Abstract
Perineural invasion (PNI) is one of the established prognostic factors in pancreatic ductal adenocarcinoma (PDAC). However, the prognostic significance of PNI in patients with PDAC who received neoadjuvant therapy and pancreaticoduodenectomy (PD) is not clear. In this study, we performed detailed examination of neural invasion in PD specimens from 212 patients with PDAC who received neoadjuvant chemoradiation (treated group) and 60 untreated patients at our institution between January 1999 and December 2007. The frequency of PNI was higher in untreated group (80%, 48/60) than the treated group (58%, 123/212). For the 123 treated cases that were positive for PNI, extra-tumoral PNI, intra-tumoral PNI, intra-pancreatic PNI only, extra-pancreatic PNI, and intra-neural invasion were identified in 86 (69.9%), 37 (30.1%), 11 (8.9%), 112 (91.1%), and 35 (28.5%) respectively. Presence of PNI correlated with tumor size, margin status, lymph node metastasis, pathologic tumor and AJCC stages in the treated group. Tumor involvement of nerves >0.8 mm correlated with higher frequency of positive margin compared to those with PNI involving nerves ≤0.8 mm, but not with other clinicopathologic parameters and survival. In treated group, the presence of PNI or intra-neural invasion correlated significantly with shorter disease-free survival (DFS) and overall survival (OS) compared to those with no PNI or PNI only respectively. PNI was an independent prognostic factor for both DFS and OS in multivariate analysis. Our results showed that PNI plays an important role in the progression of PDAC and in predicting the prognosis in this group of patients.
Keywords: pancreatic cancer, perineural invasion, intra-neural invasion, survival, prognosis
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a malignancy with a very poor prognosis. Vast majority of the patients with PDAC present with surgically unresectable disease at the time of diagnosis, due to locoregional spread or metastatic dissemination (9, 12). In patients with potentially resectable disease, subclinical metastases are often present at the time of diagnosis and surgery, which are undetected by imaging. Therefore, disease recurrence following a potentially curative pancreatectomy is common, and long-term survival rate for patients who underwent pancreatectomy is only 10%–20%(11, 25). Adjuvant treatment in the form of combined chemoradiation therapy is a standard form of treatment option for PDAC and has been shown to improve the 5-year survival and to delay the time to tumor recurrence in patients who underwent pancreatectomy (20, 22, 23). However, the overall survival for patients with PDAC has not changed significantly over the last four decades, despite significant improvements in operative techniques and advance in medical oncology (8). Recently, several tertiary referral centers worldwide, including our institution, have demonstrated the safety and feasibility of pre-operative neoadjuvant chemoradiation for PDAC (7, 15, 18, 26, 27). Evans et al. reported the median survival of 34 months and 36% 5-year survival rate for the 64 patients who underwent pancreaticoduodenectomy (PD) compare to median survival of 7 months and 0% 5-year survival for the 22 patients who did not underwent PD in a phase II trial of neoadjuvant gemcitabine-based chemoradiation in patients with PDAC (7). In another phase II trial of 90 patients who received preoperative gemcitabine and cisplatin chemotherapy in addition to gemcitabine-based chemoradiation, Varadhachary et al. reported a median survival of 31 months for the 52 patients who underwent PD compared to 10.5 months for the 27 patients who did not undergo surgical resection (27). These data suggest that neoadjuvant chemoradiation may identify patients with a favorable tumor biology who would likely benefit the most from surgery, thereby maximizing rates of postoperative survival.
Little is known about the prognostic factors for survival in patients with PDAC who received neoadjuvant chemoradiation and PD. Recently, we showed that posttherapy pathologic stage, lymph node status, and the number of positive regional lymph nodes are independent prognostic factors in this group of patients (6). PDAC characteristically spreads by infiltration of lymphatics, blood vessels and nerves. Perineural invasion (PNI) has been reported in 70.8% to 93% of the PD specimens resected for PDAC and has been shown to be associated with peritoneal dissemination and worse survival in patients with PDAC who underwent PD (1, 2, 4, 16). However, the prevalence of PNI and its prognostic significance in patients with PDAC who received neoadjuvant chemoradiation therapy and subsequent PD is not clear. In this study, we performed detailed histologic evaluation of PNI, intra-neural invasion, the size of the nerve involved by the tumor, and the location of neural invasion in 212 patients who received neoadjuvant chemoradiation therapy and PD. The results were correlated with survival and other clinical and pathological parameters. Our data showed that the presence of PNI and intra-neural invasion is important prognostic factor in this group of patients.
Materials and Methods
Patient population
Our study population consisted of 212 patients with histologically confirmed diagnosis of PDAC who received neoadjuvant chemoradiation therapy and subsequently underwent PD at our institution from January 1999 to December 2007. There were 124 male and 88 female patients with age ranging from 39 to 85 years (median age: 63 years). Thirty-nine patients (18.4%) received neoadjuvant fluoropyrimidine-based chemoradiation (group 1), 66 (31.1%) received neoadjuvant gemcitabine-based chemoradiation (group 2), 70 (33.0%) received systemic chemotherapy followed by gemcitabine-based chemoradiation (group 3), 32 (15.1%) received systemic chemotherapy followed by fluoropyrimidine-based chemoradiation (group 4) and the remaining five patients (2.4%) received neoadjuvant systemic chemotherapy alone (group 5). One hundred and thirty-six (64.2%) of these patients (groups 2 and 3) were treated on previously published protocols.(7, 27) All patients underwent restaging evaluation after completion of neoadjuvant therapy. PD was performed only in patients with resectable disease without disease progression or metastasis, and who had no contraindications to major abdominal surgery. Patients who underwent distal pancreatectomy for PDAC and those who underwent PD for other types of pancreatic tumors were excluded. 60 consecutive patients who did not receive any form of neoadjuvant therapy prior to PD (untreated group) during the same time period at our institution were used as a control group. There were 27 female and 33 male patients with age ranging from 24.9 to 80.9 years (median age: 60.9 years) in our untreated group. Among these 60 untreated patients, two had stage I disease, 13 had stage IIA disease and 45 had stage IIB disease. The study was approved by the Institutional Review Board of the University of Texas M.D. Anderson Cancer Center.
Patient clinical and follow-up information through December of 2009 was extracted from a prospectively maintained database. The clinical and follow-up data from all patients has been verified by independent review of patient medical records and the U.S. Social Security Index. Local/regional or distant recurrence at first site or sites were classified based on the computer tomography (CT) scan and biopsy confirmation of metastasis was rarely performed. Local/regional recurrence was defined as recurrence in the region of the pancreatic bed, root of mesentery, soft tissues or lymph nodes adjacent to the pancreatic bed. Distant metastases was defined as radiographic evidence of tumor spread to the liver, lungs, peritoneal cavity (including ascites), or other distant organs.
Pathologic examination
The histopathologic assessment of PNI and other pathologic parameters was done by reviewing the archival Hematoxylin and Eosin (H & E) stained slides in all the cases. The total number of slides reviewed from the pancreas and tumor ranged from 3 to 45 (the mean number of slides: 14). The cases were examined for the presence or absence of PNI, which was defined as presence of viable tumor cells in the perineural space. The presence of acellular mucin in perineural space with no viable tumor cells was considered as negative for PNI in this study. When PNI was present, the location of PNI was further classified as intra-pancreatic (defined as PNI surrounded at least two sides by benign pancreatic tissue) or extra-pancreatic [defined as PNI in soft tissue with no surrounding benign pancreatic tissue (islet of langerhans, pancreatic acinii or benign pancreatic ductules)] and intra-tumoral (PNI present within the main tumor mass) or extra-tumoral (PNI present outside the main tumor mass).
Patients who had both intra-pancreatic and extra-pancreatic invasion were classified as extra-pancreatic invasion, and who had both intra-tumoral and extra-tumoral invasion were classified as extra-tumoral invasion, respectively. The presence of intra-neural invasion was defined by histology as the presence of PNI with tumor cells invading into and/or with irregular destruction of the axon of the nerve bundle(s) as reported previously (19). The maximum diameter of the nerves involved by tumor was measured microscopically and recorded in each case. In addition, the tumor size, differentiation, lymph node status, and margin status were also reviewed and recorded. If any of the PD margins was involved by PDAC, the case was classified as margin positive. The posttherapy pathologic staging was grouped according to the American Joint Committee on Cancer (AJCC) Staging Manual, 7th edition (5).
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Sciences software (for Windows 12.0, SPSS Inc., Chicago, IL). Chi-square analysis was used to compare categorical data and analysis of variance (ANOVA) was used to compare continuous variables. Survival analyses were performed using the Kaplan-Meier method. The log-rank test was used to evaluate the statistical significance of differences in survival. Disease-free survival (DFS)was calculated as the time from the date of surgery to the date of first recurrence after surgery (in patients with recurrence)or to the date of last follow-up (in patients without recurrence). Overall survival (OS) was calculated as the time from the date of diagnosis to the date of death or the date of last follow-up (if death did not occur). The prognostic significance of clinical and pathologic characteristics was determined using univariate Cox regression analysis. Cox proportional hazards models were fitted for multivariate analysis. After interactions between the variables were examined, a backward stepwise procedure was used to derive the best-fitting model. A two-sided significance level of 0.05 was used for all statistical analyses.
Results
Correlation of nerve invasion with clinicopathologic parameters in patients who received neoadjuvant therapy
Among the 212 patients, PNI was identified in 123 (58%) patients. For the 123 cases that were positive for PNI, extra-tumoral PNI, intra-tumoral PNI, intra-pancreatic PNI only, extra-pancreatic PNI, and intraneural invasion were identified in 86 (69.9%), 37 (30.1%), 11 (8.9%), 112 (91.1%), and 35 (28.5%) respectively. The size of the involved nerves ranged from 0.1 mm to 4.0 mm with a median size of 0.8 mm and average of 1.2 mm.
Correlation of PNI with clinicopathologic features are shown in Table 1. The presence of PNI correlated significantly with posttherapy tumor size (p=0.03), resection margin status (p=0.03), posttherapy pathologic tumor stage (ypT, p=0.001), lymph node status (p=0.005), and posttherapy AJCC stage (p<0.001). During follow up, 78.1% (96/123) patients with PNI developed local recurrence or distant metastasis compared to 60.7% (54/89) of the patients who had no PNI (p=0.02). There was no significant correlation of PNI with age, gender, tumor differentiation or different neoadjuvant treatment regimens (Table 1). Among the 123 cases with PNI, the presence of intraneural invasion correlated with higher frequency of local/distant recurrence compared to those cases with PNI only. Local/distant recurrence was observed in 94.3% (33/35) cases with intraneural invasion compared to 71.6% (63/88) of the cases with PNI only (p<0.01). However no significant correlation between intra-neural invasion and other clinicopathologic parameters were observed. Using the median size (0.8 mm) of the involved nerves as cutoff, positive resection margin was observed in 15 of 58 (25.9%) cases which had PNI involving nerves of >0.8 mm compared to 4/65 (6.2%) cases that had PNI involving the nerves of ≤0.8 mm (p=0.005). However, we did not observe significant correlation of the size of the involved nerves with other clinicopathologic parameters (p>0.05, data not shown). No significant difference in any above-mentioned clinicopathologic parameters was observed between the group with intra-tumoral PNI only and the group with extratumoral PNI or between the group with intra-pancreatic PNI only and the group with extrapancreatic PNI (Fisher’s exact tests, p>0.05, data not shown).
Table 1.
Clinicopathologic features of patients with and without perineural invasion in the Treated Group
| Characteristics | No perineural invasion (n=89) | With perineural invasion (n=123) | P value |
|---|---|---|---|
| Age (yrs) | 0.91 | ||
| <60 | 36 | 52 | |
| 60–70 | 32 | 45 | |
| >70 | 21 | 26 | |
| Gender | 0.26 | ||
| Female | 41 | 47 | |
| Male | 48 | 76 | |
| Neoadjuvant therapy | 0.22 | ||
| Group 1 | 16 | 23 | |
| Group 2 | 29 | 37 | |
| Group 3 | 27 | 43 | |
| Group 4 | 17 | 15 | |
| Group 5 | 0 | 5 | |
| Tumor differentiation | 0.89 | ||
| Well-Moderate | 57 | 77 | |
| Poor | 32 | 46 | |
| Tumor size | 0.03 | ||
| ≤2cm | 41 | 38 | |
| >2cm | 48 | 85 | |
| Resection margin | 0.03 | ||
| Negative | 84 | 104 | |
| Positive | 5 | 19 | |
| Pathologic tumor stage | 0.001 | ||
| ypT1 | 10 | 1 | |
| ypT2 | 2 | 0 | |
| ypT3 | 77 | 122 | |
| Lymph node | 0.005 | ||
| Negative | 47 | 40 | |
| Positive | 42 | 83 | |
| AJCC stage | <0.001 | ||
| IA and IB | 10 | 0 | |
| IIA | 37 | 40 | |
| IIB | 42 | 83 | |
| Recurrence | 0.02 | ||
| No | 35 | 27 | |
| Local | 14 | 26 | |
| Distant | 40 | 70 |
Correlation of nerve invasion with survival in patients who received neoadjuvant therapy
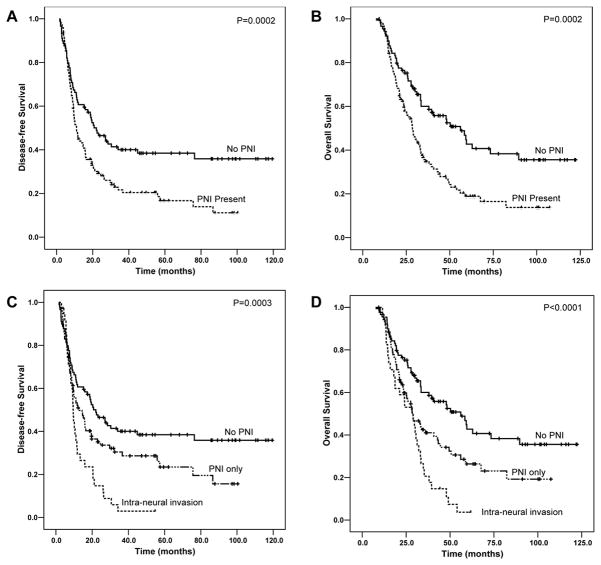
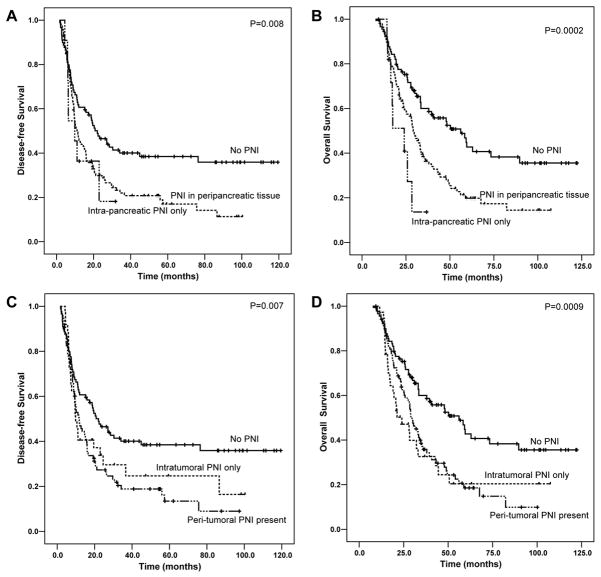
The median follow-up of all patients was 29.5 months ranging from 7.6 months to 122.3 months. Patients without PNI had a median DFS of 22.0 months [95% confidence interval (CI): 14.7–29.4 months], which was significantly longer than 11.0 months (95% CI: 8.9–13.1 months) for patients with PNI (p= 0.002, Figure 2A). Patients with PNI also had shorter OS than those without PNI. The median OS for patients without PNI was 56.1 months [95% CI: 36.7–75.6 months] compared to 28.5 months (95% CI: 24.6–32.5 months) for patients with PNI (p= 0.0002, Figure 2B). Among the 123 patients with PNI, 35 cases also showed intra-neural invasion (tumor invasion into the axon of the nerve). The presence of intra-neural invasion correlated with shorter DFS and OS compared to those patients with PNI only (Figure 2C and 2D). The mean DFS and OS were 13.4 months (95% CI: 9.8–16.9 months) and 28.1 months (95% CI: 23.6–32.6 months) respectively compared to the mean DFS of 32.9 months (95% CI: 24.7–41.2 months) and OS of 45.7 months (95% CI: 37.5–53.9 months) in patients with PNI only (p=0.01). However, no significant difference in either DFS or OS was observed between the group of patients with extra-tumoral PNI and the group with intra-tumoral PNI only and between the group of patients with extra-pancreatic PNI and the group with intra-pancreatic PNI only (p>0.05, Figure 2). There is also no difference in either DFS or OS between the group of patients with PNI involving nerves of ≤0.8 mm and the group of patients with PNI involving nerves of >0.8 mm (p>0.05, data not shown).
Figure 2.
Kaplan-Meier survival curves of disease-free survival (A and C) and overall survival (B and D) stratified by the absence or presence of perineural invasion (A and B) or intra-neural invasion (C and D) in patients with PDAC who received neoadjuvant therapy and pancreaticoduodenectomy. The patients with perineural or intra-neural invasion have shorter DFS and OS than those patients who had no perineural invasion. Only data from the treated group are shown.
The results from univariate Cox’s regression analysis for DFS and OS are shown in Table 2. Both DFS and OS were significantly associated with positive lymph node status, posttherapy AJCC tumor stage, and perineural invasion (p<0.05). The resection margin status and ypT correlated significantly with OS (p<0.05), but not DFS. There was no significant association of either DFS or OS with gender, intra-operative blood loss, tumor differentiation or tumor size (p>0.05, Table 2). In multivariate analysis, the presence of PNI was an independent prognostic factor for both OS and DFS (p<0.05). Lymph node status and resection margin status were also independent prognostic factors for OS, but not for DFS (Table 3).
Table 2.
Univariate Cox Regression Analysis of Disease-free and Overall Survival in Relation to Clinicopathologic Features in the Treated Group
| Characteristics | Number of patients | Disease -free survival
|
Overall survival
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender | 0.81 | 0.82 | |||
| Females (ref) | 88 | 1.00 | 1.00 | ||
| Males | 124 | 0.96 (0.69–1.33) | 0.96 (0.68–1.35) | ||
| Age (yrs) | 0.04 | 0.60 | |||
| <60 (ref) | 88 | 1.00 | 1.00 | ||
| 60–70 | 77 | 0.81 (0.57–1.15) | 1.28 (0.80–2.05) | ||
| >70 | 47 | 0.55 (0.35–0.87) | 1.20 (0.74– 1.95) | ||
| Blood loss | 212 | 1.00 (1.00–1.00) | 0.21 | 1.00 (1.00–1.00) | 0.10 |
| Neoadjuvant therapy | 0.09 | 0.59 | |||
| Group 1 | 39 | 1.00 | 1.00 | ||
| Group 2 | 66 | 0.66 (0.23–1.88) | 0.43 | 0.75 (0.26–2.15) | 0.59 |
| Group 3 | 70 | 0.53 (0.19–1.49) | 0.23 | 0.55 (0.20–1.54) | 0.25 |
| Group 4 | 32 | 0.84 (0.30–2.32) | 0.74 | 0.71 (0.26–1.98) | 0.52 |
| Group 5 | 5 | 1.00 (0.35–2.89) | 1.00 | 0.74 (0.25–2.17) | 0.58 |
| Tumor size | 0.15 | 0.24 | |||
| ≤2cm (ref) | 79 | 1.00 | 1.00 | ||
| >2cm | 133 | 1.28 (0.92–1.80) | 1.24 (0.87–1.76) | ||
| Margin | 0.100 | 0.008 | |||
| Negative (ref) | 188 | 1.00 | 1.00 | ||
| Positive | 24 | 1.48 (0.93–2.34) | 1.88 (1.18–3.00) | ||
| Tumor differentiation | 0.21 | 0.15 | |||
| Well-moderate (ref) | 134 | 1.00 | 1.00 | ||
| Poor | 78 | 1.24 (0.89–1.72) | 1.29 (0.92–1.81) | ||
| Pathologic tumor stage | 0.08 | 0.02 | |||
| ypT1–ypT2 (ref) | 13 | 1.00 | 1.00 | ||
| ypT3 | 199 | 1.97 (0.92–4.22) | 3.38 (1.25–9.15) | ||
| Lymph nodes | 0.007 | 0.002 | |||
| Negative (ref) | 87 | 1.00 | 1.00 | ||
| Positive | 125 | 1.58 (1.13–2.20) | 1.77 (1.24–2.53) | ||
| AJCC Stage | 0.01 | 0.003 | |||
| Stage IA and IB (ref) | 10 | 1.00 | 1.00 | ||
| Stage IIA | 77 | 1.94 (0.77–4.87) | 4.57 (1.11–18.88) | ||
| Stage IIB | 125 | 2.82 (1.14–6.95) | 6.99 (1.72–28.45) | ||
| Perineural invasion | 0.002 | <0.001 | |||
| Absent (ref) | 89 | 1.00 | 1.00 | ||
| Present | 123 | 1.69 (1.21–2.36) | 1.95 (1.36–2.78) | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; ref, reference
Table 3.
Multivariate Cox Regression Analysis of Disease-free and Overall Survival in Relation to Clinicopathologic Features in the Treated Group
| Characteristics | Number of patients | Disease -free survival
|
Overall survival
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (yrs) | 0.05 | NA | |||
| <60 (ref) | 88 | 1.00 | |||
| 60–70 | 77 | 0.85 (0.60–1.21) | |||
| >70 | 47 | 0.56 (0.36–0.89) | |||
| Margin | 0.23 | 0.04 | |||
| Negative (ref) | 188 | 1.00 | 1.00 | ||
| Positive | 24 | 1.34 (0.83–2.17) | 1.63 (1.01–2.61) | ||
| Pathologic tumor stage | 0.30 | 0.18 | |||
| ypT1–ypT2 (ref) | 13 | 1.00 | 1.00 | ||
| ypT3 | 199 | 1.54 (0.68–3.45) | 2.03 (0.72–5.73) | ||
| Lymph nodes | 0.11 | 0.02 | |||
| Negative (ref) | 87 | 1.00 | 1.00 | ||
| Positive | 125 | 1.33 (0.93–1.90) | 1.54 (1.07–2.22) | ||
| Perineural invasion | 0.003 | 0.005 | |||
| Absent (ref) | 89 | 1.00 | 1.00 | ||
| Present | 123 | 1.67 (1.19–2.34) | 1.70 (1.18–2.45) | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; ref, reference
Nerve invasion in patients who did not receive neoadjuvant therapy
To compare the frequency of nerve invasion and to evaluate the role of PNI in predicting the prognosis in patients with PDAC who did not receive neoadjuvant therapy, we examined PNI and its correlation with survival and clinicopathologic features in 60 consecutive untreated patients during the same time period at our institution. We found that PNI was present in 80% (48/60) of untreated cases, which was higher than those who received neoadjuvant therapy (58%, p=0.002). Similar to our findings in patients who received neoadjuvant therapy, the presence of PNI correlated with lymph node metastasis and AJCC stage in untreated group. Lymph node metastasis was present in 83% (40/48) of the untreated patients who had PNI compared to 42% (5/12) in those who lacked PNI (p=0.006). Local recurrence was observed in 31% (15/48) in the untreated patients who had PNI compared to 0% (0/12) in those who lacked PNI (p=0.03). However, we did not observe significant correlations of PNI with other clinicopathologic parameters, distant metastasis, DFS, or OS in the untreated group (p>0.05, data not shown).
Discussion
In this study, we examined nerve invasion in the PD specimens from 212 patients with PDAC who received neoadjuvant therapy. We found that the presence of PNI correlated with tumor size, resection margin status, posttherapy tumor (ypT) stage, lymph node status, and posttherapy AJCC stage. We also showed that the presence of PNI and intra-neural invasion correlated with both DFS and OS. The presence of PNI was an independent prognostic factor for both DFS and OS. Therefore, perineural and intra-neural invasion play an important role in the progression and the prognosis in patients with PDAC who received neoadjuvant therapy and PD.
Neoadjuvant therapy has been increasingly used in patients with PDAC as an alternative to the conventional approach, which is to perform pancreatectomy first followed by adjuvant therapy (7, 27). Several studies, including two large phase II trials from our institution, have reported median survival durations of up to 34 months and up to 36% 5-year survival rate in patients with potentially resectable PDAC who were treated with neoadjuvant chemoradiation followed by PD (7, 26, 27). At our institution, a multidisciplinary approach has been used to develop clinical trials exploring the use of protocol-based neoadjuvant therapy before surgical resection. From 1990 to 2002, the numbers of patients who underwent pancreaticoduodenectomy, distal or total pancreatectomy at our institution were 302 (92%), 20 (6%), or 7 (2%) respectively. Among these patients, 253 (84%) received neoadjuvant therapy (14). In our previous study, we showed that post-therapy pathologic AJCC stage and number of positive lymph nodes are independent prognostic factors in patients with PDAC who received neoadjuvant therapy and PD (6). However, detailed study of other histopathological factors in PD specimens after neoadjuvant chemoradiation is lacking.
PNI is an important pathway by which tumor progress and spread to the adjacent tissue or organs and has been shown to be an important prognostic factor in many types of human malignancies. In patients with PDAC who underwent PD first, but received no pre-operative neoadjuvant therapy, PNI is frequently identified in the PD specimens with the reported frequencies ranging from 70.8 to 93.0% (1, 2, 4, 16). In this study, we found that PNI was present in 80% of the untreated patients, which was significantly higher than 58% in the 212 patients with PDAC who received neoadjuvant therapy and PD. Consistent with our data, Barbier et al showed that PNI was present in 43% of the PD specimens from patients with PDAC who received neoadjuvant therapy, which is significantly lower than 93% in those patients who receive surgery first in their study (2). The presence of PNI has been shown to be associated with peritoneal dissemination and worse survival in patients who received surgery first but no neoadjuvant therapy (1, 4, 16). The prognostic value of PNI is, however, not clear in patients with PDAC who received neoadjuvant therapy and subsequent PD. Our data showed that PNI correlated with larger tumor size, higher rate of positive resection margin, posttherapy tumor (ypT) stage, higher rate of lymph node metastasis, and posttherapy AJCC stage in patients with PDAC who received neoadjuvant therapy. Using multivariate analysis, we demonstrated that presence of PNI was an independent prognostic factor for both DFS and OS in our patient population. In addition, our data also showed that the presence of intra-neural invasion correlated with poor DFS and OS compared to those patients with PNI only. Similar to our results, the presence of PNI has been shown to be associated with posttherapy pathologic stage and worse five-year survival rate in patients with locally advanced gastric cancer who received neoadjuvant therapy (13). In a cohort of 297 patients with locally advanced rectal adenocarcinoma who received neoadjuvant therapy, Guillem et al showed that the presence of PNI, along with lymphovascular invasion, tumor response >95% and lymph node metastasis are independent prognostic factors for both DFS and OS in their patient population (10). Together, these results support the notion that PNI plays an important role in tumor progression and prognosis in patients who received neoadjuvant therapy.
Consistent with our findings in the patients who received neoadjuvant therapy, we found that the presence of PNI was associated with higher frequency of lymph node metastasis and local recurrence in our untreated patient population. In contrast to the previous reports which have shown that PNI is associated with peritoneal dissemination and worse survival in patients who received surgery first but no neoadjuvant therapy (1, 4, 16), we did not observe significant correlation of PNI with other clinicopathologic parameters, distant metastasis, DFS or OS in our untreated group. However, our untreated group is small compared to other studies. The difference in the prognostic significance of PNI between the untreated group and those patients who received neoadjuvant therapy in this study may be due in part to the highly selective patient population for PD who received neoadjuvant therapy in our treated group to exclude those patients whose tumor had more aggressive clinical behavior during the neoadjuvant chemoradiation treatment.
Previous studies have shown that the nerve diameter involved by PNI correlates with the prognosis in prostatic adenocarcinoma (17), oral squamous cell carcinoma (3) and cutaneous squamous cell carcinoma (21). In this study, we observed that involvement of nerve >0.8 mm by PNI was associated with significantly higher frequency of positive resection margin. However, we did not observe any significant correlation between the size of the nerve involved by PNI with either OS or DFS using 25th, 50th, or 75th percentile of the involved nerve sizes as cutoff for survival analysis in our study patient population. In contrast to the previous study, which showed that extra-tumoral PNI is associated with the development of peritoneal metastasis after surgery (24), we did not observe any significant difference in any of the examined clinicopathologic parameters and survival between the group with intra-tumoral PNI and those with extra-tumoral PNI or between the group with intra-pancreatic PNI only and the group with extra-pancreatic PNI. This difference may be in part due to the selection of patients, who received neoadjuvant therapy before PD in our study population.
In summary, our data showed that the presence of PNI and intra-neural invasion correlated with a significantly shorter DFS and OS in patients with PDAC who received neoadjuvant therapy. The presence of PNI was an independent prognostic factor for patients with PDAC who underwent PD following neoadjuvant chemoradiation. Our results suggest that PNI and intra-neural invasion play an important role in the progression of PDAC and predicting the clinical outcome in this group of patients.
Supplementary Material
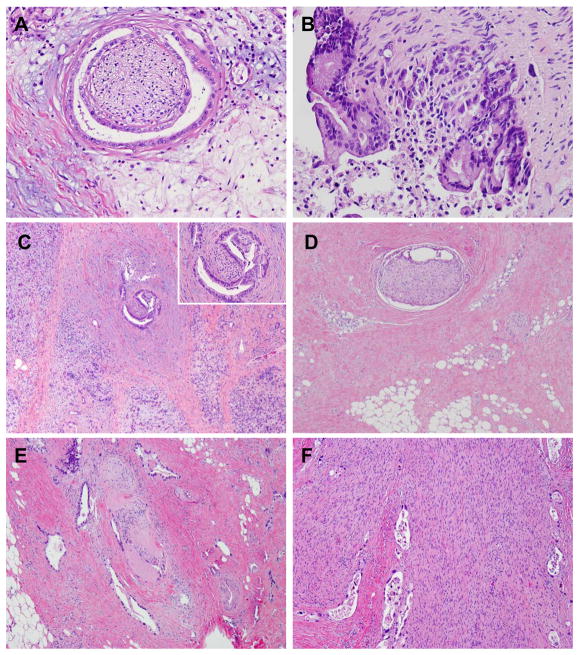
Figure 1.
Representative micrographs of perineural invasion (A), intra-neural invasion (B), intrapancreatic perineural invasion (C), extrapancratic perineural invasion (D), Intra-tumoral, extra-pancreatic perineural invasion (E), and a large nerve involved by perineural and intra-neural invasion (F). Hematoxylin & eosin stain, original magnifications: 200x for A, B and insert in panel C; 40x for C, D, and E; 100x for F.
Figure 3.
Kaplan-Meier survival curves of disease-free survival (A and C) and overall survival (B and D) stratified by the absence and presence of intra-pancreatic and extra-pancreatic perineural invasion (A and B) or the absence and presence of intra-tumoral and extra-tumoral perineural invasion (C and D) in patients with PDAC who received neoadjuvant therapy and pancreaticoduodenectomy. No significant difference in either disease-free survival or overall was observed between the group with intra-pancreatic and those with extrapancreatic perineural invasion or between the group with intra-tumoral and those with extra-tumor perineural invasion. Only data from the treated group are shown.
Acknowledgments
Supported by the National Institutes of Health grant (1R21CA149544-01A1) and G. S. Hogan Gastrointestinal Cancer Research Fund at The University of Texas M.D. Anderson Cancer Center
Footnotes
Disclosures: The authors have no conflicts of interest or funding to disclose.
References
- 1.Badger SA, Brant JL, Jones C, et al. The role of surgery for pancreatic cancer: a 12-year review of patient outcome. Ulster Med J. 2010;79:70–75. [PMC free article] [PubMed] [Google Scholar]
- 2.Barbier L, Turrini O, Gregoire E, et al. Pancreatic head resectable adenocarcinoma: preoperative chemoradiation improves local control but does not affect survival. HPB (Oxford) 2011;13:64–69. doi: 10.1111/j.1477-2574.2010.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 4.Chen JW, Bhandari M, Astill DS, et al. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford) 2010;12:101–108. doi: 10.1111/j.1477-2574.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edge S, Byrd D, Compton C, et al. AJCC Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- 6.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. doi: 10.1002/cncr.26243. [DOI] [PubMed] [Google Scholar]
- 7.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 8.Garcea G, Dennison AR, Pattenden CJ, et al. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9:99–132. [PubMed] [Google Scholar]
- 9.Greer JB, Brand RE. New developments in pancreatic cancer. Curr Gastroenterol Rep. 2011;13:131–139. doi: 10.1007/s11894-011-0175-y. [DOI] [PubMed] [Google Scholar]
- 10.Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829–836. doi: 10.1097/01.sla.0000161980.46459.96. discussion 836-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez JM, Morton CA, Al-Saadi S, et al. The natural history of resected pancreatic cancer without adjuvant chemotherapy. Am Surg. 2010;76:480–485. [PubMed] [Google Scholar]
- 12.Huguet F, Orthuon A, Touboul E, et al. Pancreatic cancer. Cancer Radiother. 2010;14 (Suppl 1):S94–102. doi: 10.1016/S1278-3218(10)70012-3. [DOI] [PubMed] [Google Scholar]
- 13.Jhawer M, Coit D, Brennan M, et al. Perineural invasion after preoperative chemotherapy predicts poor survival in patients with locally advanced gastric cancer: gene expression analysis with pathologic validation. American journal of clinical oncology. 2009;32:356–362. doi: 10.1097/COC.0b013e31818c08e8. [DOI] [PubMed] [Google Scholar]
- 14.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Scodan R, Mornex F, Girard N, et al. Preoperative chemoradiation in potentially resectable pancreatic adenocarcinoma: feasibility, treatment effect evaluation and prognostic factors, analysis of the SFRO-FFCD 9704 trial and literature review. Ann Oncol. 2009;20:1387–1396. doi: 10.1093/annonc/mdp015. [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1:469–476. [PubMed] [Google Scholar]
- 17.Maru N, Ohori M, Kattan MW, et al. Prognostic significance of the diameter of perineural invasion in radical prostatectomy specimens. Hum Pathol. 2001;32:828–833. doi: 10.1053/hupa.2001.26456. [DOI] [PubMed] [Google Scholar]
- 18.Meszoely IM, Wang H, Hoffman JP. Preoperative chemoradiation therapy for adenocarcinoma of the pancreas: The Fox Chase Cancer Center experience, 1986–2003. Surg Oncol Clin N Am. 2004;13:685–696. x. doi: 10.1016/j.soc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Mitsunaga S, Hasebe T, Kinoshita T, et al. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol. 2007;31:1636–1644. doi: 10.1097/PAS.0b013e318065bfe6. [DOI] [PubMed] [Google Scholar]
- 20.Regine WF, Abrams RA. Adjuvant therapy for pancreatic cancer: current status, future directions. Semin Oncol. 2006;33:S10–13. doi: 10.1053/j.seminoncol.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859–1866. doi: 10.1111/j.1524-4725.2009.01354.x. [DOI] [PubMed] [Google Scholar]
- 22.Squadroni M, Fazio N. Chemotherapy in pancreatic adenocarcinoma. Eur Rev Med Pharmacol Sci. 2010;14:386–394. [PubMed] [Google Scholar]
- 23.Stocken DD, Buchler MW, Dervenis C, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92:1372–1381. doi: 10.1038/sj.bjc.6602513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi S, Hasebe T, Oda T, et al. Extra-tumor perineural invasion predicts postoperative development of peritoneal dissemination in pancreatic ductal adenocarcinoma. Anticancer research. 2001;21:1407–1412. [PubMed] [Google Scholar]
- 25.Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37:1519–1524. doi: 10.1002/1097-0142(197603)37:3<1519::aid-cncr2820370340>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Turrini O, Viret F, Moureau-Zabotto L, et al. Neoadjuvant 5 fluorouracil-cisplatin chemoradiation effect on survival in patients with resectable pancreatic head adenocarcinoma: a ten-year single institution experience. Oncology. 2009;76:413–419. doi: 10.1159/000215928. [DOI] [PubMed] [Google Scholar]
- 27.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.