Abstract
This article describes an MR-safe treadmill that enables cardiovascular exercise stress testing adjacent to the MRI system, facilitating cardiac MR imaging immediately following exercise stress. The treadmill was constructed of non-ferromagnetic components utilizing a hydraulic power system. Computer control ensured precise execution of the standard Bruce treadmill protocol commonly used for cardiovascular exercise stress testing. The treadmill demonstrated no evidence of ferromagnetic attraction and did not affect image quality. Treadmill performance met design specifications both inside and outside the MRI environment. Ten healthy volunteers performed the Bruce protocol with the treadmill positioned adjacent to the MRI table. Upon reaching peak stress (98% ± 8% of age-predicted maximum heart rate (APMHR)), the subjects lay down directly on the MRI table, a cardiac array coil was placed, an intravenous line connected, and stress cine and perfusion imaging performed. Cine imaging commenced on average within 24 ± 4 s and was completed within 40 ± 7 s of the end of exercise. Subject heart rates were 86% ± 9% of APMHR at the start of imaging and 81% ± 9% of APMHR upon completion of cine imaging. The MRI compatible treadmill was shown to operate safely and effectively in the MRI environment.
Introduction
Coronary artery disease (CAD) is the number one cause of death in the United States, affecting 17.6 million individuals and leading to over 400,000 deaths annually (1). The primary non-invasive method of detecting CAD is the combination of treadmill exercise stress testing with either echocardiography (echo) or single photon emission computed tomography (SPECT). Over 10 million of these stress imaging exams are performed annually in the US (2); however both of these imaging methods have technical limitations, and SPECT has potentially harmful effects due to ionizing radiation exposure.
Cardiac magnetic resonance (CMR) provides, in a single examination, high resolution assessment of wall motion, perfusion, blood flow, and myocardial viability. Because of the MR-incompatibility of conventional exercise stress equipment, current stress CMR in clinical practice is performed using pharmacological agents that induce either inotropic stress to assess contractile response or vasodilatation to assess regional perfusion reserve. While pharmacological testing is beneficial in patients unable to exercise due to orthopedic problems or poor conditioning, exercise stress testing is preferred as it offers important physiological information independent of imaging. Exercise testing has the ability to link physical activity to symptoms and ischemia (3). Factors such as drop in blood pressure, inability to reach a workload of 6 metabolic equivalents (Mets) or completion of stage 2 of the Bruce Protocol or inability to achieve 85% of age-predicted maximum heart rate, and reproduction of exertional angina accompanied by ST depression indicate a high likelihood of CAD (4). Exercise ECG testing alone has a sensitivity of 78% and specificity of 70% for detecting CAD.
The combination of CMR with exercise stress can potentially provide comprehensive information not available from any other single diagnostic test. However, the primary challenge to implementing such an integrated exam is that traditional exercise equipment is not MRI-compatible and therefore cannot be safely operated in the MRI room. An MRI-compatible cycle ergometer that mounts to the MRI patient table (Lode BV, the Netherlands) has been available for several years and is intended to permit exercise while the patient is in the magnet bore. While this device has been used in research to investigate blood flow at elevated heart rates (5,6), it is not practical for clinical cardiovascular stress testing for several reasons and there have been no published reports of its use for detection of CAD. Cycling while lying in a flat, supine position is uncomfortable for the patient, making it nearly impossible to reach peak cardiovascular stress; leg muscle fatigue often sets in before reaching peak functional capacity (7). A perhaps even more important criticism of this technique is that it is that flat supine cycling is an unorthodox form of exercise. The Bruce treadmill stress protocol (8) has been in widespread use for over four decades and clinical cardiologists are familiar with interpreting this test. While upright or recumbent cycling may be more popular for stress testing in some European countries, treadmill testing is by far the most common stress test in the United States.
The primary challenge of combining treadmill exercise with CMR is to complete imaging rapidly while the patient is still near peak heart rate. This requires a treadmill that can be positioned in close proximity to the MRI, as well as rapid real-time imaging methods. The American Heart Association (AHA) has recommended that cardiac stress echocardiography be completed within 60 s to 120 s of peak stress, with 60 s being the preferred target (9). While no equivalent guideline exists for stress CMR, the basis of the echo guideline is that exercise induced wall motion abnormalities (WMA) generated under stress conditions begin to diminish immediately following cessation of exercise (10-14). Previous work has demonstrated the feasibility of detecting coronary artery stenoses by exercise stress cardiac MRI using a treadmill positioned just outside the MRI room (15). In this setup, the patient was required to walk about 20 feet from the treadmill to the MRI system, resulting in a time-to-image of 61±24 s from the end of exercise. Cine imaging was performed using segmented k-space acquisition, requiring the patients to breath-hold; no perfusion imaging was performed. Despite these limitations, the sensitivity and specificity to detect >70% coronary artery diameter narrowing in 27 patients were 79% and 85% respectively. However, requiring the patient to walk from outside the room to the MRI table immediately following peak exercise presents a potential safety hazard. It is also likely that the hallway or control room outside the MRI room is an impractical location to conduct a stress test. To begin to address these concerns, in previous work our group modified a conventional treadmill to allow it be positioned in the outer corner of the MRI room outside of the 5 Gauss line. This device was used to conduct treadmill exercise inside the MRI scanner room but still approximately 10 feet away from the MRI table. Studies performed on 20 healthy volunteers (16) and 43 patients referred for a clinical nuclear SPECT examination (17) demonstrated the clinical feasibility of real-time cine and perfusion CMR immediately post exercise using this treadmill positioned inside the MRI room. The setup utilized in these studies, while effective at demonstrating the potential of treadmill stress cardiac MRI, was still potentially unsafe and impractical to implement in routine clinical practice. The treadmill was driven by an electric motor, and was therefore at risk of attraction if inadvertently positioned too close to the MRI system. Also, the risk of falling persisted as patients were required to walk across the room from the treadmill to the MRI table immediately post exercise. In a typical stress echocardiography lab, the examination bed is positioned directly adjacent to the treadmill, enabling the patient to simply lie down immediately post-exercise, thereby minimizing the risk of falling, and reducing the time lapse between exercise and imaging (18).
In this paper, we describe an MRI-compatible treadmill designed specifically to safely perform exercise stress testing immediately adjacent to the MRI table, analogous to a stress-echocardiography exam.
Methods
MR-Compatible Treadmill
Treadmills are typically powered by electromagnetic motors and use ferromagnetic structural materials, precluding their use in close proximity to an MRI magnet. Objects made of ferromagnetic materials may become projectiles in the strong magnetic field surrounding the MRI machine. In addition, the operation of electromagnetic motors may be affected by the magnetic field of the MRI machine, and the magnetic field of an electric motor can affect image quality. In order to provide complete MR-conditionality for our 1.5T MRI system (MAGNETOM Avanto, Siemens Healthcare, Malvern, PA), a treadmill was constructed using non-ferromagnetic structural components, power, and control systems.
Structural Components
The basic outer frame of a conventional treadmill (Landice 8700, Randolph, NJ) made predominantly of aluminium, served as the main body of the new MR-compatible treadmill. The original handrails and all on-board electronics were removed, and a new handrail was constructed using non-ferromagnetic 316-series stainless steel tubing. Remaining ferromagnetic structural components and rollers were replaced by aluminium and 316-series stainless steel equivalents (16).
Hydraulic Power Systems
A hydraulic system was developed to remotely power the treadmill (Figure 1). The original electric motors used to drive the treadmill belt and elevate the running board were replaced with non-ferromagnetic hydraulic drive and elevation actuators. The hydraulic components were designed to use water rather than traditional oil-based hydraulic fluids to ensure simple cleanup of any accidental spill. Hydraulic hoses 40 feet in length were used to connect the drive motor and lift cylinder to an electric motor driven pump and solenoid valves located outside of the MRI room. The systems were designed for treadmill operation with patients up to 400 lb in weight up to 5.5 MPH and 20% elevation, the specifications for Stage 6 of the Bruce treadmill stress-test protocol (8).
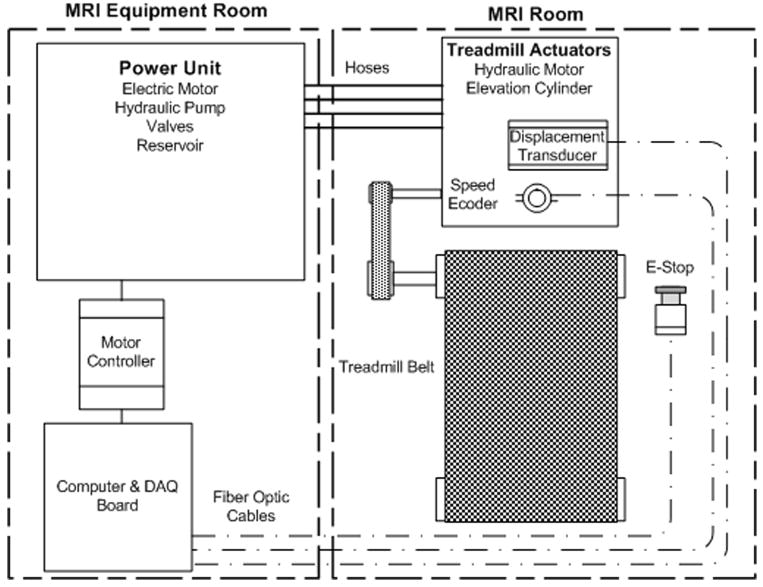
Figure 1.
Hydraulic and control schematic for MR-compatible treadmill. An electric motor driven pump outside of MRI room provides power to a hydraulic motor and cylinder mounted on the treadmill. Motor controller and computer are located outside of the MRI room. Speed and elevation feedback is transmitted through fiber-optic cables. Emergency stop (E-Stop) signal is also transmitted via optical fiber.
Drive System
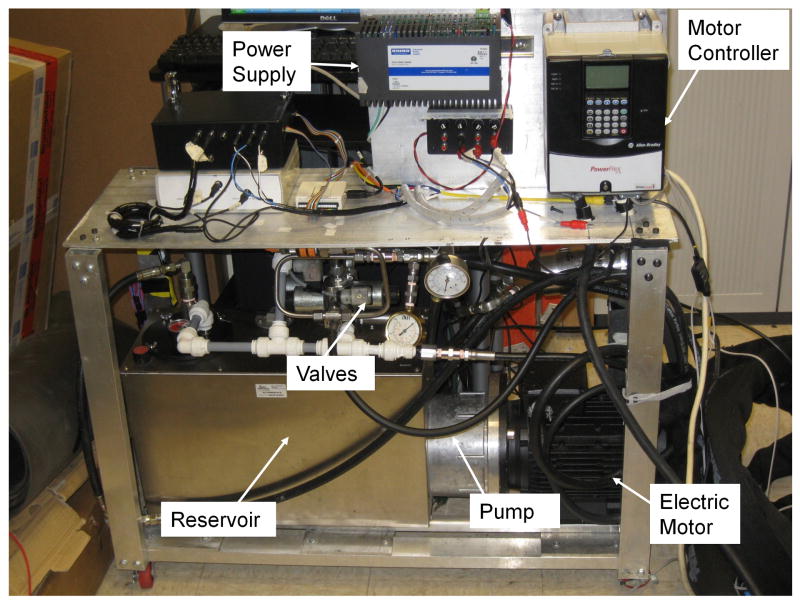
The power unit, consisting of an electrical motor driven pump, water reservoir, and valves, was located outside the MR exam room (Figure 2) in the equipment room. The pump forces ordinary tap water from the reservoir through hydraulic hoses running through a wave guide into the MRI room. The flow of fluid powers a non-ferromagnetic hydraulic motor mounted on the front of the treadmill, turning a drive shaft equipped with a non-ferromagnetic stainless steel flywheel and drive pulley. The flywheel attenuates inertial differences caused by speed change during patient footplant (Figure 3). The drive shaft is connected to the drive roller through a belt and pulley system. The motor outlet flows through a hydraulic valve capable of maintaining the appropriate pressure on the motor outlet to control speed and prevent inlet cavitation when the treadmill is operated at high elevations. Return hoses cycle the hydraulic fluid back to the reservoir. The hoses are attached to the treadmill via MR-compatible, valve-loaded quick-connects to allow for quick, clean setup and teardown.
Figure 2.
The hydraulic power pack located in the equipment room adjacent to the MRI room. An electric motor driven pump forces water through hoses run through a waveguide to the treadmill located in the MRI room. Motor controller provides feedback control of treadmill belt speed.
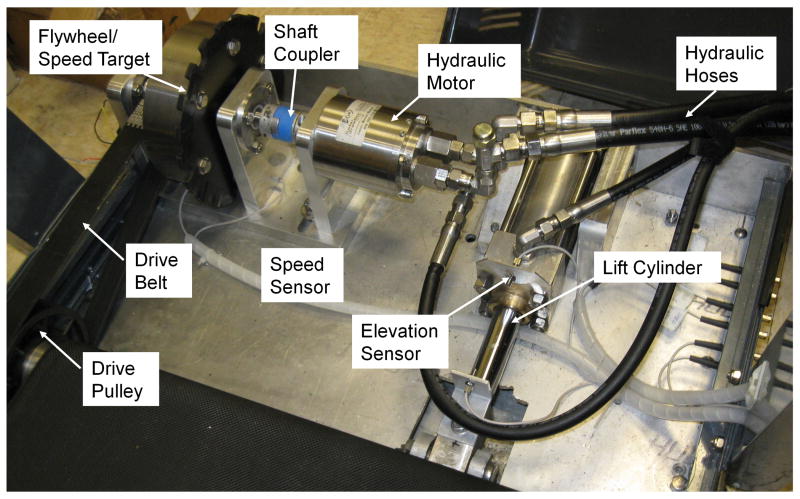
Figure 3.
MRI-compatible hydraulic drive and elevation systems mounted to the front end of the treadmill. Metal components are manufactured from either aluminium or non-ferromagnetic 316 grade stainless steel.
Elevation System
An elevation system was developed using a non-ferromagnetic hydraulic cylinder acting on a lever arm to raise and lower the treadmill (Figure 3) via the front leg assembly. Prior to each exercise test an accumulator, located on the power pack, is charged via the electric motor driven pump. At each protocol stage, a portion of water is discharged from the accumulator using solenoid valves and sent through a hose to a non-ferromagnetic stainless steel cylinder located at the front of the treadmill.
Treadmill Control
A series of valves directs the water to the appropriate hydraulic components throughout the exercise test. A custom application written in Labview (National Instruments, Austin TX) controls the speed and elevation of the treadmill, as well as providing constant visual monitoring of safety features (water level, temperature and speed limits). The control program automatically runs the treadmill through the Bruce treadmill stress protocol, as well as allowing for manual control. A feedback control system was designed to maintain treadmill speed and elevation within 5% of desired values. A through-beam optical sensor (42KL-L2LB-F4, Rockwell Automation, Milwaukee, WI) positioned adjacent to the flywheel (Figure 3) transmits through an optical chopper mounted to the flywheel to create a series of pulses with frequency proportional to treadmill belt speed. The signal feeds back into the motor controller for automatic adjustment of the electric motor (pump) speed using proportional-integral (PI) control. An optical emergency stop button (PICO-Guard, Banner Engineering Corporation, Minneapolis, MN) located on the treadmill is connected via fiber-optic cables to the motor controller. An analog photoelectric sensor (42FB-F2JKA-A2, Rockwell Automation, Milwaukee, WI) that generates a voltage proportional to distance is mounted on the cylinder body to measure cylinder stroke length (Figure 3). The signal is transmitted to a data acquisition board (USB-6008, National Instruments) located outside of the MRI room. A calibration curve was generated relating treadmill elevation angle to sensor voltage, and a LabView program written to provide feedback control of elevation. All control connections were made using plastic fiber-optic cables running through a wave guide to avoid radiofrequency (RF) interference. The program is interfaced from within the MRI room via keyboard and mouse communicating with a computer located in the MRI equipment room (Figure 4) and displayed on an in-room MR console (Siemens, Erlangen, Germany).
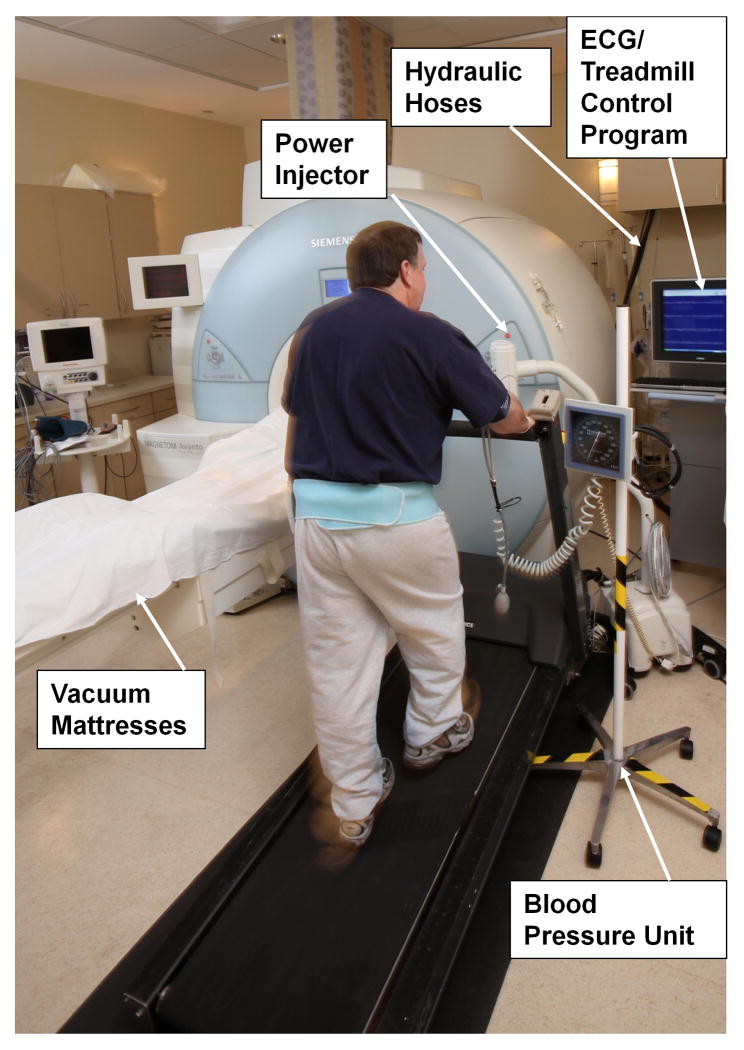
Figure 4.
Exam room layout for exercise stress MRI. The treadmill, treadmill control console, ECG display, and blood pressure meter are all within the room adjacent to the MRI system. Vacuum mattresses on patient table are used to facilitate accurate repositioning of the patient following exercise.
Patient Monitoring
Because MR-compatible, 12-lead diagnostic ECG systems are not commercially available, we developed a method to provide patient monitoring using a PC-based system (Cardiosoft, GE Healthcare, United Kingdom). The Cardiosoft program runs on a standard PC located in the MR control room and is displayed on the in-room, MR-compatible monitor used for treadmill control (Figure 4). A cable runs through the doorway between the control room and MRI room and connects the ECG acquisition module worn on a waist belt to the PC during the treadmill stress test. The cable is detached and removed from the MR room during imaging to prevent RF interference. During imaging, heart rate and rhythm are monitored with the standard 2-lead (Lead I and AVF) wireless ECG unit used for gating the MRI scans. Immediately after stress imaging, the patient table is moved out of the magnet bore and the ECG system reconnected for 12-lead monitoring during recovery. We have previously shown that the ECG signal is unaffected by magnetic fields of 70 mT or less (19). On the MRI system used in this study, the patient’s heart remained at field strength less than 70 mT both during exercise on the treadmill as well as during recovery while lying on the extended MRI table.
MR Compatibility Testing
In order to be considered MR-compatible (20), the treadmill system must demonstrate that it can be safely located in the MR environment, that the introduction of the treadmill does not affect image quality, and that the magnetic field of the MR system does not affect treadmill performance. The following tests were performed to verify MRI-compatibility.
MR Safety
All components used to construct the treadmill were specified to be made of non-ferromagnetic materials. Before bringing the treadmill into the MRI room, a 0.3T hand magnet (Next Generation Science, Lafeyette, IN) was used to inspect each component prior to assembly, and again to examine the entire assembled treadmill.
Effect of Treadmill on Image Quality
Placement of the treadmill with its significant mass of aluminum and stainless steel next to the MRI system has the potential to significantly distort the magnetic field. RF interference may be introduced by conductive wires, cables, or water-filled hoses running from outside the Faraday cage through the waveguide into the MRI room. Vibrations caused by the running treadmill may also serve as a source of image artifacts, although in our stress test procedure imaging takes place after treadmill exercise; the treadmill will not normally be running during patient scanning. The potential detrimental effects of positioning and operating a treadmill adjacent to the MRI system were investigated through a series of MRI system performance checks run with and without the treadmill positioned and operating.
The treadmill was placed immediately adjacent to the 1.5T MRI system (Figure 4) and connected to the hydraulic power pack located in the adjacent equipment room with hydraulic hoses and fiber optic cables running through the wall through a wave guide 3” in diameter and 12” in length. A series of tests were performed using service software tools (Siemens Medical, Malvern, PA) and a spherical MRI calibration phantom. All tests were performed under three conditions: without the treadmill in the room, with the treadmill adjacent to the MRI table but not running, and finally with the treadmill adjacent to the MRI table and running. A field map was generated and compared to system field homogeneity specifications to determine whether the treadmill positioned adjacent to the MRI magnet caused significant variation in the static magnetic field. An artifact calculation test was used to detect instabilities associated with the environment, such as those potentially caused by the vibrations related to the operation of the treadmill adjacent to the MRI system. This test runs a double echo spin echo sequence at two slice locations in all three orientations, strategically pulsing the gradients so that all three physical gradient coils are used for frequency, phase, and slice encoding. An automated image analysis evaluates the level of ghost artifact in the phase encoding direction relative to the phantom signal intensity and compares to system specifications. A spike test was executed to evaluate the presence of any noise spikes by running through a series of gradient pulses and checking for significant spikes in the raw k-space data. A final test evaluated external RF noise over a 500 kHz bandwidth about the center frequency of the system (63.5 MHz). A series of noise tests were run covering 10 kHz bandwidth increments to enable the frequency and potential sources of RF noise to be identified. During all tests except field homogeneity, a copper wire was laid on the MRI table running from outside the magnet bore all the way through to the other end of the magnet to act as an antenna to carry any RF noise present in the magnet room into the magnet bore to be detected by the receiver coil. This mimics the presence of a patient in the magnet bore, whose body would act as an antenna in the same fashion. The hydraulic hoses remained through the wave guide and filled with water for each test configuration with the treadmill in the MRI room. All tests were considered successful if the results were within the system performance specifications provided by the vendor.
Treadmill Performance Validation
To ensure that the treadmill met design specifications, treadmill speed and elevation performance were evaluated outside of the MRI room. The treadmill was run without load for three minute intervals corresponding to each of the first six stages of the Bruce treadmill test protocol. During each stage, average speed over a two minute interval was measured using a measuring wheel (77-193 - MW208, Stanley, New Britain, CT) and stopwatch. Elevation was determined by measuring the front height of the treadmill with a nonferromagnetic ruler and using this height to calculate the slope of the running board. Height measurements were taken at 30 s intervals during each Bruce Protocol stage to evaluate the accuracy and stability of the elevation system.
Treadmill performance was also evaluated under loaded conditions both inside and outside of the MR room to ensure design specifications were met under load, and whether the magnetic field of the MRI system affected treadmill performance. Five subjects (weight: 125 – 263 lb) performed stages 1 through 6 of the Bruce Protocol on the treadmill first outside the MRI room, and then inside with the treadmill located as shown in Figure 4. Speed and elevation were measured according to previously described procedures.
Demonstration of Exercise CMR
Ten healthy subjects (Age 23-67 years, mean = 39 ± 16, 2 females) were recruited to undergo exercise stress testing on the hydraulic treadmill located next to the MRI system, followed by MRI of the heart immediately post exercise. The study protocol was approved by the Institutional Review Board and all participants gave written informed consent. The exclusion criteria were known or suspected CAD, inability to exercise, and standard contraindications to MRI.
Each subject was first positioned on the MRI table using vacuum mattress positioning devices (Vac-Lok Cushions, MEDTEC, Orange City, IA) under the head/shoulders and legs to form a cast of the subject to facilitate accurate repositioning and avoid the need to repeat localizer scans after exercise. Slice positions were defined and real-time resting cine images (TGRAPPA parallel acceleration rate 4, TR/TE 2.3/1.0 ms, temporal resolution 47.6ms, matrix 84x160, slices: 5 short-axis (SAX), 3 vertical long-axis (VLA), 1 horizontal long-axis (HLA)) were acquired without ECG synchronization or breath-holding. Imaging sequences for both real-time cine and first-pass perfusion were queued up for immediate execution following exercise. The subject was then removed from the MRI machine, and resting 12-lead ECG recorded in the supine position while lying on the fully-extended MRI table, and upright while standing on the treadmill. Each subject performed the standard Bruce Treadmill Protocol (8) to peak stress, as determined by age-predicted maximal heart rate (APMHR = 220 – Age). Our goal was to commence imaging with the subject’s heart rate at the target of 85% of APMHR. Upon reaching maximal exercise stress as determined by heart rate, the blood pressure cuff and ECG cable were removed and the subject lay down on the vacuum mattresses on the MRI examination table. The anterior array coil was positioned on the chest, and the IV line connected to the arm for contrast agent injection before moving the table to magnet isocenter. Imaging was started from inside the MRI room by pressing the “Start” button located on the MRI scanner housing. Images of cardiac function at peak stress were acquired using the previously queued cine sequence. A perfusion scan (hybrid gradient echo, echo-planar imaging sequence (3 SAX slices, TR/TE 5.6/1.1 ms, FA 25°, BW 1955 Hz/pixel, slice thickness 10 mm, in-plane spatial resolution 3 mm) with simultaneous injection of 0.1 mmol/kg gadolinium-based contrast agent automatically started immediately following the cine scan. Contrast agent injection was administered using a pre-armed power injector located in the MRI room using a button directly attached to the injector. The perfusion acquisition was ECG triggered but did not require breath-hold. A stopwatch was used to record times from the end of exercise to the start of imaging (patient transfer time), and to the end of each phase of imaging (function and perfusion). In three subjects, the time it took the MRI table to move to isocenter was also recorded. Heart rate was recorded from the MRI gating signal, or directly from the cine images when the ECG signal was unreliable. Immediately following stress imaging, the subject was removed from the magnet bore and remained on the table for 8-10 minutes of recovery with 12-lead ECG and blood pressure monitoring. The subject was then moved back into the MRI system for recovery cine images as well as resting perfusion and delayed enhancement viability imaging using a single-shot non-breathhold scan (TR/TE 2.5/1.2 ms, FA 50°, BW 790 Hz/pixel, slice thickness 8 mm, in-plane spatial resolution 2-3 mm) covering the same slice positions used for cine imaging.
Results
MR Compatibility Testing
Inspection via a hand magnet demonstrated no perceptible magnetic attraction from any component individually or from the fully assembled unit. Likewise, the treadmill demonstrated no attraction towards the MRI system when placed immediately adjacent to the MRI table (Figure 4). All MRI diagnostic tests indicated that the MRI system was within specification prior to bringing the treadmill into the MRI room, and remained in specification with the treadmill positioned next to the MRI table both while stationary and running (Table 1). No RF noise, data spikes, or ghost artifacts were identified with the treadmill present or operating, and field homogeneity remained within specification.
Table 1.
Results of the static field homogeneity and image artifact tests show that the MRI system remains in specification with the treadmill positioned and operating next to the MRI table.
| Specification | Measured | |||||
|---|---|---|---|---|---|---|
| Low | High | Baseline | Treadmill Present | Treadmill Running | ||
| Static Field | Bpp | 0.00 | 3.00 | 1.58 | 1.64 | 1.57 |
| Homogeneity (ppm) | Brms | 0.00 | 0.40 | 0.10 | 0.11 | 0.10 |
|
| ||||||
| Image Artifact (%) | Echo 1 | 0.000 | 2.000 | 0.322 | 0.259 | 0.380 |
| Echo 2 | 0.000 | 3.000 | 0.406 | 0.377 | 0.515 | |
Treadmill Performance Validation
The treadmill met desired speeds and elevations corresponding to the first 6 stages of the Bruce protocol under unloaded conditions outside of the MRI room and did not deviate significantly from these values over the course of each protocol stage (Table 2). Performance testing with subjects running on the treadmill both inside and outside of the MRI room showed that the speed of the treadmill deviated by no greater than 4.1% from the desired speed, and by no greater than 2.38% between operation inside and outside the MR environment at any speed or weight (Table 3). Elevation performance showed a deviation of no greater than 2.80% from desired or 0.80% between measurements obtained inside and outside of the MRI room (Table 3).
Table 2.
Results of treadmill performance testing outside of the MRI room under zero-load conditions show that the system meets and remains within desired values throughout six stages of the Bruce treadmill protocol.
| Speed (MPH) | Elevation (deg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time into stage (s) | Max Deviation | ||||||||||||
| Stage | Desired | Actual | % Error | Desired | 30 | 60 | 90 | 120 | 150 | during stage | Ave | % Error | |
| 1 | 1.7 | 1.8 | 3.7 | 5.7 | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 | 0.0 | 5.8 | 1.5 | |
| 2 | 2.5 | 2.5 | 1.5 | 6.8 | 6.9 | 6.9 | 6.9 | 6.8 | 6.8 | 0.1 | 6.9 | 1.0 | |
| 3 | 3.4 | 3.4 | 0.8 | 8.0 | 8.1 | 8.1 | 8.1 | 8.1 | 8.1 | 0.0 | 8.1 | 0.7 | |
| 4 | 4.2 | 4.2 | -0.5 | 9.1 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 | 0.0 | 9.2 | 1.4 | |
| 5 | 5.0 | 5.0 | -0.8 | 10.2 | 10.3 | 10.3 | 10.3 | 10.3 | 10.3 | 0.0 | 10.3 | 0.8 | |
| 6 | 5.5 | 5.4 | -1.7 | 11.3 | 11.1 | 11.1 | 11.1 | 11.1 | 11.1 | 0.0 | 11.1 | -1.8 | |
Table 3.
Treadmill performance testing inside and outside of the MRI room. Values represent average ± standard deviation across all 5 subjects. Only minimal differences in speed and elevation performance were observed between operation outside and inside the MRI room.
| Desired | Outside MRI Room | Inside MRI Room | Inside vs. Outside | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Speed | Elevation | Speed | Elevation | Speed | Elevation | Speed | Elevation | |||||
| Actual | Error | Actual | Error | Actual | Error | Actual | Error | Difference | ||||
| Stage | (MPH) | (deg) | (MPH) | (%) | (deg) | (%) | (MPH) | (%) | (deg) | (%) | (%) | |
| 1 | 1.7 | 5.7 | 1.7 ± 0.0 | 2.2 | 5.8 ± 0.1 | 1.5 | 1.8 ± 0.0 | 4.1 | 5.8 ± 0.1 | 2.3 | 1.9 | 0.8 |
| 2 | 2.5 | 6.8 | 2.5 ± 0.1 | 2.2 | 6.9 ± 0.0 | 1.3 | 2.5 ± 0.1 | 2.3 | 6.9 ± 0.2 | 1.6 | 2.4 | -0.5 |
| 3 | 3.4 | 8.0 | 3.4 ± 0.1 | 1.8 | 8.0 ± 0.1 | 1.0 | 3.4 ± 0.1 | 1.7 | 8.1 ± 0.1 | 0.9 | 1.3 | 0.7 |
| 4 | 4.2 | 9.1 | 4.1 ± 0.1 | 1.7 | 9.2 ± 0.1 | 1.0 | 4.2 ± 0.1 | 1.6 | 9.2 ± 0.1 | 1.2 | 1.8 | 0.0 |
| 5 | 5.0 | 10.2 | 5.0 ± 0.1 | 1.1 | 10.2 ± 0.1 | 0.6 | 5.0 ± 0.1 | 1.6 | 10.2 ± 0.1 | 1.0 | 0.6 | -0.1 |
| 6 | 5.5 | 11.3 | 5.4 ± 0.1 | 1.6 | 11.0 ± 0.0 | 2.6 | 5.5 ± 0.1 | 1.7 | 11.0 ± 0.1 | 2.8 | 1.6 | -0.2 |
Exercise Stress Testing
All 10 subjects completed the treadmill exercise study, exercising for an average of 13.1 ± 1.3 minutes (approximately 1 minute into Stage 4) of the Bruce Protocol and achieving an average heart rate of 98% ± 8% of APMHR (Table 4). The average time to transfer subjects to the MRI table and start imaging was 24 ± 4 s, including 10 s for the table to move to isocenter (Table 4).
Table 4.
Timing and heart rate results for exercise CMR examination. Results are average and standard deviation over all 10 subjects. Cine and first pass imaging to peak myocardial enhancement were both completed within the 60 s recommended by the American Heart Association for completion of echocardiography imaging post-exercise.
| Time | Heart Rate (% APMHR) | |
|---|---|---|
| Bruce Protocol (min) | 13.1 ± 1.3 | 98 ± 8 |
| Start Cine (s after exercise) | 24 ± 4 | 86 ± 9 |
| Complete Cine (s after exercise) | 40 ± 7 | 81 ± 9 |
| Peak Myocardial Enhancement (s after exercise) | 52 ± 7 | - |
Stress real-time cine (Figure 5) and first-pass perfusion imaging (Figure 5) were successfully completed in 10 subjects. Real-time cine imaging covering five short-axis and four long-axis views was completed within 40 ± 7 s after the end of exercise; first-pass perfusion required an additional 10.5 ± 2 s seconds to reach peak myocardial enhancement. Heart rate at the start of stress cine imaging was 86% ± 9% of APMHR, and fell to 81% ± 9% of APMHR by the end of cine imaging. Subjects remained disconnected from the 12-lead ECG system for 103 s ± 22 s during imaging. During this time, the 2-lead wireless ECG unit used by the MRI scanner for cardiac gating allowed heart rate and rhythm to be monitored.
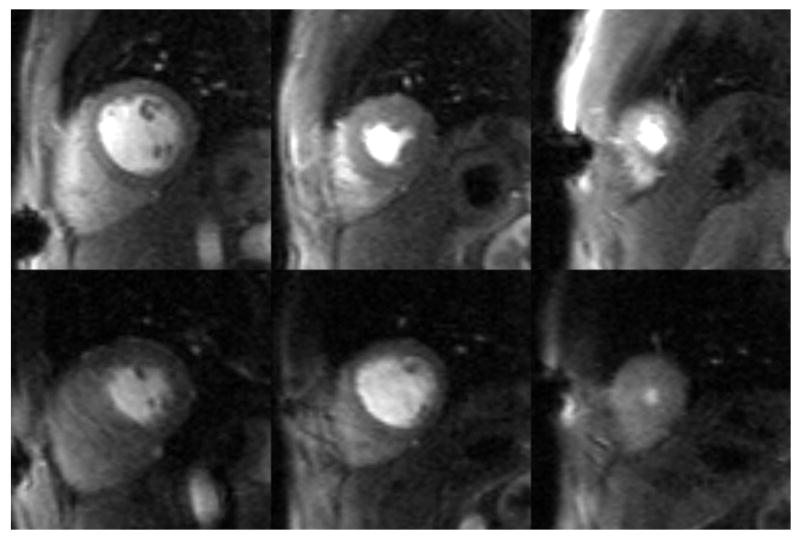
Figure 5.
Cine images obtained at rest and immediately post-exercise stress. End systolic frames are shown for the same slices positions at rest (left column) and stress (right column). Increased myocardial contraction at peak systole is seen in the stress images compared to the rest images. In this subject, resting cine images were acquired at a heart rate of 59 beats per minute, and stress cine images at 156 beats per minute.
Discussion and Conclusions
We have successfully constructed a hydraulic-powered treadmill using exclusively non-ferromagnetic components and safely operated this device in the MRI room without affecting image quality and without the magnetic field affecting treadmill performance. The treadmill met and remained within design specifications by operating within 5% of desired speed and elevation under varying loads. All image quality tests remained within vendor specifications with the treadmill operating immediately adjacent to the MRI patient table. Ten volunteers were successfully imaged immediately post-exercise.
The treadmill serves as the core component of a system designed to enable treadmill exercise stress testing safely inside the MR room, immediately adjacent to the MRI table. This setup is directly analogous to that widely used for stress echocardiography, and is designed to facilitate rapid, safe transfer of the patient directly from the treadmill onto the table for post-stress imaging. Stress imaging utilizing the MRI-compatible treadmill offers significant safety advantages over our previously reported use of an electric treadmill positioned in the outer corner of the MRI room. While the previous system did enable start of imaging 30 s after exercise, completion of cine imaging within 45 s, and time to peak myocardial enhancement within 57 s post-stress in healthy volunteers (16). Subjects were still required to walk across the room from the treadmill to the MRI table after stopping exercise, and the electric treadmill could not be considered safe in the MRI room except under the carefully-controlled conditions of a research study. A subsequent study using this same system and patients referred for exercise stress SPECT resulted in an average time to start imaging of 42.4 s, and 68 s to complete cine imaging (17); this fell outside of the preferred AHA guidelines for post-exercise imaging of myocardial wall motion. In that study, perfusion imaging was completed within 88 s of the end of exercise, and perfusion results were in agreement with wall motion in every case. Timing guidelines are not available for post-exercise perfusion imaging which is typically performed with SPECT, not echocardiography. While these previous studies demonstrated the feasibility of in-room treadmill exercise CMR, safety requirements were not met, and time to complete imaging exceeded the recommended limit in patients. The current configuration utilizing a MRI compatible treadmill positioned adjacent to the MRI table resulted in a 20% improvement in the time to start imaging (24 s vs. 30 s), an 11% improvement in the time to complete cine imaging (40 s vs, 45 s), and 8% in the time to complete first-pass perfusion (52.5 s vs. 57 s) in healthy volunteers. While the time between exercise and imaging has been considerably reduced by positioning the treadmill adjacent to the MRI system, it remains to be proven whether the target of imaging within 60 seconds will be consistently achieved in patients. Our goal of initiating imaging at 85% of MAPHR was met, however the average heart rate dropped to 81% of MAPHR by the end of cine imaging. It should be noted that these were younger, healthy subjects and therefore heart rate is expected to decrease faster following stress than in older deconditioned patients.
Advances in technology for rapid cardiac MRI are particularly important to exercise stress imaging under conditions of high heart rate and rapid, deep breathing. In our previous study a 12-element array coil was used and TSENSE acceleration rate 3 was feasible with acceptable image quality. In the current study, a 32-channel cardiac array was used and the TGRAPPA technique (21) became available; this combination enabled the use of parallel acceleration rate 4, and a resulting improvement in cine temporal resolution from 57.8 ms to 47.6 ms. It is hoped that such gains in temporal resolution will improve the accuracy of cardiac wall motion assessment in patients.
Despite successfully achieving design performance goal of executing the standard Bruce treadmill protocol with this prototype system, initial testing has revealed opportunities to improve the performance of the device and the method. Treadmill speed control was less accurate at low speeds, i.e., speeds corresponding to stage 1 of the Bruce Protocol. A larger capacity hydraulic motor would provide better control at lower speeds; this may be important in poorly conditioned patients in whom modification of the Bruce protocol is necessary. Hose compliance led to frequent adjustments of the elevation system, affecting accuracy at the highest elevations. A design revision is underway to eliminate the accumulator, enabling greater flexibility and accuracy in elevation control. A significant portion of the time between end of exercise and the start of imaging was taken up by the 10 seconds it took to move the MRI table to magnet isocenter. Patient tables on CT scanners now move much faster to facilitate rapid spiral scanning; it may be feasible to increase the MRI table translation speed while maintaining patient safety and further reduce the delay between exercise and imaging. A MR-compatible, wireless 12-lead ECG system would greatly improve the current method of patient monitoring requiring the ECG acquisition module to be disconnected and the cable removed from the room prior to scanning.
This new method of cardiac stress testing has the potential to improve diagnostic performance by providing superior and more comprehensive image information than existing exercise stress imaging modalities. Stress echocardiography is used primarily for left ventricular wall motion assessment, and stress SPECT for myocardial perfusion imaging. Exercise stress CMR can provide both stress wall motion and stress perfusion, as well as high resolution imaging of myocardial viability. Exercise CMR may also provide diagnostic and prognostic advantages over current methods of pharmacological stress MRI; the treadmill test offers important physiological information not available from pharmacological stress such as the appearance of ECG changes and symptoms with exertion, as well as evidence of abnormal changes in blood pressure and heart rate. The MRI-compatible treadmill safely brings the entire stress testing system and team into the MRI exam room. This enables direct communication and monitoring of the patient by the stress testing team throughout the exam, and the rapid and safe transfer of the patient to the MRI table. The use of conventional patient monitoring equipment, standard stress 12-lead ECG information, and methodology similar to stress echocardiography are likely to provide familiarity and ease of adoption by clinical staff.
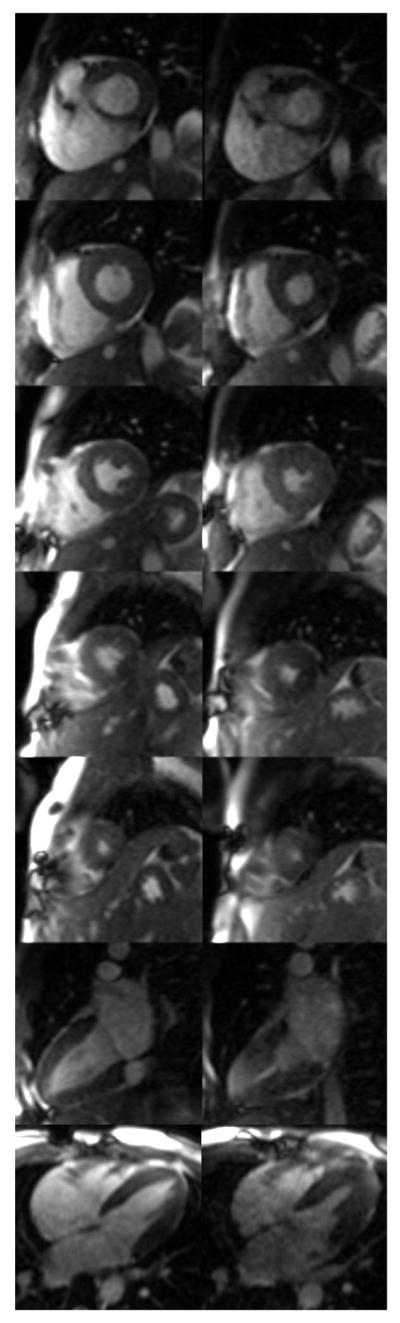
Figure 6.
Myocardial perfusion images obtained at rest (top row) and immediately post-exercise stress (bottom row). Difference in heart rate causes each slice to be sampled at a different point in the cardiac cycle between rest and stress.
Acknowledgments
The project described was supported by Award Number R41HL096212 from the National Heart, Lung, And Blood Institute and through the Global Cardiovascular Innovation Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute, the National Institutes of Health, or the Global Cardiovascular Innovation Center. The authors also wish to thank Beth McCarthy and Debbie Scandling for their assistance with coordination of volunteer testing and data analysis.
Relationships with Industry: Ownership interest: EXCMR, Ltd. (ELF, OPS, SVR, JWA), Grant support: Siemens (OPS, SVR), patent pending (ELF, OPS, SVR, JWA)
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Klem I, Heitner JF, Shah DJ, Sketch MH, Jr, Behar V, Weinsaft J, Cawley P, Parker M, Elliott M, Judd RM, Kim RJ. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47(8):1630–1638. doi: 10.1016/j.jacc.2005.10.074. [DOI] [PubMed] [Google Scholar]
- 3.Tavel ME. Stress testing in cardiac evaluation: current concepts with emphasis on the ECG. Chest. 2001;119(3):907–925. doi: 10.1378/chest.119.3.907. [DOI] [PubMed] [Google Scholar]
- 4.Hill J, Timmis A. Exercise tolerance testing. BMJ. 2002;324(7345):1084–1087. doi: 10.1136/bmj.324.7345.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hjortdal VE, Emmertsen K, Stenbog E, Frund T, Schmidt MR, Kromann O, Sorensen K, Pedersen EM. Effects of Exercise and Respiration on Blood Flow in Total Cavopulmonary Connection: A Real-Time Magnetic Resonance Flow Study. Circulation. 2003;108(10):1227–1231. doi: 10.1161/01.CIR.0000087406.27922.6B. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen EM, Kozerke S, Ringgaard S, Scheidegger MB, Boesiger P. Quantitative abdominal aortic flow measurements at controlled levels of ergometer exercise. Magnetic Resonance Imaging. 1999;17(4):489–494. doi: 10.1016/s0730-725x(98)00209-4. [DOI] [PubMed] [Google Scholar]
- 7.Niezen RA, Doornbos J, van der Wall EE, de Roos A. Measurement of aortic and pulmonary flow with MRI at rest and during physical exercise. J Comput Assist Tomogr. 1998;22(2):194–201. doi: 10.1097/00004728-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Bruce RA, Blackmon JR, Jones JW, Strait G. Exercising testing in adult normal subjects and cardiac patients. Pediatrics. 1963;32(SUPPL):742–756. [PubMed] [Google Scholar]
- 9.Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Pina IL, Rodney R, Simons-Morton DA, Williams MA, Bazzarre T. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- 10.Salustri A, Pozzoli MM, Hermans W, Ilmer B, Cornel JH, Reijs AE, Roelandt JR, Fioretti PM. Relationship between exercise echocardiography and perfusion single-photon emission computed tomography in patients with single-vessel coronary artery disease. Am Heart J. 1992;124(1):75–83. doi: 10.1016/0002-8703(92)90922-i. [DOI] [PubMed] [Google Scholar]
- 11.Iliceto S, D’Ambrosio G, Sorino M, Papa A, Amico A, Ricci A, Rizzon P. Comparison of postexercise and transesophageal atrial pacing two-dimensional echocardiography for detection of coronary artery disease. Am J Cardiol. 1986;57(8):547–553. doi: 10.1016/0002-9149(86)90832-5. [DOI] [PubMed] [Google Scholar]
- 12.Dymond DS, Foster C, Grenier RP, Carpenter J, Schmidt DH. Peak exercise and immediate postexercise imaging for the detection of left ventricular functional abnormalities in coronary artery disease. Am J Cardiol. 1984;53(11):1532–1537. doi: 10.1016/0002-9149(84)90574-5. [DOI] [PubMed] [Google Scholar]
- 13.Presti CF, Armstrong WF, Feigenbaum H. Comparison of echocardiography at peak exercise and after bicycle exercise in evaluation of patients with known or suspected coronary artery disease. J Am Soc Echocardiogr. 1988;1(2):119–126. doi: 10.1016/s0894-7317(88)80093-2. [DOI] [PubMed] [Google Scholar]
- 14.Dagianti A, Penco M, Bandiera A, Sgorbini L, Fedele F. Clinical application of exercise stress echocardiography: supine bicycle or treadmill? Am J Cardiol. 1998;81(12A):62G–67G. doi: 10.1016/s0002-9149(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 15.Rerkpattanapipat P, Gandhi SK, Darty SN, Williams RT, Davis AD, Mazur W, Clark HP, Little WC, Link KM, Hamilton CA, Hundley WG. Feasibility to detect severe coronary artery stenoses with upright treadmill exercise magnetic resonance imaging. Am J Cardiol. 2003;92(5):603–606. doi: 10.1016/s0002-9149(03)00734-3. [DOI] [PubMed] [Google Scholar]
- 16.Jekic M, Foster EL, Ballinger MR, Raman SV, Simonetti OP. Cardiac function and myocardial perfusion immediately following maximal treadmill exercise inside the MRI room. J Cardiovasc Magn Reson. 2008;10(1):3. doi: 10.1186/1532-429X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raman SV, Dickerson JA, Jekic M, Foster EL, Pennell ML, McCarthy B, Simonetti OP. Real-time cine and myocardial perfusion with treadmill exercise stress cardiovascular magnetic resonance in patients referred for stress SPECT. J Cardiovasc Magn Reson. 2010;12:41. doi: 10.1186/1532-429X-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong WF. Treadmill exercise echocardiography: methodology and clinical role. Eur Heart J. 1997;18(Suppl D):D2–8. doi: 10.1093/eurheartj/18.suppl_d.2. [DOI] [PubMed] [Google Scholar]
- 19.Jekic M, Ding Y, Dzwonczyk R, Burns P, Raman SV, Simonetti OP. Magnetic field threshold for accurate electrocardiography in the MRI environment. Magn Reson Med. 2010 doi: 10.1002/mrm.22419. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shellock FG. Magnetic resonance safety update 2002: implants and devices. J Magn Reson Imaging. 2002;16(5):485–496. doi: 10.1002/jmri.10196. [DOI] [PubMed] [Google Scholar]
- 21.Breuer FA, Kellman P, Griswold MA, Jakob PM. Dynamic autocalibrated parallel imaging using temporal GRAPPA (TGRAPPA) Magn Reson Med. 2005;53(4):981–985. doi: 10.1002/mrm.20430. [DOI] [PubMed] [Google Scholar]