Abstract
S100A8 and S100A9 regulate polymorphonuclear neutrophils (PMNs) recruitment and represent 40% of PMN cytosolic protein weight. We have shown that S100A8/S100A9 inhibit PMN oxidative metabolism. The present study was designed to elucidate the mechanisms of this anti-oxidative effect. We hypothesized that the protease activated receptor-2 (PAR-2) played a role in the down-regulation of PMN oxidative metabolism by S100A8/S100A9.
Freshly isolated PMNs were tested for their ability to oxidize dichlorofluorescin-diacetate. Functional inhibition of PAR-2 with ENMD-1068, the pepducin P2pal-21 or an antibody directed at PAR-2 cleavage/activation site, resulted in a significant inhibition of S100A8 and S100A9 anti-oxidative effect. Conversely, the controlled activation of PAR-2 potentiated S100 anti-oxidative effect.
Taken together, the data indicate that the anti-oxidative effect of S100A8/A9 is initiated by PAR-2 activation. S100A8/S100A9 may therefore dampen inflammation without interfering with its initial strength. This finding opens translational possibilities to limit deleterious PMN activation with a dual PAR-2/S100 strategy.
Keywords: neutrophil (PMN), S100A8, S100A9, calprotectin, PAR-2
1. Introduction
The recruitment of polymorphonuclear neutrophils (PMNs) into tissue and their subsequent activation is a crucial and early event in acute inflammation. Once recruited, PMNs produce and release copious amounts of reactive oxygen species (ROS) that target potential bacterial invaders. A failure in sufficient production of ROS leads to frequent and recurrent bacterial and fungal infections; as observed in chronic granulomatous disease (CGD), a disease prompted by a deficient oxidase system in PMNs (Eckert et al., 1995). Conversely, excess ROS production contributes to the pathophysiology of conditions such as impaired wound healing (Gordillo and Sen, 2003), cardiovascular disease (Cave et al., 2006), sepsis (Guo and Ward, 2007)or ischemia/reperfusion injury(Kaminski et al., 2002). The role of S100A8/A9 in the regulation of PMN oxidative metabolism is to date a controversial issue. Some reports indicate that S100A8/A9 physically interact with the oxidase complex and enhance its activity (Doussiere et al., 2002). Seemingly in contradiction with this oxidation enhancing effect, S100A8 and S100A9 have been shown by others to scavenge ROS (Lim et al., 2008) an effect which potentially would reduce oxidative stress.
In our previous studies, we have shown that calprotectin, an immune regulatory molecule, inhibited the oxidative metabolism of PMNs in-vitro, a process linked to adenosine metabolism (Sroussi et al., 2010). In this study we sought further to explore the mechanism (s) of S100A8/9 anti-oxidative effect.
Calprotectin is a heterocomplex formed by S100A8 and S100A9, two calcium binding proteins which represents 40% of PMN cytosolic proteins (by weight) (Edgeworth et al., 1991). A familiar syndrome of immune deficiency, growth retardation, and arthritis has been linked to unusually high serum levels of calprotectin (Sampson et al., 2002). As epithelial expression and circulating levels of calprotectin increase with inflammation, some have suggested that calprotectin may have a pro-inflammatory role (Youssef et al., 1999). In support of this view, calprotectin was shown to activate the recruitment of PMNs and increased their adhesion by activating the beta 2 integrin MAC-1 (Newton and Hogg, 1998). What is more, the functional blockage of calprotectin with a specific antibody reduced PMN recruitment stimulated by lipopolysaccharides (LPS) or monosodium urate monohydrate in-vivo (Ryckman et al., 2003).
Seemingly in contradiction with those findings, calprotectin produced a marked anti-inflammatory effect in a model of adjuvant-induced arthritis in rats (Brun et al., 1995). Moreover, calprotectin deactivated peritoneal macrophages (Aguiar-Passeti et al., 1997), protected the liver from LPS induced inflammation (Ikemoto et al., 2007), suppressed inflammation in experimental autoimmune myocarditis (Otsuka et al., 2009), and reduced inflammatory pain in a model of neutrophilic peritonitis (Pagano et al., 2002). Glucocorticoids were shown to induce calprotectin expression also supporting an anti-inflammatory role (Hsu et al., 2005). Additionally, we have presented evidence, in previous studies, for the ability of S100A8 and S100A9 to repel PMNs (fugetaxis) and inhibit their chemotaxis toward chemokines in-vitro (Sroussi et al., 2006; Sroussi et al., 2007). We have shown that S100A8 inhibits LPS induced recruitment of PMNs in the rat air-pouch model of inflammation in-vivo.
Reports from others indicate that the C-terminal domain of the murine S100A9 (mS100A9p) reproduced several anti-inflammatory and antinociceptive properties of the full-length S100A9 protein. mS100A9p inhibits spreading and phagocytic activity of adherent peritoneal cells (Pagano et al., 2005) and inhibits hyperalgesia induced by jararhagin (Dale et al., 2004) or by an activator of the protease-activated receptor 2 (PAR2) (Dale et al., 2006). Furthermore, mS100A9p blocked the spreading and phagocytosis induced by PAR1 agonists while it did not interfere in PAR-2 induced PMN spreading (Pagano et al.). Implication of calprotectin in PARs functions was supported by those last studies, but the nature of this interaction remains elusive. Recently, S100A8 and S100A9 were shown to act via the toll like receptor-4 (TLR-4) (Vogl et al., 2007). TLR-4 and PAR-2 (Nhu et al.) form a functionally cooperative receptor complex. This could indicate that PAR-2 may also play a role in S100A8/A9’s functions, a possibility we addressed in this study.
The PARs form a family of G-coupled receptors comprising of 4 members named PAR-1 to PAR-4. PARs are activated after proteolysis of an N-terminal portion of the receptors resulting in the unmasking of a tethered ligand (Macfarlane et al., 2001). The regulation of PARs activity is a complex process. Proteases can either activate PARs by unmasking their ligand or prevent their future proteolytic activation by cleaving its N-terminal extracellular domain inclusive of the activating motif. PAR-1, PAR-3 and PAR-4 are activated by thrombin, whereas PAR-2 activation is believed to be the result of other proteases such as PMN derived pr3, coagulation associated factor Xa or Gingipain (RgpB, HRgpA and Kgp) from Porphyromonas gingivalis, (Darveau, 2009). Experimentally, small peptides mimicking the native activating sequence of PARs are routinely used to specifically activate PARs. Those peptides are referred to as PAR-activating peptide (PAR-AP). PAR-1 and PAR-4 stimulate platelet functions related to hemostasis in humans (Kahn et al., 1999). Activation of PAR-3 and PAR-4 in the mouse triggers essentially similar functions as PAR1/PAR4 humans (Kahn et al., 1998). PAR3 has no known functions in humans and PAR-2 is broadly expressed (including on PMNs) and implicated in the pathophysiology of inflammatory, neoplastic and sensory diseases (Kawabata, 2006).
In this work we tested the hypothesis that PAR-2 was involved in S100A8 and S100A9 regulation of PMN oxidative metabolism. We present data in support of this hypothesis and implicating PAR-2 in the regulation and modulation of S100A8 and S100A9 anti-oxidative effect.
2. Methods
2.1 Expression and purification of recombinant S100 proteins
Recombinant S100A8 and S100A9 protein were produced and purified based on standard methods and as previously described (Sroussi et al., 2006; Sroussi et al., 2007). Briefly, both proteins were cloned in a pGEX-2T GST vector (Amersham, Piscataway, NJ). The proteins were expressed in Escherichia coli as GST fusion proteins. The GST tag was cleaved during the purification process. Protein concentration was assessed through a Bradford protein assay (Pierce, Rockford, IL).
Reagents
Dichlorofluorescin diacetate (DCFH-DA) and was purchased from EMD Calbiochem (San Diego, California). PAR2-AP (SLIGKV-NH2) and PAR4-AP (GYPGQV-NH2) were purchased from Anaspec (San Jose, CA). Phorbol 12-myristate 13-acetate (PMA), Phenylmethanesulfonyl fluoride (PMSF) and Cathepsin G from human sputum were purchased from Sigma-Aldrich (St. Louis, Mo). A palmitoylated peptide P2-pal-21 (palmitoyl-RMLRSSAMDENSEKKRKRAIK-CONH2) which specifically blocks PAR-2 (Covic et al., 2002) was synthesized and purified >93% by peptide 2.0 Inc (Chantilly, VA). Mouse monoclonal antibody against the activation/cleavage site of PAR-2 (SAM-11) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). ENMD-1068 was purchased from Enzo Biochem, (Plymouth Meeting, PA).
2.2 Isolation of peripheral PMNs
Peripheral neutrophils were isolated from heparinized blood donated by healthy volunteers according to a protocol approved by the University of Illinois Institutional Review Board. The cells were isolated using a histopaque gradient (Sigma-Aldrich (St. Louis, Mo)) according to the manufacturer’s instructions. Cell viability and identity was confirmed after Coomassie blue staining. Live cells and neutrophils represented at least 95% of isolated cells.
2.3 Assay for oxidative activation of neutrophils
The method for the measurement of oxidative activation of neutrophils was based on the ROS-dependent oxidation of DCFH-DA to DCF and was adapted from Ciapetti et al (Ciapetti et al., 1998). DCFH-DA crosses the cell membrane and is hydrolyzed by nonspecific esterases to nonfluorescent DCFH. Its oxidation by ROS results in the generation of highly fluorescent DCF (LeBel et al., 1992). DCFH-DA is therefore a widely accepted probe for the measurement of an overall index of oxidative activity. The assays were run in clear bottom black 96-well plates. “Edge effects” (a higher fluorescence in edge wells) were avoided by using only center wells. Briefly, 50 μl of Phosphate Buffered Saline (PBS) containing DCFH-DA was added to each well with the final DCFH-DA concentration of 10μg/ml. Just before a baseline reading 100,000 neutrophils in 50 μl PBS were placed in each well. 96-well plates were incubated at 37°C and 5% CO2 and were read at baseline (immediately after cell addition to the plates) and at indicated time points in a Spectra Max Gemini XS fluorescent plate reader. The excitation wavelength was 485 and the reading was done at 530 nm. Wells with no DCFH-DA were used to measure background fluorescence which was subtracted from each reading. Controls with no cells were also analyzed and display no increased fluorescence over time. All assays were conducted in triplicate or quadruplicate wells.
2.4 Assays for PAR-2 activation
PAR-2 activation was tested with enzyme fragment complementation assay conducted in PathHunter eXpress™ β-arrestin PAR-2 cells from DiscoveRx Corporation (Fremont, CA). In this assay recruitment of β-arrestin by the activated GPCR is detected by the reconstitution of the β-galactosidase enzyme which is split into two inactive fragments, one fused to the transfected GPCR (PAR-2) and the other to β-arrestin. The interaction of β-arrestin and the GPCR is quantified by β-galactosidase activity as measurable by the hydrolysis of a substrate and the generation of a fluorescence signal.
2.5 Data analysis
In order to avoid differences between donors, experimental procedures, and/or plate to plate variation, all experimental conditions and statistical analysis were run within one plate inclusive of all positive and negative controls. Planned comparisons were performed using t-tests and statistical significance was determined at p < 0.05. Comparisons between the S100A8, S100A9, combined proteins and control in timeline experiments were calculated with two sample t-tests using SPSS (SPSS Inc., Chicago, IL) with alpha set to ≤ 0.05 to determine statistical relevance.
3 Results
3.1 ENMD-1068 inhibits LPS but not the constitutive or PMA induced stimulation of PMN oxidative metabolism
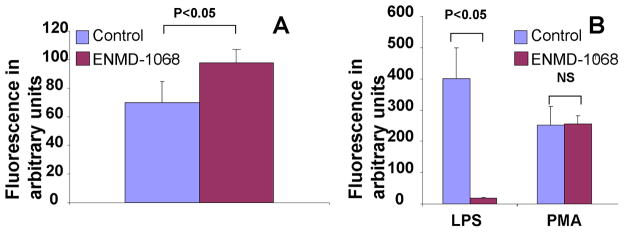
We first tested the effect of ENMD-1068, a PAR-2 specific antagonist (Kelso et al., 2007; Kelso et al., 2006) on the oxidative metabolism of PMNs. ENMD-1068 at a concentration of 5 mM caused a small but significant increase in the constitutive oxidative activation of PMNs (Fig. 1A). This effect of ENMD-1068 was dose dependent (data not shown). We next evaluated the effect of ENMD-1068 on controlled activation of PMNs. Activation of PMNs with LPS was almost completely eliminated by ENMD-1068 whereas the activation by PMA, which directly activate the NADPH oxidase remained unchanged. (Fig. 1B)
Figure 1.
Fluorescence emission of PMNs incubated with DCFH-DA probe for 1 hour in the presence or absence of ENMD-1068 at a concentration of 5 mM. The effect of ENMD-1068 was tested on the constitutive oxidative metabolism of PMNs (A) and on LPS (2.5 μg/ml) and PMA (1μM) (B) The data represent the mean ± SD and it is a representative of at least 4 experiments conducted in triplicates or quadruplicates.
3.2 ENMD-1068 blocks the anti-oxidative effect of S100A8/A9
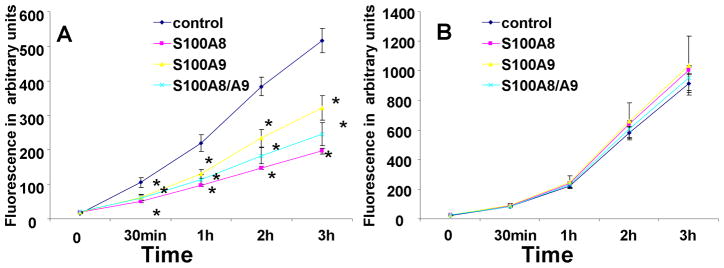
The effect of ENMD-1068 was next tested on the anti-oxidative effect of S100A8/A9. A time course study revealed that while S100A8 and S100A9, separately or together, significantly, reduced the constitutive oxidative metabolism of PMNs (Fig. 2A), this effect was not observed when PMNs were treated with ENMD-1068. This data supported a critical role for PAR-2 in S100A8/A9 effect (Fig 2B).
Figure 2.
Time course of fluorescence emission of PMNs incubated with DCFH-DA probe in the presence or absence of 10 μg/ml S100A8, S100A9 or S100A8+S100A9 (A). The assay was repeated in the presence of ENMD-1068 at a concentration of 5 mM. (B). The data represent the mean ± SD and it is a representative of at least 4 experiments conducted in triplicates or quadruplicates. *= P<0.05 comparing S100 treated to control.
3.3 A pepducin and an antibody directed at PAR-2 reduce the anti-oxidative effect of S100A8/A9
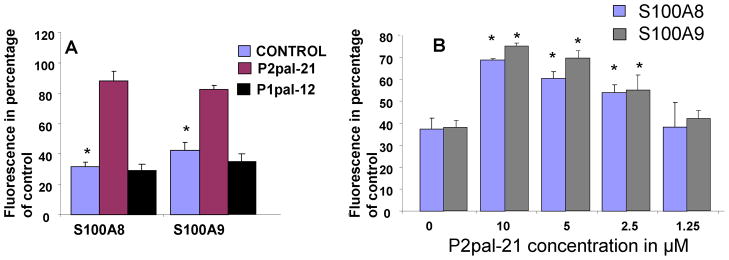
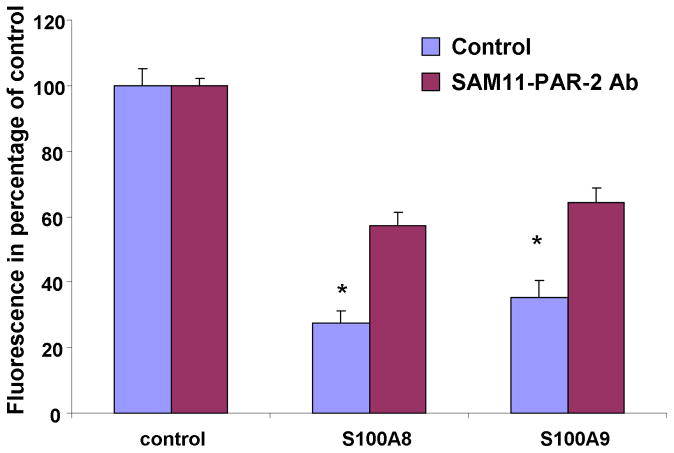
In order to confirm the involvement of PAR-2 in S100A8/A9 functions, we next evaluated the effect of two additional pharmaceutical inhibitors of PAR-2 on S100A8 and S100A9 anti-oxidative effect. First, a palmitoylated cell penetrating peptide (pepducin) with known PAR-2 antagonist activity (Covic et al., 2002) significantly inhibited S100A8 and S100A9 anti-oxidative effect (Fig 3A). The inhibition by P2-pal21 of S100A8 and S100A9 (Fig 3B) was dose dependent. Additionally, a monoclonal antibody directed at the cleavage/activation site of PAR-2 also attenuated the effect of S100A8 and S100A9 (Fig 4) and provided additional support to the results with the PAR-2 pepducin and ENMD-1068.
Figure 3.
Fluorescence emission of PMNs incubated with DCFH-DA probe for 1 hour in the presence or absence of 10 μg/ml S100A8 or S100A9 and P2pal-21 at a concentration of 10 μM. (A). Dose response to P2pal-21 with a fix concentration of 10 μg/ml S100A8 or S100A9 (B). The data represent the mean ± SD and it is a representative of at least 4 experiments conducted in triplicates or quadruplicates. *=P<0.05 comparing p2pal-21 treated to control.
Figure 4.
Fluorescence emission of PMNs incubated with DCFH-DA probe for 1 hour in the presence or absence of 10 μg/ml S100A8 or S100A9 and SAM11 anti-PAR-2 monoclonal antibody at a concentration of 25 μg/ml. The data represent the mean ± SD and it is a representative of 3 experiments conducted in triplicates. *=P<0.05 comparing SAM11 treated to control
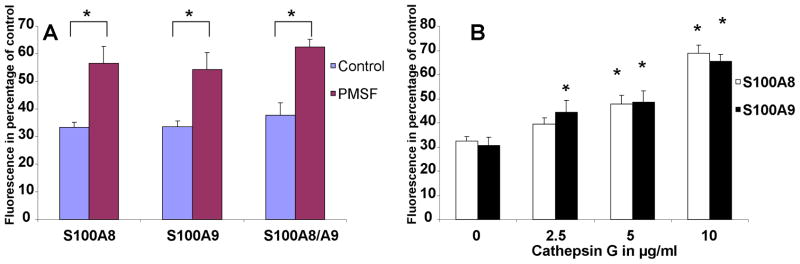
3.4 Inhibition of serine proteases reduces the anti-oxidative effect of S100A8 and S100A9
In order to offer further support for the necessary role of PAR-2 in S100A8/A9 anti-oxidative effect, we next investigated the effect of a potent and non-specific inhibitor of serine proteases, phenylmethylsulfonyl fluoride (PMSF). PARs are activated by serine proteases. We hypothesized that if PAR-2 activation was required for S100A8/A9 anti-oxidative effect, inhibiting serine proteases activity would result in a lower S100 anti-oxidative effect. The data supported this hypothesis. PMSF treatment of PMNs resulted in a reduction of the anti-oxidative effect of S100A8/A9 (Fig 5A). The role of serine protease activity was further investigated by treating the PMNs with cathepsin G, a PMN derived serine protease which has been shown to disarm PAR-2 of the extracellular domain downstream from the trypsin cleavage/activation site (Dulon et al., 2003). The data showed that Cathepsin G treatment of PMNs caused a dose-dependent reduction of the anti-oxidative effect of S100A8 and S100A9 (Fig 5B). This finding further supported a regulatory role of serine proteases in S100 effect and offered additional support for the necessary role of PAR-2.
Figure 5.
Fluorescence emission of PMNs incubated with DCFH-DA probe for 1 hour in the presence or absence of 10 μg/ml S100A8 or S100A9 with or without PMSF at a concentration of 0.4mM (A) or with various concentrations of Cathepsin G (B). The data represent the mean ± SD and it is a representative of 3 experiments conducted in triplicates. *=P<0.05 when comparing PMSF treated to control (A) or Cathepsin G treated to control without Cathepsin G (B).
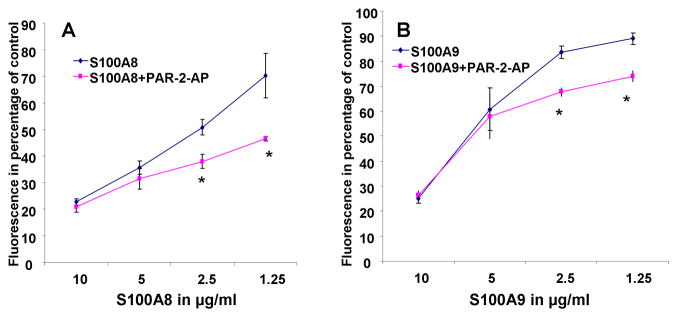
3.5 Controlled activation of PAR-2 potentiates the effect of S100A8/A9
We next tested the effect of PAR-2 activation on PMN oxidative metabolism. A PAR-2 AP was used to activate PAR-2 and the response of PMNs to LPS induced stimulation was measured. PAR-2 activation alone had no significant effect (data not shown). Conversely, PAR-2 activation caused a shift in the dose response of S100A8 (Fig. 6A) and S100A9 (Fig 6B). The potentiation of S100A8 and S100A9 anti-oxidative effect by PAR2-AP supported a crucial modulatory role for this receptor in S100A8/A9 functions.
Figure 6.
Fluorescence emission of PMNs incubated with DCFH-DA probe for 1 hour in the presence or absence of 10 μM PAR2-AP (SLIGKV) and activated by 2.5 μg/ml LPS. The effect of various concentrations of S100A8 (A) and S100A9 (B) was tested and is presented as percentage inhibition of LPS stimulation. The data represent the mean ± SD and it is a representative of 3 experiments conducted in triplicates. *=P<0.05 when comparing PAR-2-AP treated to control at various concentration of S100A8 or S100A9.
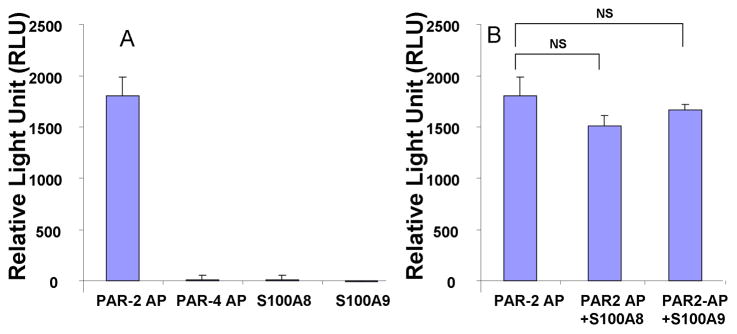
3.6 S100A8 and S100A9 do not activate or inhibit PAR-2 activation
While the anti-oxidative effect of S100A8/A9 did not mimic that of a PAR-2 agonist or antagonist, we next sought to explore whether S100A8/A9 had any effect on PAR-2 activation. Using the commercially available enzyme fragment complementation PathHunter eXpress™ β-arrestin PAR-2 cells from DiscoveRx Corporation, we assessed the ability of S100A8 or S100A9 to activate and/or inhibit PAR-2. In this cell line PAR-2 AP caused a dose dependant activation of PAR-2 as measured by β-arrestin recruitment (data not shown). Conversely neither a PAR-4 AP nor S100A8 or S100A9 activated PAR-2 (Fig 7A). The activation of PAR-2 by 10 μM concentration of PAR2-AP was also not significantly modified by S100A8 or S100A9 (Fig 7B). This would indicate that S100A8 and S100A9 did not directly activate or inhibit PAR-2 and that the regulatory effect of PAR-2 was an indirect effect.
Figure 7.
PAR-2 activation assay conducted in PathHunter eXpress™ β-arrestin PAR-2 cells. The PAR-2 AP (SLIGKV) at 10 μM activates PAR-2 whereas S100A8 or S100A9 at 10 μg/ml or the PAR-4 AP (GYPGQV) at 100 μM do not activate PAR-2 (A). S100A8 and S100A9 fail to inhibit PAR-2 activation by 10 μM PAR-2-AP. The data represent the mean ± SD and it is a representative of 3 experiments conducted in triplicates.
4. Discussion
The production and release of oxidative metabolism by PMNs is critical to the defense of the host against potentially pathogenic microorganisms. The same oxidative metabolites may also cause serious injury and contributes to the pathogenesis of numerous diseases. The production of ROS must therefore be tightly regulated so that it is sufficient to accomplish its protective task but limited to avoid unnecessary damage to the host. The data presented herein indicate that S100A8 and S100A9 down-regulate PMN oxidative metabolism and implicate a member of protease activated receptor in this process.
Using three distinct pharmaceutical approaches, we show that the activation of PAR-2 is necessary for the anti-oxidative effect of S100A8 and S100A9. ENMD-1068, p2-pal21 and a monoclonal antibody which blocks the cleavage/activation site of PAR-2 all significantly reduced or completely abolished the inhibitory effect of S100A8 and S100A9 on PMN oxidative metabolism.
PARs are activated, inhibited, or disarmed by serine proteases. PMSF, a potent inhibitor of serine proteases, significantly blocked the effect of the S100 proteins. Serine protease activity is therefore required for the anti-oxidative effect of S100A8 and S100A9. Cathepsin G is a serine protease which disarms PAR-2 by cleaving its extracellular domain inclusive of the activating peptide (Dulon et al., 2003). Our data indicate that Cathepsin G inhibits the anti-oxidative effect of S100A8 and S100A9. Those two findings support the essential role of PAR-2 but also stress the complexity of mechanisms regulating PMN oxidative metabolism and its regulation by calprotectin. Imbalance in serine proteases can result by itself in deleterious inflammation and are seen in conditions such as Wegener’s granulomatosis. In this rare condition, auto-antibodies to pr3, a PAR-2 activator are frequently detected in the circulation (Kallenberg). It is tempting to speculate that the dysregulated serine protease activity results in deleterious inflammation in part due to a lack of protective activity by molecules such as S100A8 and S100A9.
To further investigate the role of PAR-2 in S100A8/A9 effect, we next tested the effect of a controlled activation of PAR-2. We presented data indicating that PAR-2 AP potentiates the anti-oxidative effect of the two S100 proteins causing a significant shift in the dose response of PMNs to S100A8 and S100A9. On-going studies in our laboratory are attempting to utilize the potentiation of S100 effect by PAR-2 in order to design a strategy aimed at maximizing the protective effect of S100A8 and S100A9 in a model of endotoxemia.
The concentrations at which S100A8 and S100A9 inhibited PMN oxidative metabolism are within the range of concentrations recorded during inflammation (Dunlop et al., 1991) PAR-2 activation is caused by host and microbial factors (Darveau, 2009) associated with inflammation and infection. Controlled activation of PAR-2 was shown to protect against deleterious inflammation associated with redox imbalance (Napoli et al., 2000). A scenario in which PAR-2 activation coupled with an increase in calprotectin levels would together activate a biologically relevant anti-oxidative and protective effect driven by S100A8 and S100A9 is therefore plausible and the object of studies our laboratory is conducting.
As discussed in the introduction section of this manuscript, calprotectin has been attributed both anti and pro-inflammatory activities specifically related to PMN recruitment and functions. Those a-priori conflicting views on calprotectin biological functions are strikingly mirrored by studies of PAR-2.
Inhibition of PAR-2 results in a lesser response to LPS in vivo (Jesmin et al., 2006). PAR-2 activation is sufficient to induce experimental periodontitis in rats (Holzhausen et al., 2005), and a PAR-2 inhibitor (ENMD-1068) reduces joint inflammation in rodents (Kelso et al., 2006). Seemingly in conflict with the pro-inflammatory functions described above, the controlled activation of PAR-2 with PAR2-AP has resulted in reduced LPS-induced lung neutrophilia (Moffatt et al., 2002), less PMNs recovered from bronchoalveolar lavage fluid in mice challenged with intranasal LPS (Peters et al.), improved bronchorelaxation in LPS treated rats (Morello et al., 2005) and protection of cardiac functions following ischemia-reperfusion injury (Zhong and Wang, 2009), an oxidative stress and PMN associated phenomenon.
It is tempting to link those a-priori conflicting data on calprotectin and PAR-2. The effect of calprotectin and PAR-2 must be studied in a carefully designed contextual approach. It may be important to know the state of PAR-2 to predict the activity of calprotectin and vice versa. This may further be complicated by additional regulatory elements which have yet to be unmasked. The complexity of signal integration and the tricky role of regulators of inflammation such as PAR-2 have been exemplified in a study by Kaneider et al (Kaneider et al., 2007). In this study, PAR-2 was shown to turn on compensatory anti-inflammatory signals which were found to be dependent on PAR-1. In fact, PAR-2 activation resulted in a reversal of PAR-1 functions from pro to anti-inflammatory. So while PAR-2 may contribute positively to inflammation initially (Lindner et al., 2000), it may later initiate indirect anti-inflammatory signals via PAR-1 (Kaneider et al., 2007) or via S100A8/A9 as our data point to.
In our previous study, we showed that adenosine metabolites and receptors played an important role in S100A8 and S100A9 down-regulation of PMN oxidative metabolism (Sroussi et al., 2010). Here we show that PAR-2 plays a crucial role in this process. Interactions between PARs and adenosine signaling are supported by studies by Strande et al. (Strande et al., 2008). Their significance remains to be fully understood.
In conclusion, the anti-oxidative effect of S100A8/A9 requires PAR-2 activation and can be potentiated by controlled activation of this receptor. Accordingly, S100A8 and S100A9 represent a protective mechanism against oxidative stress which would take effect only after PAR-2 activation an early phase of acute inflammation. Conceptually, inflammation is less likely to be detrimental if it is initially forceful but prompt to resolve. By being activated by PAR-2, S100A8 and S100A9 contribute to dampening inflammation without interfering with its initial strength
Highlights.
While PAR-2 amplifies LPS induced oxidative metabolism of PMNs, PAR-2 activation is also required for the anti-oxidative effect of calprotectin on peripheral PMNs.
A broad inhibitor of serine protease (PMSF) and a serine protease known to disarm PAR-2 (Cathepsin G) caused a reduction in calprotectin anti-oxidative effect.
The controlled activation of PAR-2 amplifies the anti-oxidative effect of calprotectin
The role of PAR-2 in calprotectin anti-oxidative effect can not be explained by a direct effect of calprotectin on PAR-2
Acknowledgments
This work was supported by a National Institutive of Dental and Craniofacial Research at the National Institute of Health [4K22 DE017161-04, 3K22 DE017161-04S1]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguiar-Passeti T, Postol E, Sorg C, Mariano M. Epithelioid cells from foreign-body granuloma selectively express the calcium-binding protein MRP-14, a novel down-regulatory molecule of macrophage activation. J Leukoc Biol. 1997;62:852–8. doi: 10.1002/jlb.62.6.852. [DOI] [PubMed] [Google Scholar]
- Brun JG, Haland G, Haga HJ, Fagerhol MK, Jonsson R. Effects of calprotectin in avridine-induced arthritis. Apmis. 1995;103:233–40. doi: 10.1111/j.1699-0463.1995.tb01100.x. [DOI] [PubMed] [Google Scholar]
- Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- Ciapetti G, Granchi D, Verri E, Savarino L, Cenni E, Savioli F, Pizzoferrato A. Fluorescent microplate assay for respiratory burst of PMNs challenged in vitro with orthopedic metals. J Biomed Mater Res. 1998;41:455–60. doi: 10.1002/(sici)1097-4636(19980905)41:3<455::aid-jbm15>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci U S A. 2002;99:643–8. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CS, Cenac N, Britto LR, Juliano MA, Juliano L, Vergnolle N, Giorgi R. The C-terminus of murine S100A9 protein inhibits hyperalgesia induced by the agonist peptide of protease-activated receptor 2 (PAR2) Br J Pharmacol. 2006;149:374–84. doi: 10.1038/sj.bjp.0706884. Epub 2006 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CS, Goncalves LR, Juliano L, Juliano MA, da Silva AM, Giorgi R. The C-terminus of murine S100A9 inhibits hyperalgesia and edema induced by jararhagin. Peptides. 2004;25:81–9. doi: 10.1016/j.peptides.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Darveau RP. The oral microbial consortium’s interaction with the periodontal innate defense system. DNA Cell Biol. 2009;28:389–95. doi: 10.1089/dna.2009.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussiere J, Bouzidi F, Vignais PV. The S100A8/A9 protein as a partner for the cytosolic factors of NADPH oxidase activation in neutrophils. Eur J Biochem. 2002;269:3246–55. doi: 10.1046/j.1432-1033.2002.03002.x. [DOI] [PubMed] [Google Scholar]
- Dulon S, Cande C, Bunnett NW, Hollenberg MD, Chignard M, Pidard D. Proteinase-Activated Receptor-2 and Human Lung Epithelial Cells: Disarming by Neutrophil Serine Proteinases. Am J Respir Cell Mol Biol. 2003;28:339–346. doi: 10.1165/rcmb.4908. [DOI] [PubMed] [Google Scholar]
- Dunlop O, Bruun JN, Myrvang B, Fagerhol MK. Calprotectin in cerebrospinal fluid of the HIV infected: a diagnostic marker of opportunistic central nervous system infection? Scand J Infect Dis. 1991;23:687–9. doi: 10.3109/00365549109024294. [DOI] [PubMed] [Google Scholar]
- Eckert JW, Abramson SL, Starke J, Brandt ML. The surgical implications of chronic granulomatous disease. Am J Surg. 1995;169:320–3. doi: 10.1016/S0002-9610(99)80167-6. [DOI] [PubMed] [Google Scholar]
- Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266:7706–13. [PubMed] [Google Scholar]
- Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186:259–63. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- Guo RF, Ward PA. Role of oxidants in lung injury during sepsis. Antioxid Redox Signal. 2007;9:1991–2002. doi: 10.1089/ars.2007.1785. [DOI] [PubMed] [Google Scholar]
- Holzhausen M, Spolidorio LC, Vergnolle N. Proteinase-activated receptor-2 (PAR2) agonist causes periodontitis in rats. J Dent Res. 2005;84:154–9. doi: 10.1177/154405910508400209. [DOI] [PubMed] [Google Scholar]
- Hsu K, Passey RJ, Endoh Y, Rahimi F, Youssef P, Yen T, Geczy CL. Regulation of S100A8 by Glucocorticoids. J Immunol. 2005;174:2318–2326. doi: 10.4049/jimmunol.174.4.2318. [DOI] [PubMed] [Google Scholar]
- Ikemoto M, Murayama H, Itoh H, Totani M, Fujita M. Intrinsic function of S100A8/A9 complex as an anti-inflammatory protein in liver injury induced by lipopolysaccharide in rats. Clin Chim Acta. 2007;376:197–204. doi: 10.1016/j.cca.2006.08.018. Epub 2006 Aug 24. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Gando S, Zaedi S, Sakuraya F. Chronological expression of PAR isoforms in acute liver injury and its amelioration by PAR2 blockade in a rat model of sepsis. Thromb Haemost. 2006;96:830–8. [PubMed] [Google Scholar]
- Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103:879–87. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–4. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- Kallenberg CG. Pathophysiology of ANCA-associated small vessel vasculitis. Curr Rheumatol Rep. 12:399–405. doi: 10.1007/s11926-010-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation--the two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86:41–59. doi: 10.1016/s0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007;8:1303–12. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata A. Proteinase-activated receptor-2 and pain. Nippon Yakurigaku Zasshi. 2006;127:133–6. 146. doi: 10.1254/fpj.127.133. [DOI] [PubMed] [Google Scholar]
- Kelso EB, Ferrell WR, Lockhart JC, Elias-Jones I, Hembrough T, Dunning L, Gracie JA, McInnes IB. Expression and proinflammatory role of proteinase-activated receptor 2 in rheumatoid synovium: ex vivo studies using a novel proteinase-activated receptor 2 antagonist. Arthritis Rheum. 2007;56:765–71. doi: 10.1002/art.22423. [DOI] [PubMed] [Google Scholar]
- Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, Hollenberg MD, Sommerhoff CP, McLean JS, Ferrell WR. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther. 2006;316:1017–24. doi: 10.1124/jpet.105.093807. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–31. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Lim SY, Raftery M, Cai H, Hsu K, Yan WX, Hseih HL, Watts RN, Richardson D, Thomas S, Perry M, Geczy CL. S-nitrosylated S100A8: novel anti-inflammatory properties. J Immunol. 2008;181:5627–36. doi: 10.4049/jimmunol.181.8.5627. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Kahn ML, Coughlin SR, Sambrano GR, Schauble E, Bernstein D, Foy D, Hafezi-Moghadam A, Ley K. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–10. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–82. [PubMed] [Google Scholar]
- Moffatt JD, Jeffrey KL, Cocks TM. Protease-activated receptor-2 activating peptide SLIGRL inhibits bacterial lipopolysaccharide-induced recruitment of polymorphonuclear leukocytes into the airways of mice. Am J Respir Cell Mol Biol. 2002;26:680–4. doi: 10.1165/ajrcmb.26.6.4693. [DOI] [PubMed] [Google Scholar]
- Morello S, Vellecco V, Roviezzo F, Maffia P, Cuzzocrea S, Cirino G, Cicala C. A protective role for proteinase activated receptor 2 in airways of lipopolysaccharide-treated rats. Biochem Pharmacol. 2005;71:223–30. doi: 10.1016/j.bcp.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Napoli C, Cicala C, Wallace JL, de Nigris F, Santagada V, Caliendo G, Franconi F, Ignarro LJ, Cirino G. Protease-activated receptor-2 modulates myocardial ischemia-reperfusion injury in the rat heart. Proc Natl Acad Sci U S A. 2000;97:3678–83. doi: 10.1073/pnas.97.7.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the beta 2 integrin Mac-1 on neutrophils. J Immunol. 1998;160:1427–35. [PubMed] [Google Scholar]
- Nhu QM, Shirey K, Teijaro JR, Farber DL, Netzel-Arnett S, Antalis TM, Fasano A, Vogel SN. Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol. 2010;3:29–39. doi: 10.1038/mi.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka K, Terasaki F, Ikemoto M, Fujita S, Tsukada B, Katashima T, Kanzaki Y, Sohmiya K, Kono T, Toko H, Fujita M, Kitaura Y. Suppression of inflammation in rat autoimmune myocarditis by S100A8/A9 through modulation of the proinflammatory cytokine network. Eur J Heart Fail. 2009;11:229–37. doi: 10.1093/eurjhf/hfn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano RL, Dias MA, Dale CS, Giorgi R. Neutrophils and the calcium-binding protein MRP-14 mediate carrageenan-induced antinociception in mice. Mediators Inflamm. 2002;11:203–10. doi: 10.1080/0962935029000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano RL, Sampaio SC, Juliano L, Juliano MA, Giorgi R. The C-terminus of murine S100A9 inhibits spreading and phagocytic activity of adherent peritoneal cells. Inflamm Res. 2005;54:204–10. doi: 10.1007/s00011-005-1344-y. [DOI] [PubMed] [Google Scholar]
- Pagano RL, Sampaio SC, Juliano MA, Juliano L, Giorgi R. Involvement of proteinase-activated receptors 1 and 2 in spreading and phagocytosis by murine adherent peritoneal cells: modulation by the C-terminal of S100A9 protein. Eur J Pharmacol. 628:240–6. doi: 10.1016/j.ejphar.2009.11.033. [DOI] [PubMed] [Google Scholar]
- Peters T, Mann TS, Henry PJ. Inhibitory influence of protease-activated receptor 2 and E-prostanoid receptor stimulants in lipopolysaccharide models of acute airway inflammation. J Pharmacol Exp Ther. 335:424–33. doi: 10.1124/jpet.109.163253. [DOI] [PubMed] [Google Scholar]
- Ryckman C, McColl SR, Vandal K, de Medicis R, Lussier A, Poubelle PE, Tessier PA. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 2003;48:2310–20. doi: 10.1002/art.11079. [DOI] [PubMed] [Google Scholar]
- Sampson B, Fagerhol MK, Sunderkotter C, Golden BE, Richmond P, Klein N, Kovar IZ, Beattie JH, Wolska-Kusnierz B, Saito Y, Roth J. Hyperzincaemia and hypercalprotectinaemia: a new disorder of zinc metabolism. Lancet. 2002;360:1742–5. doi: 10.1016/S0140-6736(02)11683-7. [DOI] [PubMed] [Google Scholar]
- Sroussi HY, Berline J, Dazin P, Green P, Palefsky JM. S100A8 Triggers Oxidation-sensitive Repulsion of Neutrophils. J Dent Res. 2006;85:829–33. doi: 10.1177/154405910608500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroussi HY, Berline J, Palefsky JM. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol. 2007;81:818–24. doi: 10.1189/jlb.0706433. Epub 2006 Nov 30. [DOI] [PubMed] [Google Scholar]
- Sroussi HY, Lu Y, Zhang QL, Villines D, Marucha PT. S100A8 and S100A9 inhibit neutrophil oxidative metabolism in-vitro: involvement of adenosine metabolites. Free Radic Res. 2010;44:389–96. doi: 10.3109/10715760903431434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strande JL, Hsu A, Su J, Fu X, Gross GJ, Baker JE. Inhibiting protease-activated receptor 4 limits myocardial ischemia/reperfusion injury in rat hearts by unmasking adenosine signaling. J Pharmacol Exp Ther. 2008;324:1045–54. doi: 10.1124/jpet.107.133595. Epub 2007 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9. doi: 10.1038/nm1638. Epub 2007 Sep 2. [DOI] [PubMed] [Google Scholar]
- Youssef P, Roth J, Frosch M, Costello P, Fitzgerald O, Sorg C, Bresnihan B. Expression of myeloid related proteins (MRP) 8 and 14 and the MRP8/14 heterodimer in rheumatoid arthritis synovial membrane. J Rheumatol. 1999;26:2523–8. [PubMed] [Google Scholar]
- Zhong B, Wang DH. Protease-activated receptor 2-mediated protection of myocardial ischemia-reperfusion injury: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1681–90. doi: 10.1152/ajpregu.90746.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]