Abstract
Introduction
Vernakalant (VER) is a relatively atrial-selective antiarrhythmic drug capable of blocking potassium and sodium currents in a frequency- and voltage-dependent manner. Ranolazine (RAN) is a sodium channel blocker shown to exert antiarrhythmic effects in pulmonary vein (PV) sleeves. DL-sotalol (SOT) is a beta-blocker commonly used in the rhythm-control treatment of AF. This study evaluated the electrophysiological and antiarrhythmic effects of VER, RAN, and SOT in canine PV sleeve preparations in a blinded fashion.
Methods
Transmembrane action potentials (AP) were recorded from canine superfused PV sleeve preparations exposed to VER (n=6), RAN (n=6), and SOT (n=6). Delayed afterdepolarizations (DADs) were induced in the presence of isoproterenol and high-calcium concentrations by periods of rapid pacing.
Results
In PV sleeves, VER, RAN and SOT (3–30 μM) produced small (10–15 ms) increases in AP duration. Effective refractory period (ERP), diastolic threshold of excitation (DTE), and the shortest S1-S1 cycle length (CL) permitting 1:1 activation were significantly increased by VER and RAN in a rate- and concentration-dependent manner. VER and RAN significantly reduced Vmax in a concentration- and rate-dependent manner. SOT did not significantly affect ERP, Vmax, DTE or the shortest S1-S1 CL permitting 1:1 activation. All three agents (3–30 μM) suppressed DAD-mediated triggered activity induced by isoproterenol and high-calcium.
Conclusions
In canine PV sleeves, the effects of VER and RAN were similar and largely characterized by concentration- and rate-dependent depression of sodium channel-mediated parameters, which were largely unaffected by SOT. All three agents demonstrated an ability to effectively suppress DAD-induced triggers of atrial arrhythmia.
Keywords: Atrial fibrillation, Antiarrhythmic drugs, Sodium channel blocker, Electrophysiology, Pharmacology
INTRODUCTION
Vernakalant is a relatively atrial-selective antiarrhythmic drug1–3 approved for conversion of recent-onset atrial fibrillation (AF) in Europe. Vernakalant exerts its antiarrhythmic activity by blocking early activating potassium currents (i.e. IKur, Ito, IKr, IKACh) combined with frequency-and voltage- dependent inhibition of cardiac sodium channels.4 Ranolazine is a sodium channel current (INa) blocker shown to exert antiarrhythmic effects by suppressing early and delayed afterdepolarization (EAD and DAD)-induced triggered activity in pulmonary vein (PV) sleeve preparations.5 DL-sotalol (SOT) is a beta-blocker commonly used in the rhythm-control treatment of AF6–8 that also has class III antiarrhythmic effects by virtue of blocking the rapidly activating delayed rectifier (IKr) potassium channel.
PV have been shown to play a major role in the development of clinical AF by generating extrasystoles responsible for triggering AF.9 A number of experimental models have proposed DADs and late phase 3 EADs in PVs as potential triggers in the initiation of AF.5, 10–15
The present study was designed to compare in a blinded fashion the electrophysiological and antiarrhythmic effects of ranolazine, vernakalant and sotalol in canine PV sleeve preparations.
METHODS
This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No 85-23, Revised 1996) and was approved by the ACUC of the Masonic Medical Research Laboratory.
Adult mongrel dogs of either sex weighing 20–35 kg were anticoagulated with heparin (180 IU/kg) and anesthetized with sodium pentobarbital (35 mg/kg, IV). The chest was opened via a left-thoracotomy, the heart was excised and placed in a cold cardioplegic solution ([K+]0 = 8 mmol/L, 4°C).
Superfused pulmonary vein sleeve preparation
PV sleeve preparations (approximately 2.0 × 1.5 cm) were isolated from the left canine atria. The thickness of the preparation was approximately 2 mm. Left superior pulmonary veins were used preferentially in most experiments. The preparations were placed in a small tissue bath and superfused with Tyrode's solution of the following composition (mM): 129 NaCl, 4 KCl, 0.9 NaH2PO4, 20 NaHCO3, 1.8 CaCl2, 0.5 MgSO4, 5.5 glucose and bubbled with 95% O2/5% CO2 (35 ± 0.5°C). PV preparations were stimulated at a basic cycle length (BCL) of 1000 ms during the equilibration period (1 h) using electrical stimulation (1–3 ms duration, 2.5 times diastolic threshold intensity) delivered through silver bipolar electrodes insulated, except at the tips. The threshold intensity ranged from 0.5 to 1.4 mV. Transmembrane potentials were recorded using glass microelectrodes filled with 3 M KCl (10–20 MΩ DC resistance) connected to a high input-impedance amplification system (World Precision Instruments, model KS-700, New Haven, CT). The following parameters were measured: take-off potential (TOP), action potential amplitude (APA), action potential duration at 85% repolarization (APD85), effective refractory period (ERP, defined as the shortest S1-S2 interval capable of eliciting a propagated response), maximum rate of rise of action potential upstroke (Vmax), diastolic threshold of excitation (DTE), and the minimum following interval (shortest S1–S1 showing 1:1 conduction). Transmembrane action potentials were recorded at a sampling rate of 41 kHz.
Experimental protocols
The drugs tested were blinded to all involved with the conduct of the experimental study, with the blinded test agent code broken only after completion of all studies and data analysis. Fresh drug stock was prepared for all three compounds before each experiment by a person not involved in the conduct of the study and provided to the investigator as Compound A, B or C. A total of 14 dogs were used in this study. One, 2, or 3 PV preparations from each animal were used in Groups 1, 3 and 4. In each of Group 1 and 3, distinct PV preparations from the same dog were used to test different compounds. In addition, some PV preparations from the same dog were used for Groups 1 and 3. Distinct dogs were used in Groups 1 and 2 (time control experiments). Distinct dogs were used for each experiment in Groups 2 and 4 (time control experiments).
Group 1 (n=18 PV preparations, n=6 PV preparations for each compound). The effect of each test agent on the normal electrophysiology of the PV sleeve preparations was evaluated at 3 distinct concentrations (3, 10, and 30 μM). The tissues were exposed to each concentration of the test agent for a period of 20–30 min. Action potentials were recorded at basic cycle lengths (BCLs) of 2000, 1000, 500 and 300 ms and the distinct EP parameters (APA, TOP, APD85, ERP, DTE, minimum following interval (shortest S1–S1 showing 1:1 conduction) were evaluated at each BCL.
Group 2 (n=4 PV preparations from 4 dogs). Time control experiments were performed to test the stability of the preparations over a period of 2 to 4 hours after the end of the equilibration period. The time control was designed to match the period consumed by the experimental protocols.
Group 3 (n=18 PV preparations, n= 6 PV preparations for each compound). We evaluated the effect of each test agent in a recently described isoproterenol (1 μM) and high calcium (5.4 mM) model of delayed afterdepolarization (DAD)-induced triggered activity in PV sleeve preparations.5, 10, 16, 17 Trains of 10 beats were elicited at progressively faster rates in order to induce DADs and triggered activity. DADs were elicited during the pause by a period of rapid pacing (BCL changed from 500 to 100 ms). The effect of each concentration of test agent on the development of DADs and triggered activity was evaluated. After reproducible induction of DAD, the test agent (first 3, and then 10, 30 μM) was added to the Tyrode's solution. Inducibility protocols were repeated and measurements obtained 30 minutes after each concentration of compound.
Group 4 (n=3 PV preparations from 3 dogs). Time control experiments were conducted to assess the reproducibility of DAD and triggered activity induction over a period of 90 min in the presence of isoproterenol + high Ca2+.
Chemicals
Vernakalant, ranolazine and sotalol were provided by Merck and Co. (West Point, PA) and Cardiome Pharma Corp. (Vancouver, BC, Canada). Drugs were dissolved directly in Tyrode's solution and were administered in a blinded fashion. Isoproterenol (Sigma-Aldrich Corp., St. Louis, MO) was dissolved in distilled water to form a stock solution of 1 mM and used at a final concentration of 1 μM.
Statistics
Statistical analysis was performed using one way repeated measures analysis of variance (ANOVA) followed by Bonferroni's test. Mean values were considered to be significantly different at p < 0.05. All data are reported as mean ± SD.
RESULTS
Electrophysiological effects of vernakalant, ranolazine and sotalol in PV sleeve preparations
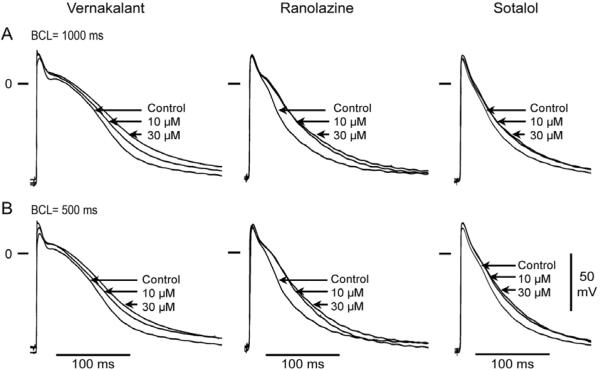
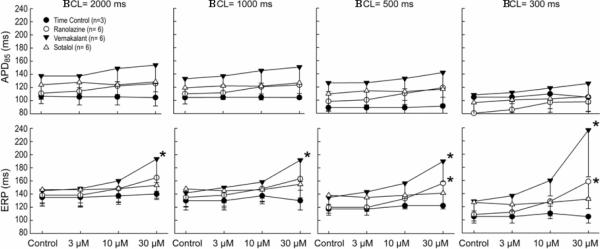
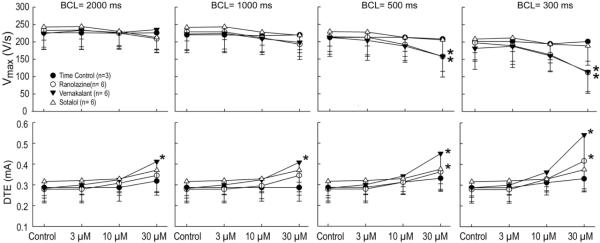
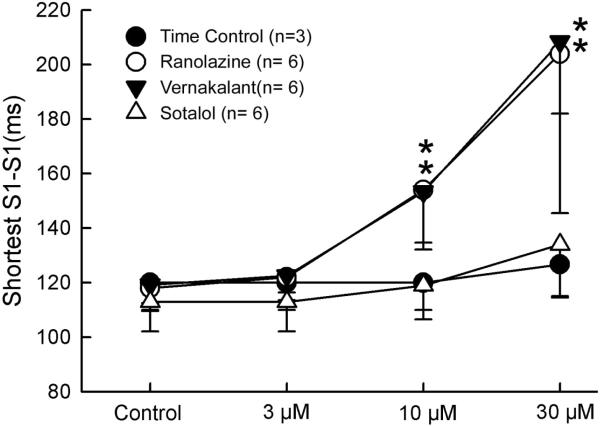
Vernakalant, ranolazine and sotalol produced small (approximately 10–15 ms) increases in APD85 (Figure 1). This effect was not statistically significant at any rate (Figure 2, upper panel). ERP was increased by vernakalant and ranolazine in a rate- and concentration-dependent manner reaching significance at a concentration of 30 μM (Figure 2, lower panel). The effect on ERP was more accentuated for vernakalant and larger at the fastest rates (500 and 300 ms) due to the development of post-repolarization refractoriness (PRR). Much of the increase in ERP produced by both vernakalant and ranolazine was due to the development of PRR, as refractoriness was prolonged beyond APD85. In contrast, ERP was largely unaffected by sotalol. Vmax was significantly reduced by vernakalant and ranolazine in a concentration- and rate-dependent manner (Figure 3, upper panel). The effects on Vmax were similar for vernakalant and ranolazine. A significant increase in DTE was observed following exposure to 30 μM of vernakalant and ranolazine at BCL of 500 and 300 ms (Figure 3, lower panel). This effect was greater for vernakalant. A significant effect on DTE was also observed for vernakalant at BCL of 1000 and 2000 ms. DTE was not affected by sotalol at any concentrations and BCLs tested. The shortest S1-S1 CL permitting 1:1 activation was significantly increased by vernakalant and ranolazine to a similar degree, but largely unaffected by sotalol (Figure 4).
Figure 1.
Representative examples of the effects of vernakalant (10–30 μM), ranolazine (10–30 μM) and sotalol (10–30 μM) on action potentials of 3 PV sleeve preparations. (A) Basic cycle length (BCL) = 1000 ms. (B) BCL = 500 ms. The three compounds produced a slight prolongation of action potential duration (APD). However, APD increase in the presence of sotalol did not appear to be concentration dependent.
Figure 2.
Composite data (n=6) of the rate-dependent effects of vernakalant (3–30 μM), ranolazine (3–30 μM) and sotalol (3–30 μM) on action potential duration at 85% repolarization (APD85, upper panel) and effective refractory period (ERP, lower panel) in pulmonary vein sleeves preparations. *- p<0.05 v. respective control. BCL = basic cycle length. Mean ± SD
Figure 3.
Composite data (n=6) of the rate-dependent effects of vernakalant (3–30 μM), ranolazine (3–30 μM) and sotalol (3–30 μM) on maximum rate of rise of action potential upstroke (Vmax) and diastolic threshold of excitation (DTE) in PV sleeve preparations. *- p<0.05 vs. respective control. BCL = basic cycle length. Mean ± SD
Figure 4.
Composite data of the effects of vernakalant (3–30 μM), ranolazine (3–30 μM) and sotalol (3–30 μM) on the shortest S1-S1 permitting 1:1 activation in PV sleeves preparations. *-p<0.05 v. respective control. Mean ± SD
In the case of ranolazine, take-off potential (TOP) was −83.3+1.6, −82.8+1.9 and −82.2+1 mV under control conditions and following 10 or 30 μM drug, respectively at a BCL of 2000 and −81.3+2.2, −80.8+2 and −79.2+1.3 mV at a BCL of 300 ms. In the case of vernakalant, TOP was −85.5+1.9, −85+1.3 and −85.7+3 mV under control conditions and following 10 or 30 μM, respectively at a BCL of 2000 and −82.8+2, −81.5+0.8 and −81+2.8 mV at a BCL of 300 ms. In the case of sotalol, TOP was −85.2+1.6, −84.6+1.5 and −84.6+1.9 under control conditions and following 10 or 30 μM, respectively at a BCL of 2000 ms and −81.8+1.5, −81.4+1.3 and −81+1.4 mV at a BCL of 300 ms. No significant change in TOP was observed under any condition studied. Vernakalant and ranolazine were observed to produce a slight concentration and rate-dependent increase in conduction time in the PV sleeves, although these changes did not reach statistical significance under any of the conditions studied.
Time control experiments indicated no changes in electrophysiological parameters over a 90 min period. These data are consistent with a potent use-dependent inhibition of the sodium channel current by vernakalant and ranolazine in PV sleeve preparations with vernakalant producing more post-repolarization refractoriness than ranolazine. Sotalol induced no significant changes in APD, ERP, Vmax, DTE and shortest S1-S1 interval.
Effects of vernakalant, ranolazine and sotalol on isoproterenol + high Ca++- induced triggered activity
In another series of experiments we assessed the ability of each drug to suppress delayed afterdepolarizations (DADs)-induced triggered activity elicited in PV sleeve preparations exposed to combined isoproterenol and high calcium. These conditions give rise to DADs in 100% of normal PV sleeves.
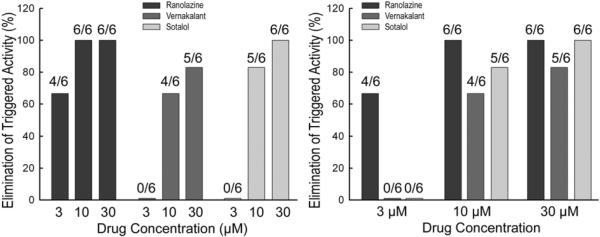
Representative action potential recordings of the effects of vernakalant, ranolazine, and sotalol on isoproterenol and high calcium-induced triggered activity are shown in Figures 5, 6 and 7, respectively. Composite data of the drug effects to eliminate triggered activity at BCL of 200 ms are shown in Figure 8. At a concentration of 3 μM, ranolazine eliminated triggered activity in 4 of 6 PV preparations, vernakalant in 0 of 6 PV preparations and sotalol in 0 of 6 PV preparations. At a concentration of 10 μM, ranolazine eliminated triggered activity in 6 of 6 PV preparations, vernakalant in 4 of 6 PV preparations and sotalol in 5 of 6 PV preparations. At a concentration of 30 μM, ranolazine eliminated triggered activity in 6 of 6 PV preparations, vernakalant in 5 of 6 PV preparations and sotalol in 5 of 6 PV preparations. Time control experiments (n=3) assessing the effects of time on isoproterenol + high Ca++-induced triggered activity in PV sleeves indicated that triggered activity persists after 30, 60 and 90 min of exposure to normal Tyrode's solution. Thus, all three compounds were effective in eliminating DAD-induced triggered activity originating in PV exposed to isoproterenol and high calcium. Importantly, no incidences of exacerbation or increased frequency of triggered activity were observed with the three test agents.
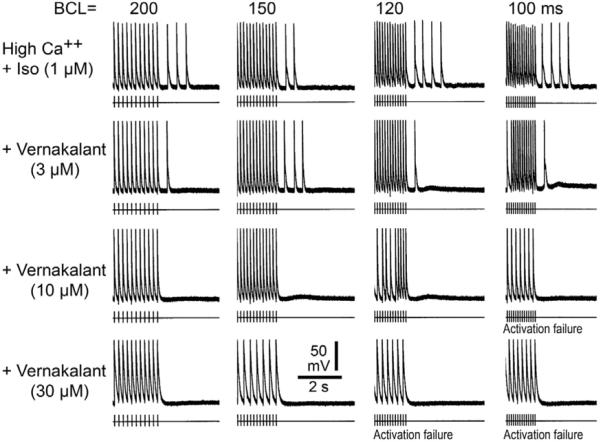
Figure 5.
Effect of vernakalant on high Ca++ + Isoproterenol (Iso) - induced delayed afterpolarizations (DADs) and triggered activity. Each panel depicts a train of 10 beats at a basic cycle length (BCL) of 200, 150, 120 and 100 ms followed by a pause. Isoproterenol and high Ca++ induce prominent rate-dependent DADs and triggered activity during the pause. Addition of vernakalant (3 μM) reduces the triggered beats; vernakalant (10 μM) suppresses triggered activity but a small DAD persists; vernakalant (30 μM) suppresses DADs and triggered activity. [Ca2+]o=5.4 mM.
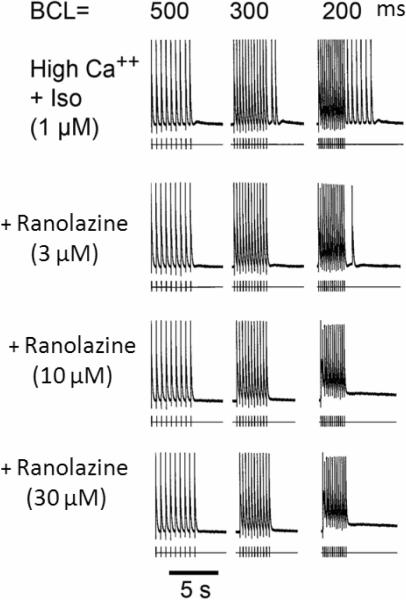
Figure 6.
Effect of ranolazine on high Ca++ + Isoproterenol (Iso) - induced delayed afterpolarizations (DADs) and triggered activity. Each panel depicts a train of 10 beats at a basic cycle length (BCL) of 500, 300, and 200 ms followed by a pause. Isoproterenol and high Ca++ induce prominent rate-dependent DADs and triggered activity during the pause. Addition of ranolazine (3 μM) suppresses the triggered beats at BCL 300 ms, but a triggered beat persists at BCL 200 ms followed by a DAD. Ranolazine (10 and 30 μM) suppresses DADs and triggered activity. [Ca2+]o=5.4 mM.
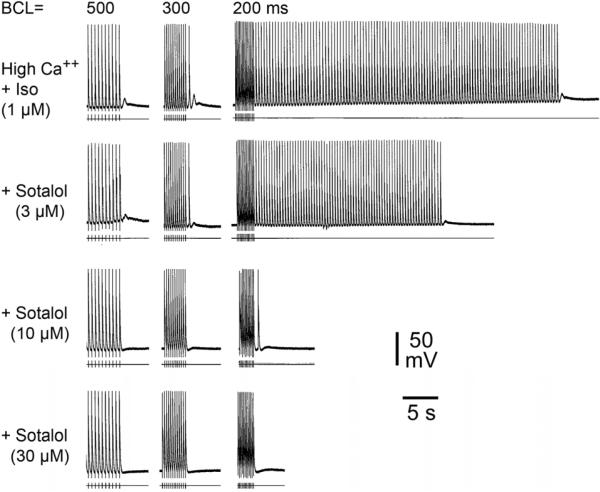
Figure 7.
Effect of sotalol on high Ca++ + Isoproterenol (Iso) - induced delayed afterpolarizations (DADs) and triggered activity. Each panel depicts a train of 10 beats at a basic cycle length (BCL) of 500, 300 and 200 ms followed by a pause. Isoproterenol and high Ca++ induce prominent rate-dependent DADs and triggered activity during the pause. Addition of sotalol (3 and 10 μM) reduces the triggered beats; sotalol (30 μM) suppresses DADs and triggered activity. [Ca2+]o=5.4 mM.
Figure 8.
Summary of the effects of vernakalant (3–30 μM), ranolazine (3–30 μM) and sotalol (3–30 μM) on suppression of isoproterenol + high Ca++ - induced triggered activity at basic cycle length of 200 ms in PV sleeves (n= 6 preparations per concentration).
DISCUSSION
Atrial fibrillation (AF) is the most common arrhythmia requiring medical attention. Ectopic beats originating from PV have been shown to be a common source of triggers for the development of AF clinically and preclinically.18–20 Accordingly, PV isolation is a commonly used procedure to eliminate the triggers arising from the pulmonary veins. The results of the present study indicate that, in canine superfused PV sleeve preparations, vernakalant and ranolazine produced significant rate-dependent changes in sodium channel-mediated electrophysiological parameters (including Vmax, ERP, DTE, and the shortest S1-S1 CL permitting 1:1 activation). The effects on ERP and DTE were more pronounced for vernakalant and the effects on Vmax and shortest S1-S1 were similar for both drugs. The greater effect on ERP observed in the presence of vernakalant might be explained by its relatively greater, albeit modest, increase in APD85. Sotalol produced no significant changes in any of the basic electrophysiologic parameters evaluated. In addition, none of the three agents were observed to accentuate DAD-induced triggered activity induced by exposure to isoproterenol and high calcium and all three agents were effective in suppressing DADs and DAD-induced triggered activity.
Triggered activity in PV sleeves
In the present study, DADs and DAD-induced triggered activity were easily induced in PV sleeve preparations following the addition of isoproterenol and high calcium. Similar findings were previously reported in canine and rabbit isolated single PV myocytes,21, 22 in isolated canine pulmonary vein sleeve preparations,5, 10, 12, 13, 23 and in coronary-perfused right atrial preparations.11, 24 In isolated rabbit PV myocytes, isoproterenol was shown to increase automaticity and induce spontaneous activity as well as EAD- or DAD-induced triggered activity.21, 22 Our data show that vernakalant, ranolazine and sotalol, in concentrations within their respective therapeutic ranges, are all capable of reducing or eliminating DAD–induced triggered activity originating in PV sleeves.
Antiarrhythmic effects of vernakalant, ranolazine and dl-sotalol
Vernakalant is a new antiarrhythmic drug that has been shown to suppress recent onset atrial fibrillation.25–27 The drug is approved in Europe. Vernakalant is a multiple ion channel blocker shown to possess relatively atrial-selective properties.3, 28–30 In experimental models, vernakalant prolongs atrial refractoriness with no effect on ventricular refractoriness or defibrillation threshold.1 In humans, vernakalant preferentially prolongs atrial refractoriness.26 The present findings in the canine PV sleeve preparation indicate that vernakalant reduces excitability, slightly prolongs APD, prolongs ERP and induces post-repolarization refractoriness, especially at rapid rates of activation. Although APD prolongation is consistent with K channel blocking activity, the effects on excitability and ERP appear to be largely mediated by vernakalant's sodium channel blocking properties. Moreover vernakalant, in concentrations within its therapeutic range, eliminates DADs in PV sleeves, pointing to an additional antiarrhythmic effect on the triggers of AF.
In canine PV sleeves, 10 μM ranolazine has previously been shown to elicit a marked reduction in excitability and sodium-channel dependent electrophysiologic parameters.5 Exposure of the PV sleeve preparations to acetylcholine (ACh), isoproterenol, high [Ca++]o or their combination together with rapid pacing induced DADs, late phase 3 EADs and triggered activity. Ranolazine (10 μM) eliminated rate-dependent DAD- and EAD-induced triggered activity and reduced afterdepolarization amplitude induced by a combination of ACh and high calcium ([Ca2+]o = 5.4 mM).5 The present data confirm the effect of ranolazine to eliminate DAD and DAD-induced triggered activity at concentrations within the therapeutic range (1–10 μM).
At therapeutic concentrations, ranolazine has been shown to produce atrial-selective depression of peak INa, causing potent use-dependent inhibition of peak INa (estimated on the basis of depression of Vmax) in canine atrial tissues,31 but not in in ventricular myocardial cells or Purkinje fibers.32–34
The ability of vernakalant and ranolazine to suppress DADs and DAD-induced triggered activity could be explained by their actions to reduce intracellular calcium loading secondary to their effects to reduce sodium loading at rapid rates. The late sodium channel blocker ranolazine has been shown to specifically inhibit sodium entry and subsequently calcium entry.35 We are not aware of similar data for vernakalant, but because of the similarity of action, vernakalant might be expected to exert a similar action. The effect of relatively pure sodium channel blockers to suppress DAD activity has been demonstrated previously in other tissues.36 Ranolazine's weak action to inhibit β-adrenergic receptors and late ICa also might be involved in the suppression of DAD-induced triggered activity.30,32–34
Like vernakalant and ranolazine, sotalol eliminates DADs and DAD-induced triggered activity in canine PV sleeves. This effect may be due in large part to the β-blocking effect of the compound.37 Sotalol is mainly used as a rhythm-control agent for the treatment of AF.38 The drug has been shown to prolong APD and refractoriness in ventricular and atrial tissues, consistent with its reported class III antiarrhythmic properties through blockade of the rapidly activating delayed rectifier (IKr) potassium channel.39 In the present studies in canine PV sleeves preparations, we did not observe significant prolongation of APD or ERP following exposure to any concentration of sotalol, suggesting that blocking IKr has a limited impact on PV repolarization. However, the effect of sotalol to block IKr, thereby prolonging ventricular repolarization and potentially inducing EADs and ventricular arrhythmias such as Torsade de Pointes arrhythmias40–42 often limits its use as an antiarrhythmic agent for the treatment of atrial arrhythmias.43
Clinical implications
In canine PV sleeves, the effects of vernakalant and ranolazine are similar and largely characterized by concentration- and rate-dependent depression of sodium channel-mediated parameters, which were largely unaffected by sotalol. All three compounds demonstrated an ability to effectively suppress DAD-induced triggers of atrial arrhythmia.
Rate control vs. rhythm control treatment of AF is still a matter of debate.30, 44 The efficacy and safety of the intravenous formulation of vernakalant for conversion of recent onset AF have been demonstrated in multiple clinical trials.45 An oral formulation of vernakalant for the prevention of AF recurrence post-cardioversion is under investigation.46 Our data suggest that the clinical efficacy demonstrated for vernakalant can be partly explained by suppression of triggered activity responsible for the induction of AF and other atrial arrhythmias. The effects of vernakalant to induce post-repolarization refractoriness in PV, as in other atrial tissues (Burashnikov and Antzelevitch, unpublished observation), also points to a mechanistic rationale for rhythm control utility in the chronic management of AF. Similarly, the antiarrhythmic properties of ranolazine described in this report could explain its anti-AF properties described in vitro31,47 and in multiple clinical studies.48–51 Sotalol was also able to suppress PV triggered activity; its effectiveness appears to be mainly due to its β-blocking activity which can also contribute to rate control management of AF. The relatively selective IKr blocking effect of sotalol may be a limitation for its clinical use due to the potential for ventricular proarrhythmia in the form of Torsade de Pointes.52
Acknowledgments
Funding: Supported by grants from Merck & Co., NHLBI- HL47678 (CA) and New York and Florida Grand Lodges F. & A.M.
Abbreviations
- ACh
acetylcholine
- AF
atrial fibrillation
- AP
action potential
- APA
action potential amplitude
- APA85
action potential duration at 85% repolarization
- BCL
basic cycle length
- CL
cycle length
- DAD
delayed afterdepolarization
- DTE
diastolic threshold of excitation
- EAD
early afterdepolarization
- ERP
effective refractory period
- IKr
rapidly activating; delayed rectifier potassium channel current
- INa
sodium channel current
- PRR
post-repolarization refractoriness
- PV
pulmonary vein
- RAN
ranolazine
- SOT
sotalol
- TOP
take-off potential
- VER
vernakalant
- Vmax
maximum rate of rise of the action potential upstroke
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Antzelevitch received research support from Merck & Co, Dr. Lynch is an employee of Merck & Co., Dr. Pourrier is an employee of Cardiome Pharma Corp. and Dr. Gibson is an employee of AAKVSL Pharma Consulting, LLC.
References
- 1.Bechard J, Gibson JK, Killingsworth CR, et al. Vernakalant selectively prolongs atrial refractoriness with no effect on ventricular refractoriness or defibrillation threshold in pigs. J Cardiovasc Pharmacol. 2011;57:302–7. doi: 10.1097/FJC.0b013e3182073c94. [DOI] [PubMed] [Google Scholar]
- 2.Ehrlich JR, Nattel S. Atrial-selective pharmacological therapy for atrial fibrillation: hype or hope? Curr Opin Cardiol. 2009;24:50–5. doi: 10.1097/HCO.0b013e32831bc336. [DOI] [PubMed] [Google Scholar]
- 3.Fedida D. Vernakalant (RSD1235): a novel, atrial-selective antifibrillatory agent. Expert Opin Investig Drugs. 2007;16:519–32. doi: 10.1517/13543784.16.4.519. [DOI] [PubMed] [Google Scholar]
- 4.Fedida D, Orth PM, Chen JY, et al. The mechanism of atrial antiarrhythmic action of RSD1235. J Cardiovasc Electrophysiol. 2005;16:1227–38. doi: 10.1111/j.1540-8167.2005.50028.x. [DOI] [PubMed] [Google Scholar]
- 5.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–26. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brachmann J, Beyer T, Schmitt C, et al. Electrophysiologic and antiarrhythmic effects of D-sotalol. J Cardiovasc Pharmacol. 1992;20(II):91–5. [PubMed] [Google Scholar]
- 7.Opolski G, Torbicki A, Kosior DA, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004;126:476–86. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 8.Reimold SC, Cantillon CO, Friedman PL, Antman EM. Propafenone versus sotalol for suppression of recurrent symptomatic atrial fibrillation. Am J Cardiol. 1993;71:558–63. doi: 10.1016/0002-9149(93)90511-a. [DOI] [PubMed] [Google Scholar]
- 9.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 10.Sicouri S, Belardinelli L, Carlsson L, Antzelevitch C. Potent antiarrhythmic effects of chronic amiodarone in canine pulmonary vein sleeve preparations. J Cardiovasc Electrophysiol. 2009;20:803–10. doi: 10.1111/j.1540-8167.2009.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. PACE. 2006;29:290–5. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Wongcharoen W, Chen YC, Chen YJ, et al. Aging increases pulmonary veins arrhythmogenesis and susceptibility to calcium regulation agents. Heart Rhythm. 2007;4:1338–49. doi: 10.1016/j.hrthm.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Lo LW, Chen YC, Chen YJ, et al. Calmodulin kinase II inhibition prevents arrhythmic activity induced by alpha and beta adrenergic agonists in rabbit pulmonary veins. Eur J Pharmacol. 2007;571:197–208. doi: 10.1016/j.ejphar.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 16.Sicouri S, Carlsson L, Antzelevitch C. Electrophysiologic and antiarrhythmic effects of AZD1305 in canine pulmonary vein sleeves. J Pharmacol Exp Ther. 2010;334:255–9. doi: 10.1124/jpet.110.166702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sicouri S, Gianetti B, Zygmunt AC, Cordeiro JM, Antzelevitch C. Antiarrhythmic effects of simvastatin in canine pulmonary vein sleeve preparations. J Am Coll Cardiol. 2011;57:986–93. doi: 10.1016/j.jacc.2010.08.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haissaguerre M, Jais P, Shah DC, et al. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409–17. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 19.Nattel S, Allessie MA, Haissaguerre M. Spotlight on atrial fibrillation-the 'complete arrhythmia'. Cardiovasc Res. 2002;54:197–203. doi: 10.1016/s0008-6363(02)00324-3. [DOI] [PubMed] [Google Scholar]
- 20.Nattel S. Combined parasympathetic-sympathetic nerve discharge and pulmonary vein afterdepolarizations: A new unifying concept with basic and clinical relevance. Heart Rhythm. 2005;2:632–3. doi: 10.1016/j.hrthm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Chen YJ, Chen SA, Chang MS, Lin CI. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000;48:265–73. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen YJ, Chen SA. Electrophysiology of pulmonary veins. J Cardiovasc Electrophysiol. 2006;17:220–4. doi: 10.1111/j.1540-8167.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 23.Sicouri S, Glass A, Carlsson L, Antzelevitch C. Electrophysiologic and antiarrhythmic effects of AZD1305 in canine pulmonary vein sleeves. Heart Rhythm. 2008;5S:S163. doi: 10.1124/jpet.110.166702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–60. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 25.Roy D, Pratt CM, Torp-Pedersen C, et al. Vernakalant hydrochloride for rapid conversion of atrial fibrillation. A phase 3, randomized, placebo-controlled trial. Circulation. 2008;117:1518–25. doi: 10.1161/CIRCULATIONAHA.107.723866. [DOI] [PubMed] [Google Scholar]
- 26.Stiell IG, Roos JS, Kavanagh KM, Dickinson G. A multicenter, open-label study of vernakalant for the conversion of atrial fibrillation to sinus rhythm. Am Heart J. 2010;159:1095–101. doi: 10.1016/j.ahj.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay BD. Vernakalant: additional evidence for safety and efficacy for new onset atrial fibrillation. J Am Coll Cardiol. 2011;52:322–3. doi: 10.1016/j.jacc.2010.08.633. [DOI] [PubMed] [Google Scholar]
- 28.Eldstrom J, Wang Z, Xu H, et al. The molecular basis of high-affinity binding of the antiarrhythmic compound vernakalant (RSD1235) to Kv1.5 channels. Mol Pharmacol. 2007;72:1522–34. doi: 10.1124/mol.107.039388. [DOI] [PubMed] [Google Scholar]
- 29.Dorian P, Pinter A, Mangat I, et al. The effect of vernakalant (RSD1235), an investigational antiarrhythmic agent, on atrial electrophysiology in humans. J Cardiovasc Pharmacol. 2007;50:35–40. doi: 10.1097/FJC.0b013e3180547553. [DOI] [PubMed] [Google Scholar]
- 30.Savelieva I, Camm J. Anti-arrhythmic drug therapy for atrial fibrillation: current anti-arrhythmic drugs, investigational agents, and innovative approaches. Europace. 2008;10:647–65. doi: 10.1093/europace/eun130. [DOI] [PubMed] [Google Scholar]
- 31.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–57. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17:S161–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antzelevitch C, Belardinelli L, Zygmunt AC, et al. Electrophysiologic effects of ranolazine: a novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004;110:904–10. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antzelevitch C, Belardinelli L, Wu L, et al. Electrophysiologic properties and antiarrhythmic actions of a novel anti-anginal agent. J Cardiovasc Pharmacol Therapeut. 2004;9(Suppl 1):S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 35.Maier LS, Hasenfuss G. Role of [Na+]i and the emerging involvement of the late sodium current in the pathophysiology of cardiovascular disease. Eur Heart J Suppl. 2006;8:A6–A9. [Google Scholar]
- 36.Rosen MR, Danilo P., Jr. Effects of tetrodotoxin, lidocaine, verapamil and AHR-266 on ouabain induced delayed afterdepolarizations in canine Purkinje fibers. Circ Res. 1980;46:117–24. doi: 10.1161/01.res.46.1.117. [DOI] [PubMed] [Google Scholar]
- 37.Singh BN, Nademanee K. Sotalol: a beta blocker with unique antiarrhythmic properties. Am Heart J. 1987;114:121–39. doi: 10.1016/0002-8703(87)90316-4. [DOI] [PubMed] [Google Scholar]
- 38.Freemantle N, Lafuente-Lafuente C, Mitchell S, Eckert L, Reynolds M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide and propafenone, for the management of atrial fibrillation. Europace. 2011;13:329–45. doi: 10.1093/europace/euq450. [DOI] [PubMed] [Google Scholar]
- 39.Campbell TJ. Cellular electrophysiological effects of D- and DL-sotalol in guinea-pig sinoatrial node, atrium and ventricle and human atrium: differential tissue sensitivity. Br J Pharmacol. 1987;90:593–9. doi: 10.1111/j.1476-5381.1987.tb11210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson E, Scherlag BJ, Lazzara R. Early afterdepolarizations produced by d,l-sotalol and clofilium. J Cardiovasc Electrophysiol. 1997;8:667–78. doi: 10.1111/j.1540-8167.1997.tb01830.x. [DOI] [PubMed] [Google Scholar]
- 41.Sasyniuk BI, Brunet S. Torsade de pointes induced by quinidine, d-sotalol, and E-4031 in the isolated rabbit heart: Importance of interval dependent dispersion of repolarization. PACE. 1995;18:II–904. [Google Scholar]
- 42.Sicouri S, Moro S, Elizari MV. d-Sotalol induces marked action potential prolongation and early afterdepolarizations in M but not epicardial or endocardial cells of the canine ventricle. J Cardiovasc Pharmacol Ther. 1997;2:27–38. doi: 10.1177/107424849700200104. [DOI] [PubMed] [Google Scholar]
- 43.Hohnloser SH. Proarrhythmia with class III antiarrhythmic drugs: types, risks, and management. Am J Cardiol. 1997;80:82G–9G. doi: 10.1016/s0002-9149(97)00717-0. [DOI] [PubMed] [Google Scholar]
- 44.Marinelli A, Capucci A. Antiarrhythmic drugs for atrial fibrillation. Expert Opin Pharmacother. 2011;12:1201–15. doi: 10.1517/14656566.2011.554400. [DOI] [PubMed] [Google Scholar]
- 45.Camm AJ. Antiarrhythmic drugs for the maintenance of sinus rhythm: Risks and benefits. Int J Cardiol. doi: 10.1016/j.ijcard.2011.06.012. In Press. [DOI] [PubMed] [Google Scholar]
- 46.Torp-Pedersen C, Raev DH, Dickinson G, et al. A randomized placebo-controlled study of vernakalant (oral) for the prevention of atrial fibrillation recurrence post-cardioversion. Circ Arrhythm Electrophysiol. doi: 10.1161/CIRCEP.111.962340. In Press. [DOI] [PubMed] [Google Scholar]
- 47.Antzelevitch C, Burashnikov A. Atrial-selective sodium channel block as a novel strategy for the management of atrial fibrillation. J Electrocardiol. 2009;42:543–8. doi: 10.1016/j.jelectrocard.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murdock DK, Kersten M, Kaliebe J, Larrian G. The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a review of experience with implications for possible “pill in the pocket” approach to atrial fibrillation. Indian Pacing Electrophysiol J. 2009;9:260–7. [PMC free article] [PubMed] [Google Scholar]
- 49.Murdock DK, Reiffel JA, Kaliebe JW, Larrian G. The conversion of paroxysmal of initial onset of atrial fibrillation with oral ranolazine: implications for “pill in the pocket” approach in structural heart disease. J Am Coll Cardiol. 2010;55:A6.E58. [Google Scholar]
- 50.Antzelevitch C, Burashnikov A, Sicouri S, Belardinelli L. Electrophysiological basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8:1281–90. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murdock DK, Passman RS, Subacius H, Miles RH. Ranolazine verses amiodarone for atrial fibrillation prophylaxis following coronary bypass surgery. J Am Coll Cardiol. 2011;57:E155. [Google Scholar]
- 52.Hohnloser SH, Singh BN. Proarrhythmia with class III antiarrhythmic drugs: definition, electrophysiologic mechanisms, incidence, predisposing factors and clinical implications. J Cardiovasc Electrophysiol. 1995;6:920–36. doi: 10.1111/j.1540-8167.1995.tb00368.x. [DOI] [PubMed] [Google Scholar]