Abstract
Incorporation of antigens by dendritic cells (DCs) increases when antigens are targeted to endocytic receptors by monoclonal antibodies (mAb). We have previously demonstrated in the mouse that mAb against C-type lectins administered intradermally are taken up by epidermal Langerhans cells (LCs), dermal Langerinneg DCs and dermal Langerin+ DCs in situ. However, the relative contribution of these skin DC subsets to the induction of immune responses after antigen targeting has not been addressed in vivo.
We show here that murine epidermal LCs and dermal DCs transport intradermally injected mAb against the lectin receptor DEC-205/CD205 in vivo. Skin DCs targeted in situ with mAb migrated through lymphatic vessels in steady state and inflammation. In the skin-draining lymph nodes, targeting mAb were found in resident CD8α+ DCs and in migrating skin DCs. More than 70% of targeted DCs expressed Langerin, including dermal Langerin+ DCs and LCs. Numbers of targeted skin DCs in the nodes increased 2-3-fold when skin was topically inflamed by the TLR7 agonist imiquimod.
Complete removal of the site where ovalbumin-coupled anti-DEC-205 had been injected decreased endogenous cytotoxic responses against ovalbumin peptide-loaded target cells by 40-50%. Surprisingly, selective ablation of all Langerin+ skin DCs in Langerin-Diphtheria-Toxin-Receptor knock-in mice did not affect such responses, independent of the adjuvant chosen. Thus, in cutaneous immunization strategies where antigen is targeted to DCs, Langerin+ skin DCs play a major role in transport of anti-DEC-205 mAb, although Langerinneg dermal DCs and CD8α+ DCs are sufficient to subsequent CD8+ T cell responses.
INTRODUCTION
Dendritic cells (DCs) are critically involved in the generation of immunity induced by vaccines and pathogens (1). Cutaneous DC subsets include epidermal Langerhans cells (LCs), and dermal DCs, which subdivide into Langerinneg and Langerin+ populations (2-5). These three DC subsets are positioned to take up intradermal vaccine, process it and carry it to the draining lymph nodes in order to stimulate antigen-specific T cells. Despite this, recent data has shed doubt on their immunogenic role in vivo (6-8). In particular, the contribution of CD8α+ DCs residing in draining lymph nodes has to be taken into account, because soluble antigens can reach them via the lymphatic flow (9) or by transfer from emigrating skin DCs (10).
All DC subsets express C-type lectin receptors that facilitate uptake and processing of antigenic proteins (11). This ability has been exploited to improve immune responses by targeting antigens to DCs (12,13). The best-studied example is DEC-205/CD205, which is expressed at highest levels by dermal DCs, LCs and CD8α+ DCs (14-16). When protein antigens are coupled to anti-DEC-205 mAb and mice are immunized with these conjugates, endogenous T cell-dependent immune responses (17-19) are dramatically enhanced in vivo. This requires the concomitant administration of DC-activating agents, such as Toll-Like Receptor (TLR) ligands or agonistic anti-CD40 mAb.
In many of the above-cited studies, immunisation with anti-DEC-205 conjugates was performed by injection into the subcutaneous tissue of the footpad. Despite extensive research performed with antibodies targeting DEC-205, only limited characterisation of the DC subsets involved in the induction of immune responses is available (17,20,21). We have previously reported that epidermal LCs and both subsets of dermal DCs are able to capture anti-DEC-205 mAb in situ, and that the model antigen ovalbumin (OVA) coupled to these mAb is presented by LCs to CD4+ and CD8+ transgenic T cells in vitro (16). Thus, we wished to complement these observations with additional studies in vivo on the transport of antigen within mAb targeting to DEC-205 and the subsequent development of endogenous immune responses. This appears important in view of the differential roles that epidermal LCs, dermal DCs, and lymph node-resident CD8α+ DCs seem to play (10,22,23). We compared the contribution of these subsets in the transport of anti-DEC-205 targeting mAb and in the induction of antigen-specific, endogenous cytotoxic responses, in steady state and inflammation. Moreover, the role of Langerin+ DC populations was specifically addressed by employing a mouse model allowing conditional depletion of Langerin-expressing cells (24).
MATERIALS AND METHODS
Mice
Mice of inbred strain C57BL/6 and BALB/c were purchased from Charles River Laboratories (Sulzfeld, Germany) and used at 2 to 6 months of age. Langerin-DTR-EGFP mice were provided by Dr. B. Malissen, Marseille, France (25). All experimental protocols were approved by the Austrian Federal Ministry of Science and Research, Department for Genetic Engineering and Animal Experimentation (#66.011/16-II/106/2008).
Antibodies and reagents
Targeting antibodies were detected with goat anti-rat immunoglobulins G (IgG; H+L) coupled with APC (BD-Pharmingen) for FACS analyses. For immunofluorescence in the murine dermis, we employed chicken anti-rat IgG coupled to Alexa Fluor 594™ (Invitrogen) that limits background staining of dermal extracellular matrix. Anti-mouse LYVE-1 polyclonal antibody (rabbit IgG, Upstate Cell Signaling Solutions, Lake Placid, NY) was used to detect dermal lymphatic vessels, and was visualized with swine anti-rabbit Ig / FITC (Dako Cytomation A/S, Glostrup, Denmark). Phenotypical analyses of murine DCs were performed with mAb against MHC class II (anti-I-A/I-Ediverse, clone 2G9), CD11c (clone HL3), CD8α (clone Ly-2), CD103 (clone M290) (all from BD-Pharmingen), CD11b (clone M1/70; eBiosciences, San Diego, CA) and Langerin/CD207 mAb (clone 929F3; Dendritics, Lyon, France). When possible, viable cells were determined by exclusion of 7-AAD-positive dead cells (Becton-Dickinson).
Targeting antibodies
The NLDC145 mAb (BMA Biomedicals, Augst, Switzerland) recognizes murine DEC-205 (26). Isotype control mAb was rat IgG2a (R&D Systems, Minneapolis, MN). For functional assays, ovalbumin-conjugated anti-DEC-205 mAb (aDEC/OVA) or ovalbumin-conjugated isotype control (ISO/OVA; mouse IgG1) were used (27).
In vivo targeting experiments
0.5-1μg of purified rat IgG2a or anti-DEC-205 mAb diluted in 25μL PBS were injected into the ear pinna of anaesthetized C57BL/6, BALB/c or Langerin-DTR-EGFP mice. Where indicated, ears were treated immediately after injection with Aldara™ cream, containing 5% imiquimod (a kind gift of Meda Pharma, Vienna, Austria), or left untreated. Alternatively, targeting antibodies were diluted in 25μL of H2O containing 25μg of polyinosinic–polycytidylic acid (poly(I:C); Sigma-Aldrich), and injected into ears. In some experiments, ears were removed 4 hours after injection of mAb, as described elsewhere (28).
Preparation of lymph node cell suspensions
Lymph nodes were harvested at the indicated times, and cell suspensions were obtained by digestion with 0.5 mg/mL Collagenase P and 120μg/mL DNAse I (Roche Applied Science, Hamburg, Germany), for 25min at 37°C.
Flow cytometry analyses
Permeabilisation was performed with Cytofix/perm kit (Becton-Dickinson). Uncoupled targeting antibodies were detected with goat anti-rat IgG /APC (BD Pharmingen). DC subsets were then identified by counterstaining with fluorochrome-coupled mAb, or biotinylated mAb plus fluorochrome-coupled streptavidin. Most of our experimental data was acquired on a FACSCalibur (Becton-Dickinson), allowing four-parameter analyses. Some experiments requiring six-parameter analyses were performed on a FACSCanto (Becton-Dickinson).
Langerhans cell ablation in Langerin-DTR-EGFP mice
Depletion of Langerin+ cells was performed 48 hours before immunization by injecting 0.5μg diphtheria toxin (DT) i.p. (25).
Whole skin explant cultures
48 hours after depletion of Langerin+ DCs with 0.5μg DT, Langerin-DTR-EGFP mice were injected with 1μg anti-DEC-205 or isotype control. Four hours later, mice were killed, ears were cut off at the base, and ear skin was split into dorsal and ventral halves (29). The dorsal halves were cultured in 24-well plates (one per well), for 4 days, in complete culture medium. Emigrant DCs were harvested and investigated by flow cytometry.
In vivo killing assays
Groups of at least three mice were immunized with the indicated targeting mAb and adjuvants. One week after immunization, mice were injected i.v. with spleen cells differentially labelled with 20 or 200 nM CFSE (Invitrogen), and loaded with 10 or 100 nM OVA257–264 (OVA-peptide SIINFEKL), respectively. As an internal control, unloaded spleen cells labelled with 10 μM Cell-Tracker Orange (CTO; Invitrogen) were mixed with CFSE-labelled cells. From each target cell population, we injected 3–6 × 106 cells, meaning a total of 9–18 × 106 target cells per mouse. The auricular lymph nodes draining the immunization site and the blood were collected 48 h after injection of target cells. Percentage of OVA-specific killing was calculated as described elsewhere (30).
Statistical analyses
All experiments involved groups of at least three mice, and were performed at least twice with similar results. Error bars represent standard deviations. P-values are from two-tailed Student’s t-tests.
RESULTS
Morphological aspects of migration of targeted skin DCs through dermal lymph vessels in vivo
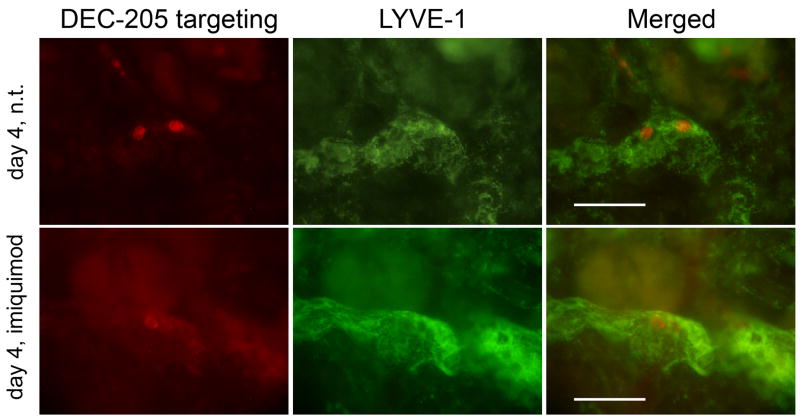
Previously, we demonstrated that LCs and several dermal DC subsets could be targeted by anti-DEC-205 mAb (clone NLDC145) in situ following intradermal injection into a live mouse (16). In a next step, we determined here whether in situ-targeted DCs would migrate from the skin to the draining lymph nodes. Four days after intradermal injection, immunofluorescence microscopy allowed the detection of DCs loaded with anti-DEC-205 mAb within dermal lymphatic vessels (Figure 1). Topical application of a cream containing the TLR7 ligand imiquimod results in skin inflammation and subsequent migration of skin DCs towards draining lymph nodes (31-33). Targeted cells were also visible in lymph vessels of imiquimod-treated skin (Figure 1, lower panel). Targeted DCs detected by this method were probably still interacting with lymphatic endothelial cells. Because such events are very rare, microscopy does not allow reliable quantification of differences between imiquimod-treated and untreated skin. Nevertheless, this suggests that inflammation is not essential for at least a basal rate of emigration of in situ-targeted skin DCs.
Figure 1. Targeted DCs migrate through the dermis in steady state and inflamed skin.
1μg IgG2a control or anti-DEC-205 mAb were injected intradermally into the ears of C57BL/6 mice. The injection site was then topically treated with imiquimod cream or left untreated (n.t.). Four days after injection, dermal sheets were prepared and fixed. Targeted cells were detected by anti-rat IgG / Alexa Fluor 594™. Dermal lymph vessels were revealed by anti-LYVE-1 antibody, specific for lymphatic endothelial cells. Scale bar represents 100μm. Results are representative of two independent experiments.
Intradermally injected anti-DEC-205 antibodies are transported by CD11clow Langerin+ DCs/LCs and CD11b+ Langerinneg dermal DCs to skin-draining lymph nodes
We wished to determine the origin of targeted lymph node cells. DEC-205 is found on skin-derived DCs and CD8α+ DCs residing in lymph nodes (2,14,23,34). Skin-derived CD8αneg CD11b+ DCs in lymph nodes are discriminated from lymph node-residing DCs by their mature phenotype (i.e., expression of costimulatory molecules, high levels of MHC class II…) (35). Because skin-derived DCs migrate from the periphery, they display the chemokine receptor CCR7 (Figure 2A). CD8α+ DCs residing in lymphoid organs express high levels of CD11c and lack CD11b and CCR7 expression (Figure 2A). Importantly, some CD8α+ DCs express Langerin, in proportions that are variable depending on the mouse strain (15,25,33,36,37). CD11bhigh CD11cneg macrophages and granulocytes did not bind the targeting antibody and were excluded from our analysis (Figure 3A, 4B, and data not shown).
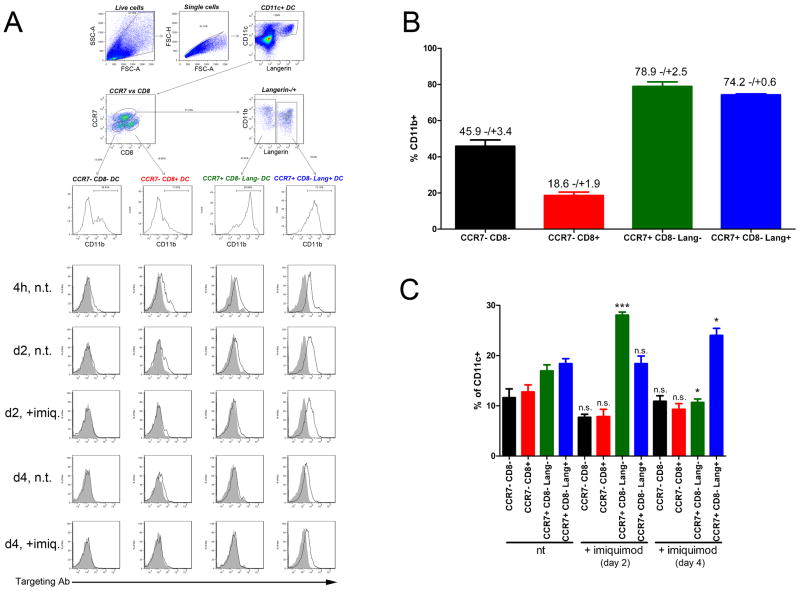
Figure 2. Detailed analysis of targeted DC subsets.
1μg IgG2a control or anti-DEC-205 mAb were injected intradermally into the ears of C57BL/6 mice. The injection site was immediately treated with imiquimod cream, or left untreated. At the indicated time points, targeting antibody was detected on permeabilised lymph nodes cells by anti-rat IgG / APC, and the different subset of DCs were distinguished by staining for CD11c, CD11b, CCR7, CD8α and Langerin. (A, upper panel) Gating strategy for CD11c+ CCR7neg CD8αneg CD11b-/+ LN DCs, CD11c+ CCR7neg CD8α+ CD11blow LN DCs, CD11c+ CCR7+ CD8αneg Langerinneg CD11b+ dermal DCs and CD11c+ CCR7+ CD8αneg Langerin+ CD11b+ LCs / dermal DCs. (A, lower panel) Detection of DEC-205 targeting antibody (empty histogram) and IgG2a isotype control (filled histogram) on four different DC subsets. Results are representative of two independent experiments. (B) Percentage of CD11b+ cells among the four different DC subsets, irrespective of targeting. (C) Relative proportions of each DC subset in untreated mice or mice treated with imiquimod cream. Data from (B) and (C) are pooled from two independent experiments, with at least 2 mice per condition. Significance values apply to the comparison of identical populations (i.e., same color columns) with or without imiquimod treatment.
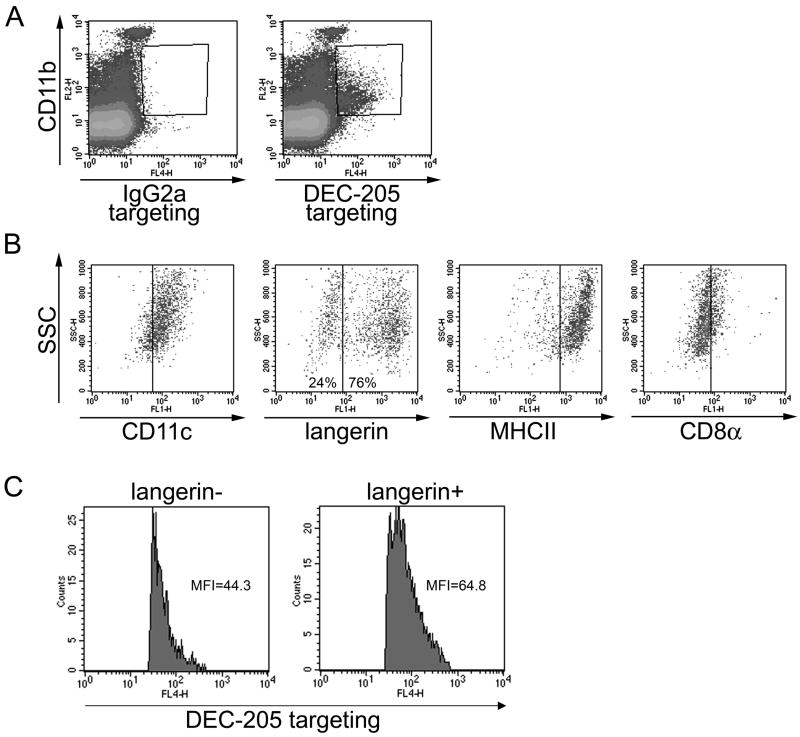
Figure 3. Targeted cells in draining lymph nodes are mature, skin-derived DCs.
1μg IgG2a control or anti-DEC-205 mAb were injected intradermally into the ears of C57BL/6 mice. Two days later, targeting antibodies were detected in permeabilised lymph nodes cells by anti-rat IgG / APC. (A) CD11b expression and levels of targeting antibody were evaluated in lymph node cells. (B) CD11b+ targeting antibody+ cells were gated and expression of MHC class II, CD11c, Langerin and CD8α was evaluated. Percentages of Langerinneg and Langerin+ cells among gated cells are shown. (C) Comparison of the amount of anti-DEC-205 mAb detected in Langerinneg and Langerin+ subsets of CD11b+ targeting antibody+ lymph node cells. MFI: mean fluorescence intensity. Results are representative of at least three independent experiments.
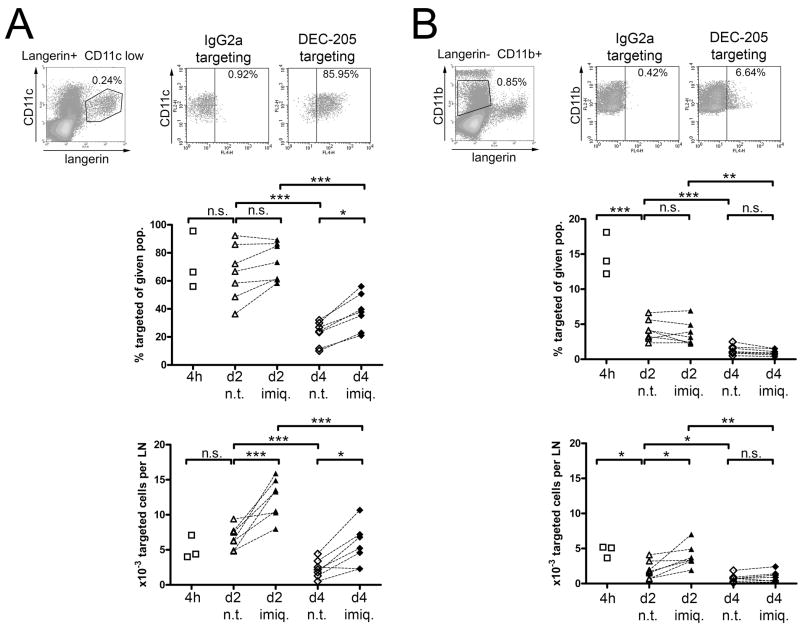
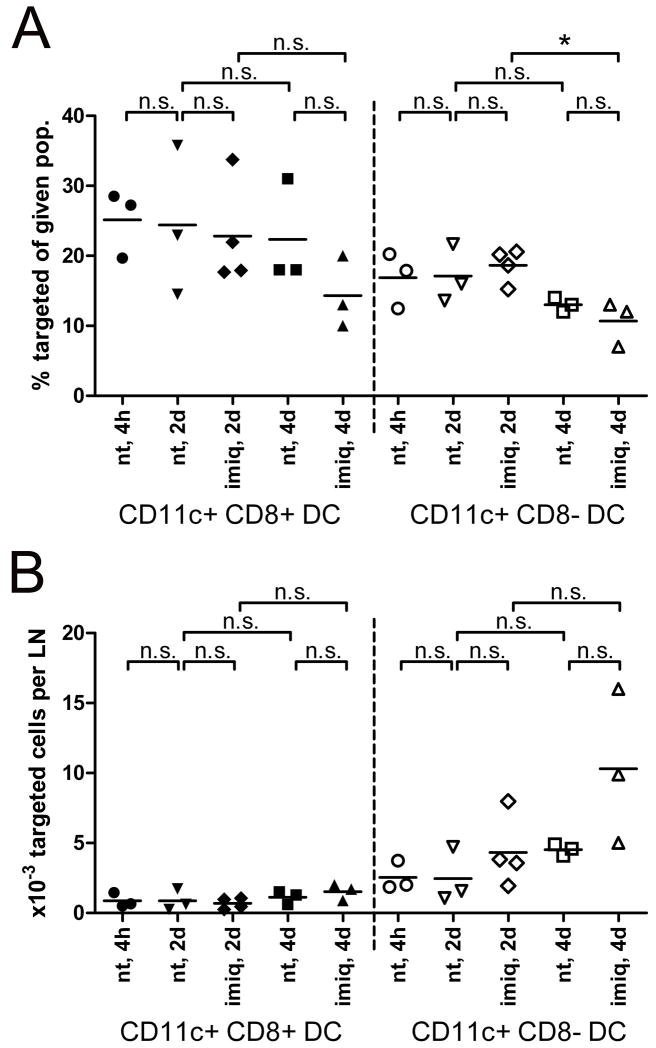
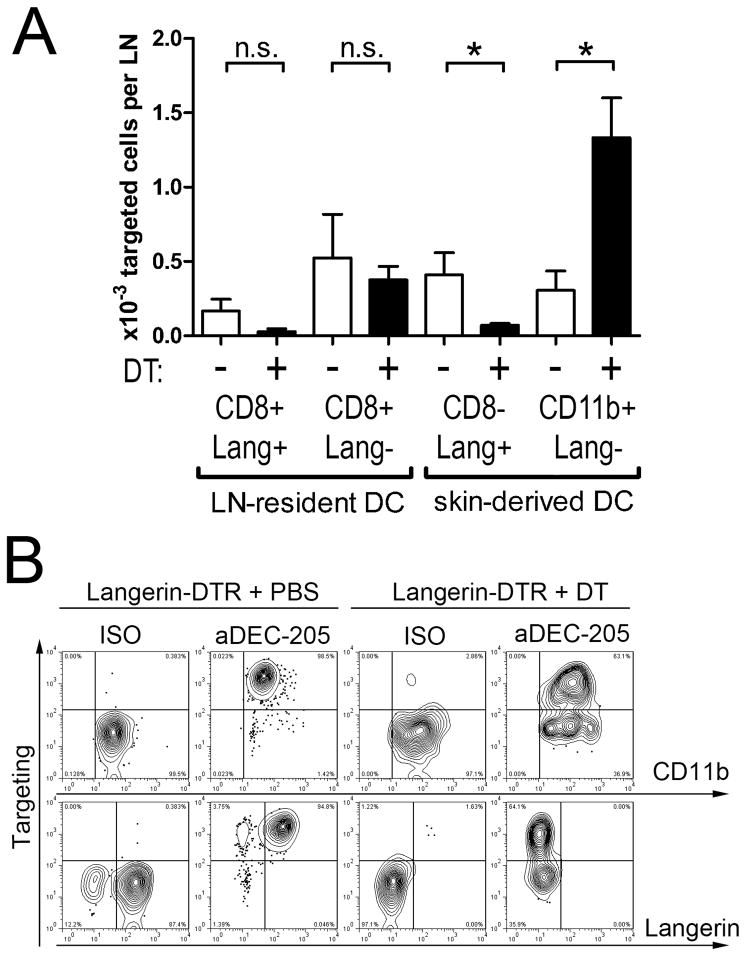
Figure 4. Langerin-positive DCs represent the major carrier of targeting antibodies to skin-draining lymph nodes.
1μg IgG2a control or anti-DEC-205 mAb were injected intradermally into the ears of C57BL/6 mice. The injection site was immediately treated with imiquimod cream, or left untreated. At the indicated time points, targeting antibody was detected on permeabilised lymph nodes cells by anti-rat IgG / APC. CD11clow Langerin+ DCs (A) or CD11b+ Langerinneg DCs (B) were gated, and the proportion of targeted cells among each population was measured (upper graphs). The absolute numbers of targeted cells from these subsets were determined 4h, 2 days, or 4 days after injection of targeting antibodies, under steady state (n.t., untreated injection site) or inflamed conditions (imiq., imiquimod-treated site) (lower graphs). Results are representative of three to seven independent experiments.
Two days after anti-DEC-205 injection, nearly all targeted cells displayed CD11b (Figure 3A), CD11c, high levels of MHC class II, but only low levels of CD8α (Figure 3B). Interestingly, targeted cells were split into two populations, with more than 70% expressing Langerin (Figure 3B). Labelling by targeting antibody was slightly stronger in Langerin+ DCs than in Langerinneg DCs, as shown by mean fluorescence intensity (Figure 3C). Thus, Langerin+ DCs appear as the predominant, though not exclusive, population responsible for transport of anti-DEC-205 antibodies from the skin to the draining lymph nodes.
These results correlate with a more detailed analysis of DEC-205-targeting in individual DC subsets over time (Figure 2). Targeting antibodies were mostly detected in CCR7+ CD11b+ CD8αneg Langerinneg/+ skin-derived DCs, but also CCR7neg CD11bneg CD8α+ lymph node-resident DCs (Figure 2A). Consistent with our previous observations, anti-DEC-205 mAb were retrieved in much higher amounts in Langerin+ DCs at any given time point.
Active transport of targeting antibodies by skin DCs prevails over passive transport via lymphatic flow
We next quantified the influx of skin-derived Langerinneg and Langerin+ DCs from untreated vs. imiquimod-treated skin at different time points. Based on our own results (Figure 2 and 3) and previous publications (15,25,33), we separated skin-derived, DEC-205-targeted cells between CD11clow Langerin+ LCs / dermal DCs (Figure 4A), and CD11b+ Langerinneg dermal DCs (Figure 4B).
Intradermally injected targeting antibodies may “passively” reach the draining lymph nodes through lymphatic vessels, without being carried there by emigrating skin DCs. Migrating skin DCs need at least 6 hours to reach draining lymph nodes upon inflammation (10,25,38). In addition, 2 hours are sufficient to visualize draining of intradermally injected Patent Blue dye towards lymph nodes, and the cutaneous bleb resulting from intradermal injection is resolved in less than 4 hours (data not shown). Accordingly, substantial numbers of targeted cells of both Langerin+ and Langerinneg skin DCs could be detected in the lymph nodes 4 hours after injection of anti-DEC-205 mAb (Figure 4 and Figure 2A). Of note, at this early time point, anti-DEC-205 antibodies could also be found on CCR7neg CD8α+ lymph node-resident DCs (Figure 2A). Thus, passive flow through the lymph makes targeting antibody available for lymph node DCs shortly after intradermal injection.
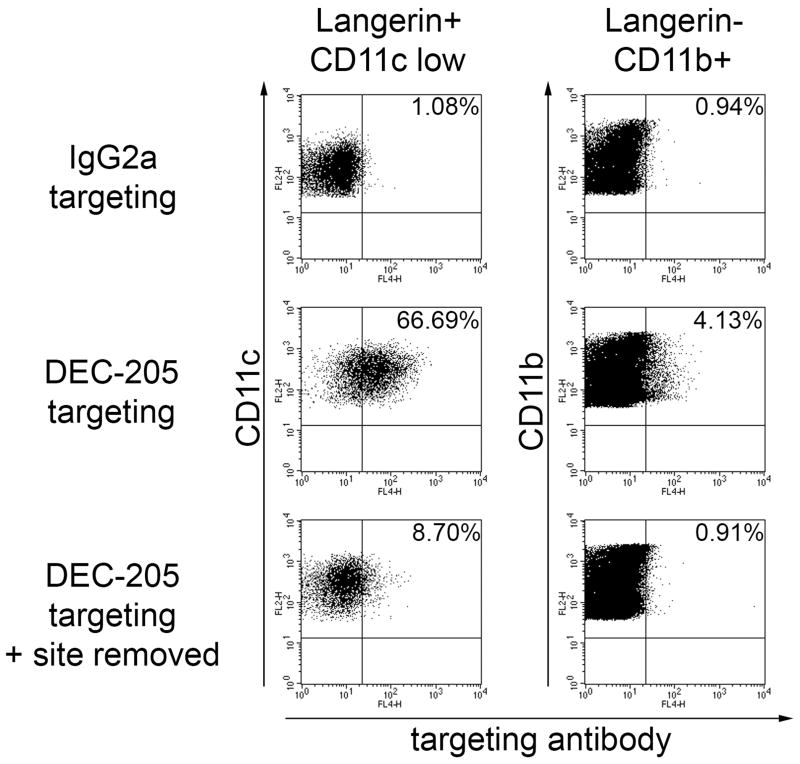
The question remained whether DCs bearing targeting antibodies 2 days after injection were loaded within the lymph node at early time points by passive flow of mAb, or rather represented skin DCs which were targeted in the periphery before emigrating to the lymph nodes. To address this, we removed the injection site (i.e., the ear) after 4 hours (39). Two days later, in draining lymph nodes, both Langerin+ and CD11b+ DC subsets were devoid of targeting antibodies (Figure 5). Thus, the presence of targeting antibody in lymph nodes at later time points does not result from the survival of DCs targeted in the first hours after injection, but requires migration of peripheral DCs, which have taken up targeting antibody in the skin.
Figure 5. Removal of the injection site leads to a shortage of skin-derived DCs carrying targeting antibodies.
1μg IgG2a control or anti-DEC-205 mAb were injected intradermally into the ears of C57BL/6 mice. Where indicated, ears injected with anti-DEC-205 mAb were cut off 4 hours after injection. Two days later, cells of the corresponding draining lymph nodes were collected, permeabilised, and the presence of targeting antibodies was detected by anti-rat IgG / APC on Langerin+ CD11clow and Langerinneg CD11b+ populations. Results are representative of two independent experiments.
Migration of targeted DCs towards lymph nodes is enhanced by epicutaneous application of imiquimod
In order to avoid induction of antigen-specific tolerance, targeting antibodies are generally applied together with adjuvants. These reagents trigger migration towards lymph nodes of peripheral DCs in their mature state, which is required to induce immunity rather than tolerance (40). We observed the consequences of local skin inflammation triggered by imiquimod, based on its known effect on the migration of DCs (33).
In the steady state, numbers of targeted Langerin+ DCs were equivalent between 4h and day 2 after injection, while numbers of targeted dermal Langerinneg DCs decreased (Figure 4A and 4B). After four days, very few targeted cells could be retrieved from draining lymph nodes, and the majority of them belonged to the Langerin+ subset (Figure 4A and 4B). The percentage of targeted cells among Langerin+ DCs remained high from 4h until day 2 post-injection (Figure 4A). On the other hand, fewer cells from the Langerinneg subset displayed DEC-205, and this proportion quickly dropped between 4h and 2 days (Figure 4B). The proportion of lymph node resident CD8α+ DCs displaying targeting mAb was stable over time (Figure 2A and 6A). However, the absolute number of targeted cells from this subset remained low as compared to CD8αneg DCs (Figure 6B).
Figure 6. Resident CD8α+ DCs in skin-draining LN are targeted via passive transport of intradermally injected aDEC/OVA.
1μg IgG2a control or anti-DEC-205 mAb were injected intradermally into the ears of BALB/c mice. The injection site was immediately treated with imiquimod cream, or left untreated. Targeting antibody was detected on permeabilised lymph nodes cells by anti-rat IgG / APC, 4h, 2 days or 4 days after injection of targeting antibodies, under steady state (n.t., untreated injection site) or inflamed conditions (imiq., imiquimod-treated site). CD11c+ CD8α+ DCs or CD11c+ CD8αneg DCs were gated, and the proportions (A) and absolute numbers (B) of targeted cells among each population was determined. Each data point represents measurements obtained from pooled auricular lymph nodes of 1-3 mice. Results displayed are pooled from six independent experiments.
Two days after imiquimod was applied to the site of anti-DEC-205 injection, overall numbers of DCs, regardless of targeting, were moderately increased in the corresponding skin-draining lymph nodes (Figure 7A). However, absolute numbers of skin-derived targeted cells were significantly increased (Figure 4A and 4B), although this increase was very limited for Langerinneg dermal DCs. This is most likely the consequence of an enhanced migration of targeted DCs from inflamed dermis and epidermis, as suggested by shifting proportions of the different DC subsets in the lymph nodes (Figure 2C). Under these conditions, Langerin+ DCs clearly outnumbered Langerinneg dermal DCs as carriers of targeting antibodies. At day 4, migratory Langerinneg DCs displaying targeting antibody did not significantly differ between imiquimod-treated and untreated skin, suggesting that imiquimod-dependent migration of this DC subset to lymph nodes was resolved by this time (Figure 4B). Altogether, extensive six-parameter FACS analyses confirmed these observations (Figure 2A).
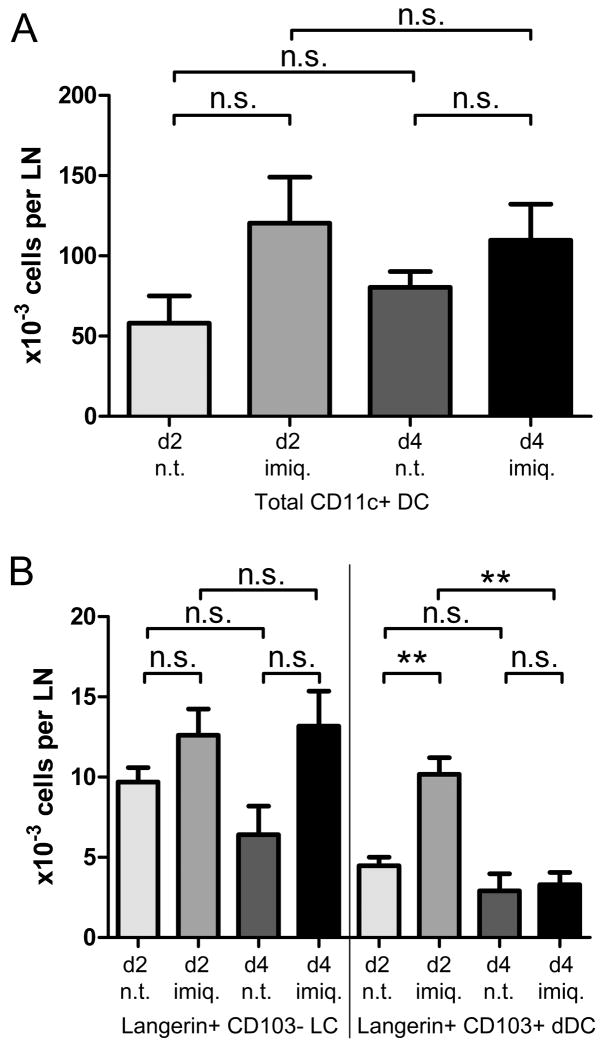
Figure 7. Variations in the total numbers of DC populations in skin-draining lymph nodes.
1μg IgG2a control or anti-DEC-205 mAb were injected intradermally into the ears of C57BL/6 mice. The injection site was immediately treated with imiquimod cream, or left untreated. (A) Total numbers, regardless of targeting, of CD11c+ DCs. (B) Numbers of Langerin+ CD103neg LCs and Langerin+ CD103+ dDCs, also irrespective of targeting. Results were pooled from at least four independent experiments, with 3-4 mice per condition.
CD103+ Langerin+ dermal DCs participate in the transport of targeting antibodies
The prevailing ability of Langerin+ DCs to transport targeting antibodies led us to first investigate the relative contributions of epidermal LCs and dermal Langerin+ DCs. Epidermal LCs are devoid of the integrin αE/CD103, whereas most Langerin+ dermal DCs are CD103+ (2). We have previously shown in skin explant models that dermal Langerin+ DCs are potent carriers of anti-DEC-205 targeting antibodies (16). Targeted Langerin+ DCs belonged to both CD103neg and CD103+ subsets (Figure 8A). CD103neg LCs were the dominant population, and displayed higher amounts of the targeting antibody.
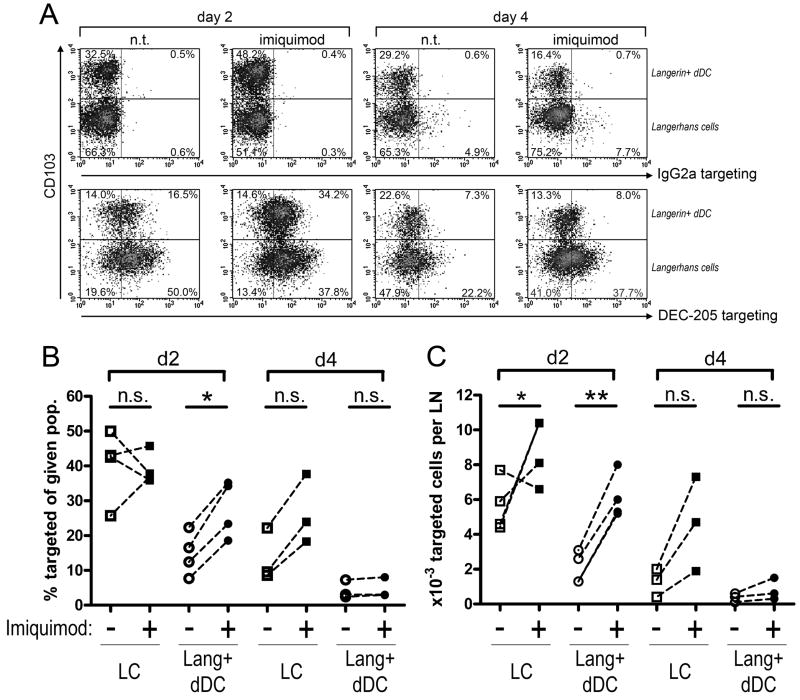
Figure 8. Dermal Langerin+ CD103+ DCs contribute to the transport of targeting antibodies.
1μg IgG2a control or anti-DEC-205 mAb were injected intradermally into the ears of C57BL/6 mice. The injection site was immediately treated with imiquimod cream, or left untreated. At the indicated time points, targeting antibody was detected on permeabilised lymph nodes cells by anti-rat IgG / APC. (A) Representative density plots show targeting efficiency and CD103 expression on CD11clow Langerin+ cells. Note that all cells of both populations are targeted by anti-DEC-205 antibody. Percentages indicate the respective proportions of CD103neg and CD103+ populations. FACS stainings were used to determine, in the CD103neg and CD103+ populations, (B) the proportion of anti-DEC-205-targeted cells displaying the highest levels of targeting antibody, and (C) the absolute numbers of targeted cells per lymph node. Results are representative of at least three independent experiments.
Strikingly, the proportion of CD103+ Langerin+ dermal DCs increased sharply two days after treatment with imiquimod (~30% CD103+ in untreated mice vs. ~50% in imiquimod-treated; Figure 8A). In line with this, a significant increase of total Langerin+ dermal DCs was observed. (Figure 7B). This suggests that migration of the Langerin+ dermal DCs occurs faster than that of epidermal LCs. As a consequence, the imiquimod-dependent increase in targeted Langerin+ DCs observed at day 2 mainly relied on the dermal-derived subset (Figure 8B). Conversely, at day 4, imiquimod treatment induced a reproducible, though non-significant, increase of total (Figure 7B) as well as targeted LCs (Figure 8C).
Antibody uptake and transport is handled by Langerinneg CD11b+ dermal DCs in the absence of Langerin+ skin DCs
Treatment of Langerin-DTR-EGFP mice with diphtheria toxin (DT) results in a fast and highly specific depletion of all Langerin-expressing DCs (25). It has been repeatedly demonstrated that, as soon as two days after DT treatment, no Langerin+ DC persists in the skin, leaving only Langerinneg dermal DCs. We determined how DT-induced depletion affects targeting of DCs within lymph nodes draining the injection site. 48 hours after DT treatment, anti-DEC-205 mAb were injected i.d. and the relevant lymph nodes analysed 4 hours later. Langerin+ skin-derived DCs were depleted as expected, excluding their participation in early antibody uptake (data not shown). As a consequence, numbers of targeted Langerin+ DCs in the nodes dropped markedly (Figure 9A). Conversely, we observed an intriguing increase of targeted Langerinneg dermal DCs.
Figure 9. Depletion of Langerin+ DCs enhances targeting by anti-DEC-205 of Langerinneg CD11b+ dermal DCs.
1μg IgG2a control or anti-DEC-205 mAb were injected intradermally into the ears of Langerin-DTR-EGFP mice, which were injected i.p. with PBS or 0.5μg DT two days before. Four hours later, the mice were sacrificed; ears and auricular lymph nodes were collected. (A) Targeting antibody was detected after permeabilisation by anti-rat IgG / APC, and the cells were counterstained with Langerin, CD8α, CD11c and CD11b. The number of targeted cells was calculated for CD8α+ Langerin+, CD8α+ Langerinneg, CD8αneg Langerin+ and CD11b+ Langerinneg DCs. (B) Whole ear skin explants were cultured for 4 days on complete medium. Emigrating cells were collected in the supernatant. Targeting antibody was detected after permeabilisation by anti-rat IgG / APC, and the cells were counterstained with CD11b, Langerin and CD11c. Plots depicts cells gated for CD11c expression. Results are representative for three independent experiments.
In Langerin-DTR-EGFP mice, part of the CD8α+ DCs in lymph nodes express Langerin and might therefore be eliminated similarly to LCs and dermal Langerin+ DCs (25,41). However, less than 200 Langerin+ CD8α+ DCs per lymph node displayed targeting mAb in untreated mice. This small population disappeared upon DT treatment, while targeted Langerinneg CD8α+ DCs were observed in similar numbers in PBS- and DT-treated mice (Figure 9A).
Finally, to ascertain which skin DC subset was responsible for antigen transport, ears of Langerin-DTR-EGFP mice, treated with PBS or DT 48 hours before, were injected with uncoupled anti-DEC-205 mAb or isotype control. Four hours later, ears were removed, whole skin explants were cultured for 4 days, and emigrating DCs were harvested from the culture medium. DEC-205-targeted skin DCs emigrating from these explants were mostly Langerin+ CD11b+ in PBS-treated mice (Figure 9B). On the other hand, no Langerin+ DCs came out of skin explants from DT-treated mice. Still, a substantial part of the remaining Langerinneg CD11b+ DCs had taken up anti-DEC-205 mAb.
Immune responses triggered by targeting antigen to DEC-205 are enhanced by topical application of TLR agonists
To address the functional consequences of targeting antibody transport on immune responses, we used anti-DEC-205 coupled to the model antigen OVA (aDEC/OVA). An often used method evaluates how targeting antibodies influence the proliferation of transferred OVA-specific CD4+ and CD8+ T cells obtained from TCR-transgenic mice (17). However, such methods are exceedingly sensitive, as demonstrated by the minute amounts of antigen sufficient to induce transgenic T cells to proliferate (42). For this reason, we restricted our analysis to unmanipulated C57BL/6 mice, and quantified endogenous, primary immune responses in vivo by monitoring the killing of target cells previously loaded with the OVA-derived MHC class I peptide SIINFEKL (30). Immunization with 0.5μg of aDEC/OVA into each ear (representing ~400ng of OVA per mouse) resulted in poorly efficient cytotoxic responses in the absence of adjuvant (Figure 10A). On the other hand, treating the injection site with the TLR7 agonist imiquimod or the TLR3 agonist poly(I:C) (Figure 10A) notably increased the percentage of killing of target cells. As an additional control, we examined the responses obtained after targeting with an isotype-matched, OVA-coupled nonspecific antibody (ISO/OVA), in the presence of imiquimod as an adjuvant. The killing rates yielded by this reagent were always very low as compared to the cytotoxic responses resulting from aDEC/OVA and imiquimod (Figure 10B).
Figure 10. Ovalbumin-coupled anti-DEC-205 requires TLR agonists to trigger efficient endogenous killing responses.
(A) C57BL/6 mice were left untreated (control), or injected with 0.5μg aDEC/OVA per ear alone (untreated, n.t.) or together with topical imiquimod treatment or 25μg poly(I:C). One week later, fluorescently labelled target cells loaded with low (CTOneg CFSElow; “SIINFEKL low”) or high (CTOneg CFSEhigh; “SIINFEKL high”) doses of MHC class I peptide SIINFEKL derived from OVA were injected intravenously. By comparing their numbers to that of unloaded targets (CTO+ CFSEneg; “unpulsed”), percentages of specific lysis were calculated two days later in the blood (data not shown) and in skin-draining lymph nodes, with similar results. Numbers quantify events present in a given gate. (B) C57BL/6 mice were left untreated (control) or immunized with 0.5μg OVA-coupled anti-DEC-205 (aDEC/OVA; black bars) or isotype-matched, nonspecific mAb (ISO/OVA; white bars) per ear alone (n.t.), or together with topical imiquimod treatment. One week later, fluorescently labelled target cells loaded with the MHC class I peptide SIINFEKL derived from OVA were injected intravenously. The efficiency of cytotoxic responses was measured, as in (A), in the blood and lymph nodes, one or two days after target cells transfer. Bar graphs show lysis in imiquimod treated mice only. Results are pooled data from at least two experiments, with 3 to 4 mice per group.
Immune responses triggered by targeting antigen to DEC-205 depend on skin DCs, but not Langerin+ DC subsets
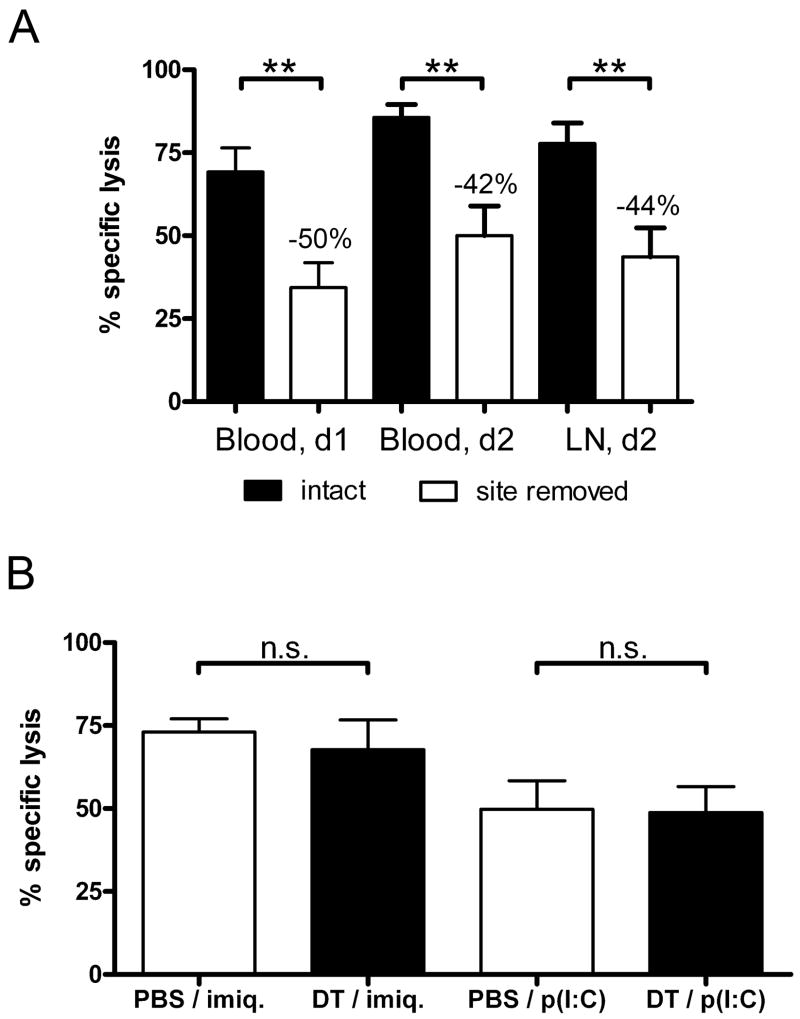
Next, we aimed at correlating our data regarding transport of targeting mAb with cytotoxic responses in vivo. To eliminate the contribution of all skin-derived DCs in this transport, the injection site was removed 4 hours after immunization by cutting off the ears, as previously described (39,43). We observed a clear decrease of the killing rates following this procedure (Figure 11A). This inhibition, though incomplete, suggested that epidermal or dermal DCs targeted in situ before migrating to skin-draining lymph nodes handle a significant part of the antigen cross-presentation to CD8+ T cells.
Figure 11. Skin DCs, but not Langerin+ DCs, critically contribute to endogenous killing responses induced by ovalbumin-coupled anti-DEC-205.
(A) C57BL/6 mice were immunized with 0.5μg OVA-coupled anti-DEC-205 (aDEC/OVA) per ear and treated with topically applied imiquimod cream. Where indicated (white bars), ears were cut off 4 hours after injection. One week later, target cells loaded with the MHC class I peptide SIINFEKL derived from OVA were injected intravenously. Percentages of specific lysis were measured one or two days later in skin-draining lymph nodes and in the blood, with similar results. (B) Langerin-DTR-EGFP mice were injected i.p. with diphtheria toxin (DT) or PBS. Two days later, the mice were immunized with 0.5μg aDEC/OVA per ear and treated with imiquimod (imiq.) or 25μg poly(I:C). The efficiency of cytotoxic responses was measured in the lymph nodes, as in (A), two days after target cells transfer. Results are pooled data from at least two experiments, with 3 to 4 mice per group.
The major contribution of Langerin+ DCs in active transport of anti-DEC-205 targeting antibody led us to examine how depletion of these cells would affect the development of T cell responses. Therefore, we used Langerin-DTR-EGFP mice to selectively deplete Langerin+ DCs by DT treatment 48h before injecting aDEC/OVA, together with imiquimod or poly(I:C). Surprisingly, no significant decrease of cytotoxic responses could be observed in DT-treated mice, regardless of the adjuvant provided (Figure 11B).
DISCUSSION
Targeting antigens to DCs with the help of specific antibodies has great potential for the development of effective vaccines (12,13,17). We carried our previous observations further and studied in detail what happens when antibodies selectively targeting DCs are applied to the skin in vivo. Although intradermally injected antibodies quickly and passively diffused via lymph vessels, we demonstrate that active transport by skin-derived DCs is critical to a prolonged presence of targeting antibodies in skin-draining lymph nodes and to optimal cytotoxic responses.
Transport of antigen to the draining lymph nodes from inflamed or steady-state skin
The outcome of immune responses initiated by targeting antigen to skin DCs as opposed to DCs within secondary lymphoid organs has not been directly compared so far. Antigen uptake by DCs with different origin or maturation status influences the development of adaptive immune responses (39,44). Interestingly, our data indicate that active transport of protein antigen provided by skin DCs, though potentiated by inflammation, also occurs in the steady state. Concomitantly, passive flow through the lymph brings cell-free targeting mAb to draining lymph nodes within a few hours, where they bind their target cells. However, removal of the injection site proves that, despite this early transfer of targeting antibodies to lymph node DCs, migration of skin DCs is essential to the persistence of antigen in the lymph nodes. Prolonged antigen presentation is a key regulating element in adaptive immune responses (45). In particular, constitutive migration of Langerin+ DCs appears responsible for the durable availability of targeting antibodies in skin-draining lymph nodes. However, other interpretations cannot be excluded, such as different degradation rates of endocytosed mAb by Langerin+ DCs, as compared to dermal Langerinneg DCs, or a superior survival of Langerin+ DCs.
Another aim of our study was to compare targeting efficiency in steady state and inflammation. Indeed, DC-activating stimuli, provided together with targeting reagents to avoid development of tolerance (40,46), could act by increasing the number of targeted DCs migrating from the skin, or by activating DCs targeted in draining lymph nodes. Because maturation and migration are intimately linked, the literature does not clearly favour one hypothesis or the other. Whereas targeting of DCs within lymph nodes is limited in numbers and in duration, the migration of targeted skin DCs, greatly enhanced by inflammatory stimulation, contributes for most of targeting mAb transport. We expect this to affect antigen presentation and T cell immunity, although the extent of this impact remains to be determined.
Proinflammatory stimuli are not necessary to initiate immune responses, such as the first cycles of naïve T cell proliferation (28,40,46). The DCs targeted within skin-draining lymph nodes in the first hours following immunization may be sufficient to generate these early events. However, inflammation is needed to avoid the deletion of expanded T cells (40) or the generation of regulatory T cells (47). This correlates with our observations that optimal endogenous cytotoxic responses require emigration of DCs targeted in the skin.
A role for CD8α+ lymph node DCs and Langerinneg CD103neg dermal DCs in immune responses through intradermal DEC-205 targeting
Langerin+ skin DCs are essential for the generation of cytotoxic anti-tumor immunity in the mouse (48). There is also evidence for an important role of human LCs in cytotoxic T cell responses (49,50). Surprisingly, cytotoxic responses following targeting of antigen to DEC-205 persisted when only Langerin+ cells were missing, but were severely impaired when all cutaneous DC subsets were removed by excision of the immunization site, i.e. the ear. In the latter situation, the residual response may be attributed to the diffusion of targeting mAb into an area wider than the removed ear, or to lymph node-residing CD8α+ DCs, which also express DEC-205 and are targeted via the lymph in the first hours following intradermal injection. Importantly, cross-presentation to CD8+ T cells has been repeatedly attributed to CD8α+ DCs (51). Although, in the Langerin-DTR-EGFP strain, some of the CD8α+ DCs express Langerin (25,41), DT treatment does not seem to significantly affect targeting of CD8α+ DCs. Therefore, we cannot formally exclude a contribution of this subset.
Our data also suggests a role for Langerinneg dermal DCs. We have shown that anti-DEC-205 targets Langerinneg CD103neg CD11b+ skin DCs (16,52). This subset was previously described as being unable to cross-present endogenous (52) or viral antigens (53), as opposed to the Langerin+ CD103+ subset, and it was even suggested to induce regulatory T cells (54). However, the outcome might be different when antigens are directly targeted to an endocytic receptor, as described in our report. Indeed, targeting DEC-205 in murine ear skin results in uptake by Langerinneg dermal DCs (16), which then reach lymph nodes. Removal of the injection site, which strongly impairs CD8+ T cell responses, leads to shortage of DEC-205-targeted dermal DC, but should not affect the early targeting of CD8α+ DCs residing in draining lymph nodes. An alternative explanation for the decreased responses upon ear removal might be that optimal immune responses require late skin-derived inflammatory molecules drained from the inflamed injection site.
Although uptake of anti-DEC-205 mAb was evident in CD11b+ Langerinneg dermal DCs from whole skin explants culture (16), we detected only few targeted Langerinneg dermal DCs in lymph nodes. This might be due to efficient degradation of the targeting antibodies. Moreover, Langerinneg dermal DCs remain available in the dermis after DT treatment, and their numbers in skin-draining lymph nodes seem to increase quickly when LCs and Langerin+ dermal DCs are absent. Therefore, Langerinneg dermal DCs could take a significant part of the immunogenic stimulation in our experimental settings. Altogether, our results call for a more detailed comparison of the function of Langerinneg dermal DCs with CD8α+ DCs in cross-presentation and CD8+ T cell immunity.
Acknowledgments
The authors would like to dedicate this publication to the memory of Dr. Ralph M. Steinman, for his continued support, encouragement and helpful discussions. We thank Martin Thurnher and his team at the Cell Therapy Unit lab of Oncotyrol for allowing ample use of the FACScanto flow cytometer.
Abbreviations
- DC(s)
dendritic cell(s)
- LC(s)
Langerhans cell(s)
- DT
diphtheria toxin
- EGFP
enhanced GFP
- DTR
DT receptor
Footnotes
This work was funded by the Austrian Science Fund (FWF project–P21487 to P.S.) and by the COMET Center ONCOTYROL (project #1.3, Cell Therapy Unit), which is funded by the Austrian Federal Ministries BMVIT/BMWFJ (via FFG) and the Tiroler Zukunftsstiftung/Standortagentur Tirol (SAT). Additional support came from the Austrian National Bank (Jubiläumsfond grant No. 13479 to N.R.). J.I. is supported by an NIH grant AI13013 to Ralph M. Steinman.
References
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Henri S, Guilliams M, Poulin LF, Tamoutounour S, Ardouin L, Dalod M, Malissen B. Disentangling the complexity of the skin dendritic cell network. Immunol Cell Biol. 2010;88:366–375. doi: 10.1038/icb.2010.34. [DOI] [PubMed] [Google Scholar]
- 3.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan RS, Smith CM, Belz GT, Van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 7.Zhao XY, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pior J, Vogl T, Sorg C, Macher E. Free hapten molecules are dispersed by way of the bloodstream during contact sensitization to fluorescein isothiocyanate. J Invest Dermatol. 1999;113:888–893. doi: 10.1046/j.1523-1747.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 10.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory Dendritic Cells Transfer Antigen to a Lymph Node-Resident Dendritic Cell Population for Efficient CTL Priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Sousa CR. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 12.Romani N, Thurnher M, Idoyaga J, Steinman RM, Flacher V. Targeting of antigens to skin dendritic cells: possibilities to enhance vaccine efficacy. Immunol Cell Biol. 2010;88:424–430. doi: 10.1038/icb.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 14.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, Burnham K, Saeland S, Handman E, Shortman K. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 15.Idoyaga J, Suda N, Suda K, Park CG, Steinman RM. Antibody to Langerin/CD207 localizes large numbers of CD8alpha+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci U S A. 2009;106:1524–1529. doi: 10.1073/pnas.0812247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flacher V, Tripp CH, Stoitzner P, Haid B, Ebner S, Del Frari B, Koch F, Park CG, Steinman RM, Idoyaga J, Romani N. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J Invest Dermatol. 2010;130:755–762. doi: 10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii SI, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, Huang Y, Schlesinger SJ, Park CG, Nussenzweig MC, Granelli-Piperno A, Steinman RM. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boscardin SB, Hafalla JCR, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, Morrot A, Zavala F, Steinman RM, Nussenzweig RS, Nussenzweig MC. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahnke K, Qian YJ, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–4869. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 21.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J Immunol. 2006;177:2276–2284. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- 22.Kissenpfennig A, Malissen B. Langerhans cells - revisiting the paradigm using genetically engineered mice. Trends Immunol. 2006;27:132–139. doi: 10.1016/j.it.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 23.He Y, Zhang J, Donahue C, Falo LD., Jr Skin-Derived Dendritic Cells Induce Potent CD8(+) T Cell Immunity in Recombinant Lentivector-Mediated Genetic Immunization. Immunity. 2006;24:643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 25.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhé C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: Dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Kraal G, Breel M, Janse M, Bruin G. Langerhans’ cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, Park CG, Steinman RM. Cutting Edge: Langerin/CD207 Receptor on Dendritic Cells Mediates Efficient Antigen Presentation on MHC I and II Products In Vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 28.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 29.Stoitzner P, Holzmann S, McLellan AD, Ivarsson L, Stössel H, Kapp M, Kämmerer U, Douillard P, Kämpgen E, Koch F, Saeland S, Romani N. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J Invest Dermatol. 2003;120:266–274. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- 30.Hermans IF, Silk JD, Yang J, Palmowski MJ, Gileadi U, McCarthy C, Salio M, Ronchese F, Cerundolo V. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J Immunol Methods. 2004;285:25–40. doi: 10.1016/j.jim.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Palamara F, Meindl S, Holcmann M, Luhrs P, Stingl G, Sibilia M. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173:3051–3061. doi: 10.4049/jimmunol.173.5.3051. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki H, Wang BH, Shivji GM, Toto P, Amerio P, Tomai MA, Miller RL, Sauder DN. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Invest Dermatol. 2000;114:135–141. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 33.Flacher V, Douillard P, Ait-Yahia S, Stoitzner P, Clair-Moninot V, Romani N, Saeland S. Expression of langerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology. 2008;123:339–347. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson NS, El Sukkari D, Belz GT, Smith CM, Steptoe RJ, Heath WR, Shortman K, Villadangos JA. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187–2194. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- 35.Ruedl C, Koebel P, Karjalainen K. In vivo-matured Langerhans cells continue to take up and process native proteins unlike in vitro-matured counterparts. J Immunol. 2001;166:7178–7182. doi: 10.4049/jimmunol.166.12.7178. [DOI] [PubMed] [Google Scholar]
- 36.Douillard P, Stoitzner P, Tripp CH, Clair-Moninot V, Ait-Yahia S, McLellan A, Eggert A, Romani N, Saeland S. Mouse lymphoid tissue contains distinct subsets of Langerin / CD207+ dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- 37.Cheong C, Idoyaga J, Do Y, Pack M, Park SH, Lee H, Kang YS, Choi JH, Kim JY, Bonito A, Inaba K, Yamazaki S, Steinman RM, Park CG. Production of monoclonal antibodies that recognize the extracellular domain of mouse Langerin/CD207. J Immunol Methods. 2007;324:48–62. doi: 10.1016/j.jim.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shklovskaya E, Roediger B, Fazekas de St GB. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo. J Immunol. 2008;181:418–430. doi: 10.4049/jimmunol.181.1.418. [DOI] [PubMed] [Google Scholar]
- 39.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 40.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farrand KJ, Dickgreber N, Stoitzner P, Ronchese F, Petersen TR, Hermans IF. Langerin+ CD8alpha+ dendritic cells are critical for cross-priming and IL-12 production in response to systemic antigens. J Immunol. 2009;183:7732–7742. doi: 10.4049/jimmunol.0902707. [DOI] [PubMed] [Google Scholar]
- 42.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meza-Sanchez D, Perez-Montesinos G, Sanchez-Garcia J, Moreno J, Bonifaz LC. Intradermal immunization in the ear with cholera toxin and its non-toxic beta subunit promotes efficient Th1 and Th17 differentiation dependent on migrating DCs. Eur J Immunol. 2011;41:2894–2904. doi: 10.1002/eji.201040997. [DOI] [PubMed] [Google Scholar]
- 44.Henri S, Siret C, Machy P, Kissenpfennig A, Malissen B, Leserman L. Mature DC from skin and skin-draining LN retain the ability to acquire and efficiently present targeted antigen. Eur J Immunol. 2007;37:1184–1193. doi: 10.1002/eji.200636793. [DOI] [PubMed] [Google Scholar]
- 45.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 46.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoitzner P, Green LK, Jung JY, Price KM, Tripp CH, Malissen B, Kissenpfennig A, Hermans IF, Ronchese F. Tumor immunotherapy by epicutaneous immunization requires langerhans cells. J Immunol. 2008;180:1991–1998. doi: 10.4049/jimmunol.180.3.1991. [DOI] [PubMed] [Google Scholar]
- 49.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palucka K, Ueno H, Zurawski G, Fay J, Banchereau J. Building on dendritic cell subsets to improve cancer vaccines. Curr Opin Immunol. 2010;22:258–263. doi: 10.1016/j.coi.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 52.Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, Viret C, Azukizawa H, Kissenpfennig A, Malissen B. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 54.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(-) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]