Abstract
The extent to which characteristic adolescent behaviors are associated with pubertal changes or driven by more general, puberty-independent developmental alterations is largely unknown. Using physiological and hormonal markers of puberty, this experiment characterized pubertal timing across adolescence and examined the relationships among these variables and novelty-directed behaviors. Males and females were tested for response to novelty at P28, P32, P36, P40, P44, P48 and P75, and examined for balano-preputial skinfold separation and sperm presence (males) or vaginal opening (females), followed by blood collection for hormonal assessments. Despite earlier pubertal maturation in females, with maturation generally completed by P36 in females and P44 in males, novelty-directed behavior peaked at P32 and P36 in both sexes, and was unrelated to pubertal measures. These data support the suggestion that the ontogenetic peak in this behavior during adolescence is not notably puberty-dependent.
Keywords: puberty, adolescence, novelty-seeking, Sprague-Dawley rats, gonadal hormones, adolescent-typical behavior, sexual maturation, testosterone, estradiol
Introduction
Adolescence is a unique transitional period between childhood and adulthood which is characterized by numerous behavioral, hormonal and neural changes in humans as well as nonhuman mammals (see Spear, 2000, 2007 for review). Among the age-typical behavioral characteristics of adolescence evident across a variety of mammalian species are increases in peer-directed social interactions and novelty/sensation seeking, along with generally higher levels of alcohol consumption than are evident at maturity (see Spear, 2000, 2010, for review). Encompassed within the broad adolescent period is puberty – i.e., the processes that eventually culminate in reproductive maturation, including sex-typical increases in gonadal steroids and other physiological changes such as the emergence of secondary sexual characteristics, as well as seemingly associated behavioral alterations such as increased interest in the opposite sex and sexual desire. Although the terms adolescence and puberty have sometimes been used interchangeably, they are not synonymous, with puberty a relatively temporally restricted phase within the broader adolescent period.
Prototypic adolescence in humans is often considered to be the second decade of life (Petersen, Silbereisen, & Sorensen, 1996), with females often maturing earlier than males. Given the broad developmental period subsumed, some have divided adolescence into early (ages: ~10-14 yrs), middle (~15-17 yrs) and late (~18-25 yrs) stages (e.g., Baumrind, 1987; Feldman & Elliot, 1990; Arnett, 2000), with specific physical, hormonal, and neurobehavioral changes associated with each phase (Feldman & Elliot, 1990). Likewise, a conservative age range during which both male and female rats appear to exhibit adolescent-typical neurobehavioral characteristics has been defined as postnatal (P) day 28-42 (Spear & Brake, 1983; Spear, 2000; Odell, 1990), although females tend to progress into adolescence slightly earlier, and animals of both sexes, especially males, continue to show some signs of adolescence for some time thereafter. There has been little systematic investigation of the time course of adolescence in rats and the unfolding of puberty during this transition.
Although in some individuals the onset of adolescence seems contemporaneous with the onset of certain pubertal signs (e.g., Petersen et al., 1996), there is substantial individual variation in both the age of onset and rate of progression through puberty within the broader adolescent period (e.g., Dubas, 1991; Sun, Schubert, Chumlea, Roche, Kulin, Lee, Himes & Ryan, 2002). This timing is not inconsequential, with long-lasting differences associated with early or late pubertal onset (e.g., Michaud, Suris & Deppen, 2006; Zehr, Culbert, Sisk & Klump, 2007; Negriff, Susman & Trickell, 2010). Among children in the United States, the mean age of entering puberty in girls is approximately 11 yrs of age, continuing to completion by approximately 16 yrs of age, with this process delayed about a year on average in boys (Sun et al, , 2002). In rats, the peri-pubertal period has been suggested to subsume the interval from about P30-40 in females and P35-55 in males (Ojeda & Skinner, 2006), with physical markers of sexual maturation observed from P32-34 and P45-48 in females and males, respectively (Lewis, Barnett, Freshwater, Hoberman & Christian, 2002).
Some of the age-typical behavioral and neural characteristics of adolescence may depend upon the rise in gonadal hormones at puberty, and hence would be considered puberty-dependent. Yet, some changes during this same general developmental period may be driven by more general, puberty-independent ontogenetic processes. Studies have only recently begun to systematically examine these possibilities. Although it is difficult to parse the relative contributions of pubertal versus non-pubertal maturation processes to adolescent-typical neurobehavioral alterations in human subjects, this distinction is beginning to be addressed by relating effects not only to chronological age, but also to objective measures of pubertal development, such as Tanner’s stage classification of secondary sex characteristics (Marshall & Tanner, 1968), or subjective self-report measures such as the Pubertal Development Scale (Martin, Kelly, Rayens, Brogli, Brenzel, Smith & Omar, 2002). Using this approach, for example, adolescent-typical increases in sensation-seeking have been suggested to be pubertally-related in humans, based on findings that self-reported sensation seeking was highly correlated with pubertal stage, but not age, when examining individuals varying widely in pubertal status across a relatively narrow age range (11-14 yrs old; Martin et al., 2002). The results of similarly designed studies have provided some initial support for the suggestion that pubertal status may also play a role in cognitive processing of emotion and social stimuli as well as motivation and arousal (Steinberg, 2008; Dahl & Gunnar, 2009; Blakemore, Burnett & Dahl, 2010; Forbes & Dahl, 2010; Forbes, Phillips, Ryan & Dahl, 2011). However, it is difficult to dissociate the relative contribution of pubertal status versus age in these studies, especially when, as in the majority of these studies, the subjects examined were similar in chronological age, but varied markedly in pubertal status, thereby biasing for detection of pubertal status effects over age effects (see Spear, 2009 for discussion).
In animal models, examining puberty-dependent versus puberty-independent neurobehavioral changes can be assessed through pre-pubertal removal of the gonads, thereby creating animals that do not experience the increases in gonadal hormones associated with puberty. However, few studies have used this strategy to examine the role of rising sex hormones on behaviors during the adolescent period, focusing instead on the potential organizational role of these rising hormone levels for influencing later sex-typical behaviors in adulthood (e.g., Schulz & Sisk, 2006; Romeo, Schulz, Nelson, Menard & Sisk, 2003; Primus & Kellogg, 1989, 1990). Yet, in a recent a study conducted in our laboratory, pre-pubertal gonadectomy was not found to influence the high alcohol consumption characteristic of the adolescent period in male or female rats nor later novelty-directed behavior (Vetter-O’Hagen & Spear, 2011; Vetter-O’Hagen & Spear, under revision).
Although the results of the latter study support the suggestion that changes in novelty-seeking may be independent of increases in pubertal hormones, it is possible that examining this behavior in relation to a variety of pubertal indices may illuminate subtle pubertal effects on this behavior. Even though prior work has characterized hormonal changes associated with puberty (Dohler & Wuttke, 1974; Korenbrot, Huhtaniemi & Weiner, 1977) and physical markers of puberty onset and sexual maturation in rats (Lewis et al., 2002), there has been little attempt to systematically compare physical and hormonal indicators of pubertal onset with behavioral measures that may be puberty-related in both males and females within the same study. Consequently, the purpose of this experiment was to characterize pubertal timing in male and female rats across adolescence and into adulthood using physical markers of genital development and pubertal rises in plasma gonadal hormone levels, relate these measures to ontogenetic responses to novelty, and examine the relationships among the relative developmental timing of these physical, hormonal and behavioral measures.
Materials and Methods
Subjects
A total of 164 male and female Sprague-Dawley rats bred in our colony at Binghamton University were used as experimental subjects in the present experiment. Each litter was culled to 8-10 pups on the day after birth (postnatal day (P) 1), with 6 animals of one sex and 4 of the other kept whenever possible. Offspring were weaned at P21 and housed with a same-sex littermate until the time of experimental testing. All animals were kept in a temperature-controlled vivarium on a 14:10 light/dark cycle (lights on at 0700) and given ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. Animals were treated in accordance with guidelines for animal care established by the National Institute of Health (Institute of Laboratory Animal Research, 2010), using protocols approved by Binghamton University Institutional Animal Care and Use Committee. Throughout this experiment, only one animal per litter was assigned to any experimental condition.
Experimental Design
A total of 164 animals were used across the 2 (sex) × 7 (ontogenetic time-point) between subjects factorial design of this study, with both male and female animals examined using a cross-sectional design at the following ages: P28, P32, P36, P40, P44, P48 and P75. Eight males were placed into the youngest age group, with 10 males examined at the other ages. Eight females were placed into the two youngest age groups, with 16 females assigned to the other age groups to allow assessment of estradiol and progesterone levels at multiple stages of the estrous cycle.
Procedure
The novel chamber consisted of a Plexiglas chamber (30 × 20 × 20 cm for the 5 youngest age groups and 45 × 30 × 30 cm for the two oldest age groups) containing clean wood shavings and divided along the long axis into 2 equally sized compartments by a clear Plexiglas partition containing an aperture (7 × 5 cm for adolescents and 9 × 7 cm for adults) allowing the experimental animal to move between compartments. By using a two-compartment apparatus, animals could choose how much time to spend on the same side of the chamber as the novel object. This may be important given that the response of rats to situations involving free-choice novelty differs from that associated with inescapable novelty, with the latter situation thought to be particularly likely to be stressful (see Klevaur & Bardo, 1999). A video camera mounted approximately 60 cm above each chamber was used to record the novelty testing sessions. All testing was conducted under low light conditions (3 lux).
On the test day (i.e. P28, P32, P36, P40, P44, P48 or P75), animals were weighed and then placed individually for 30 min into the novel chamber. After the first 25 min in this test context, a novel object (a new cotton ball approximately 3 cm in diameter) was placed into one side of the chamber for 5 min. The entire 30-min session was videotaped for later analysis of behavior in the novel context as well as toward the novel object. Behaviors scored included latency to contact the novel object, time spent sniffing the novel object, time spent in contact with the novel object and the proportion of time spent on the same side as the novel object; cross-over data, blocked into 5 min time bins, was also used as an index of activity during the 25-min period prior to the introduction of the novel object. All animals were tested between 1000 and 1300 hours.
Immediately after the novelty test, animals were euthanized via rapid decapitation, with trunk blood collected and stored at −80°C until the time of analysis of circulating plasma testosterone in males and estradiol in females. Samples were also analyzed for corticosterone and progesterone to examine possible age and sex differences in stress-related hormone release after exposure to novelty.
Following blood collection, carcasses were examined for external signs of sexual maturation. Balano-preputial skinfold separation (i.e. cleavage of the prepuce from the glans of the penis) in males and vaginal opening in females was assessed at each ontogenetic time-point. Males were examined for BPS by attempting to manually retract the prepuce from the glans with gentle pressure (de Jong & van der Schoot, 1979; Korenbrot et al., 1977; Wisner, Stalvey &Warren, 1983). Skinfold cleavage was scored as none (0), incomplete (1) or complete (2). The presence or absence of sperm in the seminiferous tubules was assessed by the diffusion method. Briefly, the testes were removed and the epididymis was nicked in a few sites with a scalpel blade, avoiding blood vessels, and then placed into a test tube containing approximately 10 ml of physiologic saline. The sperm were allowed to diffuse into the saline solution for 5 min. A sample of the saline solution was then placed onto a glass slide for determination of the presence or absence of sperm under the light microscope.
In females, vaginal opening was assessed by visual inspection and defined as a complete separation of the membranous sheath covering the vaginal orifice. If vaginal opening was complete, vaginal smears were collected using a lavage technique to index stage of estrous cycle. A disposable pipette containing 30 μl of saline (0.9% w/v) was shallowly inserted into the vagina; the fluid was then flushed into the vagina and recollected into the pipette. The sample was then placed onto a glass slide and while still wet was examined under a light microscope (x40 magnification) for determination of estrous cycle phase. The proportion of nucleated epithelial cells to non-nucleated cornified cells to leukocytes was utilized to assign estrous cycle phase (Becker, Arnold, Berkley, Blaustein, Eckel, Hampson, Herman, Marts, Sadee, Steiner, Taylor & Young, 2005; Yener, Turkkani, Aslan, Aytan & Cantug Caliskan, 2007) using the following criteria: diestrus 1 samples contain primarily leukocytes; diestrus 2 samples contain leukocytes and round non-nucleated cells; proestrus samples contain a predominance of nucleated epithelial cells; and estrus samples contain primarily non-nucleated cornified cells. For the purposes of data analysis, diestrus 1 & 2 were combined because they both represent the follicular phase of the estrous cycle and have similar hormonal profiles (i.e., estradiol and progesterone; Becker et al., 2005).
Hormone Analyses
Testosterone, estradiol and progesterone levels were assessed via radioimmunoassay using a 125I RIA double antibody kit from MP Biomedicals (Solon, OH), with specificities of 100% for each hormone assayed. Testosterone assay sensitivity was 0.03 ng/ml, with inter- and intra-assay coefficients of variation of 10.1% and 6.6%, respectively. Sensitivity of the estradiol assay was 7.2 pg/ml, with inter-assay coefficients of 9% and intra-assay coefficients of 7.3%. Progesterone assay sensitivity was 0.11 ng/ml, with inter-assay coefficients of 7.9% and intra-assay coefficients of 5.9%. Samples and standards for each gonadal hormone assay were run in duplicate, using a Packard Cobra II Autogamma Counter, with disintegrations per min averaged against a standard curve. Corticosterone levels were assessed by radioimmunoassay using a tritium-based kit supplied by MP Biomedicals (Solon, OH), with 100% specificity for rat corticosterone. The sensitivity of this assay was 12 ng/ml, with inter- and intra-assay coefficients of variation of 9% and 7% respectively. Standards and samples for corticosterone were assayed in duplicate, with disintegrations per min averaged against a standard curve performed with each assay and input into GraphPad Prism 2.0 software for calculations of ng/ml in each sample.
Data Analyses
Since vaginal opening (in females) and sperm presence (in males) data were dichotomous and categorical in nature, these data were analyzed using non-parametic Fisher’s Exact Probability Tests comparing the number of animals displaying either vaginal opening or sperm presence in each age group to the number displaying these characteristics at every other age, using an adjusted α = .0024 in order to avoid inflating the possibility of Type 1 errors. For analysis of BPS data, which was rated on a scale of 0-2 and hence ordinal in nature, the Kruskal-Wallis H test was used, followed by Mann-Whitney U tests comparing ratings at each age to ratings at every other age (again, using an α of .0024).
Hormone, novelty, and weight data were analyzed using 2 sex × 7 age (P28, P32, P36, P40, P44, P48 & P75) analyses of variance (ANOVAs). The distribution of females in each phase of estrous across age was examined using a Pearson’s Chi-Square test. In order to explore the potential impact of phase of estrous on hormonal and behavioral data among immature and mature females, data from P32, P36, P40 & P44 females were grouped and compared to data for females examined at P48 & P75 using 2 age (i.e. P32-P44 vs. P48-P75) × 3 stage of estrous cycle (proestrus, estrus, diestrus 1 & 2) factorial ANOVAs. Levene’s tests were used to test for homogeneity of variance (HV) in each data set. Data violating this assumption were normalized via log(10) (n+1) transformations as necessary prior to analysis by ANOVA, as detailed in the Results. For ease of interpretation, non-transformed data are shown in all figures and tables. Fisher’s LSD tests were used to determine the locus of significant main effects and interactions in all ANOVAs.
In order to examine the contributions of puberty above and beyond those of age per se, relationships between the hormonal and physical measures of pubertal onset and the behavioral data were explored using partial correlations controlling for age.
Results
Genital Development
In females, vaginal opening was not observed in any females at P28, whereas by P32, 25% of females tested exhibited vaginal opening, with generally all females exhibiting vaginal opening from P36 on (with the exception of one female at P40; see Fig. 1 – left panel). The number of animals exhibiting vaginal opening at P32 was not significantly greater than at P28, but was significantly less than all of the older age groups (p< .001 for all comparisons, Fisher’s Exact Test), with the older ages not differing significantly from one another. Males at the youngest 3 age groups did not show complete BPS or sperm presence; whereas 60% of males showed both complete separation and sperm presence by P40, 90% by P44 and 100% by P48 and older (see Fig. 1 – right panel). In the analysis of BPS, age was a significant variable (H(6, 68) = 59.00, p< .0025), with the number of animals showing either complete or incomplete separation at P40, P44, P48, and P75 significantly greater than the three youngest age groups. In the analysis of sperm presence, the number of animals showing sperm presence in the three oldest age groups was significantly greater than the three youngest age groups (p < .001 for all comparisons, Fisher’s Exact Test), with P40 males not differing significantly from either the older or younger groups.
Figure 1.
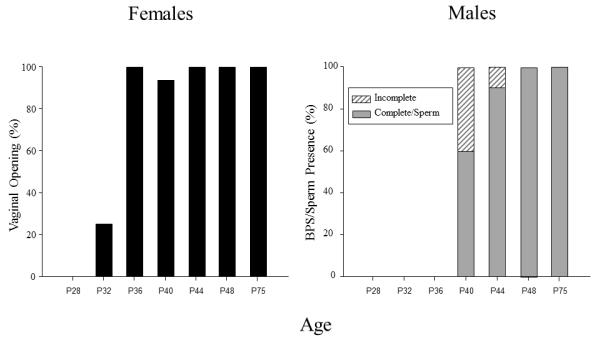
The percent of females at each age exhibiting vaginal opening is shown on the left, whereas the percent of males at each age showing either complete or incomplete balano-preputial skin fold separation (BPS) of the penis is shown on the right. The percent of males at each age exhibiting sperm presence in the seminiferous tubules was the same as the percent of males at each age showing complete BPS, thus, these data are represented by the same bars.
Plasma Hormone Levels
Estradiol was first analyzed in all females collapsed across stage of estrous. When analyzed in this way, a main effect of age was revealed (F(6, 88) = 9.27, p< .01), with estradiol levels increasing gradually across age to reach adult levels by P48 (see Fig. 2 – left panel). Postnatal day 40 females were significantly elevated above the youngest age, but still significantly lower than both P48 and P75 and not different from P44 females. In males, the analysis of testosterone levels revealed a main effect of age (H(6,68) = 56.48, p< .0025), with levels being undetectable at the three youngest ages (P28, 32 & 36; see Fig 2 – right panel). Testosterone levels were low but significantly greater than zero at P40 and increased dramatically from P40 to P44, followed by additional significant rises from P44 to P48 and from P48 to P75.
Figure 2.
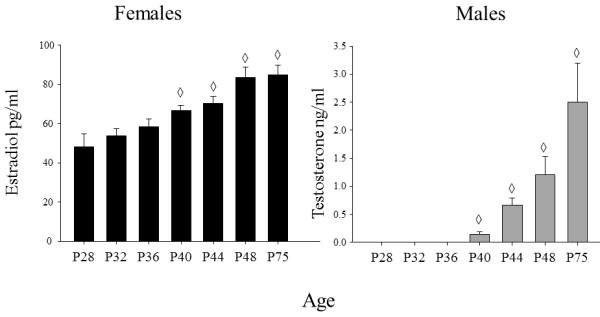
Mean estradiol in females (pg/ml) and mean testosterone in males (ng/ml) are shown across the ages examined. The ◇ symbol indicates the ages at which hormone levels were significantly different from P28. The error bars indicate standard error of the mean.
In the overall ANOVAs of progesterone and corticosterone levels, baseline sex differences were evident, with these hormones significantly greater in females than males [main effects of sex: F(1, 150)= 32.52, p< .01; F(1, 150) = 42.61, p< .01, respectively]. However, progesterone and cort data violated HV assumptions and transformations were not successful in improving data homogeneity, likely due, at least in part, to these sex differences. Consequently, all progesterone and cort data were analyzed separately by sex. Although the progesterone data in females still violated the HV assumption, data homogeneity was improved by the log(10)(n+1) transformation and hence the data were analyzed in this way. In these analyses, the only age effect that emerged was in the analysis of the female progesterone data, (F(6, 89) = 3.54, p< .01), with progesterone levels in females low at P28 and P32, followed by an increase from P32 to P40, with levels not differing significantly from adults by this age (see Fig. 3).
Figure 3.
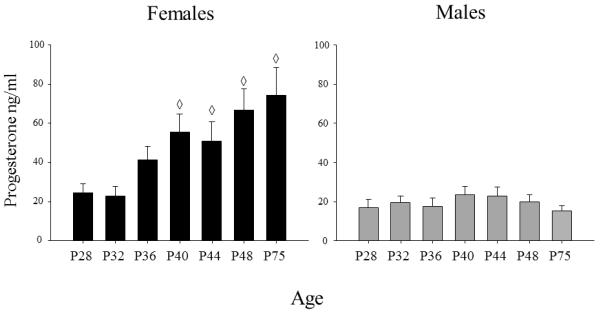
Mean progesterone in males and females (ng/ml) is shown across the ages examined. The ◇ symbol indicates the ages at which hormone levels were significantly different from P28. The error bars indicate standard error of the mean.
Phase of Estrous Effects
The percent of females in each estrous stage at each age is shown in Fig.4. When the distribution of females in each phase of estrous was analyzed across age from P36 on, females at P36, P40 & P44 were found to differ significantly from P48 & P75 females, with fewer females in estrous at the younger ages than at the older ages (X2 = 11.52, p< .01). Since the number of females in each phase differed across age, with some phases poorly represented at certain ages, the impact of phase of estrous on the hormonal and behavioral data was examined by grouping estrous cycle data for females at P32, P36, P40 & P44 and comparing those data with that estrous cycle data from females combined across the two older age groups (i.e., P48 & P75). When the estradiol and progesterone data were analyzed in this way, main effects of age (F(1, 75) = 10.67, p< .01; F(1,75) = 5.62, p<.05, respectively) emerged, with greater levels of both hormones seen in the older than younger group of females. Main effects of estrous stage also were evident for both hormones (F(2,75) = 11.64, p<.01; F(2, 75) = 4.42, p<.05, respectively), with estradiol levels higher during proestrus than in estrus or diestrus 1 & 2, whereas progesterone levels were lower during estrus than during both proestrus and diestrus 1 & 2. As can be seen in Fig. 5, these cycle effects tended to be more pronounced in the older age groups for both hormones, although age did not interact significantly with cycle in either analysis.
Figure 4.
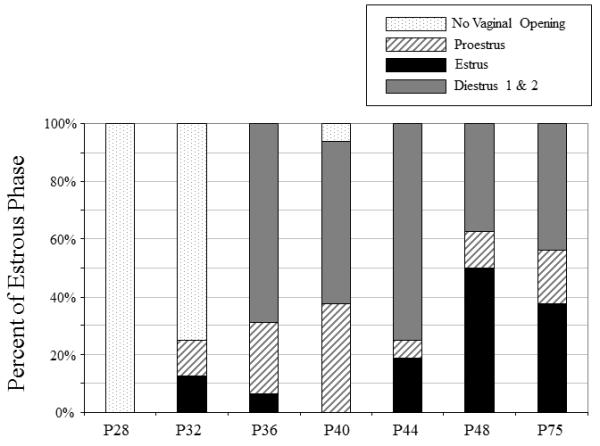
The percentage of females in each phase of the estrous cycle is shown across age; percentage of females not displaying vaginal opening is also noted where indicated.
Figure 5.
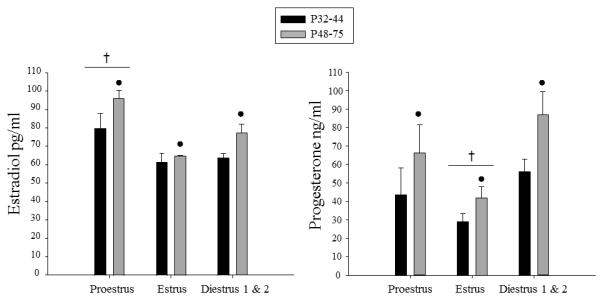
Mean estradiol and progesterone are shown across the phases of estrous in immature (P32-44) and mature (P48-75) females. The ● symbol indicates significant effects of age, whereas the † symbol represents significant differences in hormone levels between the phase indicated and the other two phases of the estrous cycle. The error bars indicate standard error of the mean.
Body Weight
In order to examine patterns of relative weight gain across age, daily percent body weight gain was estimated by subtracting each animals’ weight at a particular age from the mean of the next younger age, then dividing by that mean as well as the number of days between the two ages (i.e., 4 days between all ages, with the exception of 27 days between P48 and P75), then multiplying by 100. For example, to determine the estimated daily percent body weight gain between P28 and P32, the following formula was used: [(((n1 weight(P32) − mean weight(P28))/mean weight(P28))/4)*100)]. A main effect of sex (F(1, 136) = 9.75, p< .01) and age (F(5, 136) emerged in the analysis of these data, with males showing greater percent body weight gain than females (see Fig. 6). The greatest daily body weight gain was observed on P32, followed by a significant decline at P36, with gains at P32-36 significantly greater than at P40-48 and gains at all ages from P32-48 greater than at P75. Although a trend for an age × sex interaction did not reach significance, as can be seen in Fig. 6, body weight gains tended to decline earlier in females (at P32 to P36) than in males (between P36 and P40).
Figure 6.
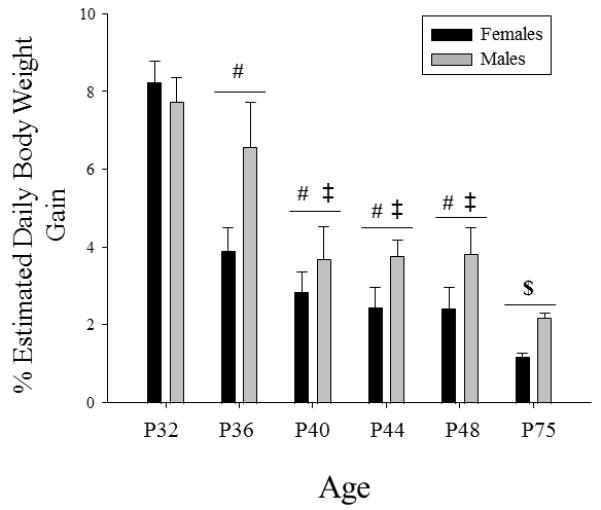
Mean estimated percent daily body weight gain is shown by sex across age. The # symbol indicates a significant difference from P32, whereas the ‡ indicates a significant difference from P36, and the $ symbol indicates a significant difference from all other ages. The error bars indicate standard error of the mean.
Response to Novelty
In the ANOVA of time spent sniffing the novel object, a main effect of age (F(6, 150)= 2.13, p≤ .05) was revealed, with sniffing of the novel object peaking at P32 & P36 (see Fig. 7 – left panel). Novel object sniffing at P36 was significantly elevated over both the youngest age (P28) and two oldest ages (P48 & P75), with sniffing at P32 also being significantly greater than at the oldest age. A trend for a similar age effect was also observed in the analysis of time spent in contact with the novel object, with contact peaking at P36, although this tendency did not reach statistical significance (F(6, 150) = 1.92, p = .08; see Fig. 7 – right panel). No sex effects emerged in the analyses focused on response to the novel object, despite a trend for females to show less sniffing of and contact with the novel object than males at P28 (see Fig. 7). There were no significant effects of sex or age in analyses of latency to contact the novel object and the proportion of time spent on the side of the chamber containing the novel object (data not shown).
Figure 7.
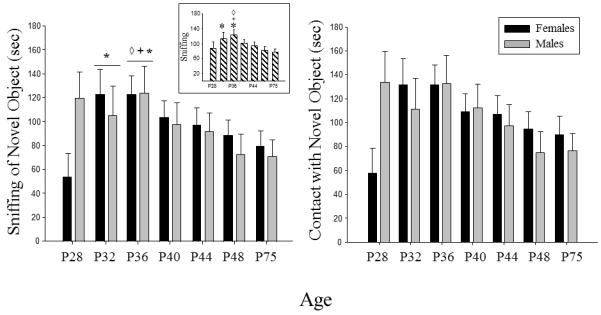
Mean time spent sniffing the novel object and mean time spent in contact with the novel object are shown by sex across the ages examined. The ◇ symbol indicates a significant difference from P28, whereas the + symbol represents a significant difference from P48, and the * symbol indicates a significant differences from P75 when collapsed across sex. The insert on the left shows the main effect of age collapsed across sex in the analysis of time spent sniffing the novel object. The error bars indicate standard error of the mean.
The analysis of crossover data during the first 25 min of the familiarization session revealed only a significant main effect of sex (F(1, 150) = 3.80, p≤ .05), with females (M= 8.91, SEM= 0.41) regardless of age, showing a greater number of crossovers than males (M= 7.71, SEM= 0.46). Additionally, a main effect of bin (F(4, 600) = 209.51, p < .01) also emerged, with activity dramatically decreasing from the first 5 min to the second 5 min of the familiarization session and continuing to gradually decrease across the remaining bins (data not shown).
The 2 age group × 3 cycle phase ANOVAs of the novelty data in females revealed no main effects or interactions involving estrous cycle (data not shown).
Partial Correlations Among the Hormonal, Physical and Behavioral Data
As can be seen in Table 1, in the correlational analyses of the female data controlling for age, a significant positive correlation between vaginal opening and estradiol levels emerged (r2 = 0.21, p< .05), along with a significant negative correlation between vaginal opening and percent daily body weight gain (r2 = −0.42, p< .01), with females displaying vaginal opening more likely to have higher estradiol levels and the rate of body weight gain declining with sexual maturity. Estradiol levels were also significantly positively correlated with corticosterone levels (r2 = 0.21, p< .05). The analysis of the novelty-directed behavioral measures revealed that sniffing of the novel object, contact with the novel object, latency to contact the novel object and proportion of time spent on the same side as the novel object were all significantly positively correlated with each other (see Table 1 for r2 values), but not with any of the hormonal or physical measures (other than a negative correlation between time spent on the same side as the novel object and corticosterone levels; r2 = −0.25, p< .05). Crossover data from the 25 min prior to the novel object test (i.e. total crossovers) was not correlated with any of the variables.
Table 1.
Partial Correlations Controlling for Age in Females
| Vaginal Opening |
% Body Weight Gain |
Estradiol | Progesterone | Corticosterone | Latency | Sniffing | Contact | Time on Same Side |
Total Crossovers |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Vaginal Opening | --- | |||||||||
| % Body Weight Gain | −0.42** | --- | ||||||||
| Estradiol | 0.21* | 0.18 | --- | |||||||
| Progesterone | 0.15 | −0.12 | −0.05 | --- | ||||||
| Corticosterone | −0.03 | −0.08 | 0.21* | 0.11 | --- | |||||
| Latency | −0.07 | −0.07 | −0.12 | 0.03 | −0.01 | --- | ||||
| Sniffing | 0.10 | 0.07 | 0.06 | −0.16 | 0.02 | −0.62** | --- | |||
| Contact | 0.09 | 0.08 | 0.04 | −0.16 | 0.01 | −0.60** | 0.99** | --- | ||
| Time on Same Side | 0.06 | 0.02 | −0.03 | −0.16 | −0.25* | −0.33** | 0.57** | 0.58** | --- | |
| Total Crossovers | −0.18 | 0.04 | −0.13 | −0.18 | −0.03 | 0.17 | −0.12 | −0.12 | −0.17 | --- |
Indicates significance at the p< .05 level
Indicates significance at the p< .01 level
As shown in Table 2, in the analyses of the male data (controlling for age), BPS was positively correlated with sperm presence (r2 = 0.88, p< .01) and both of these variables were negatively correlated with percent daily body weight (r2 = −0.40, p<.01, and r2 = −0.32, p<.05, respectively). Progesterone was also positively correlated with corticosterone (r2 = 0.46, p<.01). As in the female data, all of the novelty behaviors were significantly correlated with each other (see Table 2 for r2 values), but no other variables, other than a significant negative correlation of corticosterone and time spent on the same side as the novel object (r2 = −0.26, p< .05). Total crossovers prior to the novel object test were not correlated with any other measure.
Table 2.
Partial Correlations Controlling for Age in Males
| BPS | Sperm | % Body Weight Gain |
Testosterone | Progesterone | Corticosterone | Latency | Sniffing | Contact | Time on Same Side |
Total Crossovers |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| BPS | --- | ||||||||||
| Sperm | 0.88** | --- | |||||||||
| % Body Weight Gain |
−0.40** | −0.32* | --- | ||||||||
| Testosterone | −0.01 | 0.05 | 0.06 | --- | |||||||
| Progesterone | 0.200.10 | −0.27 | −0.07 | --- | |||||||
| Corticosterone | 0.14 | 0.11 | −0.23 | −0.22 | 0.46** | --- | |||||
| Latency | −0.08 | 0.03 | 0.15 | −0.05 | 0.12 | −0.03 | --- | ||||
| Sniffing | 0.06 | −0.07 | 0.02 | −0.04 | −0.17 | −0.19 | −0.56** | --- | |||
| Contact | −0.18 | −0.09 | 0.01 | −0.04 | −0.17 | −0.18 | −0.56** | 0.99** | --- | ||
| Time on Same Side |
−0.04 | −0.01 | 0.14 | 0.18 | −0.11 | −0.26* | −0.38** | 0.71** | 0.71** | --- | |
| Total Crossovers | −0.22 | −0.15 | −0.03 | 0.02 | −0.09 | 0.06 | 0.05 | 0.00 | 0.05 | −0.11 | --- |
Indicates significance at the p< .05 level
Indicates significance at the p< .01 level
Discussion
As expected, physical and hormonal signs of puberty were observed earlier in females, with vaginal opening beginning to occur at P32 and generally completed by P36, whereas in males, BPS and the presence of sperm was observed in a majority of males by P40, with these indices of pubertal maturation generally complete by P44. Sex hormones showed similar sex-specific ontogenetic patterns, with estradiol and progesterone levels in females detectable at the youngest age (P28) and increasing across age, whereas testosterone levels did not reach detectable levels until P40 and increased thereafter. Among vaginally open females, stage of estrous cycle impacted hormone levels, but not novelty-directed behavioral measures, with estradiol levels highest during proestrus, and progesterone levels lowest during estrus regardless of age, although more mature females (P48-75) showed higher overall levels of both hormones than their younger (P32-44) counterparts. Novelty-directed behavior peaked between P32 and P36, followed by a gradual decline into adulthood; no significant impact of sex on this behavior was revealed. Partial correlations controlling for age found no significant relationship between the hormonal or physical measures of puberty and novelty-directed behavioral data, suggesting that the developmental peaks in this behavior were not related to pubertal onset.
In the present study, general ontogenetic increases in sex steroid hormones were observed in females, with significant levels of estradiol and progesterone seen at the youngest age examined (P28) that rose thereafter to reach levels significantly greater than the youngest age by P40. When levels of estradiol and progesterone of younger (P32-44) and older (P48-75) females were compared across estrous phase, the same phase of estrous effects were present in both age groups, with hormone levels greater overall in more mature females relative to their younger counterparts. These results suggest that the general ontogenetic increases observed in both estradiol and progesterone are developmental in nature and not simply driven by different phases of the estrous cycle being expressed across age. It is unclear why the distribution of estrous cycle phases varied somewhat across age among vaginally open females, with possibilities ranging from either random sampling differences or age differences in cycle timing and patterning. Examining more females per age group could have helped distinguish these possibilities, although phase of estrous is best determined when females are examined for multiple consecutive days and at least 2 cycles are observed (Becker et al., 2005) -- a technique not possible in the current study given the cross-sectional nature of the design.
Among males, BPS and sperm presence were observed in a majority of males at the same age that testosterone was first detectable, suggesting a low threshold for this androgen’s action on genital tissue and sperm development. The fact that testosterone was not detectable until P40 was surprising given reports of low levels of plasma testosterone throughout the juvenile period in some studies (Dohler & Wuttke, 1975; Gupta, Rager, Zarzycki & Eichner, 1975). Exposure to a mild stressor, however, was found to suppress the low levels of testosterone normally evident in pre-pubertal males (Romeo, Lee, Chhua, McPherson & McEwen, 2004). Based on the slightly elevated levels of corticosterone observed following the novelty test in the present study, it is possible that the mild stress of this testing may have suppressed the already low levels of testosterone in pre-pubertal males to levels below the threshold of detection. Alternatively, the assay used in the present study may not have been sensitive enough to detect low levels of testosterone in the youngest males. Nonetheless, physical changes in male genitalia were observed at the same time as increases in plasma testosterone. Future studies examining a broader range of hormones across the pubertal transition, such as prolactin and DHT, may be helpful in understanding the constellation of hormonal changes associated with puberty that influence genital development and other end-points of sexual maturation.
Peaks in estimated percent daily body weight gains were greatest at P32 and P36 when collapsed across sex, although there was a trend for an earlier post-peak decline in body weight gain in females (P32-P36) than in males (from P36-P40) that did not reach significance. Remarkably similar and more robust findings were obtained in prior longitudinal work in our laboratory where daily body weight gain in females was found to asymptote between P23-P34 before declining, whereas body weight gains in males were greatest between P27-38 (Vetter & Spear, 2007). Taken together, these data suggest that the accelerated rate of growth typical of the adolescent period (Nance, 1983; Marshall & Tanner, 1968) occurs during the early to mid adolescent period, around the time of puberty onset in both sexes, findings consistent with prior work (Kennedy & Mitra, 1963; Nazian & Cameron, 1999). In line with other evidence linking this growth spurt to pubertal processes in humans and in animal models (e.g., Plant & Witchel, 2006), results from the partial correlational analyses where age was controlled revealed significant negative correlations between percent daily body weight gain and vaginal opening in females, and between weight gains and BPS and sperm presence in males.
Novelty-directed behavior, specifically, sniffing of the novel object and, to a lesser extent, contact with the novel object, peaked between P32 and P36 at levels elevated above that of younger animals (P28) and began to decline after P36, with adult-like levels reached by P40. These results are reminiscent of prior work in our laboratory showing that adolescents exposed to a novel object from P33-37 spent more time interacting with the novel object than adults, with no sex differences emerging during adolescence (Douglas, Varlinskaya & Spear, 2003). Similar findings of greater novelty-seeking behavior in adolescents than adults have been reported in other groups as well (Stansfield & Kirstein, 2006; Abreu-Villaca, Queiroz-Gomes, Dal Monte, Filgueiras & Manhaes, 2006; Adriani et al., 1998), although in these studies adolescents were generally examined only at one age, making it difficult to determine the developmental time-course of novelty-seeking behavior through adolescence and across the transition into adulthood.
The peak in novelty behaviors at P32-36, prior to the completion of the physical and hormonal signs of puberty suggests that the greater novelty-seeking behavior of adolescents may be independent of puberty-related behavior changes. The lack of significant sex differences in novelty-directed behaviors during development in the present study and others (Douglas et al., 2003; Abreu-Villaca et al., 2006) adds further support for the independence of novelty-seeking and puberty. If pubertal changes played a major role in this behavior, sex differences would be expected, with females showing a different ontogenetic profile than males due to their earlier progression through puberty. Perhaps the most compelling evidence for the independence of novelty-seeking and pubertal processes in our animals was the results of partial correlation analyses exploring the relationship between hormonal and physical signs of pubertal development and novelty behaviors (controlling for the effects of age) in which these puberty measures were not correlated with novelty-directed behaviors. It is not simply the case, however, that these partial correlational analyses were insensitive to detection of hormonal influences. First, similar conclusions were reached in analyses showing that phase of estrous did not influence novelty-directed behaviors during adolescence (or in adulthood). Moreover, the partial correlation analyses did reveal significant negative associations between levels of another hormone – corticosterone – and time spent on the same side of the chamber as the novel object in both sexes. Although unrelated to pubertal processes per se, these associations between corticosterone and avoidance of the side containing the novel object may reflect individual differences in anxiety responses to novelty that, if confirmed, could prove a promising avenue for additional study.
The lack of pubertal associations with novelty-seeking measures seen in the present study contrasts with the limited literature available to date in human adolescents examining the role of puberty on the related construct of sensation-seeking (McCourt et al., 1993). One such study examining adolescents and young adults (ages 10-30 yrs old) showed that whereas sex differences in self-reported sensation-seeking were not present in the sample as a whole, when a subset of participants 16 and younger were analyzed, sex differences emerged, with males reporting greater self-reported sensation-seeking than females. Additionally, pubertal status was predictive of sensation-seeking in analyses controlling for age in males, but not in females (Steinberg, Albert, Cauffman, Banich, Graham & Woolard, 2008), although no sex differences or effects of pubertal status were observed in self-reported impulsivity or risky decision making (Martin et al, 2002; Gardner & Steinberg, 2005; Steinberg et al., 2008). These studies in human subjects suggest that adolescent-typical increases in sensation-seeking may be in part related to pubertal status, whereas other related constructs such as risk-taking and impulsivity may be more reflective of puberty-independent behavior changes during adolescence. Perhaps the novel object test used in the current study is more closely related to laboratory tests of human risk-taking behavior than to constructs indexed by self-reported sensation-seeking.
The data shown here may also be of relevance for considerations regarding age ranges of adolescence and puberty in the rat. The peak in novelty seeking in rats was found to occur during a time of rapid pubertal change in females, but with post-peak declines that overlapped with the maturation of the external genitalia in males. In a similar manner, sensation-seeking in human adolescents peaks during early adolescence at 12-15 (Steinberg et al., 2008), at a time during the greatest pubertal development in females, but prior to the average age of completion of male sexual maturation (i.e., ~17; Sun et al., 2002). Taken together, such data are consistent with the notion that the P28-42 period conservatively used to characterize adolescence in earlier work based on behavioral, psychopharmacological and neural characteristics (e.g., Spear & Brake, 1983; Spear, 2000) may be better considered as comparable to early/mid adolescence in humans, during which maturation of the external genitalia occurs in females but prior to complete maturation of the external genitalia in males. A later ontogenetic interval in rats (roughly subsuming ages from ~ P42-55) may be more consistent with the late adolescent/ “emerging adulthood” period in humans, a time of continued developmental declines in impulsivity (Steinberg et al, 2008), along with high rates of binge drinking, tobacco and illicit drug use (Substance Abuse and Mental Health Services Administration, 2009).
Taken together, the results of the present study provide compelling evidence that developmental changes in expression of novelty-seeking behavior seen across adolescence are not related in any simple fashion to pubertal changes in gonadal hormone secretion or other physical signs of pubertal maturation. Clearly, additional developmental research in both humans and rodents is needed to distinguish the role of puberty from pubertal-independent developmental processes in these and other critical adolescent-typical behaviors.
Acknowledgments
Funding acknowledgements: This research was supported by NIAAA grant R01-AA017355
References
- Abreu-Villaca Y, Queiroz-Gomes Fdo E, Dal Monte AP, Filgueiras CC, Manhaes AC. Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behavioural Brain Research. 2006;167(1):175–182. doi: 10.1016/j.bbr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behavioral Neuroscience. 1998;112(5):1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. American Psychologist. 1999;54(5):317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. American Psychologist. 2000;55(5):469–480. [PubMed] [Google Scholar]
- Baumrind D. A developmental perspective on adolescent risk taking in contemporary America. In: Irwin CE Jr., editor. Adolescent social behavior and health. Jossey-Bass; San Francisco, CA: 1987. pp. 93–125. CE. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146(4):1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berlin) 2004;174(3):389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Human Brain Mapping. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH, Rissman EF. The biology of puberty. Biological Reviews of the Cambridge Philosophical Society. 1986;61(2):157–195. doi: 10.1111/j.1469-185x.1986.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007;32(11):2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berlin) 2005;183(2):218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacology (Berlin) 2007;193(2):247–260. doi: 10.1007/s00213-007-0764-5. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46(3):349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Developmental Psychopathology. 2009;21(1):1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- de Jong RA, van der Schoot P. Advancement of sexual maturation in male rats by pituitary transplants. Biological Reproduction. 1979;21(5):1263–1271. doi: 10.1095/biolreprod21.5.1263. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34(3):136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Wuttke W. Serum LH, FSH, prolactin and progesterone from birth to puberty in female and male rats. Endocrinology. 1974;94(4):1003–1008. doi: 10.1210/endo-94-4-1003. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97(4):898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiology & Behavior. 2003;80(2-3):317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Dubas JS. Cognitive abilities and physical maturation. In: Lerner RM, Petersen AC, Brooks-Gunn J, editors. Encyclopedia of Adolescence. Vol. 1. Garland; New York: 1991. pp. 133–138. [Google Scholar]
- Feldman SS, Elliot GR. At the threshold: The developing adolescent. Harvard University Press; Cambridge, MA: 1990. [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain & Cognition. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: Role of pubertal maturation and relation to measures of negative affect. Developmental Neuropsychology. 2011;36(4):429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41(4):625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Gupta D, Rager K, Zarzycki J, Eichner M. Levels of luteinizing hormone, follicle-stimulating hormone, testosterone and dihydrotestosterone in the circulation of sexually maturing intact male rats and after orchidectomy and experimental bilateral cryptorchidism. Journal of Endocrinology. 1975;66(2):183–193. doi: 10.1677/joe.0.0660183. [DOI] [PubMed] [Google Scholar]
- Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. Journal of Physiology. 1963;166:408–418. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebaur JE, Bardo MT. Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preferences. Pharmacology, Biochemistry and Behavior. 1999;63(1):133–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biological Reproduction. 1977;17(2):298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Lewis EM, Barnett JF, Jr., Freshwater L, Hoberman AM, Christian MS. Sexual maturation data for Crl Sprague-Dawley rats: criteria and confounding factors. Drug & Chemical Toxicology. 2002;25(4):437–458. doi: 10.1081/dct-120014794. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annual Review of Medicine. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, et al. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of American Academy of Child & Adolescent Psychiatry. 2002;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- McCourt WF, Gurrera RJ, Cutter HS. Sensation seeking and novelty seeking. Are they the same? Journal of Nervous Mental Disease. 1993;181(5):309–312. doi: 10.1097/00005053-199305000-00006. [DOI] [PubMed] [Google Scholar]
- Michaud PA, Suris JC, Deppen A. Gender-related psychological and behavioural correlates of pubertal timing in a national sample of Swiss adolescents. Molecular and Cellular Endocrinology. 2006;254-255:172–178. doi: 10.1016/j.mce.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Nance DM. The developmental and neural determinants of the effects of estrogen on feeding behavior in the rat: a theoretical perspective. Neuroscience & Biobehavioral Reviews. 1983;7(2):189–211. doi: 10.1016/0149-7634(83)90015-5. [DOI] [PubMed] [Google Scholar]
- Nazian SJ, Cameron DF. Temporal relation between leptin and various indices of sexual maturation in the male rat. Journal Andrology. 1999;20(4):487–491. [PubMed] [Google Scholar]
- Negriff S, Susman EJ, Trickett PK. The Developmental Pathway from Pubertal Timing to Delinquency and Sexual Activity from Early to Late Adolescence. Journal of Youth Adolescence. 2010 doi: 10.1007/s10964-010-9621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell WD. Sexual maturation in the rat. In: Grumbach MM, Sizonenko PC, Aubert ML, editors. Control of the onset of puberty. Williams and Wilkins; Baltimore, MD: 1990. pp. 183–210. [Google Scholar]
- Ojeda SR, Terasawa E. Neuroendocrine regulation of puberty. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. vol. 4. Academic Press; New York: 2002. pp. 589–659. [Google Scholar]
- Ojeda SR, Skinner MK. Puberty in the rat. In: Knobil E, Neill JD, editors. Physiology of Reproduction. Elsevier Academic Press; St. Louis, MO: 2006. pp. 2061–2126. [Google Scholar]
- Petersen AC, Silbereisen RK, Sorensen S. Adolescent development: A global perspective. In: Hurrelmann K, Hamilton SF, editors. Social Problems and Social Contexts in Adolescence. Aldine de Gruyter; New York: 1996. pp. 3–37. [Google Scholar]
- Philpot RM, Wecker L. Dependence of adolescent novelty-seeking behavior on response phenotype and effects of apparatus scaling. Behavioral Neuroscience. 2008;122(4):861–875. doi: 10.1037/0735-7044.122.4.861. [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF. Puberty in Nonhuman Primates and Humans. In: Knobil E, Neill JD, editors. Physiology of Reproduction. Elsevier Academic Press; St. Louis, MO: 2006. pp. 1017–1070. [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Developmental Psychobiology. 1989;22(6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Hormones & Behavior. 1990;24(3):311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79(3):125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Schulz KM, Nelson AL, Menard TA, Sisk CL. Testosterone, puberty, and the pattern of male aggression in Syrian hamsters. Developmental Psychobiology. 2003;43(2):102–108. doi: 10.1002/dev.10125. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular and Cellular Endocrinology. 2006;254-255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The psychobiology of adolescence. In: Kline K, editor. Authoritative Communities: The Scientific Case for Nurturing the Child (The Search Institute Series on Developmentally Attentive Community and Society. Springer; New York, NY: 2007. pp. 263–280. [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Developmental Psychopathology. 2009;21(1):87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. W.W. Norton; New York: 2010. [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Developmental Psychobiology. 1983;16(2):83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Developmental Psychobiology. 2006;48(1):10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . National Survey on Drug Use and Health. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2009. [Google Scholar]
- Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110(5):911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcoholism. 2009;44(6):547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in sprague-dawley rats. Alcoholism: Clinical & Experimental Research. 2011;35(11) doi: 10.1111/j.1530-0277.2011.01555.x. DOI: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on sex- and age- typical responses to novelty and ethanol-induced social inhibition in adult male and female sprague-dawley rats. (under revision) [DOI] [PMC free article] [PubMed]
- Vetter CS, Spear LP. Age-associated trajectories of consumption and body weight gain in pair- and isolate-housed adolescent and adult sprague-dawley rats. Developmental Psychobiology. 2007;49(7):743. [Google Scholar]
- Wisner JR, Jr., Stalvey JR, Warren DW., 3rd Delay in the age of balano-preputial skinfold cleavage and alterations in serum profiles of testosterone, 5 alpha-androstane-3 alpha,17 beta-diol, and gonadotropins in adult rats treated during puberty with luteinizing hormone releasing hormone. Steroids. 1983;41(4):443–454. doi: 10.1016/0039-128x(83)90084-3. [DOI] [PubMed] [Google Scholar]
- Yener T, Tunc A. Turkkani, Aslan H, Aytan H, Caliskan A. Cantug. Determination of oestrous cycle of the rats by direct examination: how reliable? Anatomia, Histology, Embryologia. 2007;36(1):75–77. doi: 10.1111/j.1439-0264.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Culbert KM, Sisk CL, Klump KL. An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Horm Behav. 2007;52(4):427–435. doi: 10.1016/j.yhbeh.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


