Abstract
Study Objective
To evaluate the effect of ventilation strategy on markers of inflammation in patients undergoing spine surgery in the prone position.
Design
Randomized controlled trial.
Setting
University-affiliated teaching hospital.
Patients
26 ASA physical status 1 and 2 patients scheduled for elective primary lumbar decompression and fusion in the prone position.
Interventions
Patients were randomized to receive mechanical ventilation with either a tidal volume (VT) of 12 mL/kg ideal body weight with zero positive end-expiratory pressure (PEEP) or VT of 6 mL/kg ideal body weight with PEEP of 8 cm H2O.
Measurements
Plasma levels of interleukin (IL)-6 and IL-8 were determined at the beginning of ventilation and at 6 and 12 hours later. Urinary levels of desmosine were determined at the beginning of ventilation and on postoperative days 1 and 3.
Main Results
A significant increase in IL-6, IL-8, and urine desmosine levels was noted over time compared with baseline (P < 0.01). However, no significant difference in the levels of markers was seen between the groups at any time point when controlling for demographics, ASA physical status, body mass index, duration of ventilation, or estimated blood loss.
Conclusions
Although markers of inflammation are increased after posterior spine fusion surgery, ventilation strategy has minimal impact on markers of systemic inflammation.
Keywords: Lung injury, spine surgery, tidal volume
1. Introduction
In patients with acute respiratory distress syndrome (ARDS), mechanical ventilation with a lower tidal volume (VT) than is traditionally used results in decreased mortality and morbidity [1]. It remains inconclusive, however, if a beneficial effect of this strategy may be extrapolated to individuals with healthy lungs undergoing short-term ventilation for surgery. A recent study suggests that the use of lower VTs and positive end-expiratory pressure (PEEP) may limit pulmonary inflammation in mechanically ventilated patients without preexisting lung injury who undergo an elective surgical procedure of 5 hours’ duration or longer [2].
Other studies performed in healthy individuals undergoing thoracic, abdominal, and cardiac surgery failed to show a benefit of a protective ventilation strategy, suggesting that the type of surgery may also contribute to the perioperative inflammatory response [3,4]. The impact of the protective ventilation strategy has not been studied in patients undergoing spine surgery, a cohort who may be prone to lung injury secondary to intraoperative insults, including pulmonary embolization [5–9]. While it is difficult to influence factors related to surgical insults of the lung, manipulation of ventilator strategies is possible.
Data evaluating the effect of the intraoperative comparative effect of low versus traditional VT ventilation in the prone position is unavailable. Prone positioning may be an important factor when assessing the impact of various ventilation strategies on markers of inflammation as the number of changes in the physiology of hemodynamics and ventilation occur [10]. Previous studies have suggested that prone positioning produces a number of hemodynamic changes most likely due to inferior vena cava compression and increased intrathoracic pressure [11]. Prone positioning causes a more homogeneous distribution of transpulmonary pressure compared with the supine position. Pelosi et al observed a movement of lung densities from dorsal to ventral regions when patients were turned from supine to prone, and a more homogeneous distribution of alveolar inflation in the prone position [12].
The specific aim of this prospective randomized study was to compare markers of inflammation between patients receiving intraoperative low VT versus traditional VT during posterior lumbar spine fusion in the prone position. We hypothesized that use of lower VT would be associated with decreased levels of inflammation and lung catabolism compared with traditional VT.
2. Materials and methods
After obtaining approval by the Hospital for Special Surgery Institutional Review Board (Protocol no. 28117) and written, informed consent, 26 patients scheduled for elective, primary lumbar decompression and fusion of 4 spinal levels or less were enrolled in this prospective randomized controlled study conducted from February 2009 to September 2010. Excluded were patients with known previous lung pathology, use of immunosuppressants, renal failure with creatinine > 1.5 mg/dL, recent exposure to a ventilator or surgery during general anesthesia (< one yr), and ASA physical status 3 or higher.
Patients were randomly assigned by a computer-generated list of random numbers to receive either a VT of 12 mL/kg ideal body weight (n =13) and zero PEEP or 6 mL/kg and 8 cm H2O of PEEP (n =13). The allocation sequence was concealed from the research assistant in sequentially numbered, opaque, sealed, and stapled envelopes. Patients, surgeons, and research assistants who were responsible for subsequent data collection were blinded to the randomization. The ideal body weight of male patients was calculated as equal to 50 ± 0.91 (cm of height - 152.4); that of female patients was calculated as 45.5 ± 0.91 (cm of height - 152.4) [2].
All patients underwent standardized general endotracheal anesthesia. Anesthesia was induced with an intravenous (IV) injection of midazolam 5 mg, fentanyl 250 μg, propofol 2.0 mg/kg, and vecuronium 0.1 mg/kg. After intubation and placement of arterial catheters, and central catheters, if necessary, anesthesia was maintained with 0.25% to 0.5% isoflurane, nitrous oxide 50% in oxygen, continuous infusion of propofol 100 μg/kg/min, fentanyl 1.5 μg/kg/hr, and ketamine 0.15 mg/kg/hr. This combination of agents and IV anesthetics and analgesics represents routine management of spine surgical cases requiring neuromonitoring at our institution. Intermittent doses of IV vecuronium were given as necessary. The ventilatory protocol consisted of volume-controlled minute ventilation, inspiratory-to-expiratory ratio of 1:2, and a respiratory rate adjusted to achieve normocapnia [end-tidal carbon dioxide (ETCO2) between 30 and 36 mmHg] [2]. Decompression and stabilization with segmental spinal pedicular instrumentation procedures were performed as indicated according to each patient’s needs. Patient characteristics and perioperative events (fluid data, length of surgery, and ventilation complications) were recorded. Arterial blood samples were collected for gas analysis approximately 30 minutes after the start of mechanical ventilation in the prone position (baseline). Urine samples were collected at baseline and on postoperative days (PODs) 1 and 3, and analyzed for levels of desmosine to determine the level of elastin catabolism. Concomitant analysis of urine creatinine levels was performed to adjust for dilution [10]. Desmosine has been used as a marker of lung injury in the past [13], and recently it was found to be useful as a marker of lung injury during spine surgery [5].
Blood samples to measure levels of Interleukin (IL)- 6 and IL-8 were drawn at baseline, at 6, and 12 hours thereafter. Markers of inflammation spike early (between 6 and 12 hrs) after ventilator-associated lung injury and orthopedic surgery [2,14]. Interleukin-6 and Il-8 are markers of inflammation and levels were affected differently by the type of ventilation in a previous study [2].
Immediately after collection, blood samples were centrifuged at 3,000 rpm for 5 minutes, and the supernatant was frozen at less than18° Fahrenheit. For the analysis, solid-phase enzyme-labeled chemiluminescent sequential immunometric assays (Immulite; Siemens, Los Angeles, CA, USA) were used. Results are presented as picomoles of desmosine per milligram of creatinine. The primary outcome was a reduction of urine desmosine levels postoperatively in the low VT, not traditional VT group. Secondary outcomes were the reduction of IL-6 and IL-8 in the low versus traditional VT group.
2.1 Statistics
Mann-Whitney U test and Chi-square test were conducted to test the difference between the low VT and high VT groups in a bivariate analysis for continuous and categorical outcomes, respectively. Urine desmosine, IL-6, and IL-8 levels were modeled as a function of time using multiple linear regression with inference based on the generalized estimating equations (GEEs) method [15]. The low and the high VT groups were compared in a regression model while adjusting for demographics (age, gender, BMI), duration of ventilation, and estimated blood loss (EBL). Changes in outcomes over time were detected using appropriate linear contrasts. All statistical analyses were performed using SAS version 9.2.1 software (SAS Institute, Cary, NC, USA). Continuous variables are presented as means ± standard deviation and categorical variables are described as percentages. A P-value of 0.05 was considered to be significant.
To detect a reduction of 30% of urine desmosine levels, with a two-sided 5% significance level and a power of 80%, a sample size of 13 patients per group was necessary. This level of reduction was based on findings from a previous study conducted by McClintock et al, who found an approximately 30% difference in desmosine levels in patients with ARDS who were ventilated with a VT of 6 mL/kg or 12 mL/kg [13].
3. Results
Of the 26 patients enrolled in the study, one patient in the low VT group withdrew consent for blood draws after randomization and surgery. Patient demographics and intraoperative data are shown in Table 1. Table 2 details ventilatory and respiratory variables in the two patient groups.
Table 1.
Demographic and intraoperative data in the two groups
| High VT | Low VT PEEP | P-value | |
|---|---|---|---|
| Gender (M/F; n) | 6/7 | 6/7 | 1 |
| Age (yrs) | 50 ± 12 | 60 ± 15 | 0.01 |
| BMI (kg/m2) | 27 ± 5 | 28 ± 5 | 0.8 |
| ASA physical status (1/2; n) | 0/13 | 5/8 | 0.03 |
| Duration of ventilation (min) | 277 ± 47 | 307 ± 66 | 0.04 |
| Cystalloid (mL) | 2835 ± 568 | 2692 ± 1058 | 0.7 |
| Albumin (mL) | 533 ± 176 | 556 ± 157 | 0.6 |
| PRBCs (U) | 1.25 ± 0.43 | 2 ± 1 | 0.5 |
| Cell Saver (mL) | 245 ± 141 | 355 ± 231 | 0.06 |
| EBL (mL) | 692 ± 342 | 1019 ± 733 | 0.33 |
| Urine (mL) | 365 ± 152 | 350 ± 214 | 0.5 |
Values are means ± SD.
VT=tidal volume, PEEP=positive end-expiratory pressure, BMI=body mass index, PRBC=packed red blood cells, EBL=estimated blood loss. (Cell Saver; Haemonetics, Braintree, MA, USA)
The High VT group received mechanical ventilation with a VT of 12 mL/kg ideal body weight and no PEEP; the Low VT PEEP group received mechanical ventilation with a VT of 6 mL/kg ideal body weight and PEEP of 8 cm H2O
Table 2.
Ventilatory and respiratory variables
| High VT | Low VT PEEP | P-value | |
|---|---|---|---|
| VT (mL) | 755 ± 118 | 390 ± 79 | <0.0001 |
| Ventilatory rate (L/min) | 8 ± 1.4 | 12 ± 3.3 | 0.001 |
| Pawmax (cm H2O) | 23 ± 6 | 19 ± 2.3 | 0.22 |
| Pawmean (cm H2O) | 7 ± 2.5 | 9.1 ± 0.9 | 0.1 |
| ETCO2 (mmHg) | 32 ± 4.5 | 36 ± 5.8 | 0.05 |
| pH | 7.44 ± 0.05 | 7.38 ± 0.05 | 0.005 |
| PaO2 (mmHg) | 199 ± 45 | 233 ± 62 | 0.1 |
| PaCO2 (mmHg) | 35 ± 6 | 43 ± 6 | 0.002 |
Values are presented as means ± SD.
VT=tidal volume, PEEP=positive and-expiratory pressure, Pawmax=maximal airway pressure, Pawmean =mean airway pressure, ETCO2=end-tidal carbon dioxide, PaO2=arterial blood oxygen tension, PaCO2=arterial carbon dioxide tension.
The High VT group received mechanical ventilation with a VT of 12 mL/kg ideal body weight and no PEEP; the Low VT PEEP group received mechanical ventilation with a VT of 6 mL/kg ideal body weight with PEEP of 8 cm H2O
Despite randomization, patients in the low VT group were older (P = 0.01) and were ventilated slightly but significantly longer (P = 0.04) than patients in the high VT group. However, subsequent analysis of differences in markers between groups controlled for these discrepancies (see Materials and methods above). There was no difference in the two groups with respect to EBL, volume of crystalloid or albumin infused, blood transfusion, or urine output (Table 1). Patients in the low VT group tended to have a lower average pH and a higher ETCO2 and PCO2 (Table 2).
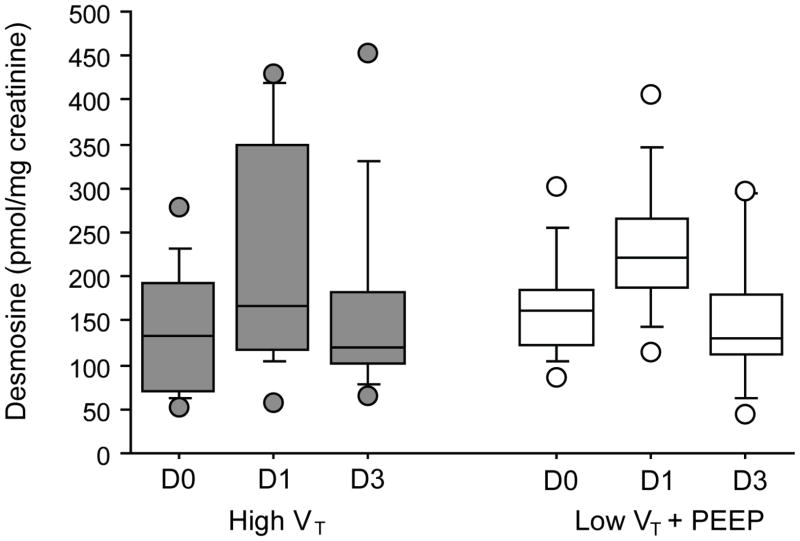
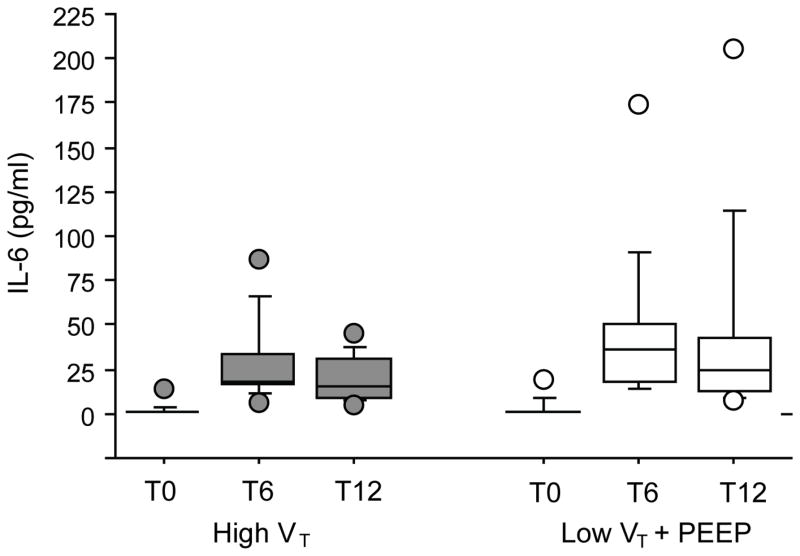
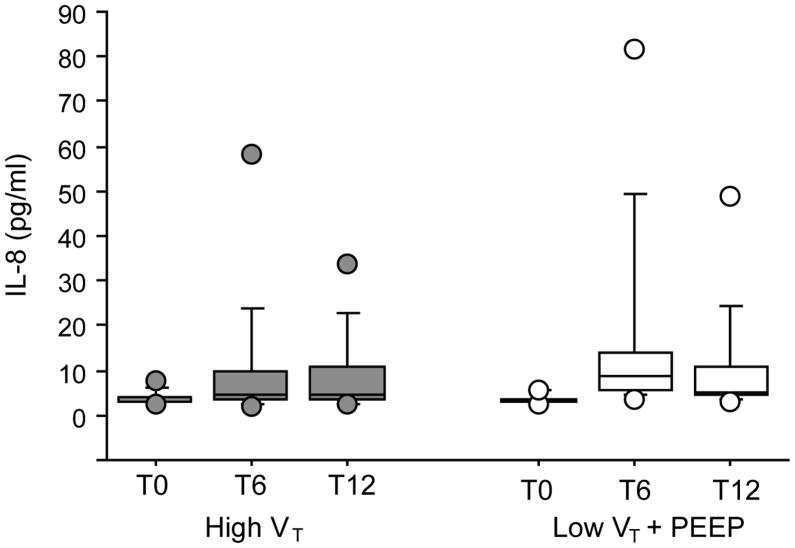
Figures 1, 2, and 3 depict perioperative trends in urine desmosine, IL-6, and IL-8 levels, respectively. In both groups, a significant increase in the levels of urine desmosine-creatinine ratios was observed on POD1 than on the day of surgery (P = 0.001); no differences compared with baseline were seen on POD3 (P = 0.53). In both groups, a significant increase in IL-6 and IL-8 also was noted at 6 and 12 hours compared with baseline (P < 0.0001 vs P = 0.001, P = 0.01 vs P = 0.01, respectively). However, no significant difference in the levels of these markers was seen between groups at any time point when controlling for patient demographics, duration of ventilation, or EBL.
Fig. 1.
Levels of urinary desmosine/creatinine over time from patients mechanically ventilated with 12 mL/kg [high tidal volume (VT); grey] or with 6 mL/kg and 8 cm H2O of positive end-expiratory pressure (PEEP; low VT; white). In both groups, a significant increase in the levels of urine desmosine-creatinine ratios was noted on postoperative day 1 (D1) versus the day of surgery (DO; P = 0.001); no differences compared with baseline were seen on postoperative day 3 (D3; P = 0.53). No differences between groups were found (P = 0.7).
Fig. 2.
Levels of interleukin (IL)-6 over time from patients mechanically ventilated with 12 mL/kg [high tidal volume (VT); grey] or with 6 mL/kg and 8 cm H2O of positive end-expiratory pressure (PEEP; low VT; white). In both groups, a significant increase compared with baseline was noted at 6 and 12 hours after the start of ventilation (P < 0.0001 and P = 0.001, respectively). No differences between groups were found (P = 0.3). T0, T6, T12=blood sampling: start of ventilation (baseline), at 6 hours, and at 12 hours, respectively.
Fig. 3.
Levels of interleukin (IL)-8 over time from patients mechanically ventilated with 12 mL/kg [high tidal volume (VT); grey] or with 6 mL/kg and 8 cm H2O of positive end-expiratory pressure (PEEP; low VT; white). In both groups, a significant increase compared with baseline was observed at 6 and 12 hours after the beginning of ventilation (P = 0.01). No differences between groups were noted (P = 0.7). T0, T6, T12=blood sampling: start of ventilation (baseline), at 6 hours, and at 12 hours, respectively.
One patient in the low VT group developed ARDS requiring prolonged mechanical ventilation. This patient also showed the highest increase in IL-6 at 12 hours (206 pg/mL) and second highest level of IL-8 at 6 hours (35.6 pg/mL) of all patients postoperatively. Urine desmosine levels reached almost double the average level on POD1 (407 pmol/mg of creatinine). No clinically apparent respiratory complications were noted in the remainder of patients.
4. Discussion
In this study of 26 patients with no preexisting lung pathology, undergoing posterior spinal fusion, we measured a significant increase in inflammatory markers in the postoperative period. However, when controlling for perioperative variables, we found no difference when using low VT versus traditional VT of ventilation on inflammatory markers and elastin catabolism.
Increasing evidence suggests that low VT ventilation with 6 – 8 mL/kg ideal body weight with moderate or high levels of PEEP decreases mortality in acute lung injury (ALI) or ARDS when compared with mechanical ventilation with high VT of at least 12 mL/kg ideal body weight. This approach also is associated with lower pulmonary and/or systemic inflammatory mediator concentrations [16–19]. However, although these studies showed that mechanical ventilation may alter outcome and course of an existing inflammatory injury in patients with ALI/ARDS, it is not entirely clear whether differences in mechanical ventilation strategy alone alters the cytokine production in patients with healthy lungs during short-term ventilation associated with surgery.
In our study of 26 healthy patients undergoing posterior spinal fusion, we found no difference in inflammatory markers using different ventilation strategies. The lack of differential response has been documented previously. Wrigge et al [3] studied the effect of high VT (12–15 mL/kg per body weight) with zero or low PEEP versus low VT (6 mL/kg) with PEEP of 10 cm H2O in patients with healthy lungs undergoing major thoracic and abdominal surgery. They observed an increase in cytokine levels [tumor necrosis factor (TNF)-α, Il-1, Il-6, IL-8, Il-10, and IL-12] in the plasma after 0, one, two, and three hours, and in tracheal aspirates after three hours of mechanical ventilation. However, no difference in levels of inflammatory markers was found between the two different ventilatory approaches.
Similarly, Koner et al [4] studied the effects of protective (6 mL/kg) and conventional (10 mL/kg) mechanical ventilation on systemic cytokine release after saw no difference in systemic TNF-α and IL-6 in the two groups of patients.
Contrary to the previously mentioned studies, in which no differences in the levels of inflammation between ventilation strategies were found, Wolthius et al [2] showed that mechanical ventilation with lower VT and PEEP attenuates the increase of IL-8, myeloperoxidase, and elastase in the bronchoalveolar lavage as compared with higher VT and no PEEP. Their findings suggesting that traditional mechanical ventilator volumes represented bigger proinflammatory stumuli in noninjured lungs.
The conflicting observations in the various studies are likely multifactorial, but may be explained by the different impact of the surgical procedure on lung injury. It is unclear whether mechanical ventilation itself is the main determinant or if it aggravates the production and/or translocation of cytokines released by an inflammatory co-stimulus such as major surgery. Significant lung injury does occur during spine surgery [5–9]. The exact mechanism of lung injury associated with spine surgery remains unclear, but Urban et al [7] showed an adverse pulmonary effect of perioperative events, noting an increase in pulmonary vascular resistance in patients during or after posterior spinal instrumentation. The same authors [6] analyzed bronchoalveolar specimens and linked the presence of lipid-laden macrophages to possible embolization of fat and debris entering the bloodstream during the surgical procedure. This mechanism of lung injury is supported by echocardiographic studies, in which 80% of spine surgery patients experienced moderate to severe embolic events during instrumentation of the spine [9]. The additional insult related to the mechanical ventilation likely contributed to the pulmonary damage but it may not have been the primary determinant of the injury. In fact, in our study we found a statistically significant increase in the levels of inflammatory markers postoperatively, but there was no difference based on the ventilation settings. This finding suggested that the surgical procedure may overwhelm any potential contribution of different ventilation strategies to the overall insult.
The lack of impact of ventilation strategy noted in our patient population as compared with patients with ARDS also may be explained by the physiologic effects of the prone position on the lungs of previously healthy subjects. Prone positioning causes a more homogeneous distribution of transpulmonary pressure, providing a more uniform alveolar ventilation, than does the supine position [12]. Several factors may contribute to this differential ability of the prone position to alter dorsal lung transpulmonary pressures, including a reversal of lung weight gradients, direct transmission of the weight of the heart to subjacent regions, direct transmission of the weight of abdominal contents to caudal regions of the dorsal lung and/or regional mechanical properties, and shape of the chest wall and lung [12]. It is therefore possible, although speculative, that prone positioning may have beneficial effects in reducing the inflammatory response to mechanical ventilation, thus making it more difficult to show differences between low and traditional volume ventilation strategies in our study.
In our cohort of patients we had one case of postoperative ARDS in a patient ventilated with low VT. Although no definitive conclusion may be drawn from this observation, and a number of factors may influence the development of perioperative ARDS, it must be noted that some researchers have linked low VT ventilation to potentially adverse findings. In an experimental animal model, Hong et al [20] recently showed that ventilation with low VT and high PEEP (6 mL/kg and PEEP of 10 cm H2O) was associated with the greatest increase in inflammatory cytokines identified in bronchoalveolar lavage. The authors found an up to 6-fold increase in the pro-inflammatory cytokines TNF-α, Il-1, Il-6, and IL-8 compared with ventilation with high VT and low PEEP (15 mL/kg and PEEP of 3 cm H2O) and low VT and low PEEP (6 mL/kg and PEEP of 3 cm H2O).
The major purpose of lung-protective mechanical ventilation strategies is to reduce regional end-inspiratory stretch, thereby decreasing alveolar damage and alveolar inflammation [21]. However, if plateau pressures in ventilated patients are low, smaller VTs may not be indicated as they may predispose to atelectasis formation [20]. In patients with normal lungs, the end-inspiratory stretch may be relatively low, even with a ventilation strategy using high VT [22]. In fact, despite the difference in VT in our groups of patients, differences in maximum and mean pulmonary ventilation pressures in patients ventilated with high VT versus those ventilated with low VT were small and not statistically significant (23 ± 6 vs 19 ± 2.3, P = 0.22, and 7 ± 2.5 vs 9.1 ± 0.1, P = 0.1, respectively).
Our study was limited by a few factors. The number of patients enrolled was relatively small. Thus, the fact that the marker levels did not reach significance may be secondary to insufficient power. Furthermore, like any study evaluating systemic inflammation, the assessment of this outcome was limited by the utilization of the number of markers used. Thus, we cannot exclude the possibility that significant differences of other inflammatory proteins may have been present.
However IL-6, IL-8, and desmosine have been described as useful indicators of acute inflammation in studies evaluating the role of inflammatory markers on ventilation [2–5]. It is also of importance to note that we measured inflammatory markers for only 12 hours and effects on markers beyond this time point remain thus elusive. However, in previous clinical studies of patients with ALI, alveolar and systemic cytokine concentrations increased within one hour after starting mechanical ventilation with low PEEP and high VT [2]. Furthermore, we measured only plasma cytokine levels. Wolthius et al [2] showed that mechanical ventilation with lower VT and PEEP attenuated the increase of IL-8, myeloperoxidase, and elastase in the bronchoalveolar lavage as compared with higher VT and no PEEP. But they found a similar increase in plasma levels of IL-6 and IL-8 in both groups of patients. Therefore, the lack of bronchoalveolar lavage cytokine measurement may have limited the ability to show a beneficial effect in the low VT group in our study.
Finally, despite randomization, differences in the characteristics of both groups in age and length of surgery were found. While the magnitude of the differences and their clinical impact remains unknown, we controlled for these variables in our analysis by choosing an appropriate model. Thus, these differences in covariates should not have influenced our results.
In conclusion, we found no differences in markers of inflammation and lung catabolism when comparing mechanical ventilation with low VT and traditional VT in patients with not preexisting lung pathology undergoing posterior spine fusion.
Acknowledgments
Funding: Department of Anesthesiology, Hospital for Special Surgery (Stavros G. Memtsoudis), and Clinical Translational Science Center (CTSC) grant: NIH UL1-RR024996 (Yan Ma).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brower RG, Lanken PN, MacIntyre N, et al. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 2.Wolthuis EK, Choi G, Dessing MC, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology. 2008;108:46–54. doi: 10.1097/01.anes.0000296068.80921.10. [DOI] [PubMed] [Google Scholar]
- 3.Wrigge H, Uhlig U, Zinserling J, et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesth Analg. 2004;98:775–81. doi: 10.1213/01.ane.0000100663.11852.bf. [DOI] [PubMed] [Google Scholar]
- 4.Koner O, Celebi S, Balci H, Cetin G, Karaoglu K, Cakar N. Effects of protective and conventional mechanical ventilation on pulmonary function and systemic cytokine release after cardiopulmonary bypass. Intensive Care Med. 2004;30:620–6. doi: 10.1007/s00134-003-2104-5. [DOI] [PubMed] [Google Scholar]
- 5.Memtsoudis SG, Starcher B, Ma Y, Buschiazzo V, Urban MK, Girardi FP. The utility of urine desmosine as a marker of lung injury in spine surgery: a pilot study. HSS J. 2010;6:160–3. doi: 10.1007/s11420-010-9158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urban MK, Jules-Elysee KM, Beckman JB, et al. Pulmonary injury in patients undergoing complex spine surgery. Spine J. 2005;5:269–76. doi: 10.1016/j.spinee.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 7.Urban MK, Urquhart B, Boachie-Adjei O. Evidence of lung injury during reconstructive surgery for adult spinal deformities with pulmonary artery pressure monitoring. Spine. 2001;26:387–90. doi: 10.1097/00007632-200102150-00015. [DOI] [PubMed] [Google Scholar]
- 8.Jules-Elysee K, Urban MK, Urquhart BL, Susman MH, Brown AC, Kelsey WT. Pulmonary complications in anterior-posterior thoracic lumbar fusions. Spine J. 2004;4:312–6. doi: 10.1016/j.spinee.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi S, Kitagawa H, Ishii T. Intraoperative pulmonary embolism during spinal instrumentation surgery. A prospective study using transoesophageal echocardiography. J Bone Joint Surg Br. 2003;85:90–4. doi: 10.1302/0301-620x.85b1.13172. [DOI] [PubMed] [Google Scholar]
- 10.Santana MC, Garcia CS, Xisto DG, et al. Prone position prevents regional alveolar hyperinflation and mechanical stress and strain in mild experimental acute lung injury. Respir Physiol Neurobiol. 2009;167:181–8. doi: 10.1016/j.resp.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Toyota S, Amaki Y. Hemodynamic evaluation of the prone position by transesophageal echocardiography. J Clin Anesth. 1998;10:32–5. doi: 10.1016/s0952-8180(97)00216-x. [DOI] [PubMed] [Google Scholar]
- 12.Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J. 2002;20:1017–28. doi: 10.1183/09031936.02.00401702. [DOI] [PubMed] [Google Scholar]
- 13.McClintock DE, Starcher B, Eisner MD, et al. National Heart, Lung Blood Institute Acute Respiratory Distress Syndrome Clinical Trial Network. Higher urine desmosine levels are associated with mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L566–71. doi: 10.1152/ajplung.00457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wirtz DC, Heller KD, Miltner O, Zilkens KW, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop. 2000;24:194–6. doi: 10.1007/s002640000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 16.Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 17.Stüber F, Wrigge H, Schroeder S, et al. Kinetic and reversibility of mechanical ventilation-associated pulmonary and systemic inflammatory response in patients with acute lung injury. Intensive Care Med. 2002;28:834–41. doi: 10.1007/s00134-002-1321-7. [DOI] [PubMed] [Google Scholar]
- 18.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 19.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–54. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 20.Hong CM, Xu DZ, Lu Q, et al. Low tidal volume and high positive end-expiratory pressure mechanical ventilation results in increased inflammation and ventilator-associated lung injury in normal lungs. Anesth Analg. 2010;110:1652–60. doi: 10.1213/ANE.0b013e3181cfc416. [DOI] [PubMed] [Google Scholar]
- 21.Kilpatrick B, Slinger P. Lung protective strategies in anaesthesia. Br J Anaesth. 2010;105 (Suppl 1):i108–16. doi: 10.1093/bja/aeq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz MJ, Haitsma JJ, Slutsky AS, Gajic O. What tidal volumes should be used in patients without acute lung injury? Anesthesiology. 2007;106:1226–31. doi: 10.1097/01.anes.0000267607.25011.e8. [DOI] [PubMed] [Google Scholar]