Abstract
The intraneuronal conversion of testosterone to estradiol constitutes a critical step in the development and sexual differentiation of the brain of many short gestation mammalian species and has been inferred to play a similar role in long gestation sheep. This conversion is catalyzed by cytochrome P450 aromatase (CYP 19), which is expressed in specific brain structures during fetal development. The present study was undertaken to examine the specific neuroanatomical distribution and relative expression of aromatase mRNA expression in the developing sheep hypothalamus. The fetal sheep is a highly tractable model system for localizing the region-specific expression of aromatase in the brain during prenatal development that can help predict regions where estrogen acts to shape neural development. Our results using real time quantitative RT-PCR revealed that aromatase mRNA was expressed throughout mid to late gestation in the fetal preoptic area and amygdala. In the preoptic area aromatase expression declined with advancing gestation, while in amygdala it increased. No sex differences were observed in either brain area. We next investigated the anatomical distribution of aromatase using in situ hybridization histochemistry and found that the pattern of mRNA expression was largely established by midgestation. High expression was observed in the medial preoptic nucleus, bed n. of the stria terminalis, and corticomedial amygdala. We also observed substantial expression in the dorsal striatum. These results extend our understanding of the developmental expression of aromatase in the fetal sheep brain and lend support to the view that it plays an essential role in sexual differentiation and maturation of the neuroendocrine, motor, and reward control systems.
Keywords: Preoptic area, Striatum, Hypothalamus, Amygdala, Aromatase, CYP19, Sheep, Steroids, Development
Introduction
The conversion of circulating testosterone (T) into estradiol (E2) by neural tissue is a critical step for mediating the differentiation and activation of brain circuits that regulate male reproductive physiology and behavior in many mammalian and avian species (1–3). According to the “aromatization hypothesis” proposed by Naftolin et al. (4), T exerts many of its effects on neural cells, in part or whole, through locally produced estrogen. Cytochrome P450 aromatase (CYP 19) is the terminal enzyme that when complexed with NADPH reductase, catalyzes the transformation of Δ4–5-androgen precursors, such as T and androstenedione, to estrogens, such as E2 and estrone (5). The presence of aromatase activity in the brain has now been shown in a wide variety of species, including mammals, birds, amphibia, and fish (6). In adult mammals, aromatase activity is most abundant in brain regions that are highly involved in the control of reproduction and sexual behavior. These aromatase-rich brain regions overlap extensively with brain areas that express estrogen receptors (ER), supporting the idea that locally formed estrogens exert effects through this transcriptional regulatory pathway. In addition, the observation that aromatase activity can be rapidly inhibited through post-transcriptional phosphorylation suggests that it may function to modulate estrogen’s rapid actions by regulating its local availability (7).
Histochemical studies have provided a more detailed picture for the distribution of aromatase protein and mRNA expression (6). In particular, aromatase is localized to specific brain nuclei and subnuclei, within the circuitry of the medial subdivision of the extended amygdala (AMYG), which is a critical conduit for steroid hormone input regulating homeostatic and reproductive brain functions (8). In addition, various other regions outside of this circuit, such as the hippocampus, regions of the cerebral cortex, midbrain, central AMYG, and spinal cord also contain appreciable aromatase supporting roles for brain-derived estrogens in learning, memory, and sensory perception (9–11).
We demonstrated previously that aromatase activity and mRNA expression in the adult sheep brain is abundant throughout the extended AMYG neuronal circuit (12,13). Especially high amounts of aromatase mRNA expression were observed in the ovine sexually dimorphic nucleus (oSDN) and the bed n. of the stria terminalis (12). The presence of aromatase mRNA in the oSDN is of particular interest because we also found the oSDN is larger in rams that prefer to mate ewes than in rams that are sexually attracted to other rams (14). We do not currently know what function is served by aromatase in this brain region, but it is obvious from other studies in several species including sheep that estrogens synthesized locally in the preoptic area and hypothalamus activate male motivational, copulatory and aggressive behaviors, and are involved in negative feedback regulation of gonadotropin secretion (2,15–17).
The highest levels of aromatase in the hypothalamus of all species studied are found during the critical period for sexual differentiation (18–24). Aromatization of testicular androgens during development is critical for the rat, in which sexual differentiation takes place largely during the early postnatal period (1). At the cellular level, locally produced E2 curtails cell death, stimulates neuronal differentiation and elaborates the growth of processes and synaptic connections leading to the organization of sexually dimorphic neural networks that, in turn, control sexual dimorphic behaviors and reproductive physiology in the adult (25–27).
High levels of aromatase activity are present in dissections of the hypothalamus-preoptic area and AMYG from fetal sheep on gestational day (GD) 65 (24). Sexual differentiation of the brain in sheep and other long gestation mammals takes place prior to birth. Prenatal treatment of females with T (androgenic and estrogenic actions) masculinizes copulatory behaviors and disrupts or abolishes the LH surge response to E2, but prenatal DHT treatment (androgenic actions only) does not (28). It has been inferred from this and similar studies (29) that aromatase is required for at least some of the organizational effects of T in sheep. We found previously that inhibition of aromatase activity during the critical period of brain sexual differentiation in sheep, days 50 through 80 of the 147 day gestation resulted in a modest, yet significant decrease in mounting behavior at 18 months, but failed to alter either sexual preferences, oSDN volume, or the LH surge response to E2 (30,31). The suggestion from this study is that prenatal aromatization may be necessary for complete masculinization, but that locally formed estrogens may act together with androgens either together or sequentially to regulate brain development.
The fetal sheep is a highly tractable model system for localizing the region-specific expression of aromatase in the brain during prenatal development that can help predict regions where estrogen acts to shape neural development. Thus, the present study was undertaken to further understanding of the nature of the complex hormone environment in which the fetal sheep brain develops by analyzing the ontogeny and neuroanatomical distribution of aromatase mRNA expression during the window of time spanning the critical period of sexual differentiation.
Materials and Methods
Animals
Ewes were bred and maintained under standard husbandry conditions at the Sheep Research Facility at Oregon State University in Corvallis, OR. The gestational stage of pregnancy was defined as the time after mating (gestational day 1 (GD 1) = 24 h after mating). Lamb fetuses of various gestational ages ± 2 days (i.e., GD 53, 65, 85, 100, 120, and 135) were delivered surgically as described previously (32). Three to six fetuses of each sex were studied at each age. All procedures complied with the principles and procedures of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of OHSU and OSU.
Tissue Collection
Brains were removed rapidly from the fetal skull, placed on their dorsal surface, trimmed into a block of tissue that extended from the rostral aspect of the optic chiasm to the mammillary bodies. For GD 53 fetuses, the entire block was fixed and sectioned coronally as described below. For all other ages, the brain block was split longitudinally down the midline. The right half of the brain block was then immersion fixed in 4% paraformaldehyde (4°C for 16 h), cryoprotected in 20% sucrose for 3 days, frozen in isopentane at −60 °C, and stored at −80°C. The left half of the brain was used to dissect out the medial preoptic area-anterior hypothalamus (MPOA-AH) and the medial basal hypothalamus. The AMYG was dissected from the medial dorsal aspect of the left temporal lobe. Fixed tissues were sectioned coronally (20 μm or 40 μm thick) into parallel series and mounted onto Superfrost microscope slides (Fisher Scientific). Slides were desiccated under vacuum and stored frozen at −80 C. Adjacent series of brain sections were either stained with thionin or processed for in situ hybridization.
In situ Hybridization
In situ hybridization was performed as described previously (12). Briefly, a sheep specific [33P] aromatase cRNA was synthesized, purified, and diluted in hybridization buffer to an activity of 1.0 × 107 dpm/ml. On the day of hybridization, the tissue sections were treated with Proteinase K (10 μg/ml), acetylated, and dehydrated. The hybridization solution with probe was then pipetted onto the sections (80 μl/slide), covered with a glass coverslip and sealed with DPX before incubation for 18 h at 58°C. Following hybridization, the slides were subjected to RNase digestion (20 μg/ml for 30 min at 37°C) and washed several times to a final stringency of 0.1x SSC at 65°C for 30 min. The slides were then dried under vacuum and exposed to Kodak Biomax MR film for one week. To illustrate the distribution of aromatase mRNA expression, autoradiograms were scanned with a Canoscan 8800F flat bed scanner (Canon USA, Inc., Lake Success, NY) at 600 dpi and digitized using an iMac (Apple, Cupertino, CA) and Adobe Photoshop software (PS3; Adobe Systems Inc., San Jose, CA). The digitized images were adjusted for contrast and brightness, also with Photoshop.
RNA Extraction and Real RT-PCR Quantification
Total RNA was extracted using Trizol (Invitrogen, Gaithersburg, MD) according to the manufacturer’s instructions. The concentration and quality of the RNA was determined using the NanoDrop ND-100 spectrophotometer (NanoDrop Technologies, Wilmington, DE). First-strand cDNA was synthesized from 0.5 μg total RNA using SuperScript™ III reverse transcriptase (RT) and Oligo dt primers in a 20 μl reaction mixture according to the instructions of the manufacturer (Invitrogen, Carlsbad, CA). The newly synthesized cDNA was stored at −20°C and used for assay of mRNA for ovine aromatase by real-time polymerase chain reaction (RT-PCR).
RT-PCR reactions were performed using AmpliTaq Gold® DNA Polymerase (Applied Biosystems, Foster City, CA) and custom primers and TaqMan® MGB probes designed using Primer Express software and synthesized by Applied Biosystems. The sequences are given in Table 1. The probe for ovine aromatase (Accession No. NM_001123000) was labeled with 6-FAM in the 5′ position. The probe for ovine glyceraldehyde-6-phosphate dehydrogenase (GAPDH; Accession No. NM_001190390.1) was labeled with VIC in the 5′ position. Primer efficiencies for aromatase and GAPDH were 95% and 85%, respectively, over five 4-fold dilutions of cRNA from adult AMYG. Separate cDNA sample aliquots were used to measure aromatase and GAPDH in triplicate. Reactions were performed in an ABI Prism 7500 Real-time polymerase chain reaction (PCR) System (Applied Biosystems) using either 900 nM aromatase primers and 200 nM probe or 300 nM GAPDH primers and 200 nM probe. The quantitative PCR conditions included a polymerase activation step at 95°C for 10 min followed by 45 cycles of 95°C for 15 s and 60°C for 60 s. Control reactions were run without cDNA template (no template control) and with total RNA not subjected to RT (-RT control) to show that the primers and probe were specific for the amplified products and that RNA samples were not contaminated with genomic DNA. Quantification of gene expression was performed by the relative standard curve method (33) and reported as fold differences relative to the mean expression level in 65-day-old male fetuses. The youngest age group, i.e. GD 65 males, was arbitrarily chosen as the calibrator. Their cycle threshold (CT) values were within the experimental range of values to be appropriate controls from plate to plate.
Table 1.
Ovine Aromatase and GAPDH primer sequences used in RT-PCR
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | TaqMan probe |
|---|---|---|---|
| Aromatase | CAGAGAAGCTGGAAGACTGCATAG | ACCACGTTTCTCGGCAAA | TTTCGCCACTGAGTTGA |
| GAPDH | TGACCCCTTCATTGACCTTCA | TTGCCATGGGTGGAATCATA | CATGGTTCTACATGTTCC |
Statistical Analysis
In each assay, the CT value for each sample, for each gene, in all groups, was determined in triplicate. The mean value for these was used to determine concentration using the relative standard curve method by interpolating sample concentration from the standard curve. Aromatase expression was normalized to GADPH and then divided by the target quantity of the calibrator, which consisted of cDNA from a pool of untreated GD 65 males. These data were analyzed by two-way ANOVA to determine whether there were main effects of sex and age. Because there were no sex differences the expression data were combined and reanalyzed by a Kruskal-Wallis 1-way ANOVA followed by Dunn’s multiple comparison test to determine what ages were different.
Results
Relative Expression of Aromatase mRNA during Fetal Development
Aromatase mRNA was detectable using quantitative RT-PCR in dissections of the MPOA-AH and AMYG. In MPOA-AH (Fig. 1A), aromatase expression differed with advancing gestational age (F[3, 24] = 4.6; p < 0.01), but did not exhibit a sex difference (F[1, 24] = 0.0007; p = 0.98) or age × sex interaction (F[3, 24] = 0.98; p = 0.42). The data for the sexes were pooled and reanalyzed by 1-way ANOVA followed by posthoc comparisons which revealed that the expression at GD 135 in the MPOA-AH was lower than all other ages. By contrast in AMYG (Fig. 1B), aromatase mRNA expression exhibited a significant change with age (F[3, 25] = 3.1; p < 0.05) that was bimodal; an increase from GD 65 to GD 100 followed by a decrease at GD 120 and increase again on GD 135. No sex difference (F[1, 25] = 0.14; p =0.71) or interaction (F[3, 25] = 0.06; p =0.98) was noted in AMYG. Data pooled between sexes and analyzed as described above revealed that aromatase expression at GD 135 was greater than all other ages.
Figure 1.
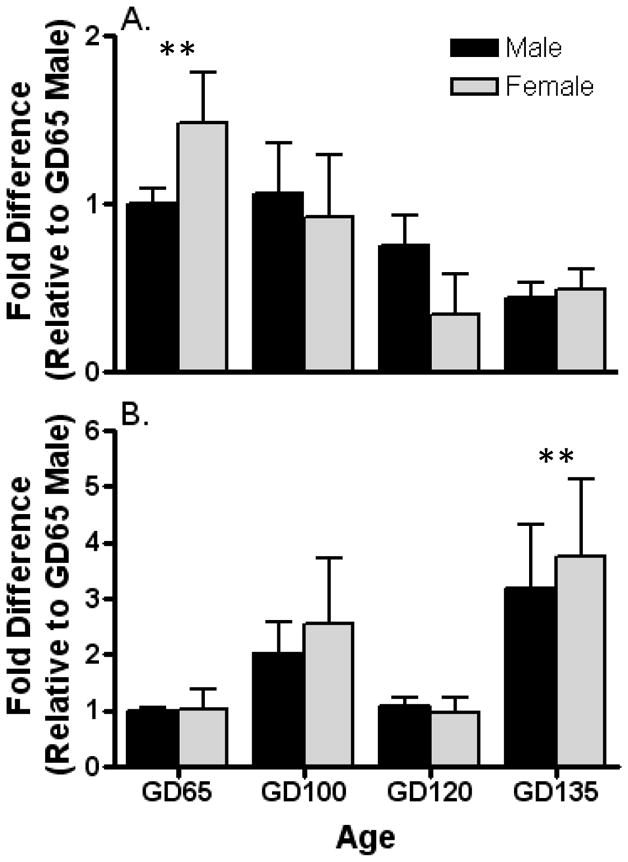
Developmental changes in expression of aromatase mRNA in the left medial preoptic area-anterior hypothalamus (a) and amygdala (b) of fetal sheep. Expression of aromatase mRNA is normalized to GAPDH mRNA expression in the same sample. Data are the mean ± SE fold differences relative to mean expression of 65-day-old male fetuses (n = 3–6 animals per group). Analysis of the data by 2-way ANOVA revealed a significant age effect (P < 0.05), but no sex effect or interaction in both the medial preoptic area and amygdala. Thus, the data for the sexes were combined and reanalyzed by a Kruskal-Wallis 1-way ANOVA followed by Dunn’s multiple comparison test. Poshoc analysis revealed that GD 135 fetuses differed from other ages by exhibiting significantly lower aromatase mRNA expression in the medial preoptic area and significantly higher expression in the amygdala (**, P < 0.05 vs. all other ages).
Neuroanatomical Distribution of Aromatase mRNA Expression in Fetal Sheep
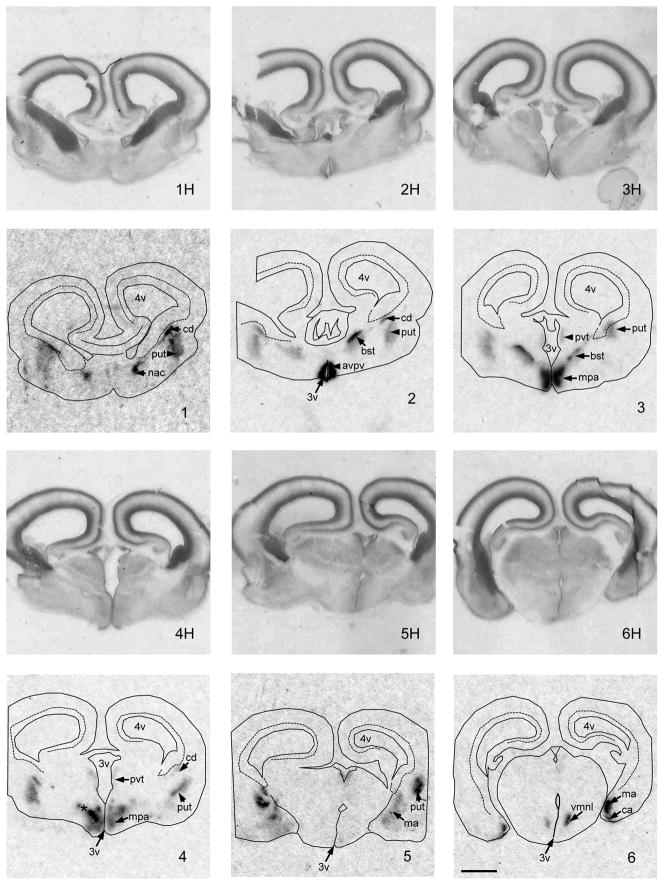
Aromatase mRNA is expressed in the sheep brain as early as the end of the first trimester of fetal development (Figure 2). At GD 53, coronal sections at the level of the striatum revealed moderate expression of aromatase mRNA in the differentiating caudate (Cd), putamen (Pd), and nucleus accumbens (Nac). At this stage in gestation, a relatively large, darkly stained subventicular zone was present dorsal and medial to the Cd, which did not express appreciable aromatase. At the level of the preoptic area, a strong signal was evident in the anterior periventricular preoptic area (AvPv), medial preoptic area (MPA), including the nascent ovine sexually dimorphic n. (oSDN) and the bed nucleus of the stria terminalis (BST). Light to moderate periventricular signal extended dorsally into the thalamus. Progressing caudally through the hypothalamus, a strong signal was detected in the ventromedial n. of the hypothalamus (VMN) and moderate to high density ISH signal was observed in the cortical (CA) and medial AMYG (MA).
Figure 2.
Images of coronal sections through the preoptic area, basal hypothalamus and amygdala of a GD 53 lamb fetus showing the expression of aromatase mRNA. Images are arranged in a rostral to caudal order. 1H–6H are the hemotoxylin stained images, that match the autoradiographic images (1–6) of aromatase mRNA expression generated by in situ hybridization using 33P-labeled cRNA probes. The following anatomic sites of aromatase mRNA expression were distinguished: avpv, anteroventral periventricular preoptic area; bst, bed n. of the strial terminalis; ca, cortical amygdala; cd, caudate n.; ma, medial amygdala; mpa, medial preoptic area; *, nascent ovine sexually dimorphic n.; nac, n. acumbens; put, putamen; pvt, periventricular thalamic area; vmn, ventromedial n. of the hypothalamus. Other abbreviations: f, fornix; 3v, 3rd ventricle; 4v, 4th ventricle. Scale bar measures 2 mm. Brightness and contrast were adjusted to optimize image.
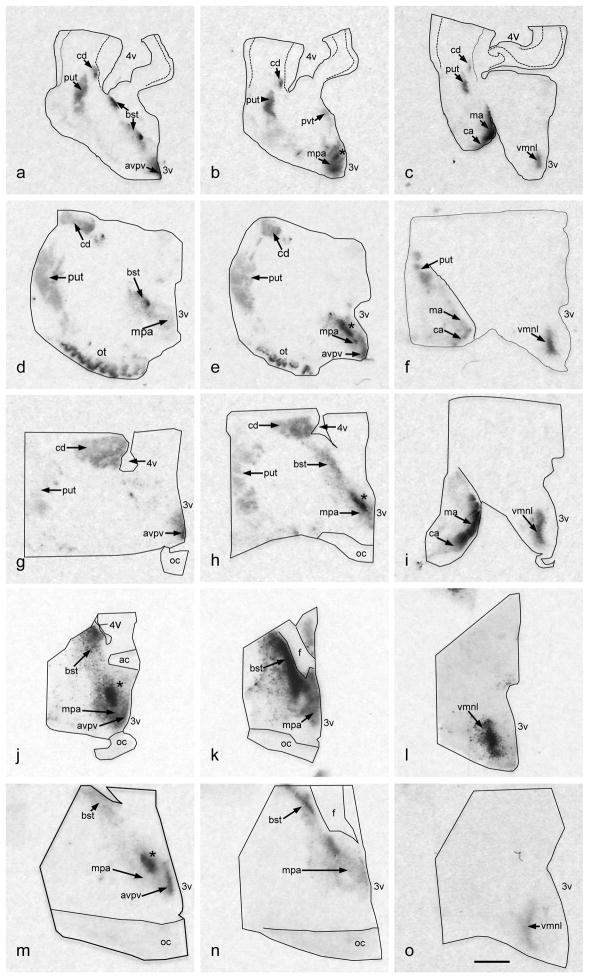
The expression pattern of aromatase mRNA in GD 65, GD 85, GD 100 sheep brain was similar to that observed at GD 53, although enhanced and enlarged (Figure 3). In particular the labeling in the striatum, MPA, BST, and VMN all appear stronger and more defined. The oSDN and the encapsulated BST (BSTe) are clearly distinguished at GD 65. Labeling in the VMN exhibits a crescent shape that corresponds to the lateral aspect of this nucleus (VMNl).
Figure 3.
Digitized autoradiographic film images of coronal sections through the right brains of fetal lambs showing the pattern of expression for aromatase mRNA at GD 65 (a–c), GD 85 (d–f), GD 100 (g–i), GD 120 (j–l) and GD 135 (m–o). Because the size of the brain increases as the fetus ages, less tissue is included in the block of coronally-sectioned tissue from older fetuses. While half of the diencephalon and the cortical medial amygdala are included in the younger fetuses (GD65–100), only the diencephalon is shown in the older fetuses (GD 120–135). For all ages, the tissue is oriented in a rostral to caudal series and the 3rd ventricle is on the lower right-hand side. The following anatomic sites of aromatase mRNA expression were distinguished: avpv, anteroventral periventricular preoptic area; bst, bed n. of the strial terminalis; ca, cortical amygdala; cd, caudate n.; ma, medial amygdala; mpa, medial preoptic area; *, nascent ovine sexually dimorphic n.; nac, n. acumbens; put, putamen; pvt, periventricular thalamic area; vmnl, ventromedial n. of the hypothalamus, ventrolateral component. Other abbreviations: ac, anterior commissure; f, fornix; oc, optic chiasm; 3v, 3rd ventricle; 4v, 4th ventricle. Brightness and contrast were adusted to optimize image. Scale bar measures 2 mm.
At later stages of gestation GD120 and GD 135, aromatase expression consolidates within the MPOA and the strongest signal is observed in the anteroventro-periventricular preoptic n., the oSDN, and the BST (Figure 3). Moderate labeling was located in the VMNl and light expression was observed in the periventricular anterior hypothalamus. At GD135 the heaviest labeling in the AMYG (Figure 4) was visible in the medial and cortical AMYG, areas that have strong reciprocal connections with the hypothalamus. Light to moderate label was also apparent in the central AMYG, piriform cortex and entorhinal cortex.
Figure 4.
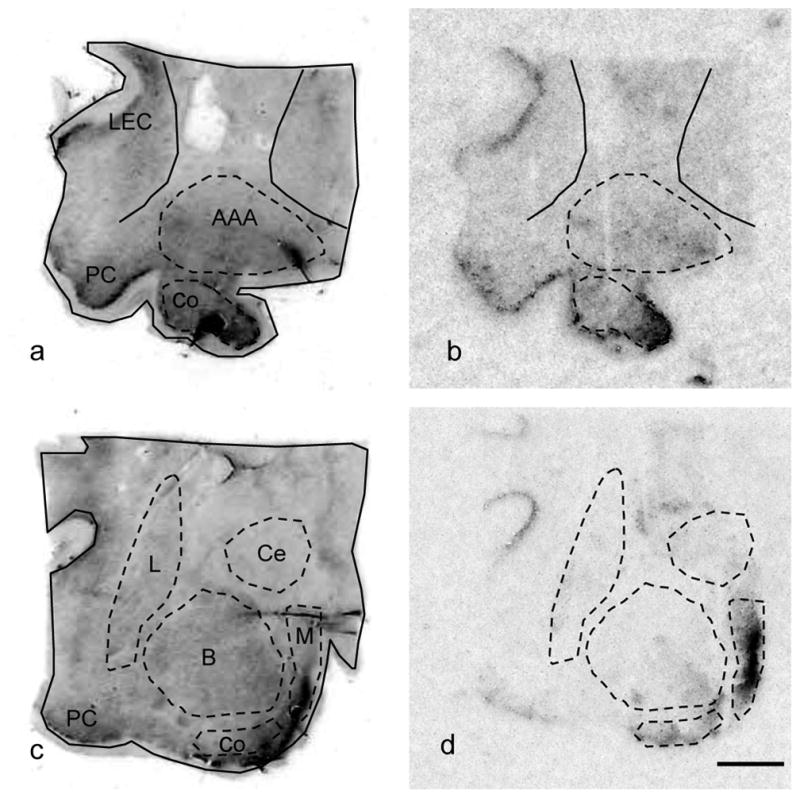
Images of coronal sections through the amygdala of GD 135 day old lamb fetus. Images a and c are hemotoxylin stained sections. Images b and d are digitized autoradiographic film images of aromatase mRNA expression. Abbreviations: AAA, anterior amygdala area; B, basal amygdala; Co, cortical amygdala; Ce, central amygdala; L, lateral amygdala; LEC, lateral entorhinal cortex; M, medial amygdala; PC, periform cortex. Brightness and contrast were adusted to optimize image. Scale bar measures 2 mm.
Discussion
Our experiment provides new information regarding the developmental and regional expression of aromatase mRNA in the developing sheep brain. Quantitative measurement of aromatase mRNA using real time PCR demonstrated that the highest expression in the MPOA occurred at midgestation and declined significantly at GD 135. In contrast, Mura et al. (20) reported that aromatase immunoreactivity is highest at GD 35 and 55 and declined dramatically by GD 80 and 115. Although we did not quantify aromatase mRNA earlier than GD 65, our results suggest that expression is fairly constant throughout midgestation and only drops significantly late in gestation at GD135 (term = 147 d). The discrepancy between the two studies may relate to the different methods used to measure aromatase expression, i.e., protein versus mRNA and could reflect differences in RNA stability or posttranslational processing, although we previously showed a high level of correlation between aromatase mRNA expression and enzyme activity in rat brain (34). Nonetheless, the high levels of aromatase expression at midgestation is indicative of the crucial timing for sexual differentiation of the brain and coincides with a sex difference in plasma testosterone concentrations (35). In short gestation species (i.e., rodents), the peak in both activity and mRNA expression of aromatase in the preoptic area occurs in late gestation and early perinatal life corresponding with the critical period for sexual differentiation of the brain (36). In long gestation species, such as guinea pigs, cattle and monkeys aromatase peaks just prior to midgestation, when the hypothalamus-preoptic area is most responsive to the effects of gonadal steroids (19,37).
Our results demonstrate that the timing for peak aromatase expression differs between the MPOA-AH and AMYG. In the AMYG, expression increased with gestational age in an apparent bimodal manner with peaks at GD 100 and 135. These results suggest that the sensitive periods for estrogen-dependent development differ between the diencephalon and AMYG in sheep. Indeed, studies in rats have shown that diencephalic aromatase expression (activity, protein and RNA) also peaks earlier in development than expression in AMYG (38–40). Aromatase is most abundant in the medial and cortical nuclei of the AMYG, which are part of the steroid-dependent sexually dimorphic brain circuitry controlling sex-odor preference and socio-sexual behaviors (41). Among other sexually dimorphic features, the medial AMYG is larger in male than in female rats and this sex difference arises later in postnatal life than the sex difference in SDN-POA volume and can be altered by steroid treatment even in adults (42,43). We do not know currently whether the AMYG in sheep exhibits sex differences similar to those found in rats.
Age differences in aromatase mRNA concentrations could be brought about by several mechanisms. They might result from differences in the expression and/or stability of the mRNA. Alternatively or in conjunction with this mechanism, they might be due to differences in the number of cells expressing the enzyme in a given area (density) either as a result of age-dependent changes in the cell composition due to differential proliferation or elimination. In rats, the highest levels of aromatase activity occur at the end of the main periods of proliferation of neurons in the MPOA-AH and AMYG (44,45) and overlap with cytological differentiation and increased rate of synaptogenesis (46,47). It would appear from the current study that, similar to the rat, aromatase peaks in the fetal sheep brain during neuronal differentiation. Additionally, studies in rats found regional and sex-dependent hemispheric asymmetries in the developmental expression of aromatase activity suggesting that asymmetries exist in steroid-dependent developmental programs (48). Although no evidence for asymmetry exists currently in sheep, this possibility should be examined in future studies.
No sex differences were observed in aromatase mRNA concentrations during the ages sampled in the current study. These results also differ from the observations of Mura et al (20) who reported that there is a greater percentage of aromatase-immunoreactive cells in the hypothalamus of female than of male sheep fetuses. In addition to the obvious differences in approach that were mentioned above, the sex differences were observed at earlier times (GD 35 and 55) than examined in the current study and it is not clear what precise region of the hypothalamus-preoptic area was sampled. Previous investigations in several other long-gestation species have also failed to find sex differences in aromatase activity in the hypothalamic and AMYG of fetuses, including our earlier study in sheep (18,22,49). In contrast, sex differences in hypothalamic aromatase activity were detected in fetal ferrets (50), and in rats, sex differences emerged during the first week of life (51). The present results suggest that even if sex differences in aromatase expression exist in the sheep brain they are not a constant feature throughout the critical period. Nevertheless, it is likely that males are exposed to higher tissue levels of estrogens because serum testosterone substrate concentrations are higher in males than in females throughout much of midgestational development (24,35,52).
Our results further demonstrated that the neuroanatomical pattern of aromatase mRNA expression was largely established by the beginning of the second trimester of gestation, as early as GD 53. At this time aromatase signal was robust in the periventricular preoptic area, medial preoptic n., bed n. of the stria terminalis, ventromedial hypothalamic n., and corticomedial AMYG. These results support our previous observation that the highest levels of aromatase activity are present in the fetal and adult MPOA-AH and AMYG (13,24) and closely matches the distribution of mRNA expression that we observed in late gestation fetal lambs and adult rams (12,53). It is also a pattern of expression that has been seen in a wide variety of fetal and adult vertebrates, as revealed by enzyme activity measurements and histochemical techniques (6). The expression of aromatase overlaps to a large degree with the circuitry of the medial extended AMYG that controls several sexual and social behaviors and which is both steroid sensitive and sexually dimorphic (41). The presence of high levels of aromatase mRNA expression in the fetal diencephalon throughout the critical period is consistent with the idea that estrogen synthesized locally by aromatase within this brain area takes part in the crucial neuronal mechanisms that masculinize and defeminize the fetal sheep brain (54).
The developmental time course of aromatase expression overlaps with the time when receptors for estrogens, androgens and progesterone are all present in the fetal sheep hypothalamus and AMYG (19,55–57). The presence of estrogen receptors provides evidence that the molecular and cellular substrates for estrogen action are intact at midgestation in the fetal lamb and agree with physiological studies that suggest aromatization is involved in masculinization of sheep sexual behavior and suppression of the LH surge (28). The simultaneous expression of all three gonadal steroid receptor genes suggest that circulating hormones produced by the fetus and/or mother may coordinately regulate developmental processes that lead to structural and functional changes in the brain important for maturation of the neuroendocrine brain. However, we previously found that inhibition of brain aromatase during the critical period was without effect on the volume of the oSDN (31) suggesting that locally formed estrogens are probably not regulating cell survival in the sheep preoptic area as they do in rats (25,58), but may be more important in the specification of a particular cellular phenotype as they appear to do in ferrets (59). Recent evidence in the aromatase knockout mouse also suggests local estrogen synthesis through aromatization plays an essential role in modulating dendritic growth, spinogenesis and synaptogenesis (60).
Substantial aromatase mRNA expression was detected in the dorsal striatum (i.e., caudate nucleus and putamen) in fetal sheep from gestational ages 53 to 100, while a less dense signal was present in the ventral striatum (i.e., olfactory tubercle and nucleus accumbens). This observation agrees with previous reports in the mouse that aromatase is expressed within the striatum (61) and ventral midbrain of mice (62) during perinatal development. The dorsal striatum receives dopaminergic input from the substantia nigra and regulates locomotor activity and is involved in stereotypical behavior (e.g., grooming and gnawing in rats). The ventral striatum receives its inputs from the ventral tegmental area. This pathway also has some influence on locomotor behavior and is involved primarily in regulating motivation, reward and reinforcement. Malfunctions of these systems lead to a number of neurological and psychiatric disorders including, addiction, schizophrenia, Parkinson’s disease, and other movement disorders. Several studies have shown that estrogen potentiates dopaminergic function in the striatum and nucleus accumbens in females but not in males (63) and exerts greater neuroprotective effects in the striatum of females than of males (64,65). These observations suggest that the striatum is sexually dimorphic and may contribute to sex biases in brain disorders. Although the origins of sexual dimorphisms in the striatum have not been as extensively researched as those in the hypothalamus, it appears that both organizational actions of hormones and genetic factors both play roles (65–67). The striatal sensitivity to estradiol is suppressed in males and, similar to the defeminization processes that occur in the hypothalamus, this could be dependent in part on the aromatization of testosterone and subsequent brain exposure to estradiol during perinatal development. Together with earlier studies in rodent, the present results demonstrating that aromatase is present in the striatum at a time when the male testis is producing testosterone support this possibility.
In conclusion, the ontogenic profile and expression of aromatase mRNA in the developing lamb brain supports the general view that locally formed estrogens are important modulators of the cellular and molecular processes that are responsible for the maturation and sexual differentiation of the brain. In this respect, it was not surprising that the highest concentrations of aromatase were circumscribed within the hypothalamus-preoptic area and corticomedial AMYG, areas of the brain that control reproductive physiology and behavior. In addition, we found that a major site of extra-hypothalamic aromatase exists within the striatum of the sheep fetus. This observation confirms a previous study in the mouse and raises the possibility that aromatase is involved in striatal development.
Acknowledgments
This work was supported by NIH R01 RR014270 (CER)
References
- 1.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 2.Hull EM, Meisel RL, Sachs BD. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 3–137. [Google Scholar]
- 3.Balthazart J, Taziaux M, Holloway K, Ball GF, Cornil CA. Behavioral effects of brain-derived estrogens in birds. Ann N Y Acad Sci. 2009;1163:31–48. doi: 10.1111/j.1749-6632.2008.03637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 5.Simpson ER. Aromatase: biologic relevance of tissue-specific expression. Semin Reprod Med. 2004;22:11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 6.Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Wood RI. Thinking about networks in the control of male hamster sexual behavior. Horm Behav. 1997;32:40–45. doi: 10.1006/hbeh.1997.1403. [DOI] [PubMed] [Google Scholar]
- 9.Boon WC, Horne MK. Aromatase and its inhibition in behaviour, obsessive compulsive disorder and parkinsonism. Steroids. 2011;76:816–819. doi: 10.1016/j.steroids.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Evrard HC. Estrogen synthesis in the spinal dorsal horn: a new central mechanism for the hormonal regulation of pain. Am J Physiol Regul Integr Comp Physiol. 2006;291:R291–R299. doi: 10.1152/ajpregu.00930.2005. [DOI] [PubMed] [Google Scholar]
- 11.MacLusky NJ, Walters MJ, Clark AS, Toran-Allerand CD. Aromatase in the cerebral cortex, hippocampus, and mid-brain: ontogeny and developmental implications. Mol Cell Neurosci. 1994;5:691–698. doi: 10.1006/mcne.1994.1083. [DOI] [PubMed] [Google Scholar]
- 12.Roselli CE, Stormshak F, Resko JA. Distribution of aromatase mRNA in the ram hypothalamus: An In Situhybridization study. J Neuroendocrinol. 2000;12:656–664. doi: 10.1046/j.1365-2826.2000.00496.x. [DOI] [PubMed] [Google Scholar]
- 13.Roselli CE, Stormshak F, Resko JA. Distribution and regulation of aromatase activity in the ram hypothalamus and amygdala. Brain Res. 1998;811:105–110. doi: 10.1016/s0006-8993(98)00995-0. [DOI] [PubMed] [Google Scholar]
- 14.Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- 15.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 16.Sharma TP, Blache D, Blackberry MA, Martin GB. Role of peripheral and central aromatization in the control of gonadotrophin secretion in the male sheep. Reprod Fertil Dev. 1999;11:293–302. doi: 10.1071/rd99084. [DOI] [PubMed] [Google Scholar]
- 17.Roselli CE. Brain aromatase: Roles in reproduction and neuroprotection. The J Steroid Biochem Mol Biol. 2007;106:143–150. doi: 10.1016/j.jsbmb.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roselli CE, Resko JA. Effects of gonadectomy and androgen treatment on aromatase activity in the fetal monkey brain. Biol Reprod. 1986;35:106–112. doi: 10.1095/biolreprod35.1.106. [DOI] [PubMed] [Google Scholar]
- 19.Peruffo A, Cozzi B, Ballarin C. Ontogenesis of brain aromatase P450 expression in the bovine hypothalamus. Brain Res Bull. 2008;75:60–65. doi: 10.1016/j.brainresbull.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Mura A, Gadau S, Lepore G, Balzano F, Zedda M, Mura E, Farina V. Expression and distribution of P450-aromatase in the ovine hypothalamus at different stages of fetal development. Neuro Endocrinol Lett. 2010;31:690–699. [PubMed] [Google Scholar]
- 21.Colciago A, Celotti F, Pravettoni A, Mornati O, Martini L, Negri-Cesi P. Dimorphic expression of testosterone metabolizing enzymes in the hypothalamic area of developing rats. Dev Brain Res. 2005;155:107–116. doi: 10.1016/j.devbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Connolly PB, Roselli CE, Resko JA. Aromatase activity in developing guinea pig brain: Ontogeny and effects of exogenous androgens. Biol Reprod. 1994;50:436–441. doi: 10.1095/biolreprod50.2.436. [DOI] [PubMed] [Google Scholar]
- 23.Hutchison JB, Wozniak A, Beyer C, Karolczak M, Hutchison RE. Steroid metabolising enzymes in the determination of brain gender. J Steroid Biochem Molec Biol. 1999;69:85–96. doi: 10.1016/s0960-0760(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 24.Roselli CE, Resko JA, Stormshak F. Estrogen synthesis in fetal sheep brain: effect of maternal treatment with an aromatase inhibitor. Biol Reprod. 2003;68:370–374. doi: 10.1095/biolreprod.102.007633. [DOI] [PubMed] [Google Scholar]
- 25.Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is determined by the perinatal hormone environment. Neurosci Lett. 1982;33:295–298. doi: 10.1016/0304-3940(82)90388-3. [DOI] [PubMed] [Google Scholar]
- 27.Houtsmuller EJ, Brand T, De Jonge FH, Joosten RNJMA, Van De Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav. 1994;56:535–541. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 28.Masek KS, Wood RI, Foster DL. Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology. 1999;140:3459–3466. doi: 10.1210/endo.140.8.6913. [DOI] [PubMed] [Google Scholar]
- 29.Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roselli CE, Schrunk JM, Stadelman HL, Resko JA, Stormshak F. The effect of aromatase inhibition on the sexual differentiation of the sheep brain. Endocrine. 2006;29:501–512. doi: 10.1385/ENDO:29:3:501. [DOI] [PubMed] [Google Scholar]
- 31.Roselli CE, Stormshak F. The neurobiology of sexual partner preferences in rams. Horm Behav. 2009;55:611–620. doi: 10.1016/j.yhbeh.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roselli CE, Estill C, Stadelman HL, Meaker M, Stormshak F. Separate critical periods exist for testosterone-induced differentiation of the brain and genitals in sheep. Endocrinology. 2011;152:2409–2415. doi: 10.1210/en.2010-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6:62. doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- 35.Pomerantz DK, Nalbandov AV. Androgen levels in the sheep fetus during gestation. Proc Soc Exp Biol Med. 1975;149:413–420. doi: 10.3181/00379727-149-38818. [DOI] [PubMed] [Google Scholar]
- 36.Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- 37.Resko JA, Roselli CE. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: observations on guinea pigs and nonhuman primates. Cell Mol Neurobiol. 1997;17:627–648. doi: 10.1023/A:1022534019718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobet SA, Baum MJ, Tang HB, Shim JH, Canick JA. Aromatase activity in the perinatal rat forebrain: effects of sex, age and intrauterine position. Dev Brain Res. 1985;23:171–178. doi: 10.1016/0165-3806(85)90038-0. [DOI] [PubMed] [Google Scholar]
- 39.Lauber ME, Lichtensteiger W. Pre- and postnatal ontogeny of aromatase cytochrome P450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology. 1994;135:1661–1668. doi: 10.1210/endo.135.4.7925130. [DOI] [PubMed] [Google Scholar]
- 40.Zhao C, Fujinaga R, Tanaka M, Yanai A, Nakahama K, Shinoda K. Region-specific expression and sex-steroidal regulation on aromatase and its mRNA in the male rat brain: immunohistochemical and in situ hybridization analyses. J Comp Neurol. 2007;500:557–573. doi: 10.1002/cne.21193. [DOI] [PubMed] [Google Scholar]
- 41.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 42.Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Mizukami S, Nishizuka M, Arai Y. Sexual difference in nuclear volume and its ontogeny in the rat amygdala. Exp Neurol. 1983;79:569–575. doi: 10.1016/0014-4886(83)90235-2. [DOI] [PubMed] [Google Scholar]
- 44.Altman J, Bayer SA. Development of the diencephalon in the rat. I Autoradiographic study of the time of origin and settling patterns of neurons of the hypothalamus. J Comp Neurol. 1978;182:945–971. doi: 10.1002/cne.901820511. [DOI] [PubMed] [Google Scholar]
- 45.Bayer SA. Quantitative 3H-thymidine radiographic analyses of neurogenesis on the rat amygdala. J Comp Neurol. 1980;194:845–875. doi: 10.1002/cne.901940409. [DOI] [PubMed] [Google Scholar]
- 46.Reier PJ, Cullen MJ, Froelich JS, Rothchild I. The ultrastructure of the developing medial preoptic nucleus in the postnatal rat. Brain Res. 1977;122:415–436. doi: 10.1016/0006-8993(77)90454-1. [DOI] [PubMed] [Google Scholar]
- 47.Lawrence JM, Raisman G. Ontogeny of synapses in a sexually dimorphic part of the preoptic area in the rat. Brain Res. 1980;183:466–471. doi: 10.1016/0006-8993(80)90482-5. [DOI] [PubMed] [Google Scholar]
- 48.Von Ziegler NI, Lichtensteiger W. Asymmetry of brain aromatase activity: region-and sex- specific developmental patterns. Neuroendocrinology. 1992;55:512–518. doi: 10.1159/000126165. [DOI] [PubMed] [Google Scholar]
- 49.George FW, Tobleman WT, Milewich L, Wilson JD. Aromatase activity in the developing rabbit brain. Endocrinology. 1978;102(1):86–91. doi: 10.1210/endo-102-1-86. [DOI] [PubMed] [Google Scholar]
- 50.Tobet SA, Shim JH, Osiecki ST, Baum MJ, Canick JA. Androgen aromatization and 5alpha-Reduction in ferret brain during perinatal development: Effects of sex and testosterone manipulation. Endocrinology. 1985;116(5):1869–1877. doi: 10.1210/endo-116-5-1869. [DOI] [PubMed] [Google Scholar]
- 51.MacLusky NJ, Philip A, Hurlbut C, Naftolin F. Estrogen formation in the developing rat brain: sex differences in aromatase activity during early post-natal life. Psychoneuroendocrinology. 1985;10:355–361. doi: 10.1016/0306-4530(85)90013-7. [DOI] [PubMed] [Google Scholar]
- 52.Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intra-uterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84:87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- 54.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roselli CE, Resko JA, Stormshak F. Expression of steroid hormone receptors in the fetal sheep brain during the critical period for sexual differentiation. Brain Res. 2006;1110:76–80. doi: 10.1016/j.brainres.2006.06.070. [DOI] [PubMed] [Google Scholar]
- 56.Schaub CE, Gersting JA, Keller-Wood M, Wood CE. Development of ER-alpha and ER-beta expression in the developing ovine brain and pituitary. Gene Expr Patterns. 2008;8:457–463. doi: 10.1016/j.gep.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Wood CE, Keller-Wood M. Ontogeny of androgen receptor expression in the ovine fetal central nervous system and pituitary. Neurosci Lett. 2008;439:153–156. doi: 10.1016/j.neulet.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- 59.Park JJ, Baum MJ, Paredes RG, Tobet SA. Neurogenesis and cell migration into the sexually dimorphic preoptic area/anterior hypothalamus of the fetal ferret. J Neurobiol. 1996;30:315–328. doi: 10.1002/(SICI)1097-4695(199607)30:3<315::AID-NEU1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 60.Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda SI, Harada N, Tsutsui K. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing purkinje cell. J Neurosci. 2007;27:7408–7417. doi: 10.1523/JNEUROSCI.0710-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Küppers E, Beyer C. Expression of aromatase in the embryonic and postnatal mouse striatum. Mol Brain Res. 1998;63:184–188. doi: 10.1016/s0169-328x(98)00279-4. [DOI] [PubMed] [Google Scholar]
- 62.Raab H, Beyer C, Wozniak A, Hutchison JB, Pilgrim C, Reisert I. Ontogeny of aromatase messenger ribonucleic acid and aromatase activity in the rat midbrain. Mol Brain Res. 1995;34:333–336. doi: 10.1016/0169-328x(95)00196-y. [DOI] [PubMed] [Google Scholar]
- 63.Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 64.Küppers E, Ivanova T, Karolczak M, Beyer C. Estrogen: A multifunctional messenger to nigrostriatal dopaminergic neurons. J Neurocytol. 2000;29:375–385. doi: 10.1023/a:1007165307652. [DOI] [PubMed] [Google Scholar]
- 65.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharm Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dewing P, Chiang CWK, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Current Biol. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 67.Stroppolo A, Tian C, Guinea B, Olm V, Sheffield R, Sommer J, Ehrlich ME. 17β-Estradiol promotes striatal medium size spiny neuronal maturation in vitro. Neuroendocrinology. 2004;79:259–267. doi: 10.1159/000079320. [DOI] [PubMed] [Google Scholar]