Abstract
Objective
To understand the different patterns of cue-induced craving and physiological reactions among recently abstinent and long- abstinent heroin-dependent patients.
Method
26 healthy adult controls (HC), 29 long-abstinent (more than 1 year, LA), and 26 recently abstinent (less than 1 month, RA) heroin-dependent individuals were exposed to heroin-related and neutral video cues, one video per session, on different days in random order. Self-reported heroin craving by a 10-point visual analog scale (VAS), physiological reactions [Skin conductance (SC), muscle electromyography (MEG), skin temperature (TEMP)] and cardiovascular arousal [heart rates (HR), systolic blood pressure (HBP) and diastolic blood pressure (LBP)] were assessed at baseline and after exposure.
Results
Both heroin-abstinent groups showed increased heroin craving, SC, MEG, HR, SBP and LBP after exposure to heroin-related video, compared to the control group and compared to exposure to the neutral video. Except the RA group showed more HR changes, changes of heroin craving, SC, MEG, HR, SBP and LBP after exposure to the heroin cue video were not different between the LA and RA groups.
Conclusions
Abstinent heroin-dependent patients had elevated craving and physiological reactions after exposure to videos containing heroin-related cues and the cue induced responses still occurred in long-abstinent patients. This phenomenon should be addressed in treatment and recovery services for heroin dependence.
Keywords: craving, cue reactions, heroin dependence, long time abstinent
1. Introduction
Reactivity to drug-related cues is a frequently observed phenomenon in drug-dependent subjects (Childress, et al., 1993; Powell, Gray, & Bradley, 1993) and it is believed to be related to substance use disorders (Koob & Kreek, 2007; Sinha, Fuse, Aubin, & O'Malley, 2000; Sinha & Li, 2007; Watson, Carpenter, Saladin, Gray, & Upadhyaya, 2010). Drug-related cue-induced response is presumed to consist of physiological and/or subjective reactions (Carter & Tiffany, 1999; Robbins, Ehrman, Childress, & O'Brien, 1997). A meta-analysis including 41 cue-reactivity studies (Carter & Tiffany, 1999) concluded that the cue-reactivity paradigm can be employed as a useful instrument to produce solid craving effects and reliable physiological reactions in drug-dependent patients. Craving, a subjective desire to use addictive drugs, plays an important role in relapse in abstinent drug-dependent persons in their natural setting (Childress, McLellan, & O'Brien, 1986), and conditioned reactivity to substance-related cues is believed to be an important factor within addictive use of alcohol (Litt, Cooney, & Morse, 2000), opiates (Powell, et al., 1993), nicotine (Chiamulera, 2005; Payne, Smith, Sturges, & Holleran, 1996; Perkins, 2009), cannabis (Hartz, Frederick-Osborne, & Galloway, 2001; Wolfling, Flor, & Grusser, 2008), and cocaine (Kosten, et al., 2006; Wolfling, et al., 2008).
Although relapse often occurred soon after detoxification, many studies show that relapse rates are still very high for patients who have been abstinent for long time (Vaillant, 1988). However, most investigations that have studied abstinence as a potential influence on cue-induced craving have examined only short-term abstinence. Some researchers thought the high reactivity to drug-related cues exist even long after resolution of acute withdrawal (Carter, et al., 2009; Robbins, et al., 1997). Recently, Gillinder Bedi, et al. (Bedi G, 2011) investigated effects of abstinence on cue-induced craving in cigarette smokers and found that cue-induced craving increases with duration of 35 day’s abstinence. Given that craving and physiological reactions in drug-related cue conditions are associated with relapse (Breese, et al., 2005; Cooney, Litt, Morse, Bauer, & Gaupp, 1997; Kosten, et al., 2006; Niaura, et al., 1988; Sinha, et al., 2011), monitoring the cue reactivity through sustained abstinence is an important consideration in the process of recovery from drug dependence (O'Brien, Childress, Ehrman, & Robbins, 1998; Sinha & Li, 2007). Therefore, it is important to understand the nature and extent of craving and other physiological reactions to drug-related cues in drug-dependent patients who have been abstinent for more than one year.
Opiates are among the most addictive substances. Many studies have showed that exposure to drug-related cues, such as drug paraphernalia, images of drug use, drug-related pictures, or drug-related videos (Ooteman, Koeter, Vserheul, Schippers, & van den Brink, 2006; Shi, et al., 2009) can reliably elicit subjective craving and physiological responses in individuals with opiate dependence. The present study is designed to examine the cue reactivity in heroin dependent patients who have been abstinent for at least 12 months, compared to recently abstinent patients in a compulsory drug-free rehabilitation program. This study should shed light on factors involved in drug dependence and provide helpful information for effective treatment of opiate dependence. We hypothesized that: (1) Heroin-dependent individuals would show increases in craving and physiology reactions in response to drug-related cues as compared to the neutral condition, and they would show greater reactivity than the healthy control group in response to drug cues. (2) Recently abstinent and long-abstinent heroin-dependent groups would show similar craving and physiological reactions to drug-related cues.
2. Methods
2.1 Subjects
A total of 56 heroin-dependent patients who were either abstinent less than one month or were abstinent at least for 12 months from a compulsory drug rehabilitation center in Shanghai were recruited. Of these 56 patients, 27 participants (8 females) were recently abstinent from heroin less than one month (Recently Abstinent group; RA), and 29 participants (11 females) were abstinent from heroin for at least 12 months (long-abstinent group; LA). These participants had been sent to the drug rehabilitation center 1–3 years previous to the study for inpatient compulsory drug rehabilitation. The rehabilitation program consisted of presentations that included didactic drug education classes, moral and legal education, physical exercise, physical labor, and psychosocial intervention. All subjects were interviewed with SCID-I by trained psychiatrists and met the criteria for heroin dependence according to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Subjects with other axis I psychiatric diagnoses were excluded from the study. Urine samples were obtained and screened for opiate use before they were sent to the drug rehabilitation center. Healthy controls (HC) were 26 adults (11 females) recruited through advertisement flyers requesting volunteers 18 years and older for a research study of psychological functioning. The research protocol was approved by the Ethic Committee of the Shanghai Mental Health Center and each subject signed the informed consent form approved by the institution IRB. Participants received a gift of 20 RMB (about 3 US dollars) for their time and effort.
2.2 Procedures
Prior to being recruited into the study, heroin-dependent patients had resided in a locked inpatient drug rehabilitation center with no access to heroin or any other drugs for a period of time. Heroin-dependent patients who were either abstinent within one month or were abstinent more than one year were invited to participate this study, and those who provided informed consent were interviewed to complete demographic, drug history, and diagnostic assessments. The laboratory session was conducted within 3 days after the recruitment interview. Participants were abstinent from heroin and were not in acute withdrawal during the laboratory sessions. When the laboratory sessions were conducted, the mean abstinence time was 17.0 (7.15) days for the RA group, and 392.4(14.74) days for the LA group. Urine testing was conducted regularly to ensure abstinence during the time participants were in the rehabilitation center.
2.3 Laboratory session
All participants received two laboratory sessions (exposure to neutral video or exposure or heroin-related video) on different days in random order. The neutral video was about the process of a middle-aged male to brush his teeth in the morning after waking up, and the heroin use video was about the process of a middle-aged heroin-dependent man smoking or injecting heroin, each video lasting 3 minutes. During the laboratory session, smoking heroin use video and injection heroin use video was exposed to smoking and injection heroin dependents respectively. Blood pressure was accessed by electronic blood pressure monitor and physiological reactions included skin conductance (SC), skin temperature (TEMP), muscle electromyography (MEG), and heart rates (HR) were monitored using multichannel biofeedback device. In the morning of each laboratory day, each participant was brought into the testing room and seated in a comfortable chair. Blood pressure cuff, electrodes, and sensors were attached and connected to a multichannel biofeedback device.
The laboratory procedure was based on the following format: a 5-min baseline period, a 3-min cue exposure period, and a 10-min recovery period. During the baseline period, participants were given instructions to relax for 5 minutes when light music was playing to clear their mind of any worrying thoughts, and to focus on deep breathing. After the baseline period, participants were given the cue exposure (neutral videotape or heroin-related videotape). During the recovery period, the participants were again instructed to relax for 10 min. Baseline craving and physiological measures were obtained before the cue exposure session and also obtained immediately after the cue presentation. Participants were allowed to leave when their physiological measures had returned to baseline levels.
2.4 Measurements
2.4.1 Clinical assessment
All subjects were screened using the patient edition of the Structured Clinical Interview for DSM-IV Axis I Disorders to ensure that they did not have a current anxiety, mood, or psychotic disorder. Diagnoses of DSM-IV opiate dependence were also established by structured interview.
2.4.2 Demographic and drug use history
Demographic information (age, years of education, marital status, etc.) and drug use history (age at onset of drug use, frequency and amount of current daily use, history of previous treatment, etc.) were collected by a self-completion form developed for the study.
2.4.3 Craving
Craving for heroin was assessed by a 10-point visual analog scale (VAS) that participants marked as “0” for “not at all” to “10” for “extremely high” in response to the question, “How much do you feel the urge to use heroin?” Craving ratings were obtained at the end of baseline period and immediately after the cue exposure period (either to the neutral or heroin videotape).
2.4.4 Physiological measurements
Physiological measurements included skin conductance (SC), muscle electromyography (MEG), skin temperature (TEMP), heart rates (HR), systolic blood pressure (HBP) and diastolic blood pressure (LBP). An electronic blood pressure monitor (OMRON, HEM-6111) was used to assess blood pressure. HBP and LBP were measured at baseline and immediately after exposure to the neutral or heroin video. A multichannel biofeedback device (Thought technology, Canada, VBFB3000) was used to monitor physiological reactions including SC, MEG, TEMP, and HR. The physiological measurements were recorded at several time points at baseline and during the cue exposure period. The mean value of each physiological measurement at each period was used for the study.
2.5 Statistics Analyses
Demographic and clinical characteristics of the two heroin-dependent groups were assessed using independent t-tests or chi-square tests. One-way ANOVAs were used to analyze baseline craving and physiological measures. 3(Group: control, LA and long RA)× 2 (cue condition: heroin or neutral) different repeated-measures ANOVAs were used to assess the group and cue effects on craving scores and physiological measures, with group as between-subjects factor and cue condition as within-subjects factor. Post hoc tests were used if the ANOVA tests found group differences. The significant level was 0.05, two-tailed.
3 RESULTS
3.1 Participant Characteristics
The participants comprised 56 heroin-dependent individuals (19 females) with a mean age (± SD) of 34.0 (8.35) years, and 26 healthy controls (11 females) with a mean age of 32.1(4.5) years. The gender composition and mean age were not different between heroin-dependent and control groups (gender: x2=0.46, p=0.47; age: t=1.1, p=0.27). All participants are Chinese Hans.
Table 1 shows that the demographic and drug use history were not different between heroin-dependent groups, except the abstinence time in the LA group was significantly longer than that in RA group [(392.4(14.7) vs17.1(3.3), t=132.5, p<0.001].
Table 1.
Demographic and drug use history for the two heroin-dependent groups
| RA group (n=27) | LA group (n=29) | x2 or t | p | |
|---|---|---|---|---|
| Age (years), mean(SD) | 31.8(7.7) | 35.1(7.1) | 1.71 | 0.09 |
| Female, n(%) | 8(29.6) | 11(37.9) | 0.51 | 0.58 |
| Education (years), mean(SD) | 11.4(2.5) | 10.7(2.2) | 1.08 | 0.28 |
| Marriage status | ||||
| Married, n(%) | 3(11.1%) | 4(13.8%) | ||
| Divorce/separated, n(%) | 8(27.6%) | 10(37.0%) | ||
| Unmarried, n(%) | 17(58.6%) | 14(51.9%) | ||
| Onset age of drug use (years) | 24.0(8.3) | 23.9(8.1) | 0.06 | 0.95 |
| Injection drug use, n(%) | 19(70.4%) | 20(69.0%) | 0.13 | 0.91 |
| Average of drug abuse time (years) | 5.7(3.7) | 7.6(3.6) | ||
| Drug use days in last 30 days (days) | 20.1(14.0) | 23.3(10.6) | 0.94 | 0.35 |
| Abstinent from last heroin use (days) | 17.1(3.3) | 392.4(14.7) | 132.5 | <0.001 |
3.2 Baseline craving and physiological measurements
One-way ANOVA tests showed that the basal levels of craving [F(2,79)=21.31, p<0.001], MEG [F(2,79)=4.82, p=0.009], SC [F(2,79)=8.68, p<0.001], HR [F(2,79)=9.66, p<0.001] were different among the three groups. The heroin-dependent groups had significantly higher basal levels of heroin craving, and SC compared with the HC group. The RA group had a higher MEG and HR compared with the HC group. Other than the RA group having higher HR [82.9(12.7) vs 74.4(8.3), t=8.72, p<0.001] than the LA group, the baseline heroin craving and physiological measurements were not different between the two groups. Because of the group main effect on baseline scores, response analyses (below) examined changes from baseline scores.
3.3 Craving and physiological responses
3.3.1 Changes from baseline measurements
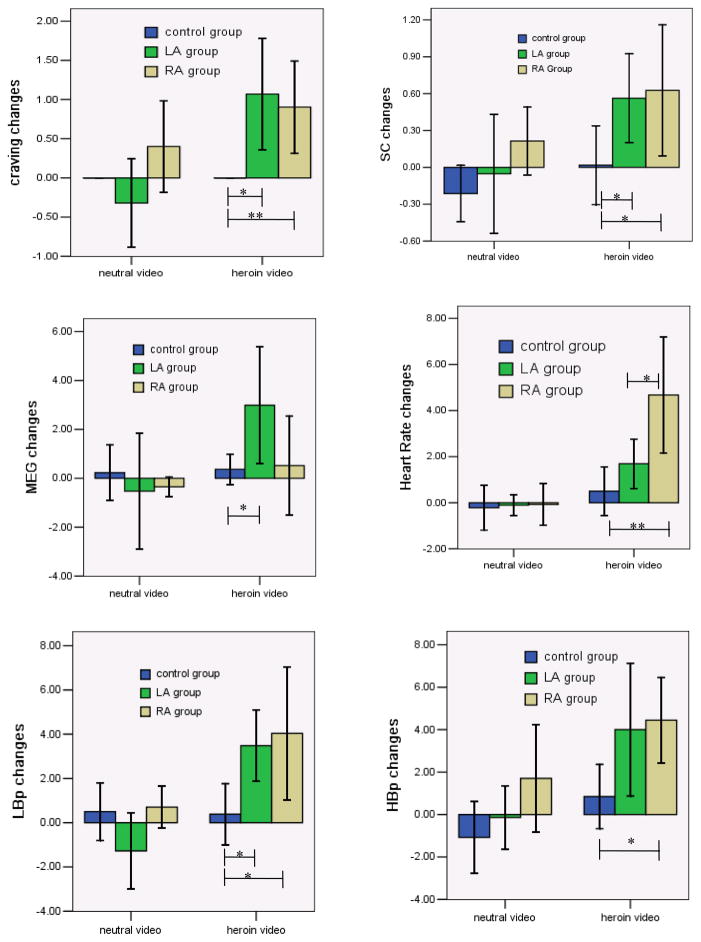
Results from repeated-measures ANOVA tests showed the main effect (changes from baseline measurements) was significant on craving [F(2,164)=11.08, p=0.001)], SC[F(2,164)6.22, p=0.014), HR[(F(2, 164)=17.02, p<0.001], HBP [F(2,164)=14.11, p<0.001], LBP[F(2,164)=13.41,p<0.001]. Both heroin-dependent groups reported significantly higher heroin craving, and showed higher SC, HR, and had higher SBP, DBP when exposed to the heroin video (paired t tests, p<0.05 ). On the other hand, the control group didn’t report heroin craving changes and showed no other physiological measurements changes when exposed to the heroin video (paired t tests, p>0.05), except they had a marginal increase in breath rates [paired t test, t (25)=2.06, p=0.05]. All three groups showed no changes in heroin craving, and in other physiological measurements in response to the neutral video (paired t tests, p>0.05). In sum, only the two heroin-dependent groups showed increased craving and elevated physiological response (SC, HR, SBP, LBP) to the heroin-related video (compared to the neutral video), but the control group didn’t have such responses.
3.3.2 Group Effects
The repeated-measures ANOVA tests showed group had significant effect on craving [F(2,164)=28.14, p<0.001], SC[F(2,164)=10.49, p<0.001], MEG [F(2, 164)=5.22, p=0.006], HR[F(2,164)=11.89, p<0.001], SBP[F(2,164)=11.89, p<0.001], SBP [F(2,164)=4.95, p=0.008]. Figure 1–6 showed the changes of craving and physiological measurements in three groups by two cue conditions.
Figure 1–6.
Craving and physiological changes in three groups by two cue conditions Note: *group differences, p<0.05; **group differences, p<0.01
Compared to the control group, both heroin groups reported more changes on heroin craving, SC, and LBP after exposure to heroin-related videos. The RA group showed more changes on HR and HBP, and the LA group showed more changes on MEG when exposed to the heroin video compared to the control group. While the RA group showed more HR changes, changes in heroin craving and other physiological measurements were not different between heroin groups when exposed to heroin video. Changes in heroin craving and other physiological measurements were not different when exposed to the neutral video among three groups. In sum, the control group and the two heroin-dependent groups responded differently to the heroin videos, but not to the neutral video. The heroin groups didn’t show different responses to either heroin video or neutral video, except the RA showed more changes on HR.
3.3.3 Cue condition effects
Significant effects of cue condition were found on craving [F(1,164)=9.41, p<0.001], MEG[F(1,164)=4.65, p=0.033], SC[F(1,164)=7.37, p=0.007], HR [F(1,164)=21.51, p<0.001], HBP[F(1,164)=11.43, p=0.001], LBP[F(1,164)= 13.91, p<0.001]. The two heroin-dependent groups had more changes on craving, MEG, SC, HR, HBP and LBP when exposed to heroin video (compared to neutral video). No differences were found in heroin craving and other physiological measures in the control group individuals when they were exposed to the heroin video compared to the neutral video (p>0.05).
4 Discussions
Consistent with our hypotheses, heroin-dependent individuals showed elevated craving and physiological responses when they were exposed to heroin-related video, compared to neutral video, and these responses were not produced in the healthy controls. Our results were also consistent with findings from previous research on users of opiates and other substances (Ehrman, Robbins, Childress, & O'Brien, 1992; Kaplan, et al., 1985). These studies have shown an increase in craving and other physiological measurements when they were exposed to drug-related cues in individuals who abuse nicotine, alcohol, opiates, cocaine, marijuana, and other substances(Childress, et al., 1986; Ehrman, et al., 1992; Fox, Bergquist, Hong, & Sinha, 2007; Fox, Hong, Siedlarz, & Sinha, 2007; Kaplan, et al., 1985; Tiffany & Drobes, 1990; Tolliver, et al.). Many theories have proposed to explain this phenomenon, the most popular model postulating a central role for associative learning mechanisms (O'Brien, et al., 1998; O'Brien, Childress, McLellan, & Ehrman, 1992). Recently, many studies have speculated that drug-dependent individuals show altered responses to drug cues, potentially due to neurobiological changes associated with chronic drug use (Buffalari & See, 2010; Fox, Hong, Siedlarz, & Sinha, 2008; Hyman, 2005; Kosten, et al., 2006). Therefore, it is important to examine and monitor the reactivity to drug-related cues in substance abuse treatment (Fox, et al., 2008; O'Brien, et al., 1998; Sinha, et al., 2009; Sinha & Li, 2007).
The primary aim of this study was to investigate to the occurrence and nature of cue reactivity in heroin-dependent individuals who had been abstinent for at lest 12 months, compared to recently abstinent heroin dependent patients (less than one month). The results confirmed our hypothesis in that we found higher cue reactivity among patients after 12 months of drug-free rehabilitation, similar to the recently detoxified patients. The recently abstinent and long-abstinent heroin-dependent patients didn’t show different responses to either heroin video or neutral video, except RA individuals showed more changes on HR. Our results also showed that the LA and RA groups had similar baseline craving and physiological measurements, except the recently abstinent heroin group had higher HR. Maybe the higher basal HR and stronger responses to heroin cues were associated with withdrawal symptoms in the recently abstinent heroin-dependent patients. To our best knowledge, this is the first study to compare cue-induced craving and physiological reactions between recently and long-abstinent heroin-dependent individuals. Our findings were consistent with several related studies in smokers, which found that elevated craving and higher physiological reactions can be reliably elicited over repeated cue reactivity sessions (LaRowe, Saladin, Carpenter, & Upadhyaya, 2007; Miranda, Rohsenow, Monti, Tidey, & Ray, 2008). Furthermore, one study found cue-induced craving increases with duration of abstinence in smokers (Bedi G, 2011). Our study indicated that long-abstinent patients in drug-free settings continued to be vulnerable to drug-related stimuli. Given that individuals in the rehabilitation center are likely to be confronted with these stimuli soon after discharge, a reduction of cue reactivity may contribute to rehabilitation and probably to the prevention of relapse.
Several study limitations are noteworthy; without prospective data, it is difficult to know if cue-elicited craving and reactivity have a causal role in the maintenance of drug use or relapse. The samples were from drug-free rehabilitation settings and findings may not be generalizable to outpatient or community settings. It will be important to determine whether the phenomenon also occurs among patients in other settings and how it relates to relapse.
Conclusions and clinical implications
The study shows that abstinent heroin-dependent patients have elevated craving and physiological reactions to heroin-related cues and that strong responses still exist in long-abstinent heroin-dependent patients. Despite above-mentioned limitations, findings from the study provide some novel information regarding drug-related reactions in long-abstinent heroin-dependent patients. Such findings may have clinical implications highlight the importance of individualized interventions. For example, cue-induced craving and physiological reactions may be assessed in a clinical setting and patients with greater reactions may be assigned to more targeted interventions such as cue exposure treatment to prevent the possibility of relapse due to cue sensitivity. Also, during the course of therapy, therapists could use the cue-induced craving and physiological reactions to evaluate patients’ progress.
Highlight.
Heroin-abstinent groups had increased heroin craving and physiological reactions to heroin related cue.
Heroin-abstinent groups had no significant craving and physiological reactions to neutral cue.
Normal controls didn’t have significant craving and physiological reactions to both cues.
Recently and long time abstinent heroin dependents showed similar craving and physiological reactions to heroin related cues
Acknowledgments
Role of Funding Sources
This study was supported by a grant from the Chinese National Nature Science Foundation (30971048), the NIH/FIC (R01TW007279) and the Science and technology commission of the Shanghai municipality (09410707000). The funders had no role in the study design, data collection, analysis, preparation of the manuscript, or selection of publications.
Footnotes
Contributors
Min Zhao conceptualized and designed the study and wrote the paper; Chenglu Fan, Jiang Du did the cue exposure experiments and collected the data. Haifeng Jiang, Hanhui Chen, and Haiming Sun collected the clinical data. All authors contributed to editing of manuscript, and all have approved the final manuscript.
Conflict of Interest
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedi GPK, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69(7):708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29(2):185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Amygdala mechanisms of Pavlovian psychostimulant conditioning and relapse. Curr Top Behav Neurosci. 2010;3:73–99. doi: 10.1007/7854_2009_18. [DOI] [PubMed] [Google Scholar]
- Carter BL, Lam CY, Robinson JD, Paris MM, Waters AJ, Wetter DW, et al. Generalized craving, self-report of arousal, and cue reactivity after brief abstinence. Nicotine Tob Res. 2009;11(7):823–826. doi: 10.1093/ntr/ntp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Chiamulera C. Cue reactivity in nicotine and tobacco dependence: a “multiple-action” model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking-associated stimuli. Brain Res Brain Res Rev. 2005;48(1):74–97. doi: 10.1016/j.brainresrev.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O'Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict. 1986;81(5):655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106(2):243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107(4):523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33(4):796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63(3):269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162(8):1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerleau OF. Reactivity to alcohol-related cues: physiological and subjective responses in alcoholics and nonproblem drinkers. J Stud Alcohol. 1985;46(4):267–272. doi: 10.15288/jsa.1985.46.267. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31(3):644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addict Behav. 2007;32(12):2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction. 2000;95(6):889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr, Rohsenow DJ, Monti PM, Tidey J, Ray L. Effects of repeated days of smoking cue exposure on urge to smoke and physiological reactivity. Addict Behav. 2008;33(2):347–353. doi: 10.1016/j.addbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97(2):133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Vserheul R, Schippers GM, van den Brink W. Measuring craving: an attempt to connect subjective craving with cue reactivity. Alcohol Clin Exp Res. 2006;30(1):57–69. doi: 10.1111/j.1530-0277.2006.00019.x. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, Sturges LV, Holleran SA. Reactivity to smoking cues: mediating roles of nicotine dependence and duration of deprivation. Addict Behav. 1996;21(2):139–154. doi: 10.1016/0306-4603(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104(10):1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Powell J, Gray J, Bradley B. Subjective craving for opiates: evaluation of a cue exposure protocol for use with detoxified opiate addicts. Br J Clin Psychol. 1993;32(Pt 1):39–53. doi: 10.1111/j.2044-8260.1993.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav. 1997;22(2):157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Shi J, Jun W, Zhao LY, Xue YX, Zhang XY, Kosten TR, et al. Effect of rapamycin on cue-induced drug craving in abstinent heroin addicts. Eur J Pharmacol. 2009;615(1–3):108–112. doi: 10.1016/j.ejphar.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34(5):1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of Adrenal Sensitivity, Stress- and Cue-Induced Craving, and Anxiety on Subsequent Alcohol Relapse and Treatment Outcomes. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152(2):140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. Imagery and smoking urges: the manipulation of affective content. Addict Behav. 1990;15(6):531–539. doi: 10.1016/0306-4603(90)90053-z. [DOI] [PubMed] [Google Scholar]
- Tolliver BK, McRae-Clark AL, Saladin M, Price KL, Simpson AN, DeSantis SM, et al. Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. Am J Drug Alcohol Abuse. 36(2):106–113. doi: 10.3109/00952991003686402. [DOI] [PubMed] [Google Scholar]
- Vaillant GE. What can long-term follow-up teach us about relapse and prevention of relapse in addiction? Br J Addict. 1988;83(10):1147–1157. doi: 10.1111/j.1360-0443.1988.tb03021.x. [DOI] [PubMed] [Google Scholar]
- Watson NL, Carpenter MJ, Saladin ME, Gray KM, Upadhyaya HP. Evidence for greater cue reactivity among low-dependent vs. high-dependent smokers. Addict Behav. 2010;35(7):673–677. doi: 10.1016/j.addbeh.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci. 2008;27(4):976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]