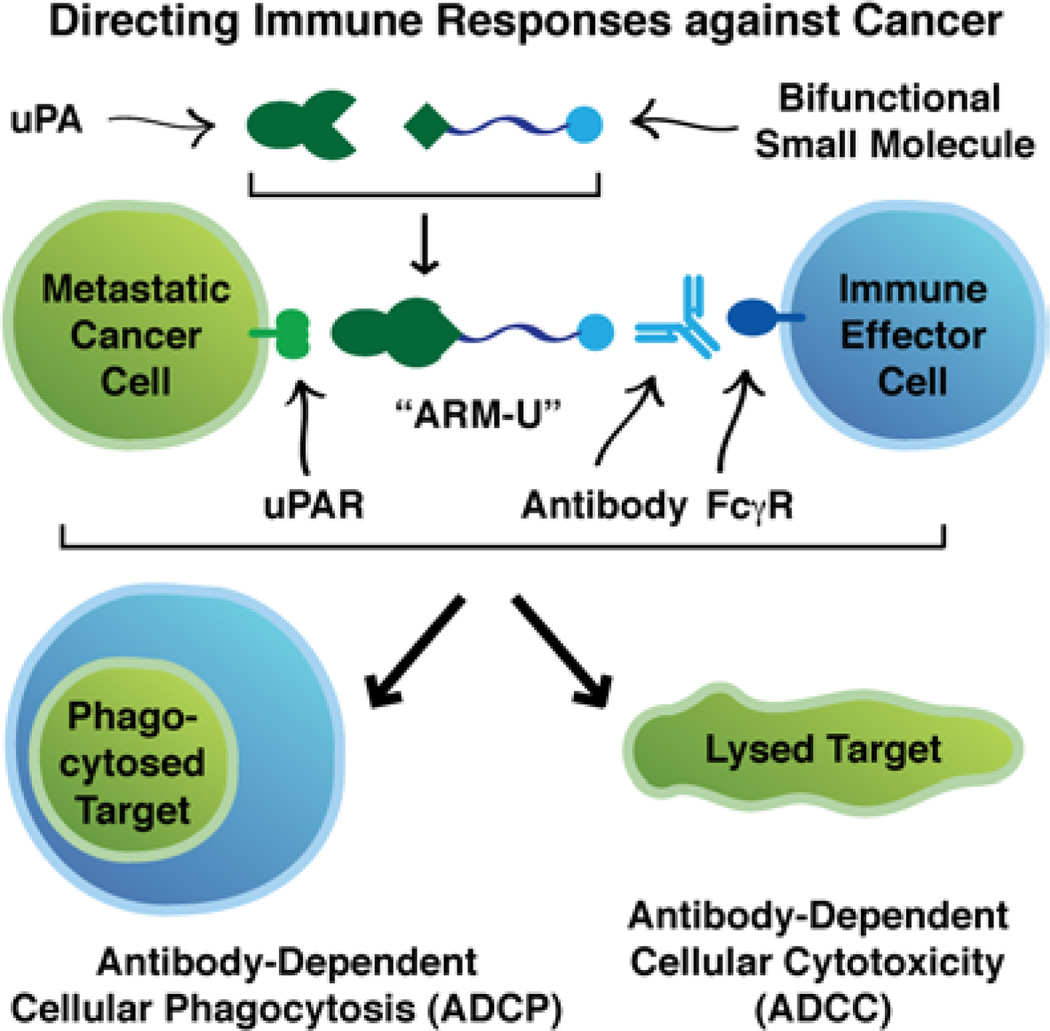
The growing field of synthetic immunology(27) aims to develop novel synthetic materials capable of modulating the human immune system. One emerging concept in this area is to use bifunctional molecules to direct normal antibody responses to attack cancer cells that are not sufficiently recognized by the immune system on its own. Indeed, pioneering work by a number of research groups has demonstrated the promise of this and related approaches in both in vitro and in vivo settings.(28,29,30,31,32,33,34,35) We report here a novel application of this strategy to direct endogenous immunological effector mechanisms to act against uPAR-expressing human cancer cells (Figure 1). We have designed and synthesized two small molecules that can convert uPA into catalytically inactive, bifunctional constructs (ARM-Us) that are capable of both recruiting antibodies and directing antibody-dependent immune responses against uPAR-expressing cancer cells. These small molecules quantitatively inhibit uPA’s enzymatic activity by covalently binding to its active site, and this covalent modification simultaneously appends either a 2,4-dinitrophenyl (DNP) moiety or a fluorescein label. The DNP antigen is of particular interest for therapeutic application because anti-DNP antibodies have been found endogenously in the plasma of most humans.(36) Here we demonstrate that ARM-U can bind with high affinity to uPAR-expressing cancer cells, recruit antibodies to these cells, and induce phagocytosis and cytotoxicity in an antibody-dependent, immune-mediated fashion. The technology reported herein represents a novel strategy to target uPAR-expressing cancers, which may find broad application in treating a variety of deadly malignancies.
Figure 1.
Schematic overview of using bifunctional ARM-U complexes to direct natural immune responses against uPAR-expressing cancer cells.
Results
ARM-U Design, Synthesis, and Evaluation
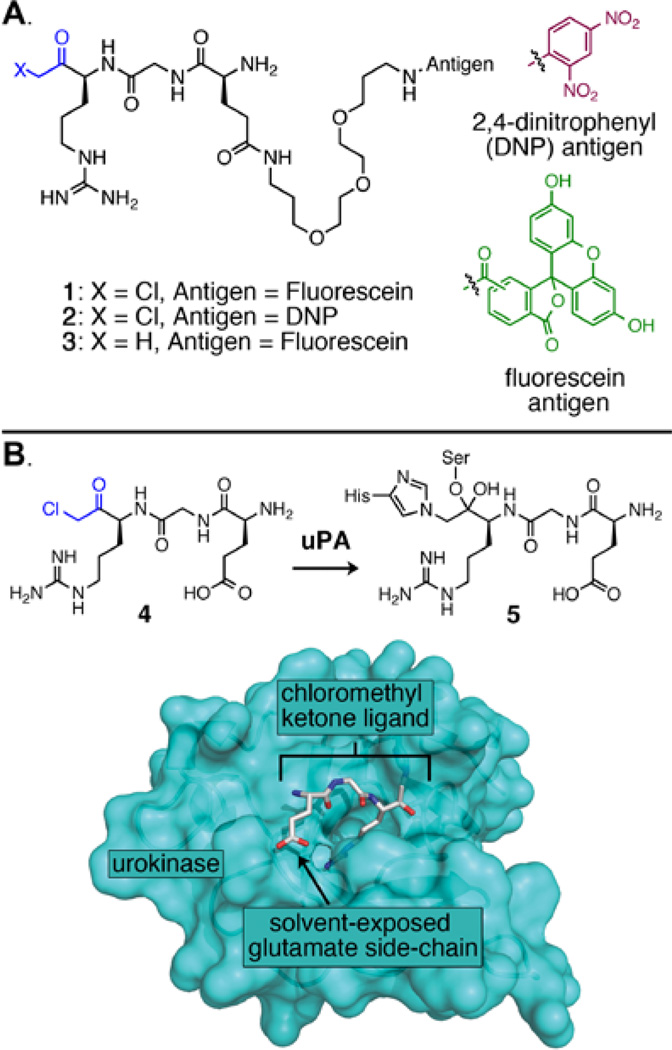
With the goal of preparing ARM-U by simultaneously inactivating uPA’s catalytic activity and site-specifically attaching an antibody-recruiting hapten to the protein, we designed chloromethyl ketones 1 and 2 (Figure 2A). These molecules were inspired by tripeptide 4, which has been shown to covalently inhibit several serine proteases including uPA.(37,38,39) Analysis of a published crystal structure of uPA bound to chloromethyl ketone inhibitor 4 (Figure 2B)(38) suggested to us that the glutamic acid side chain of the inhibitor would remain solvent-exposed following covalent binding and would therefore serve as an ideal site to attach an antibody-recruiting motif. Thus, we prepared chloromethyl ketones 1 and 2, which incorporate ethylene glycol-derived linkers to connect either fluorescein or DNP to the chloromethyl ketone tripeptide. Methyl ketone 3 cannot covalently bind uPA because it lacks the electrophilic chloromethyl group found in 1 and 2, so 3 therefore serves as a negative control.
Figure 2.
(A) Chemical structures of bifunctional small molecules described herein. (B) X-ray crystal structure of covalent adduct 5, which is formed from the interaction of uPA and chloromethyl ketone 4 (PDB ID: 1LMW).(38) The inhibitor is shown in stick representation with grey Cα atoms, and the protein is depicted as a turquoise surface.
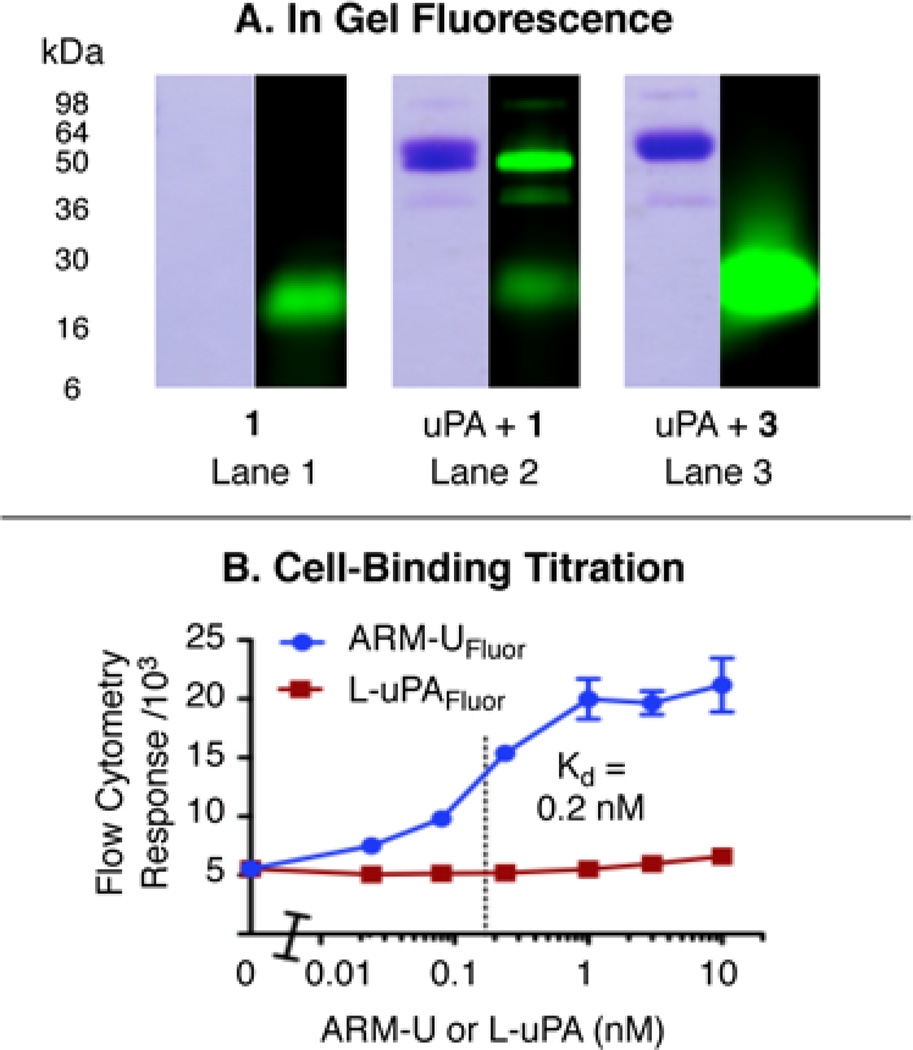
We initially evaluated the reaction between uPA and compound 1 (to form ARM-UFluor) by measuring uPA-mediated hydrolysis of a known fluorogenic substrate (Cbz-Gly-Gly-Arg-7-amino-4-methylcoumarin).(40) This analysis (Supplementary Figure S1) reveals a 97% reduction in uPA activity versus buffer-treated uPA, which suggests that compound 1 virtually quantitatively functionalizes uPA’s active site during the 1-hour, ambient-temperature incubation. We further evaluated ARM-UFluor by SDS–PAGE with in-gel fluorescence detection(39) (Figure 3A and Supplementary Figure S2). Incubating uPA with 1.5 equivalents of compound 1 (lane 2) substantially increases the fluorescence intensity of the protein’s band at 54 kDa. Incubating uPA with higher concentrations of 1 (up to 12 equivalents) does not substantially further increase the amount of fluorescence observed in this band (Supplementary Figure S3), which suggests that 1 attaches only to a single site on uPA. Furthermore, treating uPA with compound 3, which cannot covalently bind the protein (6 equiv, lane 3), does not lead to observable fluorescence changes in uPA’s band, which implies that a covalent bonding event is necessary for complex formation with uPA. Taken together, these data suggest that compound 1 forms a 1:1 covalent complex with uPA at the enzyme’s active site and provides ARM-UFluor as a chemically defined, homogeneous reagent. The antibody-recruiting complex ARM-UDNP was prepared analogously to ARM-UFlour by simply replacing chloromethyl ketone 1 with compound 2.
Figure 3.
(A) In-gel fluorescence (488 nm excitation, 532 nm emission, right) and Coomassie stain (left) analyses of mixtures of compound 1 (15 µM), uPA (10 µM), and compound 3 (60 µM). (B) Flow cytometry measurements of ARM-UFluor binding to HT-29 cells compared to L-uPAFluor as a negative control. Data points represent average values from triplicate experiments ± standard deviation.
To demonstrate that ARM-UFluor can bind anti-fluorescein antibodies, we employed an ELISA protocol.(41) ELISA wells were coated with anti-uPA antibody, treated with ARM-UFluor (or uPA as a negative control), treated with anti-fluorescein antibody, and analyzed with alkaline-phosphatase-conjugated secondary antibody. Data from these experiments (Supplementary Figure S6) indicate that anti-fluorescein antibody exhibits concentration-dependent binding to ARM-UFluor with a Kd of approximately 200 pM. These results indicate that both ends of bifunctional small molecule 1 can interact simultaneously with their respective protein targets (uPA and antibody).
We next evaluated ARM-UFluor-mediated ternary complex formation on the surface of HT-29 human colonic adenocarcinoma cells, which have been reported to express approximately 1.4 × 105 molecules of uPAR per cell.(42) Incubation of HT-29 cells with ARM-UFluor, followed by anti-fluorescein antibody and fluorescently labeled secondary antibody, gives rise to a concentration-dependent, saturable binding interaction (Figure 3B). These data indicate a Kd for the ARM-UFluor–uPAR interaction of approximately 200 pM, which is consistent with previously reported values for the uPA–uPAR interaction.(4) A bifunctional construct (L-uPAFluor) formed from 1 and low-molecular-weight uPA, which lacks the uPAR-binding domain,(4) shows negligible cell-binding ability under identical experimental conditions. Furthermore, pre-treatment of HT-29 cells with an anti-uPAR antibody that blocks the uPA binding site completely negates the cellular binding of ARM-UFluor (Supplementary Figure S9). These data support that ARM-UFluor specifically targets cells via interactions with uPAR. Furthermore, these observations are consistent with our hypothesis that modifying uPA’s active site should not perturb its receptor-binding ability because uPA’s receptor-binding and catalytic domains reside on opposite ends of the protein.
ARM-U Mediated Cell Killing
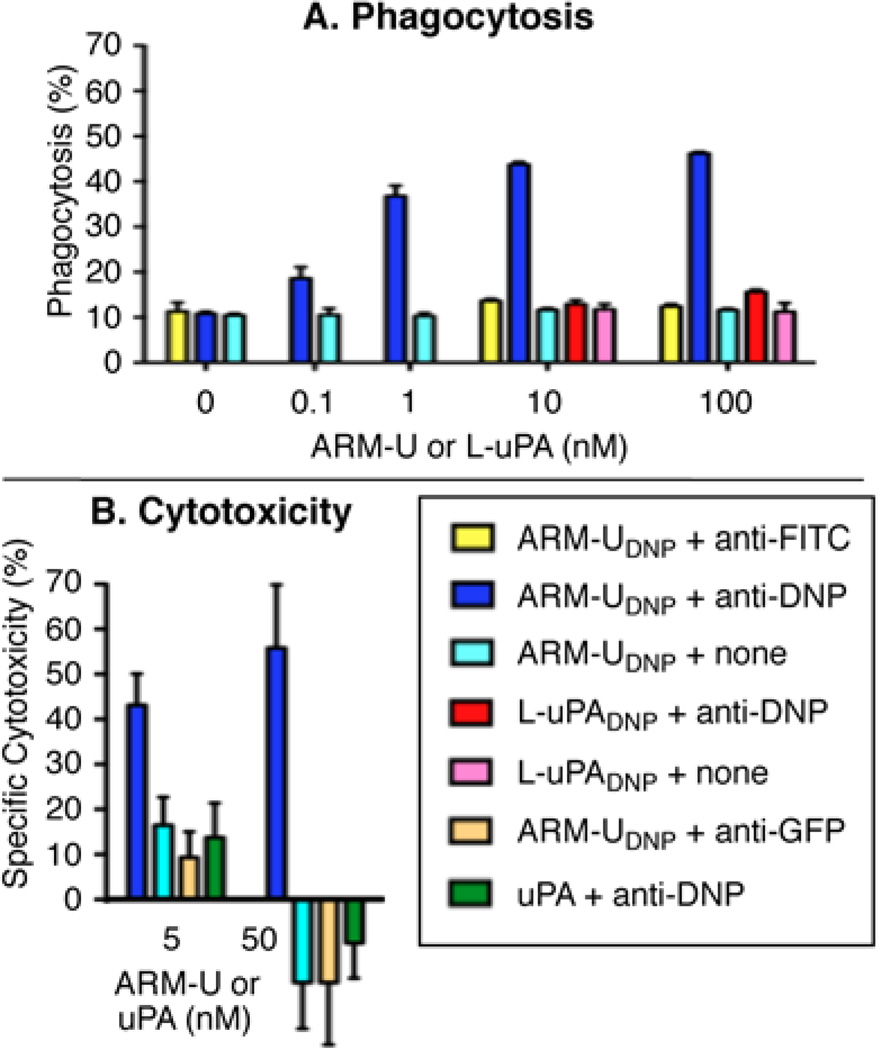
We next evaluated the ability of ARM-U-templated ternary complexes to induce immune-mediated responses against uPAR-expressing target cells. We first studied antibody-dependent cellular phagocytosis (ADCP) using a two-color flow cytometry protocol.(43) Here we measured ARM-U-dependent phagocytosis of both HT-29 cells and A172 human glioblastoma cells (which have been reported to express approximately 1.0 × 106 molecules of uPAR per cell)(42) by IFN-γ-activated U937 effector cells.(44) In this ADCP assay, target and effector cells are labeled with different fluorescent cell-membrane dyes and then incubated together with ARM-UDNP and anti-DNP antibodies. Cell mixtures are then analyzed by two-color flow cytometry, and phagocytosed cells are identified as those showing fluorescence from both target and effector cells. As previously described, these double-positive cells represent phagocytic events rather than cell–cell aggregates.(43)
As illustrated in Figure 4A, phagocytosis of A172 cells increases as a function of ARM-UDNP concentration (dark blue bars), and significant levels of phagocytosis are observed at ARM-UDNP concentrations as low as 0.1 nM. At 10 nM ARM-UDNP, the level of observed phagocytosis is similar to that observed after non-specifically labeling the target cells with high levels of antigen (trinitrophenyl sulfonic acid incubation, Supplementary Figure S10), which indicates the high efficiency of ARM-U-mediated phagocytosis. Equivalently high levels of phagocytosis are observed when the pure anti-DNP antibody is replaced with serum from rabbits that had been immunized against the DNP hapten (Supplementary Figure S11). In negative control experiments, an isotype-matched anti-fluorescein antibody (yellow bars), which does not bind DNP, was not found to promote phagocytosis, even at high ARM-UDNP concentrations. Furthermore, the adduct between low molecular weight uPA and compound 2 (L-uPADNP, red bars), which cannot bind uPAR, was also unable to promote phagocytosis even in the presence of anti-DNP antibody. In analogous assays (Supplementary Figure S10), the combination of ARM-UFluor and anti-fluorescein antibody produces high levels of phagocytosis, underscoring the requirement for a matched antigen–antibody combination. ARM-U was also capable of mediating the phagocytic uptake of HT-29 cells (Supplementary Figure S10), albeit at slightly lower levels than for A172 cells, likely due to the slightly lower levels uPAR expression on HT-29 cells.(42) Taken together, these data suggest that the observed phagocytosis is dependent upon ternary complexes formed between uPAR, ARM-U, and epitope-matched antibody on target cell surfaces.
Figure 4.
(A) Antibody-dependent phagocytosis of A172 cells by IFN-γ-primed U937 cells (16:1 effector:target). Antibody concentrations = 10 µg/mL. Data points represent average values from triplicate experiments ± standard deviation. (B) Antibody-dependent cellular cytotoxicity, as measured by the Roche xCelligence System, of A172 cells by freshly isolated PBMCs (60:1 effector:target). Antibody concentration = 27 µg/mL. At 3 h, the specific cytotoxicity was calculated as the specific decrease in cell index compared to cells treated with only anti-DNP antibody and PBMCs. Data points represent average values of triplicate experiments ± standard deviation.
We next studied the ability of ARM-U to induce antibody-dependent cellular cytotoxicity (ADCC). To this end, we utilized the Roche xCelligence System, which uses electrical impedance to measure the adhesion of live cells to gold electrodes in culture wells (“cell index”). Detachment of dying cells from these wells leads to a corresponding decrease in impedance through the gold electrodes, which has been used to measure cell death.(45,46) Treatment of A172 target cells with ARM-UDNP, anti-DNP antibody, and freshly isolated human peripheral blood mononuclear cells (PBMCs) leads to profound time-dependent decreases in cell index that are indicative of ADCC (Supplementary Figure S12). Indeed, concentrations of ARM-UDNP as low as 5 nM lead to substantial levels of cellular cytotoxicity in the presence of anti-DNP antibody (Figure 4B, dark blue bars). Control conditions employing isotype-matched anti-green-fluorescence-protein antibody (tan bars), unmodified uPA (green bars), or omitting antibody altogether (light blue bars), indicate no decreases in cell viability. Additional control experiments conducted in the absence of PBMCs reveal no antibody-dependent cytotoxic effects (Supplementary Figures S14, S15). Together, these data suggest that the combination of ARM-UDNP, effector cells, and anti-DNP antibody is required for cytotoxic effects. In light of the importance of ADCC in mediating the effects of monoclonal-antibody-based cancer therapeutics,(47) these data suggest a functional similarity between monoclonal antibodies and the ARM-U–antibody combination.
Discussion
Here we report a novel strategy for converting a cancer-promoting enzyme (uPA) into synthetic constructs (ARM-Us) capable of targeting cancer cells for immune-mediated destruction. Since the urokinase receptor is significantly upregulated on many types of cancer cells, especially the most invasive ones, ARM-U constructs have the potential to target a wide array of clinically relevant malignancies including those of the breast, colon, pancreas, and ovaries. Indeed, uPA and uPAR expression are well-documented markers for cancer aggressiveness and poor clinical outcome, and therapeutic-targeting strategies against these proteins have shown great promise.
The reported strategy has the potential to carry a number of advantages versus available protein-based anti-cancer therapeutics. uPA is readily available, and can either be isolated from human urine or produced recombinantly. Furthermore, since ARM-U reagents are formed by site-specifically modifying an endogenous human protein, these constructs are expected to be less likely to produce anaphylactoid or immunological side effects versus non-native proteins or their conjugates. Indeed, the catalytic domain of uPA (low-molecular-weight urokinase, trade name Abbokinase®) is already FDA-approved as a safe treatment for deep vein thrombosis and pulmonary embolism. Studies have shown that cytotoxic fusion proteins can target uPAR-expressing cancer cells without significant off-target damage to healthy organs,(26) and since ARM-Us are formed ex vivo with stoichiometric amounts of compounds 1 or 2, no reactive chloromethyl ketone should remain to react with off-target proteins. Furthermore, we believe that selective labeling of the active site is advantageous compared to alternative bioconjugation strategies, which could disrupt uPAR binding by modifying the uPAR-binding domain, initiate immune-complex formation by inducing aggregation of multiple antibody proteins, or create immunogenic neo-epitopes that could initiate adaptive immune responses to the conjugate itself.(48) Finally, since antibody-mediated cytotoxicity has been shown to follow a non-linear trend as a function of surface epitope expression,(34) ARM-U-mediated effects are expected to be highly selectivity for cancer cells that upregulate uPAR expression.
Conclusion
Exploiting endogenous immunological mechanisms to selectively attack and eliminate cancer cells represents a relatively new strategy that has led to numerous developments in recent years. These successes range from Provenge®, an autologous cellular immunotherapy for prostate cancer treatment,(49) to a variety of therapeutic antibodies and antibody-fusion constructs.(50,51,52,53,54) Despite these successes, relatively few examples of using small-molecules to redirect antibody-mediated immune responses against cancers have been disclosed.(28,29,30,31,32,33,34,35) Some of these approaches have shown promising results in animal studies using anti-hapten-immunized mice(29,30,35,55) or with injected antibody(31,32,33). The approach reported here differs from these prior strategies in that it targets uPAR, which is found on a broad variety of invasive cancer cells, and it also has the potential to exploit the naturally-occurring, hapten-directed class of anti-DNP antibodies for cancer cell destruction. Finally, by enhancing trafficking of cancer-associated antigens through immune cells, ARM-Us have the potential to give rise to long-lasting immunity.(29) Although we focus here on targeting one cancer marker (uPAR) using a single antibody population (anti-DNP), one can envision a collection of ARM constructs that target various cancer-associated surface proteins and a range of antibody populations. Thus, this general strategy holds promise for treating a wide range of human cancers, as well as other diseases.
Methods
Materials and Chemical Synthesis
Organic chemicals were purchased from Sigma–Aldrich or Advanced ChemTech. High and low molecular weight human urokinase proteins were purchased from American Research Products or Innovative Research. Antibody reagents were purchased from Invitrogen or Rockland. HT-29, A172, and U937 cells were purchased from ATCC, grown according to the supplier’s instructions, and used within 6 months of resuscitation. Molecules 1–3 were synthesized using standard organic chemistry procedures and characterized by standard techniques including 1H- and 13C-NMR spectroscopy, infrared (IR) spectroscopy, and mass spectrometry. Molecules 1–3 were purified to analytical purity using by preparative reverse phase HPLC.
Enzymatic Analysis
uPA (10 µM) was incubated with either compound 1 (15 µM) or buffer at ambient temperature for 1 h and then diluted to 20 nM with a solution of Cbz-Gly-Gly-Arg-7-amino-4-methylcoumarin (96 µM). Fluorescence (330 nm absorbance, 460 nm emission) was monitored at 1 minute intervals. Initial rates of enzymatic hydrolysis were calculated from the increase in fluorescence signal using the first five data points.
SDS-PAGE
Compound 1 (15 uM), compound 3 (60 uM), or buffer was mixed with uPA (10 uM) or buffer at ambient temperature for 1 h. Mixtures were loaded onto a 15% Tris-HCl SDS-PAGE gel, separated by electrophoresis, and visualized first by fluorescence (Typhoon Trio Variable Mode Imager, 488 nm excitation, 532 nm emission) and then by Coomassie stain.
ELISA
The wells of a 96-well polystyrene plate were coated with rabbit IgG anti-human-uPA, treated either with ARM-UFluor or uPA, treated with goat IgG anti-fluorescein, and treated with rabbit anti-goat-IgG conjugated to alkaline phosphatase. After addition of para-nitrophenyl phosphate, the rate of nitrophenol liberation was measured at 405 nm using a Synergy 2 Multimode Microplate Reader.
Flow Cytometry
HT-29 cells were treated with ARM-UFluor or the negative control L-uPAFluor, treated with rabbit anti-fluorescein IgG, and treated with with Alexa Fluor® 488-conjugated donkey anti-rabbit-IgG. Cells were analyzed by flow cytometry (FL-1 channel) on an Accuri C6 flow cytometer. No fluorescent signal above background was observed upon omission of the secondary antibody, indicating that the fluorescein moiety does not substantially contribute to observed fluorescent signal.
ADCP Assay
Effector cells (U937) were treated with IFN-γ for 2 days, then labeled with DiD, an FL-4 channel fluorophore. Target cells (A172 or HT-29) were labeled with DiO, an FL-1 channel fluorophore, then treated with ARM-UDNP, ARM-UFluor, L-uPADNP, L-uPAFluor, trinitrophenyl sulphonic acid (TNP-SO3H), or fluorocein isothiocyanate (FITC). Target and effector cells were mixed (16:1 effector:target), and antibody (10 µg/mL) was added. After 1 h at 37 °C, the mixtures were analyzed by two-color flow cytometry. Effector cells that had performed phagocytosis were identified as those with strong signals in both the FL-1 and FL-4 fluorescence channels. Literature precedent supports that these double positive signals represent phagocytosis events rather than cell–cell aggregates.(43) Percent phagocytosis is calculated using the following equation: Phagocytosis (%) = 100 × (double positive cells) / [(remaining target cells) + (double positive cells)].
ADCC Assay
A172 cells were seeded into the analysis chamber of the Roche xCelligence System. After 12 hours, ARM-UDNP (5 or 50 nM), uPA, or buffer was added. Rabbit anti-DNP IgG (27 µg/mL), rabbit anti-green-fluorescence-protein (GFP) IgG, or buffer was also added simultaneously. Freshly isolated human peripheral blood mononuclear cells (60:1 effector:target) were then added. The xCelligence System’s output is termed the “cell index” and is a function of the electrical impedance through gold electrodes on the surfaces of culture plates. All cell indices were normalized to 1.0 immediately following effector cell addition and were measured every 2 minutes. Normal growth is defined as that shown by target cells treated with anti-DNP antibody and effector cells, but no uPA or ARM-U. Specific cytotoxicity is calculated using the following equation: Specific cytotoxicity (%) = 100 – 100 × (cell index) / (normal growth cell index).
Supplementary Material
Acknowledgements
This work was funded by the National Institute of Health through the NIH New Innovator Award number 1DP2OD002913-01 granted to D.A.S. C.E.J. acknowledges a Leslie Warner Postdoctoral Fellowship granted by the Yale Cancer Center.
Footnotes
Associated Content
Supporting Information Available: Experimental procedures for chemical syntheses and biological experiments, scanned NMR and HPCL data for synthesized compounds, crude data from cell-killing experiments, and catalogue numbers for biological reagents. This material is free of charge via the internet at http://pubs.acs.org.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: Current status and future prospects. The Oncologist. 2011;16:5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle P, Levin B, editors. World Cancer Report 2008. Lyon: International Agency for Research on Cancer; 2008. pp. 438–443. [Google Scholar]
- 4.Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int. J. Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Duffy MJ. Urokinase-type plasminogen activator and malignancy. Fibrinolysis. 1993;7:295–302. [Google Scholar]
- 6.Saksela O, Rifkin DB. Cell-associated plasminogen activation: Regulation and physiological functions. Ann. Rev. Cell. Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- 7.Del Rosso M, Fibbi G, Pucci M, D’Alessio SA, Del Rosso A, Magnelli L, Chiarugi V. Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin. & Exp. Metastasis. 2002;19:193–207. doi: 10.1023/a:1015531321445. [DOI] [PubMed] [Google Scholar]
- 8.Jessani N, Liu Y, Humphrey M, Cravatt BF. Enzyme activity profiles of the secreted and membrane proteome that depict cancer cell invasiveness. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10335–10340. doi: 10.1073/pnas.162187599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romer J, Nielsen BS, Ploug M. The urokinase receptor as a potential target in cancer therapy. Curr. Pharm. Des. 2004;10:2359–2376. doi: 10.2174/1381612043383962. [DOI] [PubMed] [Google Scholar]
- 10.Blasi F, Carmeliet P. uPAR: A versatile signaling orchestrator. Nat. Rev. Mol. Cell. Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 11.Duffy MJ. Proteases as prognostic markers in cancer. Clin. Cancer. Res. 1996. 1996;2:613–618. [PubMed] [Google Scholar]
- 12.Dano K, Behrendt N, Brunner N, Ellis V, Ploug M, Pyke C. The urokinase receptor: Protein structure and role in plasminogen activation and cancer invasion. Fibrinolysis. 1994;8 suppl 1:189–203. [Google Scholar]
- 13.Quax PHA, van Muijen GNP, Weening�Verhoeff EJD, Lund LR, Dano K, Ruiter DJ, Verheijen JH. Metastatic Behavior of human melanoma cell lines in nude mice correlates with urokinase-type plasminogen activator, its type-1 inhibitor, and urokinase-mediated matrix degradation. J. Cell. Biol. 1991;115:191–199. doi: 10.1083/jcb.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madsen MA, Deryugina EI, Niessen S, Cravatt BF, Quigley JP. Activity-based protein profiling implicates urokinase activation as a key step in human fibrosarcoma intravasation. J. Biol. Chem. 2006;281:15997–16007. doi: 10.1074/jbc.M601223200. [DOI] [PubMed] [Google Scholar]
- 15.Sier CF, Stephens R, Bizik J, Mariani A, Bassan M, Pedersen N, Frigerio L, Ferrari A, Dano K, Brünner N, Blasi F. The level of urokinase-type activator receptor is increased in serum of ovarian cancer patients. Cancer Res. 1998;58:1843–1849. [PubMed] [Google Scholar]
- 16.Harbeck N, Kates RE, Gauger K, Willems A, Kiechle M, Magdolen V, Schmitt M. Urokinase-type plasminogen activator (uPA) and its inhibitor PAI-1: Novel tumor-derived factors with a high prognostic and predictive impact in breast cancer. Thromb. Haemost. 2004;91:450–456. doi: 10.1160/TH03-12-0798. [DOI] [PubMed] [Google Scholar]
- 17.Duffy MJ, O’Grady P, Devaney D, O’Siorain L, Fennelly JJ, Lijnen HJ. Urokinase-plasminogen activator, a marker for aggressive breast carcinomas. Cancer. 1998;62:531–533. doi: 10.1002/1097-0142(19880801)62:3<531::aid-cncr2820620315>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Herszenyi L, Plebani M, Carraro P, de Paoli M, Roveroni G, Cardin R, Tulassay Z, Naccarato R, Farinati F. The role of cystein and serine proteases in colorectal carcinoma. Cancer. 1999;86:1135–1142. doi: 10.1002/(sici)1097-0142(19991001)86:7<1135::aid-cncr6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Harvey SR, Hurd TC, Markus G, Martinick MI, Penetrante RM, Tan D, Venkataraman P, DeSouza N, Sait SNJ, Driscoll DL, Gibbs JF. Evaluation of urinary plasminogen activator, its receptor, matrix metalloproteinase-9 and von Willebrand factor in pancreatic cancer. Clin. Cancer Res. 2003;9:4935–4943. [PubMed] [Google Scholar]
- 20.Konecny G, Untch M, Pihan A, Kimmig R, Gropp M, Stieber P, Hepp H, Slamon D, Pegram M. Association of urokinase-type plasminogen activator and its inhibitor with disease progression and prognosis in ovarian cancer. Clin. Cancer Res. 2001;7:1743–1749. [PubMed] [Google Scholar]
- 21.Schmitt M, Wilhelm OG, Reuning U, Krüger A, Harbeck N, Lengyel E, Graeff H, Gänsbacher B, Kessier H, Bürgle M, Stürzebecher J, Sperl S, Magdolen V. The urokinase plasminogen activator system as a novel target for tumor therapy. Fibrinolysis & Proteolysis. 2000;14:114–132. [Google Scholar]
- 22.Ertongur S, Lang S, Mack B, Wosikowski K, Muehlenweg B, Gires O. Inhibition of the invasion capacity of carcinoma cells by WX-UK1, a novel synthetic inhibitor of the urokinase-type plasminogen activator system. Int. J. Cancer 2004. 2004;110:815–824. doi: 10.1002/ijc.20192. [DOI] [PubMed] [Google Scholar]
- 23.Ossowski L, Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983;35:611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Aaronson H, Mitola DJ, Leppla SH, Bugge TH. Potent antitumor activity of a urokinase-activated engineered anthrax toxin. Proc. Natl. Acad. Sci. U.S.A. 2003;100:657–662. doi: 10.1073/pnas.0236849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min HY, Doyle LV, Vitt CR, Zandonella CL, Stratton�Thomas JR, Shuman MA, Rosenberg S. Urokinase receptor antagonists inhibit angiogenesis and primary tumor growth in syngeneic mice. Cancer Res. 1996;56:2428–2433. [PubMed] [Google Scholar]
- 26.Vallera DA, Li C, Jin N, Mortari�Panoskaltsis A, Hall WA. Targeting urokinase-type plasminogen activator receptor on human glioblastoma tumors with diphtheria toxin fusion protein DTAT. J. Natl. Cancer Inst. 2002;94:597–606. doi: 10.1093/jnci/94.8.597. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel DA. Synthetic immunology to engineer human immunity. Nat. Chem. Biol. 2010;6:871–872. doi: 10.1038/nchembio.477. [DOI] [PubMed] [Google Scholar]
- 28.Murelli RP, Zhang AX, Michel J, Jorgensen WL, Spiegel DA. Chemical control over immune recognition: A class of antibody-recruiting small molecules that target prostate cancer. J. Am. Chem. Soc. 2009;131:17090–17092. doi: 10.1021/ja906844e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y, Low PS. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol. Immunother. 2002;51:153–162. doi: 10.1007/s00262-002-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Sega E, Low PS. Folate receptor-targeted immunotherapy: Induction of humoral and cellular immunity against hapten-decorated cancer cells. Int. J. Cancer. 2005;116:710–719. doi: 10.1002/ijc.21126. [DOI] [PubMed] [Google Scholar]
- 31.Popkov M, Gonzalez B, Sinha SC, Barbas CF., III Instant immunity through chemically programmable vaccination and covalent self-assembly. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4378–4383. doi: 10.1073/pnas.0900147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rader C, Sinha S, Popkov M, Lerner RA, Barbas CF., III Chemically programmed monoclonal antibodies for cancer therapy: Adaptor immunotherapy based on a covalent antibody catalyst. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5396–5400. doi: 10.1073/pnas.0931308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popkov M, Rader C, Gonzalez B, Sinha SC, Barbas CF., III Small molecule drug activity in melanoma models may be dramatically enhanced with an antibody effector. Int. J. Cancer. 2006;119:1194–1207. doi: 10.1002/ijc.21924. [DOI] [PubMed] [Google Scholar]
- 34.Carlson CB, Mowery P, Owen RM, Dykhuizen EC, Kiessling LL. Selective tumor cell targeting using low-affinity, multivalent interactions. ACS Chem. Biol. 2007;2:119–127. doi: 10.1021/cb6003788. [DOI] [PubMed] [Google Scholar]
- 35.Dubrovska A, Kim C, Elliott J, Shen W, Kuo T�H, Koo D�I, Li C, Tuntland T, Chang J, Groessl T, Wu X, Gorney V, Ramirez�Montagut T, Spiegel DA, Cho CY, Schultz PG. A chemically induced vaccine strategy for prostate cancer. ACS Chem. Biol. 2011;2011 doi: 10.1021/cb200222s. doi: 10.1021/cb200222s. [DOI] [PubMed] [Google Scholar]
- 36.Ortega E, Kostovetzky M, Larralde C. Natural DNP-binding immunoglobulins and antibody multispecificity. Mol. Immunol. 1984;21:883–888. doi: 10.1016/0161-5890(84)90143-3. [DOI] [PubMed] [Google Scholar]
- 37.Kettner C, Shaw E. Inactivation of trypsin-like enzymes with peptides of arginine chloromethyl ketone. Methods Enzymol. 1981;80:826–842. doi: 10.1016/s0076-6879(81)80065-1. [DOI] [PubMed] [Google Scholar]
- 38.Spraggon G, Phillips C, Nowak UK, Ponting CP, Saunders D, Dobson CM, Stuart DI, Jones EY. The crystal structure of the catalytic domain of human urokinase-type plasminogen activator. Structure. 1995;3:681–691. doi: 10.1016/s0969-2126(01)00203-9. [DOI] [PubMed] [Google Scholar]
- 39.Williams EB, Krishnaswamy S, Mann KG. Zymogen/enzyme discrimination using peptide chloromethyl ketones. J. Biol. Chem. 1989;264:7536–7545. [PubMed] [Google Scholar]
- 40.Walker B, Elmore DT. The behaviour of urokinase and porcine kidney cell plasminogen activator towards some synthetic peptides. Thromb. Res. 1984;34:103–107. doi: 10.1016/0049-3848(84)90066-5. [DOI] [PubMed] [Google Scholar]
- 41.Binnema DJ, van Iersel JJL, Dooijewaard G. Quantitation of urokinase antigen in plasma and culture media by use of an ELISA. Thromb. Res. 1986;43:569–577. doi: 10.1016/0049-3848(86)90077-0. [DOI] [PubMed] [Google Scholar]
- 42.Rajagopal V, Kreitman RJ. Recombinant toxins that bind to the urokinase receptor are cytotoxic without requiring binding to the α2-macroglobulin receptor. J. Biol. Chem. 2000;275:7566–7573. doi: 10.1074/jbc.275.11.7566. [DOI] [PubMed] [Google Scholar]
- 43.Bracher M, Gould HJ, Sutton BJ, Dombrowicz D, Karagiannis SN. Three-colour flow cytometric method to measure antibody-dependent tumour cell killing by cytotoxicity and phagocytosis. J. Immunol. Methods. 2007;323:160–171. doi: 10.1016/j.jim.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Boltz�Nitulescu G, Willheim M, Spittler A, Leutmezer F, Tempfer C, Winkler S. Modulation of IgA IgE, and IgG Fc receptor expression of human mononuclear phagocytes by 1α,25-dihydroxyvitamin D3 and cytokines. J. Leuko. Biol. 1995;58:256–262. doi: 10.1002/jlb.58.2.256. [DOI] [PubMed] [Google Scholar]
- 45.Kute TE, Savage L, Stehle JR, Jr., Kim�Shapiro JW, Blanks MJ, Wood J, Vaughn JP. Breast tumor cells isolated from in vitro resistance to trastuzumab remain sensitive to trastuzumab anti-tumor effects in vivo and to ADCC killing. Cancer Immunol. Immunother. 2009;58:1887–1896. doi: 10.1007/s00262-009-0700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J, Wang X, Xu X, Abassi YA. Dynamic and label-free monitoring of natural killer cell cytotoxic activity using electronic cell sensor arrays. J. Immunol. Methods. 2006;309:25–33. doi: 10.1016/j.jim.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Weiner GJ. Monoclonal antibody mechanisms of action in cancer. Immunol. Res. 2007;39:271–278. doi: 10.1007/s12026-007-0073-4. [DOI] [PubMed] [Google Scholar]
- 48.Christiansen J, Rajasekaran AK. Biological impediments to monoclonal antibody�based cancer immunotherapy. Mol. Cancer Ther. 2004;3:1493–1501. [PubMed] [Google Scholar]
- 49.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Eng. J. Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 50.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 51.Ross JS, Gray KE, Webb IJ, Gray GS, Rolfe M, Schenkein DP, Nanus DM, Millowsky MI, Bander NH. Antibody-based therapeutics: Focus on prostate cancer. Cancer Met. Rev. 2005;24:521–537. doi: 10.1007/s10555-005-6194-0. [DOI] [PubMed] [Google Scholar]
- 52.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer. 2006;6:714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 53.Nelson AL, Reichert JM. Development trends for therapeutic antibody fragments. Nat. Rev. Biotechnol. 2009;27:331–337. doi: 10.1038/nbt0409-331. [DOI] [PubMed] [Google Scholar]
- 54.Abès R, Teillaud J�L. Modulation of tumor immunity by therapeutic monoclonal antibodies. Cancer Met. Rev. 2011;30:111–124. doi: 10.1007/s10555-011-9282-3. [DOI] [PubMed] [Google Scholar]
- 55.Lu Y, Klein PJ, Westrick E, Xu L�C, Santhapuram HKR, Bloomfield A, Howard SJ, Vlahov IR, Ellis PR, Low PS, Leamon CP. Strategy to prevent drug-related hypersensitivity in folate-targeted hapten immunotherapy of cancer. AAPS J. 2009;2:628–638. doi: 10.1208/s12248-009-9139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.