Abstract
Background & Aims
Matriptase is a membrane-anchored serine protease encoded by Suppression of Tumorigenicity-14 (ST14) that is required for epithelial barrier homeostasis. However its functional role in inflammatory bowel disease (IBD) is unexplored.
Methods
Matriptase expression in control, Crohn's disease and ulcerative colitis tissue specimens was studied by qPCR and immunostaining. Matriptase function was investigated by subjecting St14 hypomorphic and control littermates to dextran sodium sulfate (DSS)-induced colitis and by siRNA silencing in cultured monolayers. Mice were analyzed for clinical, histological, molecular and cellular effects.
Results
Matriptase protein and ST14 mRNA levels are significantly down-regulated in inflamed colonic tissues from Crohn's disease and ulcerative colitis patients. Matriptase deficient St14 hypomorphic mice administered DSS for 7 days followed by water without DSS for 3 days develop a severe colitis with only 30% of the St14 hypomorphic mice surviving to day 14, compared with 100% of control littermates. Persistent colitis in surviving St14 hypomorphic mice was associated with sustained cytokine production, an inability to recover barrier integrity, and enhanced claudin-2 expression. Cytokines implicated in barrier disruption during IBD suppress matriptase expression in T84 epithelial monolayers and restoration of matriptase improves barrier integrity in the cytokine-perturbed monolayers.
Conclusions
These data demonstrate a critical role for matriptase in restoring barrier function to injured intestinal mucosa during colitis, which is suppressed by excessive activation of the immune system. Strategies to enhance matriptase-mediated barrier recovery could be important for intervening in the cycle of inflammation associated with IBD.
Keywords: serine protease, TTSP, DSS, colitis, IBD, matriptase
Introduction
Inflammatory bowel diseases (IBD) are chronic disorders affecting the gastrointestinal tract in humans. The etiology of IBD is not completely understood, however the pathogenesis is associated with contributions from genetic predisposition, the mucosal immune system and exposure to environmental factors (1). Genome-wide association studies for IBD susceptibility loci have revealed the importance of epithelial barrier function, and innate and adaptive immunity in disease pathogenesis. (1). A key factor in the development of mucosal inflammation associated with IBD is disturbed intestinal epithelial barrier function and increased intestinal permeability. Decreased barrier function has been shown to positively correlate with mucosal inflammation in Crohn's disease (CD) and ulcerative colitis (UC) patients (2), and increased epithelial permeability precedes clinical relapse (3; 4). Additionally, first degree relatives of CD patients who are at risk of developing disease often have increased intestinal epithelial permeability relative to the general population (5). In animal models of IBD, increased epithelial paracellular permeability can precede chronic mucosal inflammation (6), and in addition, altered epithelial barrier function has been associated with the subsequent development of colitis (7).
The integrity of mucosal epithelial barrier function is preserved by the intestinal epithelium, which regulates the trafficking of macromolecules between the environment and the host and serves as a central coordinator of communication between the immune system and the external environment (8). The intestinal epithelium is comprised of apical and subapical junctional complexes, which seal the paracellular space and regulate mucosal barrier permeability. While substantial progress has been achieved in understanding structural changes associated with barrier disruption and reassembly at the molecular level, regulatory pathways that dynamically control intestinal barrier homeostasis remain less well understood. Recently the type II transmembrane serine protease, matriptase (also known as ST14, MT-SP1, TADG-15, epithin, and SNC19 (9)), was identified as a factor critical for maintenance of epithelial barrier homeostasis. Matriptase is localized to apical junctional complexes and on the basolateral surfaces of polarized intestinal epithelium and demonstrates potent epithelial barrier protective properties (10; 11). Initial analyses of mice with complete deficiency in the matriptase gene (St14) uncovered a critical function for matriptase in skin development and function (12). Matriptase knockout mice die shortly after birth, due to a severe dehydration caused by impaired epidermal barrier. Conditional knockout of matriptase from the murine gastrointestinal tract under the direction of a Villin-Cre promoter results in persistent diarrhea, inflammation, edema, gross disruption of colonic architecture, and general loss of mucosal barrier function that leads to rapid tissue degeneration and death within a few weeks after weaning, although at birth the microarchitecture of the gastrointestinal tract appears structurally and morphologically intact (10). Constitutive expression of matriptase is required throughout adulthood to maintain intestinal barrier integrity, since inducible ablation of matriptase from the GI tract of adult mice also results in loss of mucosal barrier function and rapid tissue degeneration (10).
The importance of intestinal epithelial barrier function to the etiology of IBD and the functional link between matriptase and paracellular permeability in the gastrointestinal tract prompted us to investigate the role of matriptase in IBD. Here we report that matriptase expression is dramatically suppressed in colonic mucosa during human and experimental IBD. St14 hypomorphic mice, which express minimal levels of matriptase and are born free of intestinal disease (11; 13), are more susceptible to DSS-induced colitis, with the normally transient DSS-induced intestinal injury converted to a severe, persistent colitis, with reduced mouse survival. These data demonstrate a critical role for matriptase in the restoration of barrier function following injury of the GI tract, and suggest that matriptase dysregulation may contribute to the pathogenesis of IBD.
Materials and Methods
Human tissue specimens
TissueScan Real-Time Crohn's and Colitis Disease Panels (CCRT101) and tissue sections were purchased from Origene Technologies. cDNAs were analyzed by qPCR as below. Paraffin-embedded human tissues were immunostained using the matriptase specific monoclonal antibody S5 (14).
Animal studies
St14 hypomorphic mice have been reported previously (13). These mice possess one null allele and one allele in which a reporter gene trap is inserted into the matriptase locus and disrupts gene expression. A low level of alternative splicing in the gene trap allele results in low level synthesis of full length matriptase, which is sufficient to enable mouse survival. All experiments performed with St14 hypomorphic mice (13) were littermate controlled from mice generated from heterozygous crosses. C57BL/6J, uPA-deficient (Plau-/-) and PAR2 (F2rl1-/-) deficient mice were purchased from Jackson Laboratories. Adult mice (8-12 week old) were administered 2% (w/v) DSS (molecular weight of 36,000 – 50,000; MP Biomedicals, Lot No. 3865K) continuously for 7 days in drinking water, followed by normal drinking water for up to 8 days as described (15). Clinical disease was scored daily on a scale of 1-5 as the sum of weight loss, stool consistency and fecal occult blood (Guaiac test, Sure-Vue, Fisher HealthCare). Animal care and experimental procedures were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
Murine tissue analysis
Murine intestines were removed en bloc and colon lengths (anus to cecum) documented before (Day 0), during (Day 5), and after induction of colitis (7 days DSS followed by 3 days water: Day 3 Recovery). Intestines were cut into 1 cm segments and identical segments compared for molecular analyses. For histological examination of inflammation and damage, tissue segments were fixed in 4% paraformaldehyde, paraffin embedded, cut into 5-μm sections, and stained with hematoxylin and eosin (H&E). Microscopic injury to the colon was assessed by investigators blinded to the treatment groups and quantified based on a combined score of inflammatory cell infiltrate (range 0–4), extent of inflammatory cell infiltrate (range 0–3), and crypt damage (range 0–4). Two slides from each section of the colon were assessed per mouse, and at least three areas on each slide were examined due to the patchy nature of DSS-induced injury.
TEER
The TEER of small intestine or mid-distal colon segments mounted in microsnapwells apical side up was measured in triplicate using an EVOM Voltohmmeter (World Precision Instruments) as described (16; 17). TEER of Caco-2 monolayers was measured using “chopstick: probes (11).
Intestinal permeability to FITC-dextran
FITC-dextran (4kDa; 500μg/g body weight) was instilled by oral gavage, and the concentration of FITC-dextran in blood collected was measured after 4 or 24 hours in St14 control and hypomorphic littermates. Blood (150 μl) was collected by retro-orbital eye bleed and plasma collected by centrifugation for 15 minutes at 4°C. Plasma was diluted with PBS and the concentration of fluorescein was determined by using an excitation wavelength of 485 nm and an emission wavelength of 535 nm using serially diluted samples of the marker as a standard (range 0-50 mg/ml).
Quantitative PCR
Quantitative PCR (qPCR) was performed using Taqman Reverse Transcription and PCR reagents (Applied Biosystems). RNA was isolated from either cultured cells on transwells or St14 hypomorphic intestine using RNeasy Kits (Qiagen). qPCR was performed using pre-designed primers (Applied Biosystems): human ST14 (Hs00222707_m1); mouse St14 (Mm00487858_m1), human SPINT1/HAI-1 (Hs00173678_m1), mouse Ifnγ (Mm00801778_m1), mouse Il6 (Mm00446191_m1), mouse TNF (Mm99999068_m1), mouse Il10 (Mm00439614_m1), mouse Nos2 (Mm00440485_m1), mouse Il13 (Mm00434204_m1) and signals normalized to mouse Gapdh (Mm99999915_g1) or human GAPDH (Hs99999905_m1) as indicated.
Cell culture and siRNA transfection
The human cell lines T84 and Caco-2 [passage 35-45] (ATCC) were grown on Transwell filters (Costar) and TEER measured as described (11). siRNA transfections were performed using Stealth™ siRNAs (Invitrogen) targeting matriptase (siM2, #St14-HSS110268) and the %GC matched negative control (siCtl) (11). The basal surfaces of confluent monolayers grown on transwells were exposed to the indicated concentrations of inflammatory cytokines, 5% DSS, or 10% macrophage conditioned media (prepared by differentiation of THP-1 cells with 5ng/ml phorbol 12-myristate 13-acetate (PMA) for up to 72hrs, then treating with 10ng/ml LPS for 24 hrs). Following cytokine treatments, cell monolayers were treated on the basolateral surfaces with or without 5nM recombinant human matriptase serine protease domain (18; 19) for 8hrs in serum free medium.
Protein Lysis and Immunoblotting
Total proteins were obtained from murine intestinal tissue segments by solubilization in RIPA buffer containing Roche complete protease inhibitor cocktail and phosphatase inhibitors (PhosphoStop, Roche). Cultured cells were lysed in 1% Triton X-100 containing protease inhibitor cocktail (Roche). Proteins were resolved by SDS-PAGE, immunoblotted and stripped and reprobed for GAPDH. Antibodies were rabbit anti-human matriptase (Calbiochem), sheep anti-mouse matriptase and goat anti-HAI-1 (R&D Systems), rabbit anti-claudin-4 and rabbit anti-human claudin-2 (Zymed), rabbit anti-mouse claudin-2 (American Research Products), rabbit anti-GAPDH (Cell Signaling Technologies).
Statistics
Representative results of at least three independent experiments are shown. Data is represented as mean ± SEM. Statistical analyses were performed using the Student's t-test or where indicated, the Mann Whitney U test. The Kaplan-Meier Survival Analysis was used to compare the mortality of St14 hypomorph and control mice. A threshold of p < 0.05 was considered significant.
Results
Matriptase is down-regulated in colonic epithelium of IBD patients
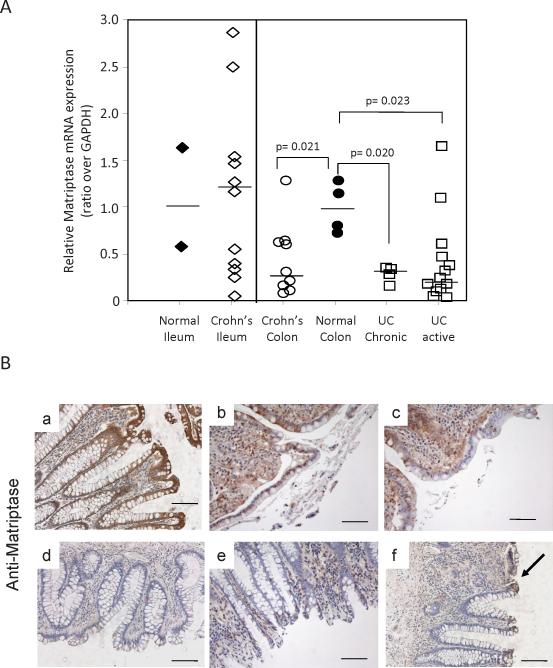
Matriptase expression was examined in ileal and colonic tissue biopsies from CD and UC patients and compared with non-inflamed tissues. Acute disease was associated with a ~4-fold decrease in ST14 mRNA levels in the colonic specimens, suggesting that colitis is associated with reduced matriptase levels (Figure 1A). The colitis tissue specimens also showed increased levels of claudin-2 and reduced epithelial cell adhesion molecule (EpCam) expression (Figure S1), previously associated with IBD (20-22). Immunostaining for matriptase protein expression in paraffin embedded colonic tissues from CD (Figure 1B(b&c), and UC (Figure 1B(d-f) patients also revealed a substantial reduction in matriptase protein expression in inflamed colonic epithelium, in contrast to the strong matriptase staining that is observed in normal colonic crypts (Figure 1B(a). Patchy matriptase staining was sometimes detected in UC tissue where it was associated with the highly differentiated colonocytes at the top of the crypts (Figure 1B(f), arrow), and in normal crypts contained within CD tissues (Figure 1B(c)).
Figure 1.
Matriptase is diminished in colonic epithelium of IBD patients. (A) qPCR analysis of human matriptase mRNA expression relative to GAPDH in 21 CD and 21 UC tissues collected from different individuals, compared with 6 normal tissues. Bars represent the average of mRNA expression normalized to GAPDH expression. (B) Immunostaining for human matriptase protein expression in normal human colon (a) and colonic tissues from CD (b,c) and UC patients (d-f). Arrow indicates area of patchy positive staining. Scale Bars, 100μM
Matriptase deficiency leads to persistent, severe inflammatory DSS-induced colitis
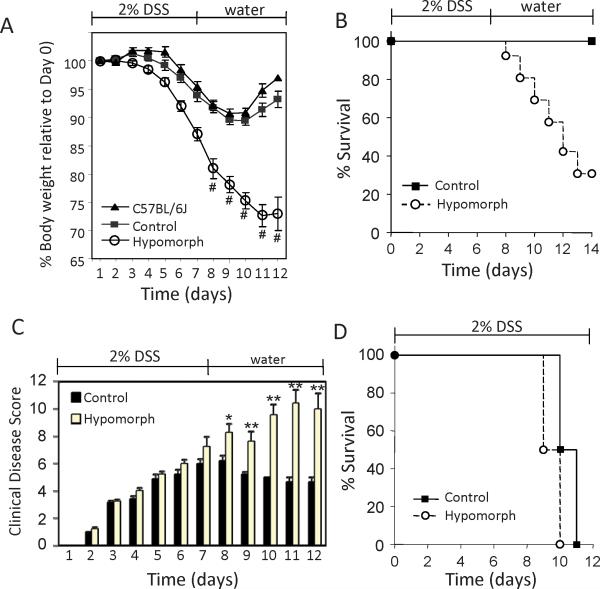
To investigate the functional importance of reduced matriptase in colitis, St14 hypomorphic mice, which express only minimal levels of intestinal matriptase (<1%) (11; 13), were subjected to colitis induced by dextran sodium sulfate (DSS). Oral administration of DSS is injurious to intestinal epithelium and causes an acute colitis which mimics human IBD in many respects (23; 24), but is self limiting, and eventually resolves after DSS removal. Age and sex-matched St14 hypomorphic and littermate control mice were administered 2% DSS in drinking water for 7 days and on day 8, were switched back to water only and the mice allowed to recover until up to day 14. The St14 hypomorphic mice lost weight much more rapidly than their control littermates or the C57BL/6J group through day 8, and continued to lose weight after switching to water (Figure 2A). By day 14, only 30% of the St14 hypomorph group had survived, whereas 100% survival was observed in the littermate control and C57BL/6J groups (Figure 2B).
Figure 2.
St14 hypomorphic mice are substantially more susceptible to DSS-induced colitis. Wild type C57BL/6J (n=4), St14 hypomorphs (Hypomorph; n=24) and heterozygote control littermates (Control; n=22) were administered DSS in drinking water for 7 days and on day 8, were returned to water only. (A) Body weight of St14 hypomorph and control groups during the course of treatment. # indicate average of data from surviving mice only. (B) Survival. All wild type C57BL/6J and Control mice survived, whereas only 8 St14 hypomorphic mice survived to day 14. p=0.0085, Kaplan Meier Survival analysis. (C) Scores of clinical disease. n=16 per group; *p <0.05, **p <0.005, Mann Whitney U Test. (D) Survival of St14 hypomorph and littermate control mice exposed to continuous DSS in drinking water. n=8 per group.
During administration of DSS, mice develop an acute colitis characterized by bloody diarrhea, ulcerations, and inflammatory infiltrates. Semi-quantitative score of clinical disease revealed that all mice exposed to DSS developed comparable signs of colitis through day 7 (Figure 2C). Following removal of the injurious DSS stimulus on day 8, a progressive reduction in clinical symptoms was observed in littermate control mice consistent with mucosal recovery. In contrast, symptoms of clinical disease were more severe and persisted in the surviving St14 hypomorphic mice, with an average clinical disease score on day 11 of 10.4 for the St14 hypomorphic mice compared with 4.7 for the littermate control group (Figure 2C). These data show that matriptase deficiency prolongs disease severity, thereby hastening mortality due to colitis.
DSS is toxic to intestinal epithelial cells of the basal crypts and directly affects the integrity of mucosal barrier, resulting in recurring pathogen challenge as long as the DSS is present (25). Mice subjected to 2% DSS continuously in the drinking water for up to 11 days showed similar survival rates (Figure 2D) and clinical disease scores (data not shown), indicating that the persistent inflammation associated with St14 hypomorphic mice was not caused by a more robust mucosal immune response to the DSS induced injury. Taken together, the data indicate that matriptase enhances recovery from DSS-induced colitis.
Severe ulceration and enhanced inflammation in DSS-treated St14 hypomorphic mice
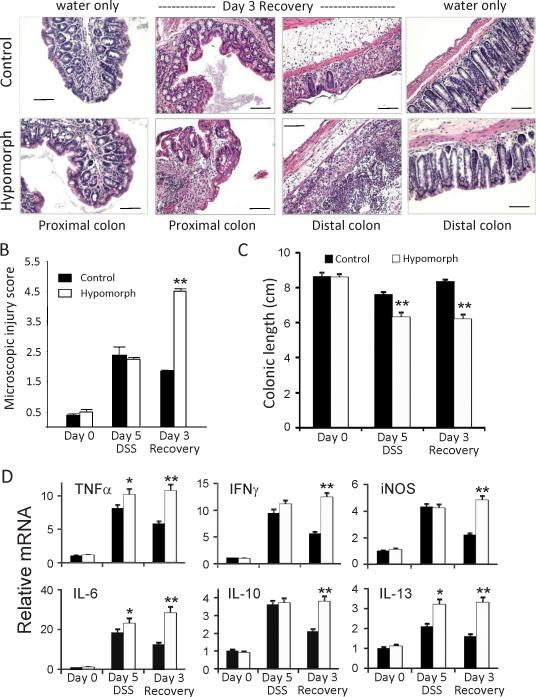
Microscopic injury to the intestinal mucosa was investigated by removing tissue segments from identical regions of the small intestine, and the mid-proximal and mid-distal colons of littermate control and surviving St14 hypomorphic mice at day 5 during DSS (acute phase) and at day 3 of the recovery phase (7 days DSS and 3 days water) for histological examination. H&E stained tissues were scored for microscopic injury based on the degree of acute inflammation, extent of inflammatory infiltration and crypt damage as described in the methods. No differences in the microscopic appearance of the gastrointestinal tracts of mice exposed to water alone were observed (Figure 3A); however, after 5 days treatment with DSS, there was substantial microscopic injury that was severe in the proximal and distal colons of both the St14 hypomorphic and littermate control genotypes, that was characterized by epithelial sloughing, and inflammatory infiltrates in the mucosa and submucosa (data not shown). By day 3 of the recovery phase however, microscopy injury in the proximal colons had substantially reduced in littermate control mice (Figure 3B), indicating resolution of inflammation after the discontinuation of DSS. In contrast, the mean microscopic injury score of the proximal colons of the surviving St14 hypomorphic group continued to increase after day 5 (2.2 vs 4.5) and was substantially worse than the littermate controls at Day 3 of the recovery phase (4.5 vs 1.8 (Figure 3B). Photomicrographs taken at Day 3 of the recovery phase showed substantial inflammatory infiltrates persistent in the proximal colons of St14 hypomorphic mice compared with their corresponding control littermates (Figure 3A).
Figure 3.
Intestinal tissue recovery is impaired in St14 hypomorphic mice. (A) Representative H&E stained sections of colonic tissue segments from St14 hypomorphic and littermate control mice after 7 days of DSS and 3 days water (Day 3 Recovery), showing the persistence of inflammatory infiltrates in the proximal and distal colons of St14 hypomorphic mice compared with the well-defined colonic crypts, and a normal submucosa layer of water alone-treated mice. Scale Bars, 100μM. (B) Comparison of microscopic injury in the proximal colons of St14 hypomorphic and littermate control mice on the indicated days, showing persistent injury in St14 hypomorphic mice. n=4-5; **p <0.005, Mann Whitney U Test. (C) Colonic lengths measured during the acute and recovery phases of DSS induced injury. n=7; **p <0.005, Mann Whitney U Test. (D) Comparison of relative mRNA levels of cytokines and other mediators in distal colons of St14 hypomorph (open bars) and littermate control mice (solid bars) during the DSS protocol, measured by qPCR analyses. n = 3-4 mice per group. *p <0.05, **p <0.005, unpaired Student t test.
Inflammation-induced colonic shortening was monitored as an indicator of crypt damage and severe inflammation. In littermate control mice, colon lengths decreased during DSS treatment, but returned to the lengths of untreated mice after 3 days recovery (Figure 3C). A significantly greater decrease in colon lengths was observed during DSS treatment of St14 hypomorphic mice compared with littermate controls (26% vs 12%), and in contrast to the littermate controls, no recovery was observed in hypomorphic mice after 3 days return to water (Figure 3C). These data demonstrate that matriptase deficiency prolongs mucosal inflammatory responses after intestinal injury.
Inflammatory responses of St14 hypomorphic mice remain elevated after removal of DSS
Chronic inflammation is associated with increased production of inflammatory cytokines in the lamina propria of IBD patients (26; 27). Investigation of cytokines and inflammatory mediators produced in response to DSS-induced injury showed that after 5 days exposure to DSS, both littermate control and St14 hypomorphic mice display dramatic increases in TNFα, IL-6, IL-10, IL-13, IFNγ, and iNOS, whereas the baseline levels of these mediators are low in both strains (Figure 3D). In addition, St14 hypomorphic mice display small, but significantly increased levels of TNFα, IL-6 and IL-13, relative to their littermate controls. After DSS withdrawal for 3 days, inflammatory cytokine levels remain elevated in surviving St14 hypomorphic mice, in contrast to littermate control mice, in which cytokine levels are diminishing (Figure 3D). These data suggest that the enhanced susceptibility to DSS-induced colitis in St14 hypomorphic mice is due, at least in part, to an impaired ability to resolve the mucosal inflammation. Thus, matriptase deficiency transforms the otherwise self-limited, DSS-induced colitis to a chronic, IBD-like colitis.
Persistent colitis in St14 hypomorphic mice is associated with an inability to recover colonic TEER
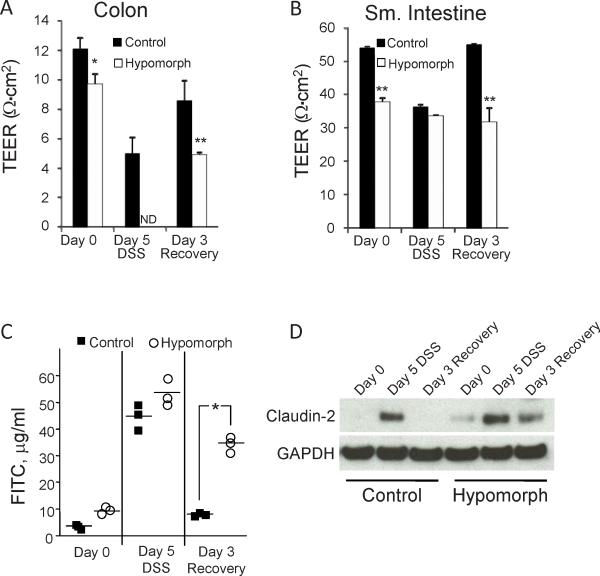
A ‘leaky’ mucosal barrier as a consequence of loss of barrier function provided by epithelial cells is thought to underlie persistent and destructive mucosal inflammation in IBD and other intestinal disorders (28; 29). St14 hypomorphic mice show enhanced intestinal barrier permeability as measured by a 20% reduction in baseline TEER of the distal colons of St14 hypomorphic mice (Figure 4A)(11) and ~2-fold higher levels of serum FITC-dextran after gavage (Figure S1). In control mice after 5 days DSS treatment, a 40% decrease in TEER in the distal colon is observed, which recovers after DSS withdrawal for 3 days to 70% of the baseline TEER, indicative of mucosal barrier recovery (Figure 4A). The instability of the St14 hypomorph colonic tissue segments recovered from mice exposed to DSS for 5 days did not allow for experimental determination of TEER; however, after withdrawal of DSS for 3 days, TEER measurements of surviving St14 hypomorphs remained 45% lower than littermate control mice (Figure 4A). The low TEER of the surviving St14 hypomorphs is similar to the TEER measured for littermate control mice during the acute phase (Day 5) of DSS treatment (4.9 vs 5.0), highlighting the persistent permeability associated with St14 hypomorphic mice. These data suggest that the inability to completely restore the colonic epithelial barrier in St14 hypomorphic mice likely leads to persistent inflammation and impaired mucosal recovery following DSS insult.
Figure 4.
Recovery of colonic TEER is hampered in St14 hypomorphs after DSS challenge. Ex vivo intestinal permeability was measured in (A) distal colon and (B) jejunal segments of St14 hypomorph and littermate control mice during DSS treatment on Day 5 and Day 3 Recovery. ND, TEER was not able to be determined. n = 5-7 mice per group. *p <0.05, **p <0.005, unpaired Student t test. (C) Enhanced permeability of St14 hypomorphs to FITC dextran during DSS treatment. Plasma FITC dextran concentrations in St14 hypomorph and littermate control mice were measured 4 hrs after FITC dextran administration by oral gavage. Permeability in St14 hypomorphs remains high after withdrawal of DSS. n=3 per group; *p <0.05, Wilcoxon Signed-Rank test. (D) Immunoblot analysis of the expression of the permeability marker protein, claudin-2, in jejunum tissue segments during the DSS protocol.
Increased small intestinal permeability during DSS induced colitis
While the distal colon is most affected by the epithelial damage induced by DSS, increased permeability in the small intestine occurs as a consequence of the injury and accompanying mucosal inflammatory responses (30). The jejunal TEER of littermate control mice decreased by ~30% after 5 days of DSS treatment, resulting in TEER similar to the baseline jejunal TEER measured in St14 hypomorphic mice (11)(Figure 4B). After withdrawal of DSS for 3 days, the jejunal TEER of littermate control mice recovered to normal levels, whereas the jejunal TEER of St14 hypomorphic mice remains low, reflecting the persistence of mucosal inflammatory responses in these mice. Measurement of in vivo macromolecular permeability by FITC-dextran flux revealed increased plasma levels of 4kDa FITC-dextran in both St14 control and hypomorphs during DSS administration (Figure 4C). However, 3 days after DSS withdrawal, permeability to FITC-dextran was significantly higher in surviving St14 hypomorphs than littermate control which had returned to near untreated levels (Figure 4C). The increased jejunal permeability during DSS treatment was not reflected in changes in the microscopic appearance of H&E stained jejunal tissues from St14 hypomorphic mice compared with control animals (Figure S3); however, it was associated with increased levels of the permeability-associated tight junction protein, claudin-2. Protein levels of claudin-2 have been reported to be elevated in jejunal tissues of St14 hypomorphic mice (11), and were substantially increased during the acute phase (Day 5) of DSS-induced injury in both St14 hypomorphic and littermate control mice (Figure 4D). Day 3 recovery was associated with absence of claudin-2 expression in control mice, whereas enhanced claudin-2 persisted in St14 hypomorph mice. These data suggest that the barrier dysfunction in St14 hypomorphic mice is similar to the level of dysfunction associated with acute colitis induced by DSS.
Matriptase expression is down-regulated in intestinal mucosa of control mice during DSS induced injury
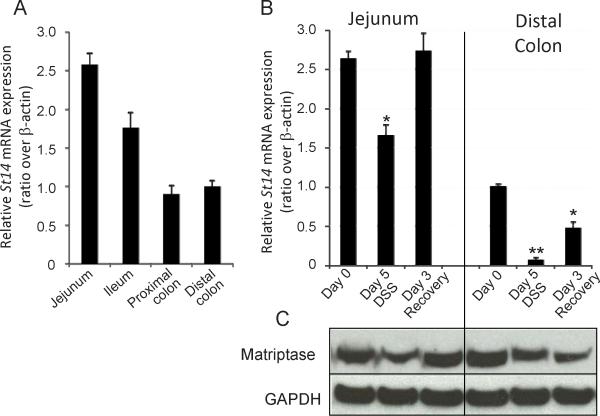
Matriptase is normally expressed throughout the murine gastrointestinal tract (Figure 5A). Upon exposure to DSS (Day 5 DSS), St14 mRNA expression was reduced by 50% in the jejunum, and in the colon by 90%. After withdrawal of DSS for 3 days, matriptase mRNA expression recovered to normal levels in the small intestine, and were increasing in the colon, reaching 50% of normal levels by day 3 (Figure 5B). Matriptase protein levels in intestinal tissue segments paralleled the changes in mRNA expression (Figure 5C). The down-regulation of matriptase during DSS exposure and the restoration of matriptase expression during the recovery phase (Figure 5B) mirror the changes in intestinal epithelial permeability measured by ex vivo TEER (Figure 4A&B).
Figure 5.
Matriptase expression changes in recovering intestinal epithelium. (A) qPCR analysis of St14 mRNA expression along the normal mouse GI tract. (B) qPCR analysis of St14 expression in segments of the jejunum and mid-distal colons of control mice during DSS-induced colitis. RNA signals are normalized to β-actin and expressed relative to the distal colon. n=3 mice per group, *p <0.05, **p <0.005. (C) Immunoblot of the jejunum and mid-distal colons of control mice (as in (B)) during DSS-induced colitis. Shown is the 70kDa full length matriptase.
Repair of T84 epithelial barrier disruption by DSS involves restoration of matriptase
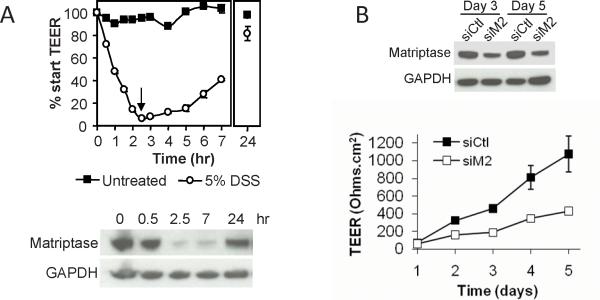
To model the effect of DSS on colonic epithelium, polarized human colonic T84 monolayers were treated with DSS, and then allowed to recover by switching to media without DSS. DSS exposure for 2.5 hrs disrupts the epithelial barrier as measured by TEER and is associated with decreased matriptase expression, mimicking DSS-induced barrier disruption in vivo. Upon removal of the DSS, TEER is recovered over 24 hrs and involves the restoration of matriptase levels (Figure 6A). Barrier recovery in T84 monolayers requires the presence of matriptase, since knockdown of matriptase by siRNA silencing inhibits TEER development (Figure 6B)(11). These data suggest matriptase contributes to the resealing of epithelial breaches and reformation of inter-epithelial tight junctions in injured epithelia.
Figure 6.
DSS induces decreased matriptase in colonic T84 epithelial monolayers. Polarized T84 monolayers (start TEER ~900 Ohms.cm2) were treated with or without 5% DSS in serum-free DME for 2.5 hrs, the DSS was removed, and the monolayers cultured in serum containing media for an additional 24 hrs. (A) (Upper panel) Changes in TEER. TEER was completely abolished after 2.5 hrs of DSS treatment. Upon removal of DSS (arrow), TEER values increased and approached that of untreated cultures by 24 hrs. (Lower panel) Immunoblot analysis showing that matriptase protein expression is significantly reduced (over 90%) upon exposure to DSS, and recovers upon removal of the DSS. (B) Matriptase siRNA knockdown in T84 monolayers compromises tight junction function. (Upper panel) Immunoblot analysis showing 80% knockdown of matriptase protein expression by 3 days. (Lower panel) TEER development is impaired in matriptase silenced T84 monolayers compared with control cells (siCtl vs siM2).
Matriptase is down-regulated by cytokines implicated in the pathogenesis of IBD
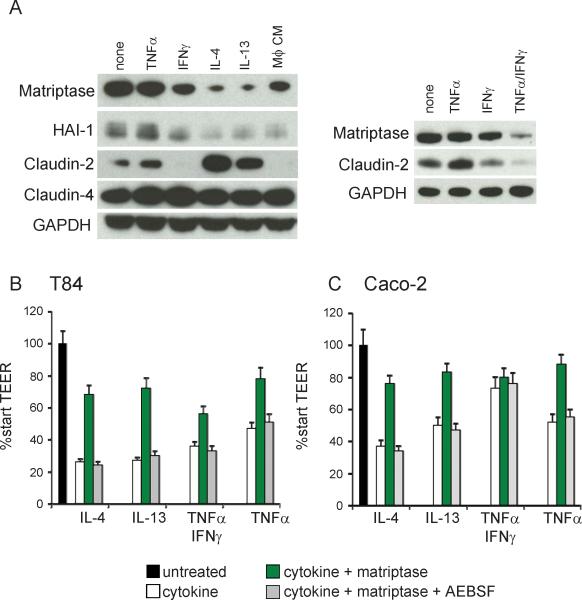
IBD is characterized by an activated mucosal immune system in which increased inflammatory cytokines contribute to epithelial barrier dysfunction (26; 27; 31). Exposure of T84 or Caco-2 monolayers to the IBD-associated inflammatory cytokines (IL-4, IL-13, TNFα/IFNγ), or a cytokine cocktail produced by activated macrophages, results in increased expression of claudin-2, indicative of increased barrier permeability, and down regulation of matriptase expression (Figures 7A and S2). The HGF activator inhibitor-1, HAI-1, which is a regulator of matriptase activity (32), is similarly down regulated by inflammatory cytokines (Figure 7A and S4), and its expression is decreased in human IBD tissues (Figure S5). These data suggest that cytokines produced during inflammatory processes associated with IBD could prevent matriptase-mediated restoration of intestinal epithelial barrier function. Indeed, when 5nM recombinant matriptase was added to T84 or Caco-2 monolayers compromised by exposure to cytokines, there was a significant improvement in the development of TEER of the cytokine-perturbed epithelial monolayers (Figure 7B&C). T he restoration of barrier function was dependent on matriptase catalytic activity, since co-treatment with the serine protease inhibitor, AEBSF, prevented the increased TEER development (Figure 7B&C). These data suggest that cytokine-mediated loss of matriptase may be an important contributor to barrier dysfunction during inflammatory colitis.
Figure 7.
Loss of matriptase contributes to intestinal epithelial barrier disruption by inflammatory cytokines. (A) Polarized T84 monolayers on transwell filters were treated daily on the basolateral side with 10ng/ml TNFα, IL-4, or IL-13, 100U/ml IFNγ, a combination of TNFα and IFNγ, 10% macrophage (Mϕ) conditioned media or left untreated. Protein lysates were analyzed at 48hrs by immunoblotting for matriptase, HAI-1, claudin-2, claudin-4, and GAPDH. (B) Polarized T84 or (C) Caco-2 monolayers were treated for 48hrs with the indicated cytokines as in (A), then replaced with 5nM recombinant matriptase or media alone control. TEER was measured after 8 hrs.
Discussion
Compromised epithelial barrier function is considered an important pathophysiologic basis for IBD. The increased permeability enhances the transport of microbial flora and other antigenic material from the intestinal lumen into the submucosa, resulting in a recurring cycle of inflammation and mucosal injury (29). A consequence of excessive activation of the immune system can be the weakening of countering mechanisms critically required to restore normal homeostasis. Here we show that matriptase, an enzyme critical for maintenance of epithelial homeostasis, is a key factor in the restoration and maintenance of intestinal barrier function after injury, and that its down-regulation by inflammatory cytokines generated by activation of the immune system contributes to perpetuation of disease severity and impaired mucosal recovery.
Mouse models of IBD have shown that a compromised epithelium is sufficient to cause intestinal inflammation, and that resident microbial flora is necessary for colitis induction (33). An initiating event, whether genetic, immune or environmental that enhances barrier permeability can initiate activation of the innate immune system given the exceptional challenge provided by the dense microbial flora unique to the gut. Indeed, complete genetic deficiency of matriptase abrogates epithelial barrier function resulting in acute and severe inflammation along with massive tissue destruction (10). Although the minimal levels of matriptase expressed by St14 hypomorphic mice weaken barrier function, they do not result in spontaneous disease in the absence of an initiating trigger. Thus the ST14 hypomorph represents a pathologically relevant model of disease susceptibility in patients who have increased intestinal epithelial permeability and are at risk of developing disease.
In the DSS model of colitis, an initiating trigger, e.g. exposure to DSS, causes damage to the epithelial layer and initiates activation of innate immune responses (25). Persistent inflammation associated with DSS injury in the ST14 hypomorphic mice was not caused by a more robust inflammatory response but was related to the inability to recover barrier function after the DSS insult was removed. Matriptase down-regulation occurs secondary to the inflammatory response, and in control mice, restoration of normal matriptase levels during the recovery phase promotes colitis resolution. In St14 hypomorphic mice, the inability to restore normal matriptase levels prevents gut barrier recovery and perpetuates the destructive inflammation. These data highlight the critical role of host barrier protective mechanisms in the resolution of colitis, which can become suppressed by excessive activation of the immune system that drives tissue damage.
The specific molecular pathways initiated by matriptase activity in the intestinal epithelium are incompletely understood (11). Matriptase colocalizes with E-cadherin to apical junctional complexes and evidence suggests that it is required for inter-epithelial junction formation (10; 11; 34). Matriptase has also been implicated in the control of epithelial-cell turnover by regulating cell-cell and/or cell-substratum adhesions (35), and in the removal of aged epithelial cells in the small intestine through detachment from the basement membrane component laminin (36). Substrates that are targeted by matriptase proteolytic activities in other cell systems (37), pro-urokinase-type plasminogen activator (pro-uPA) and protease activated receptor-2 (PAR-2) do not appear to mediate matriptase intestinal barrier protective activities, since mice deficient in uPA or PAR-2 do not show similar susceptibility to DSS-induced colitis (Figure S6). Altered pericellular activation of HGF may be involved in this phenotype since matriptase is an activator of pro-HGF (38) and HGF/c-met signaling is important for repair of injured mucosa (39; 40). Interestingly, conditional knockout of HAI-1 in the intestine of mice was shown recently to enhance susceptibility to DSS-induced colitis, possibly caused in part by deregulated matriptase activity (41).
Increased inflammatory cytokine production is associated with mucosal inflammation, and studies in vitro and in animal models demonstrate that inflammatory cytokines associated with IBD cause tight junction barrier dysfunction (42). Matriptase is down-regulated in inflamed intestinal epithelia of IBD patients (Fig 1A), and by the inflammatory cytokines IL-13 and IL-4 in in vitro cultures (Figure 7A). IL-13 enhances barrier permeability in part by stimulating the synthesis of claudin-2 (20; 26; 43) and we have shown that matriptase deficiency results in increased claudin-2 expression in both Caco-2 monolayers and in St14 hypomorph intestinal tissues (11). Endogenous mechanisms that regulate matriptase expression and activation in the gut are not currently known. Nonetheless, in vitro data suggest that restoration of matriptase expression and activity could interrupt the cytokine-mediated inflammatory cycle to promote epithelial repair and disease resolution.
While dysregulated inflammation induced by immunogen exposure is considered a major cause of mucosal tissue damage and injury in IBD, the present findings highlight the critical importance of host barrier repair mechanisms for restriction of the offending insult and its destructive consequences. Intestinal permeability has been shown to predict and possibly cause relapse in patients suffering from IBD (3; 44). A better understanding of the role of matriptase in controlling mucosal homeostasis by regulating the integrity and permeability of epithelial barrier function will be important since matriptase-based therapeutic strategies could have application for preventing, treating or altering the natural progression of IBD.
Supplementary Material
Acknowledgements
We thank Elizabeth Smith for assistance with histopathology and Dr. Chen Yong Lin, University of Maryland, for the matriptase specific S5 monoclonal antibody. This work was supported in part by grants from the National Institutes of Health: DK48373 (AF), AI/DK49316 (TSD), CA098369 and HL084387 (TMA), HL07698 (SNA) and the NIH Intramural program (THB).
Grant Support: This work was supported in part by grants from the National Institutes of Health: DK48373 (AF); AI/DK49316 (TSD); DK081376, CA098369, and HL084387 (TMA); the Maryland Stem Cell Research Fund 0145-00 (SNA); HL07698 (SNA); the NIH Intramural Program (THB); and the Canadian Institutes of Health Research (RL). MB was supported by a CJ Martin Training Fellowship (#384359) from the National Health and Medical Research Council, Australia.
Abbreviations
- CD
Crohn's Disease
- DSS
dextran sodium sulfate
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- TEER
transepithelial electrical resistance
- TJ
tight junction
- TNF
tumor necrosis factor
- UC
Ulcerative Colitis
Footnotes
Disclosures: The authors have nothing to disclose.
Supplementary Material – see attached pdf file for online supplementary material.
References
- 1.Podolsky DK. The current future understanding of inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2002;16:933–943. doi: 10.1053/bega.2002.0354. [DOI] [PubMed] [Google Scholar]
- 2.Murphy MS, Eastham EJ, Nelson R, Pearson AD, Laker MF. Intestinal permeability in Crohn's disease. Arch Dis Child. 1989;64:321–325. doi: 10.1136/adc.64.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 4.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 5.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 6.Olson TS, Reuter BK, Scott KG, Morris MA, Wang XM, Hancock LN, Burcin TL, Cohn SM, Ernst PB, Cominelli F, Meddings JB, Ley K, Pizarro TT. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resta-Lenert S, Smitham J, Barrett KE. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a-/- mice. Am J Physiol Gastrointest Liver Physiol. 2005;289:G153–G162. doi: 10.1152/ajpgi.00395.2004. [DOI] [PubMed] [Google Scholar]
- 8.Antalis TM, Shea-Donohue T, Vogel SN, Sears C, Fasano A. Mechanisms of disease: protease functions in intestinal mucosal pathobiology. Nat Clin Pract Gastroenterol Hepatol. 2007;4:393–402. doi: 10.1038/ncpgasthep0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.List K, Bugge TH, Szabo R. Matriptase: potent proteolysis on the cell surface. Mol Med. 2006;12:1–7. doi: 10.2119/2006-00022.List. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.List K, Kosa P, Szabo R, Bey AL, Wang CB, Molinolo A, Bugge TH. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am J Pathol. 2009;175:1453–1463. doi: 10.2353/ajpath.2009.090240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, Lin CY, List K, Szabo R, Fasano A, Bugge TH, Antalis TM. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci U S A. 2010;107:4200–4205. doi: 10.1073/pnas.0903923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.List K, Haudenschild CC, Szabo R, Chen W, Wahl SM, Swaim W, Engelholm LH, Behrendt N, Bugge TH. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene. 2002;21:3765–3779. doi: 10.1038/sj.onc.1205502. [DOI] [PubMed] [Google Scholar]
- 13.List K, Currie B, Scharschmidt TC, Szabo R, Shireman J, Molinolo A, Cravatt BF, Segre J, Bugge TH. Autosomal ichthyosis with hypotrichosis syndrome displays low matriptase proteolytic activity and is phenocopied in ST14 hypomorphic mice. J Biol Chem. 2007;282:36714–36723. doi: 10.1074/jbc.M705521200. [DOI] [PubMed] [Google Scholar]
- 14.Oberst M, Anders J, Xie B, Singh B, Ossandon M, Johnson M, Dickson RB, Lin CY. Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo. Am J Pathol. 2001;158:1301–1311. doi: 10.1016/S0002-9440(10)64081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shea-Donohue T, Thomas K, Cody MJ, Aiping Z, Detolla LJ, Kopydlowski KM, Fukata M, Lira SA, Vogel SN. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008;14:117–124. doi: 10.1177/1753425908088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 17.Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desilets A, Beliveau F, Vandal G, McDuff FO, Lavigne P, Leduc R. Mutation G827R in matriptase causing autosomal recessive ichthyosis with hypotrichosis yields an inactive protease. J Biol Chem. 2008;283:10535–10542. doi: 10.1074/jbc.M707012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desilets A, Longpre JM, Beaulieu ME, Leduc R. Inhibition of human matriptase by eglin c variants. FEBS Lett. 2006;580:2227–2232. doi: 10.1016/j.febslet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol. 2008;23(Suppl 2):S146–S150. doi: 10.1111/j.1440-1746.2008.05405.x. [DOI] [PubMed] [Google Scholar]
- 22.Furth EE, Li J, Purev E, Solomon AC, Rogler G, Mick R, Putt M, Zhang T, Somasundaram R, Swoboda R, Herlyn D. Serum antibodies to EpCAM in healthy donors but not ulcerative colitis patients. Cancer Immunol Immunother. 2006;55:528–537. doi: 10.1007/s00262-005-0026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melgar S, Karlsson L, Rehnstrom E, Karlsson A, Utkovic H, Jansson L, Michaelsson E. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol. 2008;8:836–844. doi: 10.1016/j.intimp.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Melgar S, Karlsson A, Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1328–G1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 25.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 26.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788:864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 28.Dignass AU, Baumgart DC, Sturm A. Review article: the aetiopathogenesis of inflammatory bowel disease--immunology and repair mechanisms. Aliment Pharmacol Ther. 2004;20(Suppl 4):9–17. doi: 10.1111/j.1365-2036.2004.02047.x. [DOI] [PubMed] [Google Scholar]
- 29.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 30.Fries W, Mazzon E, Squarzoni S, Martin A, Martines D, Micali A, Sturniolo GC, Citi S, Longo G. Experimental colitis increases small intestine permeability in the rat. Lab Invest. 1999;79:49–57. [PubMed] [Google Scholar]
- 31.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo R, Kosa P, List K, Bugge TH. Loss of matriptase suppression underlies spint1 mutation-associated ichthyosis and postnatal lethality. Am J Pathol. 2009;174:2015–2022. doi: 10.2353/ajpath.2009.090053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang JK, Lee MS, Tseng IC, Chou FP, Chen YW, Fulton A, Lee HS, Chen CJ, Johnson MD, Lin CY. Polarized epithelial cells secrete matriptase as a consequence of zymogen activation and HAI-1-mediated inhibition. Am J Physiol Cell Physiol. 2009;297:C459–C470. doi: 10.1152/ajpcell.00201.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuzuki S, Murai N, Miyake Y, Inouye K, Hirayasu H, Iwanaga T, Fushiki T. Evidence for the occurrence of membrane-type serine protease 1/matriptase on the basolateral sides of enterocytes. Biochem J. 2005;388:679–687. doi: 10.1042/BJ20041639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mochida S, Tsuzuki S, Inouye K, Fushiki T. A recombinant catalytic domain of matriptase induces detachment and apoptosis of small-intestinal epithelial IEC-6 cells cultured on laminin-coated surface. J Biochem. 2010;148:721–732. doi: 10.1093/jb/mvq108. [DOI] [PubMed] [Google Scholar]
- 37.Antalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochem J. 2010;428:325–346. doi: 10.1042/BJ20100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen KA, Qiu D, Alves J, Schumacher AM, Kilpatrick LM, Li J, Harris JL, Ellis V. Pericellular activation of hepatocyte growth factor by the transmembrane serine proteases matriptase and hepsin, but not by the membrane-associated protease uPA. Biochem J. 2010;426:219–228. doi: 10.1042/BJ20091448. [DOI] [PubMed] [Google Scholar]
- 39.Ido A, Numata M, Kodama M, Tsubouchi H. Mucosal repair and growth factors: recombinant human hepatocyte growth factor as an innovative therapy for inflammatory bowel disease. J Gastroenterol. 2005;40:925–931. doi: 10.1007/s00535-005-1705-x. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol. 2011;26(Suppl 1):188–202. doi: 10.1111/j.1440-1746.2010.06549.x. [DOI] [PubMed] [Google Scholar]
- 41.Kawaguchi M, Takeda N, Hoshiko S, Yorita K, Baba T, Sawaguchi A, Nezu Y, Yoshikawa T, Fukushima T, Kataoka H. Membrane-Bound Serine Protease Inhibitor HAI-1 Is Required for Maintenance of Intestinal Epithelial Integrity. Am J Pathol. 2011 doi: 10.1016/j.ajpath.2011.06.038. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henderson P, van Limbergen JE, Schwarze J, Wilson DC. Function of the intestinal epithelium and its dysregulation in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:382–395. doi: 10.1002/ibd.21379. [DOI] [PubMed] [Google Scholar]
- 43.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 44.Porras M, Martin MT, Yang PC, Jury J, Perdue MH, Vergara P. Correlation between cyclical epithelial barrier dysfunction and bacterial translocation in the relapses of intestinal inflammation. Inflamm Bowel Dis. 2006;12:843–852. doi: 10.1097/01.mib.0000231571.88806.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.