Abstract
Modifying surfaces of nanoparticles (NPs) with polyethylene glycol (PEG), the so called PEGylation, is the most commonly used method for reducing premature clearance of NPs from the circulation. However, several reports point out that PEGylation may negatively influence the performance of NPs as a drug carrier. Alternative surface modification strategies, including substitute polymers, conditional removal of PEG, and biomimetic surface modification, may provide solutions for the limitations of PEG.
Introduction
Chemotherapy is an indispensable modality in the treatment of most solid tumors, but its side effects often limit its benefits and impact the patients’ quality of life. Moreover, repeated chemotherapy can induce multidrug resistance (MDR) in the tumors,1–3 which then require extremely high doses of anticancer drugs, often making the regimens intolerable due to the significant toxicity.2
To address the shortcomings of existing chemotherapeutic agents, many researchers are developing new drug delivery methods that can transport medicines specifically to the target tumors without damaging healthy tissues or organs. A popular strategy is to use nanoparticles (NPs) such as polymeric NPs, liposomes, and micelles as a drug carrier. Upon systemic administration, the NP formulations display different pharmacokinetics than free drugs and enhance accumulation and release of drugs in tumors. Due to their ability to accumulate in tumors, NPs are also explored as a promising diagnostic tool.
NP delivery to solid tumors relies on the unique anatomical and physiological features of tumors and their environment. As a tumor grows, demands for nutrients, gas exchange, and waste removal of the highly metabolic cancer cells escalate as does their need for new blood vessels.4–6 The formation of new blood vessels involves extension of existing vasculature toward the tumor tissues4, 7 and recruitment of the progenitor endothelial cells.8 Soluble growth factors such as vascular endothelial growth factor and basic fibroblast growth factor promote expansion of the tumor vasculature.8–11 Due to the imbalance between pro- and antiangiogenic factors, growth of tumor blood vessels is poorly regulated, resulting in disorderly expansion of the vasculature.11–13 Consequently, tumor vasculature tends to have defective architecture with pore sizes ranging from 100 to 780 nm,14 allowing for extravasation of NPs within this size range. Another common feature of solid tumors is poor lymphatic drainage,15, 16 which prevents efficient removal of macromolecules, including NPs, from tumors.11, 17 This phenomenon, the so called “enhanced permeability and retention (EPR) effect,17–21 has been the foundation of most NP-based tumor-targeting strategies22 since the first report in 1986.15
“STEALTH COATING”: A STRATEGY TO INCREASE BIOAVAILABAILABILITY OF NANOPARTICLES
To take advantage of the EPR effect, NPs need to circulate for a prolonged period. One significant obstacle to the long-term circulation of NPs is clearance by the reticuloendothelial system (RES),23, 24 whose main role is to protect the body from the invasion of extraneous particles. The removal of NPs is initiated by interactions between foreign particles and the phagocytic cells in the blood (e.g., monocytes, neutrophils) and tissues (e.g., Kupffer cells, dendritic cells, macrophages).25–27 This process is facilitated by adsorption of plasma proteins (opsonins), such as IgG or complement fragments, onto the particle surface, which labels the NPs as a foreign substance.28–30 The opsonized NPs are ultimately eliminated by receptor-mediated phagocytosis.30, 31
Many studies have shown that NPs administered intravenously are cleared from the blood by RES within minutes, if the NP surface is not protected from opsonization.23, 24, 32, 33 The circulation half-life of NPs depends on their size,34 shape,35, 36 surface chemistry,37, 38 surface charge,34, 39 and chemical composition of NP matrix.36 Hydrophobic and/or charged NPs have shorter circulation half-lives due to significant opsonization.34, 39 Therefore, NPs developed for systemic application are almost always coated with an electrically neutral hydrophilic surface layer, the so called “stealth coating”. The circulation half-life of such NPs can be thus extended to >40 hours by the stealth coating.40
PEGYLATION FOR STEALTH COATING
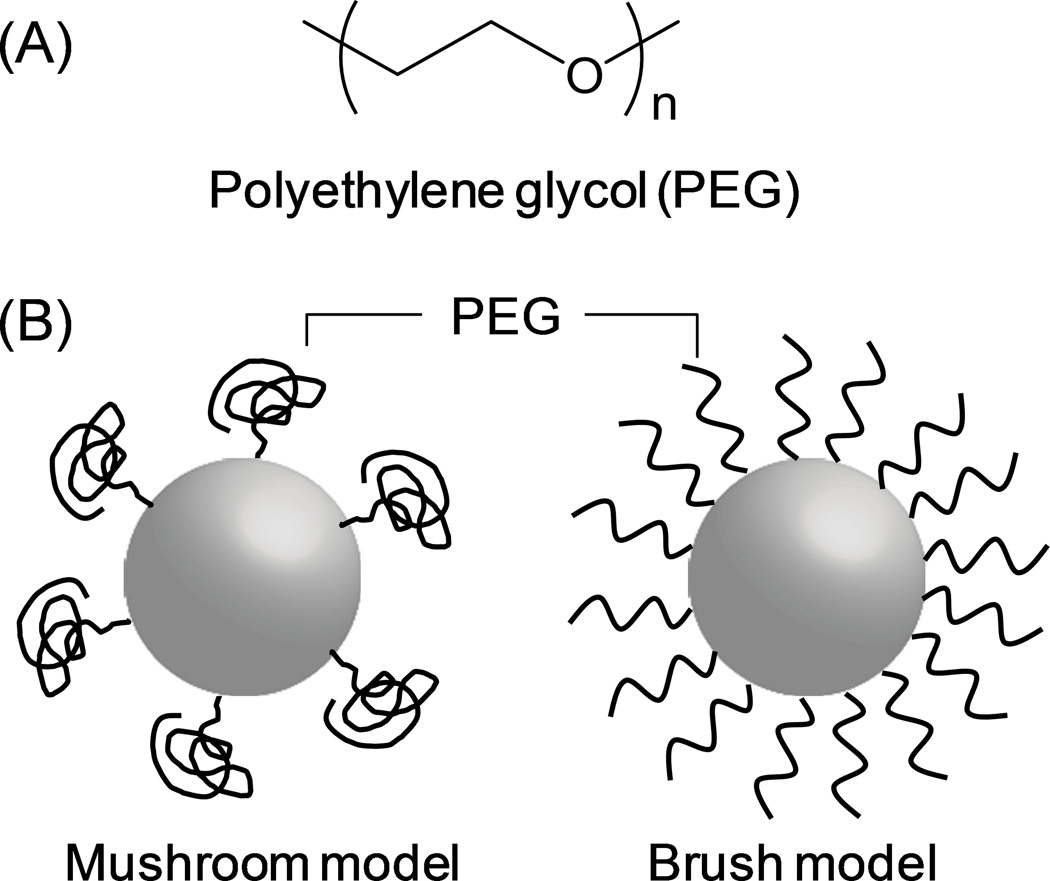
In the past three decades, the majority of surface stabilization of NPs has been carried out with non-ionic hydrophilic polymers and/or surfactants. In particular, polyethylene glycol (PEG) (Fig. 1A) is used in the majority of current studies. In 1977, Abuchowski et al reported that covalent conjugation of 2 kDa or 5 kDa PEG (”PEGylation”) to bovine liver catalase decreased immunogenicity of the protein and increased its circulating time in blood.41 Since then PEG (typically 5 kDa42) has been widely used in a variety of NP systems, such as liposomes,43–45 polymeric NPs,29, 46, 47 and micelles.48, 49
Fig. 1.
(A) PEG structure; (B) model of PEGylated NPs.
PEG forms a flexible layer on the surface of NPs,50 preventing the adsorption of opsonins51, 52 via steric hindrance53 and their subsequent uptake by phagocytic cells.54 The PEG surface layer is often described as a mushroom or a brush model (Fig. 1B). When the surface PEG density is relatively low (for example, 0.5–0.7 mol% PEG 5kDa in liposomes), it forms a mushroom-like structure to maximize surface coverage.55, 56 As the PEG density increases, the PEG chains extend to avoid overlap with other PEG molecules, resulting in a brush model.42, 56
The reported optimal PEG content varies by system. For example, 10 wt% PEG density was considered optimal for poly(lactic acid) (PLA) NPs39 or poly(lactide-co-glycolide) (PLGA) NPs57 with respect to the particle dispersibility and stealth effect. On the other hand, Gref et al reported that the optimal PEG surface coverage for PLA, PLGA, and polycaprolatone NPs was 5 wt%, and that higher levels of PEG content did not further reduce protein adsorption.42 Five to seven mol% PEG, which is at the borderline between mushroom and brush, is reported as optimal for obtaining thermodynamically stable liposomes.56 In this range, PEG dehydrates the lipid head group and decreases defects in lipid bilayer. Further increase in PEG content (>10 mol%) destabilizes liposomes due to repulsion between the neighboring PEG chains.56
The effects of PEGylation on the prolongation of the NP circulation are well established. Verrecchia et al reported that PEGylated NPs showed higher plasma concentration and lower accumulation in the liver than non-PEGylated NPs.58 At 6 hours after injection, 10% of PEGylated PLA NPs, but only 0.4% of non-PEGylated PLGA NPs remained in circulation.58 Uptake of PEGylated PLA NPs by the liver was significantly lower than that of non-PEGylated PLA NPs (11% vs. 20% of injected dose).58
EXAMPLES OF PEGYLATED NPS
Liposomes
Liposomes consist of one or more concentric spheres of lipid bilayers, which are separated by aqueous compartments.59 Liposomal doxorubicin (Doxil® or Caelyx®) is indicated for the treatment of patients with metastatic breast cancer, ovarian cancer, or Kaposi sarcoma.60 For long-term circulation of liposomes, surface protection with PEG is an essential part of the preparation. Liposome PEGylation is performed by mixing PEG–lipid conjugates and other lipid components at a fixed ratio.
Polymeric NPs
Polymeric NPs are made of hydrophobic and mostly biodegradable polymers. They contain drugs in the polymer matrix and release them by diffusion and/or matrix degradation. PEGylated polymeric NPs are prepared either by making NPs with block co-polymers of PEG and hydrophobic polymer61 or grafting PEG on the surface of pre-formed NPs. When PEG is grafted on the surface of pre-formed NPs, PEG with amine termini is covalently conjugated to reactive functional groups (e.g., carboxylic groups) exposed on the NP surface.62 Alternatively, PEG can be attached to the NP surface using avidin-biotin interaction.63
Polymeric micelles
Polymeric micelles are formed by self-assembly of amphiphilic block copolymers, which consist of hydrophilic and hydrophobic segments. When dispersed in water along with hydrophobic drugs, the amphiphilic block copolymer assembles into 30–50 nm spheres with a core-shell structure, in which drugs and hydrophobic segments form the core and the hydrophilic segment interfaces with the aqueous medium.64 Polymeric micelles usually form at a relatively low concentration compared to surfactant micelles and thus are more stable in circulation.65 PEGylation of polymeric micelles is achieved by employing PEG as the hydrophilic segment. Polymeric micelle formulations are currently in clinical trials for the treatment of various cancers.66–68
THE “PEG DILEMMA”
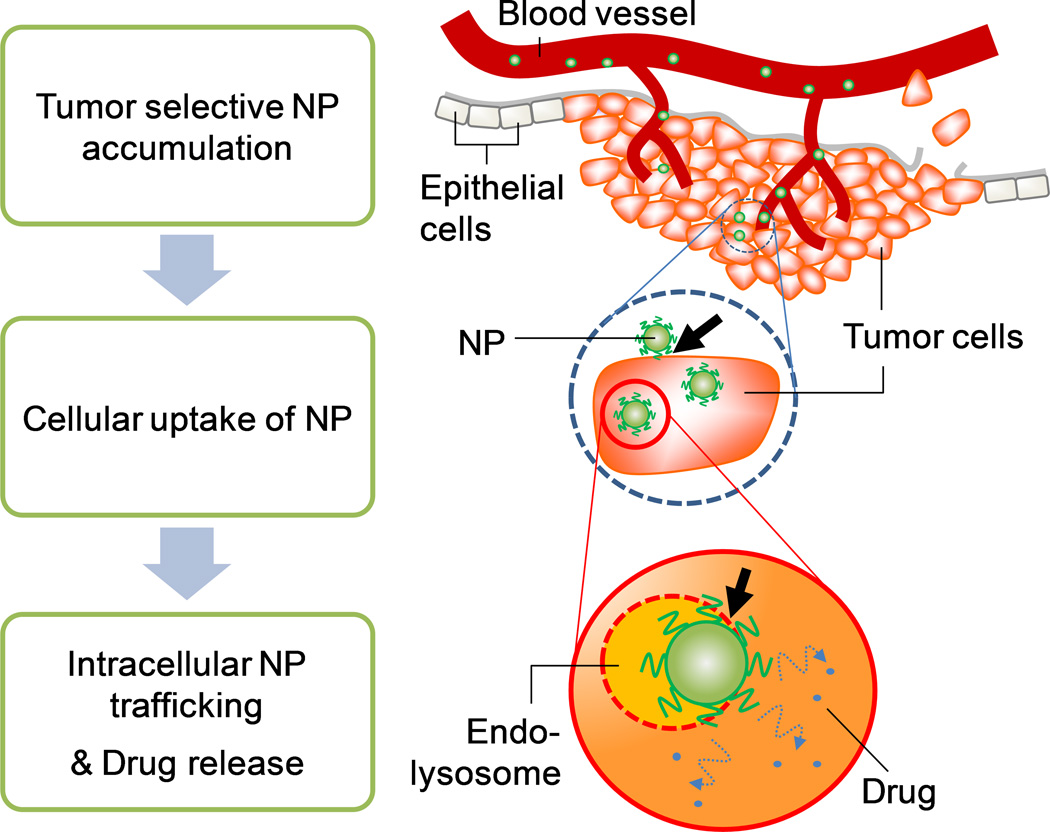
Interference with cellular uptake and endosomal escape of NPs
While PEG has been successfully used for surface protection of various NPs, recent studies recognize its disadvantages.69–74 Drug delivery using NPs often involves cellular uptake of NPs, especially when the drug does not freely enter the target cells by itself (e.g., nucleic acids) or when it is constantly removed from the cells (e.g., multidrug resistance). The extravasated NPs are still multiple steps away from cellular entry (Fig. 2). NPs go through (i) transport in the extracellular matrix, (ii) attachment to the cell membrane via receptors,75 (iii) internalization into the cells,76 (iv) escape from intracellular vesicles and drug release to the cytosol, and (v) transport to target organelles.77 The so-called “PEG dilemma” is that the PEG coating, an essential part of a NP until extravasation, interferes with NP-cell interactions and the endosomal escape of NPs after extravasation.78 Although the extent of PEG interference may vary with particle types or sizes, it significantly compromises intracellular delivery of drugs or genes by various NPs.69, 79
Fig. 2.
Schematic description of NP-based drug delivery to tumors. Arrows indicate steps negatively influenced by PEG layer on NP surface.
For example, PEGylated liposomal doxorubicin showed less tumor accumulation than non-PEGylated liposomes, indicating PEG interference with the cell-liposome interactions.80 Moreover, Hatakeyama et al discussed in their recent review article that PEGylated multifunctional envelope-type nano device (MEND) showed a significantly lower gene expression than unmodified MEND.69 Mishra et al also demonstrated that PEGylation of non-viral gene vectors (branched polyethyleneimine (bPEI) or β-cyclodextrin-containing polymer) led to significant reduction in gene expression.81 Electron microscopy revealed that the gene vectors with bare cationic surfaces entered cells as large aggregates, whereas PEGylated bPEI NPs remained small and discrete, both outside and inside the cells.81 These results indicate that PEGylated NPs are less effective in entering cells and escaping intracellular vesicles. Remaut et al investigated the intracellular fate of oligonucleotides delivered by PEGylated liposomes using fluorescence resonance energy transfer (FRET) microscopy.82 PEG layer interfered with endosomal escape of liposomes, resulting in degradation of the oligonucleotides.82
Immune responses
In addition to its effects on the cellular uptake and endosomal escape, PEGylation raises other concerns. Recently, immune reactions to PEGylated liposomes have been reported.71, 73, 74, 83–88 Ishida et al demonstrated that PEGylated liposomes were rapidly cleared from blood upon repeated injections.73, 83, 89, 90 The accelerated blood clearance (ABC) of the second dose of PEGylated liposomes was caused by the binding of PEG-specific IgM, produced by the first dose of liposomes,73, 87 and the subsequent activation of the complement system.73, 91 The liposomes cleared from the circulation accumulated in the liver and to a lesser degree in the spleen.85, 88 Induction of the IgM response depended on the spleen84 but not on T cells,74 indicating that the immune response against PEGylated liposomes was mediated by splenic B cells in a T-cell independent manner. On the other hand, the ABC response was not seen with doxorubicin-loaded PEGylated liposomes and/or high dose of PEGylated liposomes, presumably due to their detrimental effects on splenic B cells71 or depletion of blood opsonins.92 In this case, a significant fraction of NPs tend to accumulate in the spleen, and the NPs delivering cytotoxic drugs may avoid accelerated clearance by inhibiting the proliferation of the splenic B cells. However, the immune response to PEGylated nanomedicines can be a significant issue for the delivery of other drugs or for low-dose applications, adversely affecting their pharmacokinetics and biodistribution profiles. For instance, PEG-asparaginase administered for the treatment of acute lymphoblastic leukemia was reported to be cleared rapidly in one third of the treated patients, potentially decreasing effectiveness of the treatment.93
ALTERNATIVE STRATEGIES FOR PROTECTING NP SURFACES
Alternative polymers
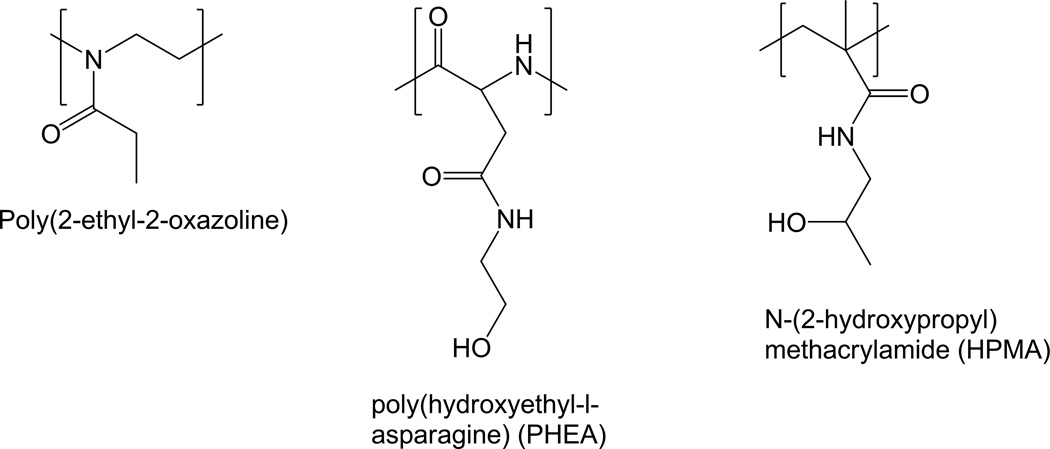
Polyoxazolines
Polyoxazolines (POZ) have been explored as a hydrophilic segment in amphiphilic block-co-polymer. Poly(2-ethyl-2-oxazoline) (Fig. 3) was coupled with poly(caprolactone),94, 95 poly(aspartic acid),96 or poly(1,3-trimethylene carbonate)97 to form polymeric micelles. POZ was also used in preparing liposomes and shown to be comparable to PEG in stealth effects.98 Poly(2-methyl-2-oxazoline) was grafted to poly(l-lysine) as a PEG alternative for non-viral gene delivery.99 A recent study reported a series of POZ-based amphiphiles, demonstrating versatility of synthetic manipulation of POZ and cytocompatibility of the POZ-based polymers.100
Fig. 3.
Structures of poly(2-ethyl-2-oxazoline), PHEA, and HPMA.
Poly(amino acids)
Poly(amino acids) such as poly(hydroxyethyl l-glutamine) or poly(hydroxyethyl-l-asparagine) (PHEA) (Fig. 3) have been developed as potential stealth polymers.101 Unlike PEG, poly(amino acids) are readily degraded by proteases and thus may reduce the risk of accumulation and related toxicity.102 These polymers were able to prolong the blood circulation of NPs to a similar extent as PEG.101, 102 In particular, PHEA-coated liposomes were superior to PEGylated liposomes in resisting ABC after repeated administration and in maintaining the stealth effect at low lipid doses.103
N-(2-hydroxypropyl)methacrylamide (HPMA)
First synthesized by Kopecek et al,104 HPMA (Fig. 3) and its derivatives have been widely explored as macromolecular drug carriers.105 HPMA polymers have many attractive features for drug delivery, including biocompatibility, hydrophilicity, and ability to accommodate structural modifications.105, 106 HPMA has been conjugated to various drugs107, 108 and targeting moieties.109, 110 HPMA conjugation increases the circulation time of low molecular weight drugs, allowing for EPR-mediated tumor accumulation.105 To facilitate intracellular drug release, drugs are conjugated via an enzymatically cleavable peptide linker (e.g., GFLG).111
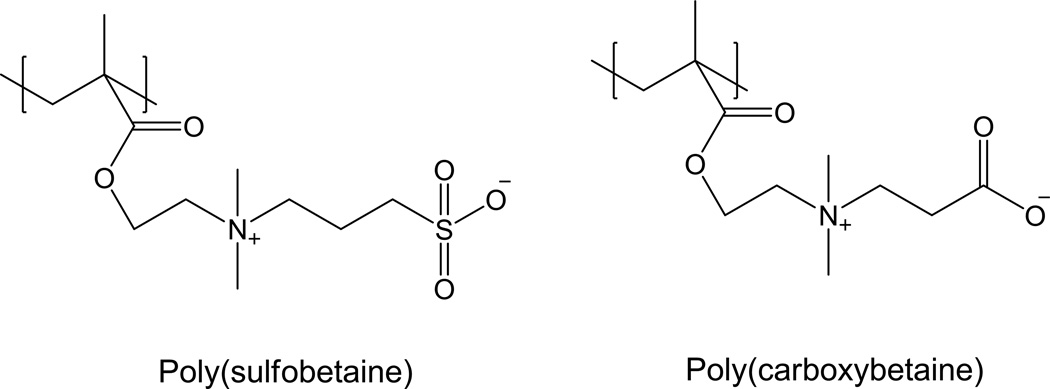
Polybetaines
Betaines such as sulfobetaine and carboxybetaine are zwitterionic molecules, which bind water molecules via electrostatic interactions,112, 113 more strongly than those relying on hydrogen bonding.114 Polymers based on betaines (Fig. 4) greatly reduce non-specific protein adsorption,115 bacterial adhesion and biofilm formation116, 117 on various surfaces. Moreover, poly(carboxybetaine) has multiple functional groups amenable to multivalent conjugations, providing a useful platform for multi-functional nanomedicines.112 For these reasons, polybetaines have generated certain interest as alternative non-fouling materials for NP modification. For example, poly(carboxybetaine) has been used to modify a variety of NPs such as silica,118 gold,119 iron oxide,120 PLGA,121 and hydrogel NPs.122, 123 These NPs showed excellent size stability in protein solutions including serum, indicating strong resistance to non-specific protein adsorption.118–121, 123
Fig. 4.
Structures of poly(sulfobetaine) and poly(carboxybetaine)
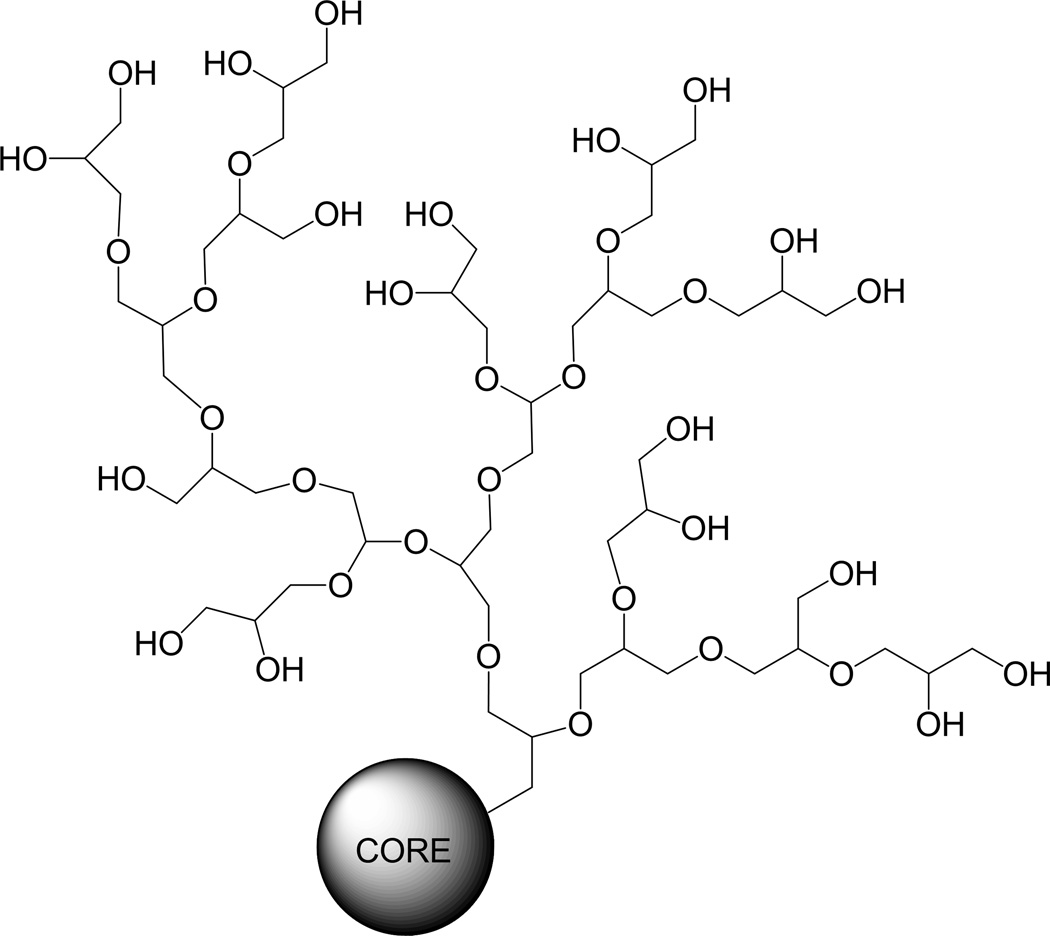
Polyglycerols
Polyglycerols (also known as polyglycidols) (Fig. 5) are biocompatible and flexible hydrophilic aliphatic polyether polyols, prepared in branched or linear forms.124, 125 The anti-fouling effect of hyperbranched polyglycerols is comparable to PEG, while they are less susceptible to oxidation or thermal stress than PEG.126 In addition, polyglycerols contain multiple hydroxyl groups, which can be further functionalized.126 The long plasma half-lives of hyperbranced polyglycerols (33 hours for 106 kDa and 57 hours for 540 kDa) indicate their promises as stealth polymers.127 Polyglycerols were used to prolong liposome circulation128 and to prevent protein adsorption to gold surface.126 A recent study reported a new liposome system covered with a block-copolymer of PEG and hyperbranched polyglycerol, where the polyglycerol moieties facilitated multivalent functionalization of the liposome.129
Fig. 5.
Structure of hyperbranched polyglycerol127.
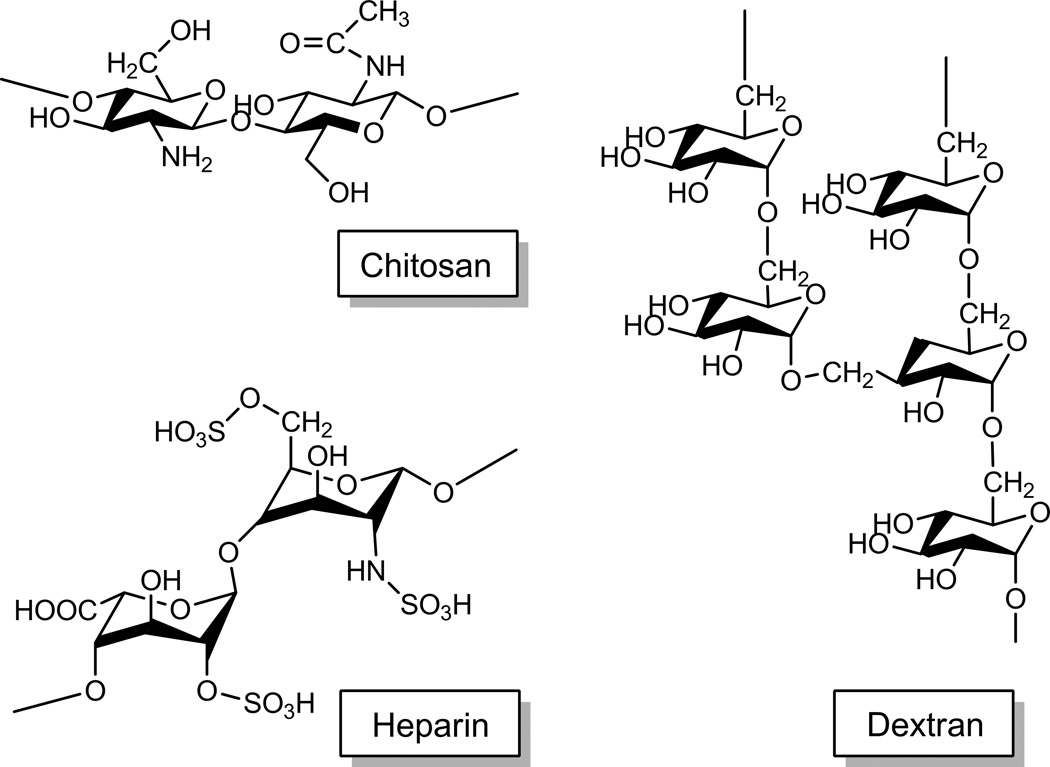
Polysaccharides
NPs are prepared with derivatives of chitosan,130, 131 dextran,132, 133 hyaluronic acid,134 and heparin,135–137 in which the polysaccharides (Fig. 6) provide hydrophilic shells on the NP surface. Advantages of polysaccharides are biodegradability, low immunogenicity and toxicity,135, 138, 139 and abundant functional groups useful for conjugation of drugs or cell-interactive ligands. These polysaccharide-based NPs extend circulation times of the loaded drugs and enhance their accumulation in tumors. In particular, chitosans assume a positive charge and thus can improve cellular interactions of NPs at weakly acidic pH. This property can be utilized to achieve specific drug delivery to acidifying tumors.
Fig. 6.
Structures of chitosan, heparin, and dextran.
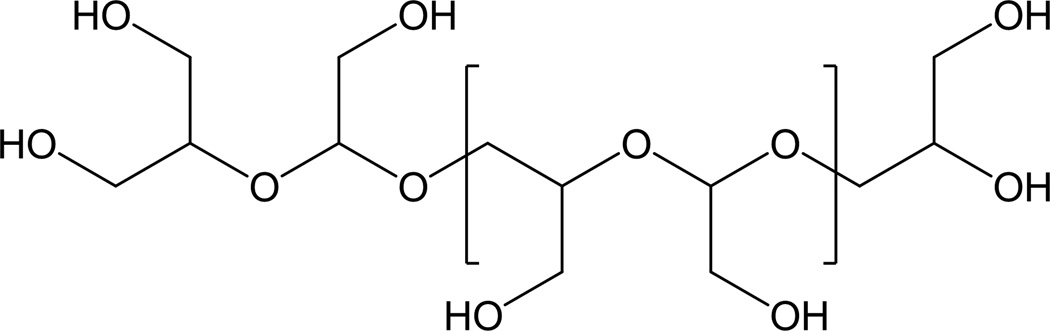
Papisov at al proposed to use acyclic hydrophilic polyacetals (Fig. 7) derived from polycarbohydrates to replace PEG.140 The advantages of hydrophilic polyacetals, as compared with PEG, are biodegradability and availability of readily modifiable functional groups.140 Moreover, a polylysine grafted with polyacetal had a much longer blood half-life than a polylysine grafted with dextran, the original polysaccharide that polyacetal was derived from.140 This difference was attributed to elimination of rigid stereospecific structures of dextran.140
Fig. 7.
Poly(hydroxymethylethylene hydroxymethylformal)
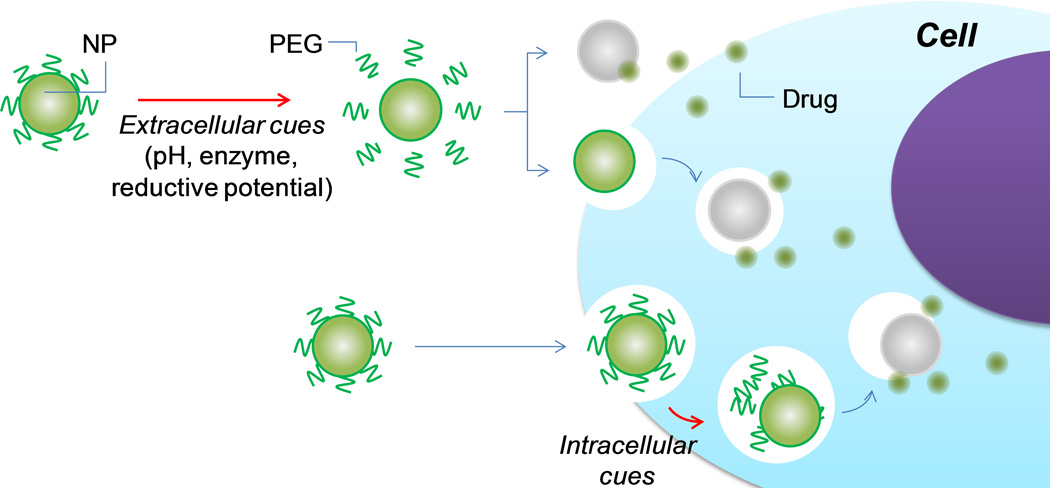
Conditional removal of PEG effect
Triggered by cellular cues (Fig. 8)
Fig. 8.
DePEGylation of NPs by cellular cues
pH change
Environmental features unique to tumors and extracellular matrices have been exploited as a way of removing the protective effect of PEG in a tumor-specific manner. One such feature is the slight acidity of tumors,141–143 induced by the increased glycolysis and plasma membrane proton-pump activity of tumor cells, which results in greater lactic acid production than in normal cells and in leakage of acid into the extracellular milieu.144, 145 Moreover, many solid tumors develop hypoxia,146, 147 which further induces or selects for hyperglycolytic cells, enhancing local acidosis.148 Therefore, many tumor tissues show weakly acidic pH,149–151 compared to the blood and normal tissues (pH 7.4).
pH-sensitive PEGylated liposomes have been proposed to take advantage of the tumor microenvironment pH.152–154 Sawant et al reported a liposome system with a pH-sensitive hydrazone linker between the surface and PEG layer.154 The PEG layer shielded liposomes at pH 7.4 and detached at pH 5–6 revealing a cell adhesive peptide, which helped liposomes to interact with cells at the acidic pH.154 pH-sensitive micelles have been developed for a similar purpose. Lee et al reported polymeric micelles composed of poly(l-histidine)-PEG diblock copolymer,155 where poly(1-histidine) (molecular weight: 5 kDa) lost hydrophobicity at pH 7 due to the ionization of the imidazole group.156 Such a transition in the ionization status of the polymer allowed gradual destabilization of the micelles and release of the encapsulated drug at acidic pH. Stability of micelles, and hence the pH at which the micelle destabilization is triggered, could be controlled by blending a pH-insensitive PLA-PEG block copolymer156 and/or introducing a pH-insensitive polymer block (poly(l-phenylalanine)) to the existing pH-sensitive polymer.157, 158
Depending on the trigger pH, pH-responsive PEG removal can enhance endosomal escape of NPs. Late endosomes and lysosomes have a pH of 5–5.5.159 pH-sensitive micelles or liposomes that destabilize at this pH allow for endosomal drug release and disruption of endosomal membrane.142, 160 Mohajer et al showed that the enhancement of endosomal drug release using pH-sensitive micelles helped cytosolic delivery of anticancer drugs and significantly increased the intracellular drug levels in MDR tumor cells.161 A pH-sensitive micelle system, combined with a cell-interactive ligand (e.g., folate), suppressed the growth rate of MDR ovarian tumors, to a much greater extent than pH-insensitive micelles.158 The effectiveness of the pH-sensitive micelles in MDR tumors was attributed to accelerated endosomal drug release, which enabled a high cytosolic drug concentration and increased intracellular drug diffusion, providing room for the subsequently delivered drug.158 In another example, a pH-sensitive hydrazone linker was used in DNA-lipopolyplexes to induce endosomal removal of PEG (dePEGylation) and increase gene transfection efficiency.162 First, cholesterol linked to PEG via a pyridine hydrazone linker was included in the liposomes. DNA-lipopolyplexes were then prepared by mixing DNA-polyethylenimine complex with the pH-sensitive liposomes.162 Lipopolyplexes were aggregated in pH 5.4, indicating acid-induced removal of PEG from the lipid surface.162 Because of the intracellular dePEGylation, the lipopolyplexes with cleavable PEG were 40 times more effective in gene delivery compared to those with non-cleavable PEG.162
Enzymatic stimuli
Another unique property common to many solid tumors is overexpression of proteinases, such as matrix metalloproteinases (MMPs),163 which play a critical role in invasion of tumor cells and angiogenesis.164 Several studies have employed MMPs to trigger PEG cleavage.163, 165, 166 Terada et al reported a galactosylated liposome system containing PEG-peptide-dioleoylphosphatidyl ethanolamine (DOPE) ternary conjugate.165 PEG was removed as the peptide linker was cleaved by MMP-2. Upon removal of PEG, galactose moieties on liposome surface was exposed and recognized by asialoglycoprotein receptors on cancer cells. The ligand-receptor interaction increased cellular uptake of liposomes and cytotoxicity of the liposomes containing an anti-cancer drug.165 Hatakeyama et al used the MMP-2 sensitive MEND for delivery of plasmid DNA167 or siRNA.166 The MMP-sensitive MEND was superior to the MMP-insensitive PEGylated MEND in cellular uptake and endosomal escape.166 The in vitro results translated to relatively higher tumor accumulation and gene silencing effect in tumors.166
Enzymatically cleavable PEG linkers may be employed not only in drug/gene delivery but also in imaging agents. A quantum dot (QD) decorated with a cell-penetrating peptide was PEGylated via a MMP-2 cleavable peptide linker.168 Cellular uptake of QDs occurred in response to the presence of MMP-2, as a result of MMP-2 induced dePEGylation of QDs.168 Similarly, gold nanorods were coated with PEG via a peptide linker cleaved by urokinase-type plasminogen activator (uPA), specifically expressed in malignant tumors. In the presence of uPA, the PEG-peptide-modified gold nanorods showed more efficient cell-binding and tumor accumulation than control nanorods with a non-cleavable linker.163 On the other hand, a challenge in enzymatically cleavable PEG systems is to find an optimal PEG density. While PEG density should be sufficiently high to protect the NPs during circulation, dense PEG chains can interfere with enzyme access to the substrate linker.163
Reductive potential
Another unique feature of many tumors is the reductive environment due to overexpression of reductase enzymes169, 170 or release of glutathione following cell death.171 Ren et al reported a micelle system consisting of PEG-polyleucine diblock copolymer, in which PEG and polyleucine were linked via the disulfide bond.172 The micelles dissociated and released the incorporated drug in response to a reducing agent, which caused disulfide cleavage.172 Cytotoxicity of doxorubicin loaded in these reducible micelles was significantly enhanced when cells were pretreated with 10 mM glutathione.172 Based on the same principle, PEG was linked to nucleoside-lipid via disulfide bond.173 Micelles and liposomes prepared with the reducible PEG-nucleolipid showed changes in particle size and surface charge upon treatment with a reducing agent, reflecting reduction-induced dePEGylation. Detachment of PEG increased cellular uptake of micelles and liposomes.173
Triggered by external cues
Thermal stimuli
Thermal stimuli can be applied to enhance drug delivery from NPs after their accumulation at tumor site.174, 175 Li et al reported a thermosensitive liposome system containing relatively high contents (5 mol%) of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-PEG (2000 Da) (DSPE-PEG2000) as compared to typical PEGylated liposomes (< 5 mol%).174 The thermosensitivity came from the balanced membrane stability. High density of surface-grafted polymers (PEG) disturbed membrane integrity due to the transition in PEG configuration.176 Liposomes containing 5 mol% DSPE-PEG2000 had the optimal balance between stability and temperature sensitivity.174 This liposome system released the payload in response to mild hyperthermia at 42°C,174 providing a way of increasing drug release at target tissues in a stimulus-dependent manner. Stability of PEGylated liposomes can be alternatively controlled by the particle size.177 Hossann et al demonstrated that particle size was inversely proportional to the drug release rate.177 This is because membrane curvature and permeability of phospholipids significantly increase as the vesicle size decreases.178
Ultrasonic stimuli
Ultrasound may also be used to trigger drug release from NPs. Ultrasound of various intensities is widely used for diagnostic imaging or therapeutic intervention.179–181 For these purposes, ultrasounds provide thermal179 or mechanical effects.182 Ultrasonic heating has been employed to trigger the drug release by increasing the permeability of liposomal membrane.183 Alternatively, pulsed ultrasound has been used to mechanically trigger the drug release from NPs.181, 183, 184 Rapoport et al achieved tumor selective drug delivery by local tumor insonation.182 This approach involved systemic administration of nanoemulsions, consisting of paclitaxel and echogenic liquid (perfluoropentane) stabilized with PEG-PLA or PEG-PCL block copolymers.182 Following tumor accumulation (4 hours after the injection), pulsed ultrasound was applied to trigger droplet-to-bubble transition of perfluoropentane and subsequent drug release.182 The ultrasonic stimulus also contributed to drug diffusion in the tumor matrix and intracellular drug uptake via perturbation of cell membrane.185
Biomimetic stealth coating
Red blood cell (RBC) membrane has been used for stealth coating of polymeric NPs.186 PLGA NPs were formed first and then co-extruded with RBC-membrane-derived vesicles through a porous membrane. Thus formed RBC-membrane-camouflaged NPs were well dispersed in serum and showed a longer circulation time than those covered with PEG-lipid.186 Clinical translation of this approach may face some challenges related to immunogenicity or disease transmission. On the other hand, this study points to the critical roles of transmembrane proteins in controlling half-lives of circulating particles. A synthetic surface recapitulating their functions may provide a new biomimetic stealth coating that overcomes limitations of PEG.
Conclusions and Perspectives
For delivery of NPs to tumors to be effective, it is critical to produce long-circulating NPs. PEGylation is the most widely used technique to achieve this; however, the popularity of PEG does not necessarily mean that it is the best polymer for stealth coating. Recent studies find that PEG can interfere with processes subsequent to the extravasation of NPs, which are required for successful drug delivery to target tissues, and that PEGylated NPs may be subject to immune surveillance after the first dose. Several approaches to replacing PEG or modifying PEGylation are worth noting. Many alternative polymers have advantages over PEG in biodegradability or ability to accommodate functional groups. These features may be useful for improving cell-NP interactions in a target-specific manner and/or avoiding immune responses, which remain a topic for future studies. pH- or enzyme-triggered removal of PEG, another promising strategy to overcome the PEG dilemma, has proven effective in several recent studies. The remaining challenge is to produce sensitive and selective linkers, which can respond to minute changes in microenvironment or to alternative biological cues. Despite potential challenges in clinical translation, biomimetic stealth coating is a stimulating idea, which taps into the nature’s secret for controlling the longevity of RBCs.
Acknowledgments
This study was supported by the NIH (CA135130), NSF (DMR-1056997), and in part by a grant from the Lilly Endowment, Inc. to College of Pharmacy, Purdue University. The authors also thank Dr. Michael D. Tsifansky for a critical reading of the manuscript.
Contributor Information
Zohreh Amoozgar, Department of Industrial and Physical Pharmacy, College of Pharmacy, Purdue University, 575 Stadium Mall Drive, West Lafayette, IN 47907, USA.
Yoon Yeo, Department of Industrial and Physical Pharmacy, College of Pharmacy, Purdue University, 575 Stadium Mall Drive, West Lafayette, IN 47907, USA; Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA.
References
- 1.Peer D, Margalit R. Fluoxetine and reversal of multidrug resistance. Cancer Letters. 2006;237:180–187. doi: 10.1016/j.canlet.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Luqmani YA. Mechanisms of Drug Resistance in Cancer Chemotherapy. Medical Principles and Practice. 2005;14:35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- 3.Kuo T-C, Lu H-P, Chao CCK. The tyrosine kinase inhibitor sorafenib sensitizes hepatocellular carcinoma cells to taxol by suppressing the HURP protein. Biochemical Pharmacology. 2011;82:184–194. doi: 10.1016/j.bcp.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Clinical Applications of Research on Angiogenesis. New England Journal of Medicine. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 5.Eddy HA, Casarett GW. Development of the vascular system in the hamster malignant neurilemmoma. Microvascular Research. 1973;6:63–82. doi: 10.1016/0026-2862(73)90007-1. [DOI] [PubMed] [Google Scholar]
- 6.Rak J, Yu JL. Oncogenes and tumor angiogenesis: The question of vascular"supply" and vascular"demand". Seminars in Cancer Biology. 2004;14:93–104. doi: 10.1016/j.semcancer.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Narang AS, Mahato RI. Biological and Biomaterial Approaches for Improved Islet Transplantation. Pharmacological Reviews. 2006;58:194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 8.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial Progenitor Cells Control the Angiogenic Switch in Mouse Lung Metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. Tumor Angiogenesis: Therapeutic Implications. New England Journal of Medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK. Tumor angiogenesis and accessibility: Role of vascular endothelial growth factor. Seminars in Oncology. 2002;29:3–9. doi: 10.1053/sonc.2002.37265. [DOI] [PubMed] [Google Scholar]
- 11.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization. Microvascular Research. 74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D, Folkman J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 13.Fukumura D, Xavier R, Sugiura T, Chen Y, Park E-C, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, et al. Tumor Induction of VEGF Promoter Activity in Stromal Cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 14.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proceedings of the National Academy of Sciences. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Research. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 16.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of Functional Lymphatics within a Murine Sarcoma: A Molecular and Functional Evaluation. Cancer Research. 2000;60:4324–4327. [PubMed] [Google Scholar]
- 17.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 18.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Advances in Enzyme Regulation. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 19.Maeda H. SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Advanced Drug Delivery Reviews. 2001;46:169–185. doi: 10.1016/s0169-409x(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 20.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. European Journal of Pharmaceutics and Biopharmaceutics. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. International Immunopharmacology. 2003;3:319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- 22.Peer D, Karp JM, Hong S, FaroKhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 23.Kao YJ, Juliano RL. Interactions of liposomes with the reticuloendothelial system effects of reticuloendothelial blockade on the clearance of large unilamellar vesicles. Biochimica et Biophysica Acta (BBA) - General Subjects. 1981;677:453–461. doi: 10.1016/0304-4165(81)90259-2. [DOI] [PubMed] [Google Scholar]
- 24.Senior JH. Fate and behavior of liposomes in vivo: a review of controlling factors. Critical reviews in therapeutic drug carrier systems. 1987;3:123–193. [PubMed] [Google Scholar]
- 25.Pratten MK, Lloyd JB. Pinocytosis and phagocytosis: the effect of size of a particulate substrate on its mode of capture by rat peritoneal macrophages cultured in vitro. Biochimica et Biophysica Acta (BBA) - General Subjects. 1986;881:307–313. doi: 10.1016/0304-4165(86)90020-6. [DOI] [PubMed] [Google Scholar]
- 26.Bartneck M, Keul HA, Zwadlo-Klarwasser G, Groll Phagocytosis Independent Extracellular Nanoparticle Clearance by Human Immune Cells. Nano Letters. 2009;10:59–63. doi: 10.1021/nl902830x. [DOI] [PubMed] [Google Scholar]
- 27.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical Studies To Understand Nanoparticle Interaction with the Immune System and Its Potential Effects on Nanoparticle Biodistribution. Molecular Pharmaceutics. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moghimi SM. Opsono-recognition of liposomes by tissue macrophages. International Journal of Pharmaceutics. 1998;162:11–18. [Google Scholar]
- 29.Essa S, Rabanel JM, Hildgen P. Characterization of rhodamine loaded PEG-g-PLA nanoparticles (NPs): Effect of poly(ethylene glycol) grafting density. International Journal of Pharmaceutics. 2011;411:178–187. doi: 10.1016/j.ijpharm.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 30.Moghimi SM, Patel HM. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system - The concept of tissue specificity. Advanced Drug Delivery Reviews. 1998;32:45–60. doi: 10.1016/s0169-409x(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 31.Moghimi SM, Hamad I. Liposome-Mediated Triggering of Complement Cascade. Journal of Liposome Research. 2008;18:195–209. doi: 10.1080/08982100802309552. [DOI] [PubMed] [Google Scholar]
- 32.Semple SC, Chonn A, Cullis PR. Interactions of liposomes and lipid-based carrier systems with blood proteins: Relation to clearance behaviour in vivo. Advanced Drug Delivery Reviews. 1998;32:3–17. doi: 10.1016/s0169-409x(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 33.Medina OP, Pillarsetty N, Glekas A, Punzalan B, Longo V, Gönen M, Zanzonico P, Smith-Jones P, Larson SM. Optimizing tumor targeting of the lipophilic EGFR-binding radiotracer SKI 243 using a liposomal nanoparticle delivery system. Journal of Controlled Release. 2011;149:292–298. doi: 10.1016/j.jconrel.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K. Long-circulating poly(ethylene glycol)-poly(-lactide) block copolymer micelles with modulated surface charge. Journal of Controlled Release. 2001;77:27–38. doi: 10.1016/s0168-3659(01)00451-5. [DOI] [PubMed] [Google Scholar]
- 35.Longmire MR, Ogawa M, Choyke PL, Kobayashi H. Biologically Optimized Nanosized Molecules and Particles: More than Just Size. Bioconjugate Chemistry. 2011;22:993–1000. doi: 10.1021/bc200111p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canelas DA, Herlihy KP, DeSimone JM. Top-down particle fabrication: control of size and shape for diagnostic imaging and drug delivery. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009;1:391–404. doi: 10.1002/wnan.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheng Y, Liu C, Yuan Y, Tao X, Yang F, Shan X, Zhou H, Xu F. Long-circulating polymeric nanoparticles bearing a combinatorial coating of PEG and water-soluble chitosan. Biomaterials. 2009;30:2340–2348. doi: 10.1016/j.biomaterials.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 38.Albanese A, Sykes EA, Chan WCW. Rough around the Edges: The Inflammatory Response of Microglial Cells to Spiky Nanoparticles. ACS Nano. 2010;4:2490–2493. doi: 10.1021/nn100776z. [DOI] [PubMed] [Google Scholar]
- 39.Sheng Y, Yuan Y, Liu C, Tao X, Shan X, Xu F. In vitro macrophage uptake and in vivo biodistribution of PLA–PEG nanoparticles loaded with hemoglobin as blood substitutes: effect of PEG content. Journal of Materials Science: Materials in Medicine. 2009;20:1881–1891. doi: 10.1007/s10856-009-3746-9. [DOI] [PubMed] [Google Scholar]
- 40.Moghimi SM, Hunter AC, Murray JC. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacological Reviews. 2001;53:283–318. [PubMed] [Google Scholar]
- 41.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. Journal of Biological Chemistry. 1977;252:3582–3586. [PubMed] [Google Scholar]
- 42.Gref R, Lück M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Müller RH. [`]Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids and Surfaces B: Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 43.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Letters. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 44.Allen TM, Hansen C. Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1991;1068:133–141. doi: 10.1016/0005-2736(91)90201-i. [DOI] [PubMed] [Google Scholar]
- 45.Allen TM, Hansen CB, de Menezes DEL. Pharmacokinetics of long-circulating liposomes. Advanced Drug Delivery Reviews. 1995;16:267–284. [Google Scholar]
- 46.Shan X, Liu C, Yuan Y, Xu F, Tao X, Sheng Y, Zhou H. In vitro macrophage uptake and in vivo biodistribution of long-circulation nanoparticles with poly(ethylene-glycol)-modified PLA (BAB type) triblock copolymer. Colloids and Surfaces B: Biointerfaces. 2009;72:303–311. doi: 10.1016/j.colsurfb.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 47.Ebrahimnejad P, Dinarvand R, Jafari MR, Tabasi SAS, Atyabi F. Characterization, blood profile and biodistribution properties of surface modified PLGA nanoparticles of SN-38. International Journal of Pharmaceutics. 2011;406:122–127. doi: 10.1016/j.ijpharm.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 48.Bhattarai N, Bhattarai SR, Khil MS, Lee DR, Kim HY. Aqueous solution properties of amphiphilic triblock copolymer poly(p-dioxanone-co--lactide)-block-poly(ethylene glycol) European Polymer Journal. 2003;39:1603–1608. [Google Scholar]
- 49.Lee S-W, Yun M-H, Jeong SW, In C-H, Kim J-Y, Seo M-H, Pai C-M, Kim S-O. Development of docetaxel-loaded intravenous formulation, Nanoxel-PM(TM) using polymer-based delivery system. Journal of Controlled Release. 2011 doi: 10.1016/j.jconrel.2011.06.012. In Press, Uncorrected Proof. [DOI] [PubMed] [Google Scholar]
- 50.Heald CR, Stolnik S, Kujawinski KS, De Matteis C, Garnett MC, Illum L, Davis SS, Purkiss SC, Barlow RJ, Gellert PR. Poly(lactic acid)-poly(ethylene oxide) (PLA-PEG) nanoparticles: NMR studies of the central solidlike PLA core and the liquid PEG corona. Langmuir. 2002;18:3669–3675. [Google Scholar]
- 51.Moffatt S, Cristiano RJ. Uptake characteristics of NGR-coupled stealth PEI/pDNA nanoparticles loaded with PLGA-PEG-PLGA tri-block copolymer for targeted delivery to human monocyte-derived dendritic cells. International Journal of Pharmaceutics. 2006;321:143–154. doi: 10.1016/j.ijpharm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Kenausis GL, Vörös J, Elbert DL, Huang N, Hofer R, Ruiz-Taylor L, Textor M, Hubbell JA, Spencer ND. Poly(l-lysine)-g-Poly(ethylene glycol) Layers on Metal Oxide Surfaces: Attachment Mechanism and Effects of Polymer Architecture on Resistance to Protein Adsorption. The Journal of Physical Chemistry B. 2000;104:3298–3309. [Google Scholar]
- 53.Drobek T, Spencer ND, Heuberger M. Compressing PEG Brushes. Macromolecules. 2005;38:5254–5259. [Google Scholar]
- 54.Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Advanced Drug Delivery Reviews. 1995;17:31–48. [Google Scholar]
- 55.Du H, Schiavi S, Levine M, Mishra J, Heur M, Grabowski GA. Enzyme therapy for lysosomal acid lipase deficiency in the mouse. Human Molecular Genetics. 2001;10:1639–1648. doi: 10.1093/hmg/10.16.1639. [DOI] [PubMed] [Google Scholar]
- 56.Tirosh O, Barenholz Y, Katzhendler J, Priev A. Hydration of Polyethylene Glycol-Grafted Liposomes. Biophysical Journal. 1998;74:1371–1379. doi: 10.1016/S0006-3495(98)77849-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beletsi A, Panagi Z, Avgoustakis K. Biodistribution properties of nanoparticles based on mixtures of PLGA with PLGA-PEG diblock copolymers. International Journal of Pharmaceutics. 2005;298:233–241. doi: 10.1016/j.ijpharm.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 58.Verrecchia T, Spenlehauer G, Bazile DV, Murry-Brelier A, Archimbaud Y, Veillard M. Non-stealth (poly(lactic acid/albumin)) and stealth (poly(lactic acid-polyethylene glycol)) nanoparticles as injectable drug carriers. Journal of Controlled Release. 1995;36:49–61. [Google Scholar]
- 59.Sarmento B, Mazzaglia D, Bonferoni MC, Neto AP, do Céu Monteiro M, Seabra V. Effect of chitosan coating in overcoming the phagocytosis of insulin loaded solid lipid nanoparticles by mononuclear phagocyte system. Carbohydrate Polymers. 84:919–925. [Google Scholar]
- 60.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: Therapeutic applications and developments. Clinical Pharmacology & Therapeutics. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 61.Gref R, Minamitake Y, Peracchia M, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 62.Suh J, Choy K-L, Lai SK, Suk JS, Tang BC, Prabhu S, Hanes J. PEGylation of nanoparticles improves their cytoplasmic transport. Int J Nanomedicine. 2007;2:735–741. [PMC free article] [PubMed] [Google Scholar]
- 63.Poon Z, Chang D, Zhao X, Hammond PT. Layer-by-Layer Nanoparticles with a pH-Sheddable Layer for in Vivo Targeting of Tumor Hypoxia. ACS Nano. doi: 10.1021/nn200876f. null-null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aliabadi HM, Shahin M, Brocks DR, Lavasanifar A. Disposition of Drugs in Block Copolymer Micelle Delivery Systems: From Discovery to Recovery. Clinical Pharmacokinetics. 2008;47:619–634. doi: 10.2165/00003088-200847100-00001. [DOI] [PubMed] [Google Scholar]
- 65.Jones M-C, Leroux J-C. Polymeric micelles - a new generation of colloidal drug carriers. European journal of pharmaceutics and biopharmaceutics. 1999;48:101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 66. http://clinicaltrials.gov/ct2/show/NCT00912639.
- 67. http://clinicaltrials.gov/ct2/show/NCT01023347.
- 68. http://clinicaltrials.gov/ct2/show/NCT00886717.
- 69.Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: A strategy for overcoming the PEG dilemma. Advanced Drug Delivery Reviews. 2011;63:152–160. doi: 10.1016/j.addr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Fella C, Walker GF, Ogris M, Wagner E. Amine-reactive pyridylhydrazone-based PEG reagents for pH-reversible PEI polyplex shielding. European Journal of Pharmaceutical Sciences. 2008;34:309–320. doi: 10.1016/j.ejps.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: Effect of doxorubicin-encapsulation and high-dose first injection. Journal of Controlled Release. 2006;115:251–258. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 72.Ishida T, Harashima H, Kiwada H. Liposome Clearance. Biosience Reports. 2002;22:197–224. doi: 10.1023/a:1020134521778. [DOI] [PubMed] [Google Scholar]
- 73.Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. Journal of Controlled Release. 2006;112:15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 74.Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. Journal of Controlled Release. 2007;122:349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 75.Gupta AK, Berry C, Gupta M, Curtis A. Receptor-mediated targeting of magnetic nanoparticles using insulin as a surface ligand to prevent endocytosis. NanoBioscience, IEEE Transactions on. 2003;2:255–261. doi: 10.1109/tnb.2003.820279. [DOI] [PubMed] [Google Scholar]
- 76.Khalil IA, Kogure K, Akita H, Harashima H. Uptake Pathways and Subsequent Intracellular Trafficking in Nonviral Gene Delivery. Pharmacological Reviews. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 77.Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. Journal of Cell Science. 2010;123:1183–1189. doi: 10.1242/jcs.066399. [DOI] [PubMed] [Google Scholar]
- 78.Du H, Chandaroy P, Hui SW. Grafted poly-(ethylene glycol) on lipid surfaces inhibits protein adsorption and cell adhesion. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1997;1326:236–248. doi: 10.1016/s0005-2736(97)00027-8. [DOI] [PubMed] [Google Scholar]
- 79.Romberg B, Hennink W, Storm G. Sheddable Coatings for Long-Circulating Nanoparticles. Pharmaceutical research. 2008;25:55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong RL, Huang CJ, Tseng YL, Pang VF, Chen ST, Liu JJ, Chang FH. Direct comparison of liposomal doxorubicin with or without polyethylene glycol coating in C-26 tumor-bearing mice: Is surface coating with polyethylene glycol beneficial? Clinical Cancer Research. 1999;5:3645–3652. [PubMed] [Google Scholar]
- 81.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. European Journal of Cell Biology. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 82.Remaut K, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Pegylation of liposomes favours the endosomal degradation of the delivered phosphodiester oligonucleotides. Journal of Controlled Release. 2007;117:256–266. doi: 10.1016/j.jconrel.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 83.Ishida T, Maeda R, Ichihara M, Irimura K, Kiwada H. Accelerated clearance of PEGylated liposomes in rats after repeated injections. Journal of Controlled Release. 2003;88:35–42. doi: 10.1016/s0168-3659(02)00462-5. [DOI] [PubMed] [Google Scholar]
- 84.Ishida T, Ichihara M, Wang X, Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. Journal of Controlled Release. 2006;115:243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Wang XY, Ishida T, Ichihara M, Kiwada H. Influence of the physicochemical properties of liposomes on the accelerated blood clearance phenomenon in rats. Journal of Controlled Release. 2005;104:91–102. doi: 10.1016/j.jconrel.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. Journal of Controlled Release. 2007;119:236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 87.Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: Effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. Journal of Controlled Release. 2005;105:305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 88.Judge A, McClintock K, Phelps JR, MacLachlan I. Hypersensitivity and Loss of Disease Site Targeting Caused by Antibody Responses to PEGylated Liposomes. Mol Ther. 2006;13:328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 89.Ishida T, Masuda K, Ichikawa T, Ichihara M, Irimura K, Kiwada H. Accelerated clearance of a second injection of PEGylated liposomes in mice. International Journal of Pharmaceutics. 2003;255:167–174. doi: 10.1016/s0378-5173(03)00085-1. [DOI] [PubMed] [Google Scholar]
- 90.Dams ETM, Laverman P, Oyen WJG, Storm G, Scherphof GL, van der Meer JWM, Corstens FHM, Boerman OC. Accelerated Blood Clearance and Altered Biodistribution of Repeated Injections of Sterically Stabilized Liposomes. Journal of Pharmacology and Experimental Therapeutics. 2000;292:1071–1079. [PubMed] [Google Scholar]
- 91.Hamada I, Hunter AC, Szebeni J, Moghimi SM. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Molecular Immunology. 2008;46:225–232. doi: 10.1016/j.molimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- 92.Laverman P, Brouwers AH, Th M Dams E, Oyen WJG, Storm G, van Rooijen N, Corstens FHM, Boerman OC. Preclinical and Clinical Evidence for Disappearance of Long-Circulating Characteristics of Polyethylene Glycol Liposomes at Low Lipid Dose. Journal of Pharmacology and Experimental Therapeutics. 2000;293:996–1001. [PubMed] [Google Scholar]
- 93.Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, Garratty G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 94.Kim C, Lee SC, Kang SW, Kwon IC, Jeong SY. Phase-transition characteristics of amphiphilic poly(2-ethyl-2-oxazoline)/poly(ε-caprolactone) block copolymers in aqueous solutions. Journal of Polymer Science Part B: Polymer Physics. 2000;38:2400–2408. [Google Scholar]
- 95.Cheon Lee S, Kim C, Chan Kwon I, Chung H, Young Jeong S. Polymeric micelles of poly(2-ethyl-2-oxazoline)-block-poly([var epsilon]-caprolactone) copolymer as a carrier for paclitaxel. Journal of Controlled Release. 2003;89:437–446. doi: 10.1016/s0168-3659(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 96.Wang C-H, Wang W-T, Hsiue G-H. Development of polyion complex micelles for encapsulating and delivering amphotericin B. Biomaterials. 2009;30:3352–3358. doi: 10.1016/j.biomaterials.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 97.C Kim SCL, Shin J-H, Yoon J-S, Kwon IC, Jeong SY. Amphiphilic diblock copolymers based on poly(2-ethyl-2-oxazoline) and poly(1,3-trimethylene carbonate): synthesis and micellar characteristics. Macromolecules. 2000;33:7448–7452. [Google Scholar]
- 98.Zalipsky S, Hansen CB, Oaks JM, Allen TM. Evaluation of blood clearance rates and biodistribution of poly(2-oxazoline)-grafted liposomes. Journal of Pharmaceutical Sciences. 1996;85:133–137. doi: 10.1021/js9504043. [DOI] [PubMed] [Google Scholar]
- 99.von Erlach T, Zwicker S, Pidhatika B, Konradi R, Textor M, Hall H, Lühmann T. Formation and characterization of DNA-polymer-condensates based on poly(2-methyl-2-oxazoline) grafted poly(l-lysine) for non-viral delivery of therapeutic DNA. Biomaterials. 2011;32:5291–5303. doi: 10.1016/j.biomaterials.2011.03.080. [DOI] [PubMed] [Google Scholar]
- 100.Luxenhofer R, Sahay G, Schulz A, Alakhova D, Bronich TK, Jordan R, Kabanov AV. Structure-property relationship in cytotoxicity and cell uptake of poly(2-oxazoline) amphiphiles. Journal of Controlled Release. 2011;153:73–82. doi: 10.1016/j.jconrel.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Metselaar JM, Bruin P, de Boer LWT, de Vringer T, Snel C, Oussoren C, Wauben MHM, Crommelin DJA, Storm G, Hennink WE. A Novel Family of l-Amino Acid-Based Biodegradable Polymer− Lipid Conjugates for the Development of Long-Circulating Liposomes with Effective Drug-Targeting Capacity. Bioconjugate Chemistry. 2003;14:1156–1164. doi: 10.1021/bc0340363. [DOI] [PubMed] [Google Scholar]
- 102.Romberg B, Metselaar JM, Baranyi L, Snel CJ, Bünger R, Hennink WE, Szebeni J, Storm G. Poly(amino acid)s: Promising enzymatically degradable stealth coatings for liposomes. International Journal of Pharmaceutics. 2007;331:186–189. doi: 10.1016/j.ijpharm.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 103.Romberg B, Oussoren C, Snel CJ, Carstens MG, Hennink WE, Storm G. Pharmacokinetics of poly(hydroxyethyl-l-asparagine)-coated liposomes is superior over that of PEG-coated liposomes at low lipid dose and upon repeated administration. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2007;1768:737–743. doi: 10.1016/j.bbamem.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 104.Kopeček J, Šprincl L, Lím D. New types of synthetic infusion solutions. I. Investigation of the effect of solutions of some hydrophilic polymers on blood. Journal of Biomedical Materials Research. 1973;7:179–191. doi: 10.1002/jbm.820070206. [DOI] [PubMed] [Google Scholar]
- 105.Kopecek J, Kopecková P. HPMA copolymers: Origins, early developments, present, and future. Advanced Drug Delivery Reviews. 2010;62:122–149. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lammers T, Subr V, Ulbrich K, Peschke P, Huber PE, Hennink WE, Storm G. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials. 2009;30:3466–3475. doi: 10.1016/j.biomaterials.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 107.Rihová B, Etrych T, Pechar M, JelInková M, Stastný M, Hovorka O, Kovár M, Ulbrich K. Doxorubicin bound to a HPMA copolymer carrier through hydrazone bond is effective also in a cancer cell line with a limited content of lysosomes. Journal of Controlled Release. 2001;74:225–232. doi: 10.1016/s0168-3659(01)00320-0. [DOI] [PubMed] [Google Scholar]
- 108.Erez R, Segal E, Miller K, Satchi-Fainaro R, Shabat D. Enhanced cytotoxicity of a polymer-drug conjugate with triple payload of paclitaxel. Bioorganic & Medicinal Chemistry. 2009;17:4327–4335. doi: 10.1016/j.bmc.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 109.Rihova B, Kopeckova P, Strohalm J, Rossmann P, Vetvicka V, Kopecek J. Antibody-directed affinity therapy applied to the immune system: in vivo effectiveness and limited toxicity of daunomycin conjugated to HPMA copolymers and targeting antibody. Clin Immunol Immunopathol. 1988;46:100–114. doi: 10.1016/0090-1229(88)90010-4. [DOI] [PubMed] [Google Scholar]
- 110.Pike DB, Ghandehari H. HPMA copolymer-cyclic RGD conjugates for tumor targeting. Advanced Drug Delivery Reviews. 2010;62:167–183. doi: 10.1016/j.addr.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 111.Rejmanová P, Kopeček J, Pohl J, Baudyš M, Kostka V. Polymers containing enzymatically degradable bonds, 8. Degradation of oligopeptide sequences in N-(2-hydroxypropyl)methacrylamide copolymers by bovine spleen cathepsin B. Die Makromolekulare Chemie. 1983;184:2009–2020. [Google Scholar]
- 112.Jiang S, Cao Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Advanced Materials. 2010;22:920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 113.Shao Q, He Y, White AD, Jiang S. Difference in hydration between carboxybetaine and sulfobetaine. J Phys Chem B. 2010;114:16625–16631. doi: 10.1021/jp107272n. [DOI] [PubMed] [Google Scholar]
- 114.Chen S, Jiang S, Mo Y, Luo J, Tang J, Ge Z. Study of zwitterionic sulfopropylbetaine containing reactive siloxanes for application in antibacterial materials. Colloids Surf B Biointerfaces. 2011;85:323–329. doi: 10.1016/j.colsurfb.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Z, Vaisocherova H, Cheng G, Yang W, Xue H, Jiang S. Nonfouling behavior of polycarboxybetaine-grafted surfaces: structural and environmental effects. Biomacromolecules. 2008;9:2686–2692. doi: 10.1021/bm800407r. [DOI] [PubMed] [Google Scholar]
- 116.Cheng G, Li G, Xue H, Chen S, Bryers JD, Jiang S. Zwitterionic carboxybetaine polymer surfaces and their resistance to long-term biofilm formation. Biomaterials. 2009;30:5234–5240. doi: 10.1016/j.biomaterials.2009.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li G, Cheng G, Xue H, Chen S, Zhang F, Jiang S. Ultra low fouling zwitterionic polymers with a biomimetic adhesive group. Biomaterials. 2008;29:4592–4597. doi: 10.1016/j.biomaterials.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 118.Jia G, Cao Z, Xue H, Xu Y, Jiang S. Novel zwitterionic-polymer-coated silica nanoparticles. Langmuir. 2009;25:3196–3199. doi: 10.1021/la803737c. [DOI] [PubMed] [Google Scholar]
- 119.Yang W, Zhang L, Wang S, White AD, Jiang S. Functionalizable and ultra stable nanoparticles coated with zwitterionic poly(carboxybetaine) in undiluted blood serum. Biomaterials. 2009;30:5617–5621. doi: 10.1016/j.biomaterials.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 120.Zhang L, Xue H, Gao C, Carr L, Wang J, Chu B, Jiang S. Imaging and cell targeting characteristics of magnetic nanoparticles modified by a functionalizable zwitterionic polymer with adhesive 3,4-dihydroxyphenyl-l-alanine linkages. Biomaterials. 2010;31:6582–6588. doi: 10.1016/j.biomaterials.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 121.Cao Z, Yu Q, Xue H, Cheng G, Jiang S. Nanoparticles for drug delivery prepared from amphiphilic PLGA zwitterionic block copolymers with sharp contrast in polarity between two blocks. Angew Chem Int Ed Engl. 2010;49:3771–3776. doi: 10.1002/anie.200907079. [DOI] [PubMed] [Google Scholar]
- 122.Zhang L, Xue H, Cao Z, Keefe A, Wang J, Jiang S. Multifunctional and degradable zwitterionic nanogels for targeted delivery, enhanced MR imaging, reduction-sensitive drug release, and renal clearance. Biomaterials. 2011;32:4604–4608. doi: 10.1016/j.biomaterials.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 123.Cheng G, Mi L, Cao Z, Xue H, Yu Q, Carr L, Jiang S. Functionalizable and ultrastable zwitterionic nanogels. Langmuir. 2010;26:6883–6886. doi: 10.1021/la100664g. [DOI] [PubMed] [Google Scholar]
- 124.Kainthan RK, Janzen J, Levin E, Devine DV, Brooks DE. Biocompatibility Testing of Branched and Linear Polyglycidol. Biomacromolecules. 2006;7:703–709. doi: 10.1021/bm0504882. [DOI] [PubMed] [Google Scholar]
- 125.Kainthan RK, Hester SR, Levin E, Devine DV, Brooks DE. In vitro biological evaluation of high molecular weight hyperbranched polyglycerols. Biomaterials. 2007;28:4581–4590. doi: 10.1016/j.biomaterials.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 126.Siegers C, Biesalski M, Haag R. Self-Assembled Monolayers of Dendritic Polyglycerol Derivatives on Gold That Resist the Adsorption of Proteins. Chemistry – A European Journal. 2004;10:2831–2838. doi: 10.1002/chem.200306073. [DOI] [PubMed] [Google Scholar]
- 127.Kainthan RK, Brooks DE. In vivo biological evaluation of high molecular weight hyperbranched polyglycerols. Biomaterials. 2007;28:4779–4787. doi: 10.1016/j.biomaterials.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 128.Maruyama K, Okuizumi S, Ishida O, Yamauchi H, Kikuchi H, Iwatsuru M. Phosphatidyl polyglycerols prolong liposome circulation in vivo. International Journal of Pharmaceutics. 1994;111:103–107. [Google Scholar]
- 129.Hofmann AM, Wurm F, Hühn E, Nawroth T, Langguth P, Frey H. Hyperbranched Polyglycerol-Based Lipids via Oxyanionic Polymerization: Toward Multifunctional Stealth Liposomes. Biomacromolecules. 2010;11:568–574. doi: 10.1021/bm901123j. [DOI] [PubMed] [Google Scholar]
- 130.Kim K, Kim JH, Park H, Kim Y-S, Park K, Nam H, Lee S, Park JH, Park R-W, Kim I-S, et al. Tumor-homing multifunctional nanoparticles for cancer theragnosis: Simultaneous diagnosis, drug delivery, and therapeutic monitoring. Journal of Controlled Release. 2010;146:219–227. doi: 10.1016/j.jconrel.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 131.Fan L, Li F, Zhang H, Wang Y, Cheng C, Li X, Gu C-h, Yang Q, Wu H, Zhang S. Co-delivery of PDTC and doxorubicin by multifunctional micellar nanoparticles to achieve active targeted drug delivery and overcome multidrug resistance. Biomaterials. 2010;31:5634–5642. doi: 10.1016/j.biomaterials.2010.03.066. [DOI] [PubMed] [Google Scholar]
- 132.Mehvar R. Dextrans for targeted and sustained delivery of therapeutic and imaging agents. Journal of Controlled Release. 2000;69:1–25. doi: 10.1016/s0168-3659(00)00302-3. [DOI] [PubMed] [Google Scholar]
- 133.Li Y-L, Zhu L, Liu Z, Cheng R, Meng F, Cui J-H, Ji S-J, Zhong Z. Reversibly Stabilized Multifunctional Dextran Nanoparticles Efficiently Deliver Doxorubicin into the Nuclei of Cancer Cells. Angewandte Chemie International Edition. 2009;48:9914–9918. doi: 10.1002/anie.200904260. [DOI] [PubMed] [Google Scholar]
- 134.Choi KY, Chung H, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Jeong SY. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials. 2010;31:106–114. doi: 10.1016/j.biomaterials.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 135.Park K, Ki Lee S, Hyun Son D, Ah Park S, Kim K, Won Chang H, Jeong E-j, Park R-W, Kim I-S, Chan Kwon I, et al. The attenuation of experimental lung metastasis by a bile acid acylated-heparin derivative. Biomaterials. 2007;28:2667–2676. doi: 10.1016/j.biomaterials.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, Xin D, Hu J, Liu K, Pan J, Xiang J. A model ternary heparin conjugate by direct covalent bond strategy applied to drug delivery system. Bioorganic & Medicinal Chemistry Letters. 2009;19:149–152. doi: 10.1016/j.bmcl.2008.10.132. [DOI] [PubMed] [Google Scholar]
- 137.Hou L, Fan Y, Yao J, Zhou J, Li C, Fang Z, Zhang Q. Low molecular weight heparin-all-trans-retinoid acid conjugate as a drug carrier for combination cancer chemotherapy of paclitaxel and all-trans-retinoid acid. Carbohydrate Polymers. 2012 In Press, Corrected Proof. [Google Scholar]
- 138.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Advanced Drug Delivery Reviews. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 139.Li H, Niu R, Yang J, Nie J, Yang D. Photocrosslinkable tissue adhesive based on dextran. Carbohydrate Polymers. 2012 doi: 10.1016/j.carbpol.2012.09.057. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 140.Papisov MI. Biopolymers from Polysaccharides and Agroproteins. Vol. 786. American Chemical Society; 2001. Acyclic Polyacetals from Polysaccharides: Biomimetic Biomedical "Stealth" Polymers; pp. 301–314. [Google Scholar]
- 141.Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. Journal of Controlled Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pHe specific TAT exposure and multidrug resistance. Journal of Controlled Release. 2008;129:228–236. doi: 10.1016/j.jconrel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Min KH, Kim J-H, Bae SM, Shin H, Kim MS, Park S, Lee H, Park R-W, Kim I-S, Kim K, et al. Tumoral acidic pH-responsive MPEG-poly([beta]-amino ester) polymeric micelles for cancer targeting therapy. Journal of Controlled Release. 144:259–266. doi: 10.1016/j.jconrel.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 144.Montcourrier P, Silver I, Farnoud R, Bird I, Rochefort H. Breast cancer cells have a high capacity to acidify extracellular milieu by a dual mechanism. Clinical & Experimental Metastasis. 1997;15:382–392. doi: 10.1023/a:1018446104071. [DOI] [PubMed] [Google Scholar]
- 145.Swallow CJ, Grinstein S, Rotstein OD. A vacuolar type H(+)-ATPase regulates cytoplasmic pH in murine macrophages. J. Biol. Chem. 1990;265:7645–7654. [PubMed] [Google Scholar]
- 146.Brown JM, Helmut S, Bernhard B. Methods in Enzymology. Vol. Volume 435. Academic Press; 2007. Tumor Hypoxia in Cancer Therapy; pp. 295pp. 297–321. [DOI] [PubMed] [Google Scholar]
- 147.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nature Reviews Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 148.Raghunand N, Gatenby RA, Gillies RJ. Microenvironmental and cellular consequences of altered blood flow in tumours. Br J Radiol. 2003;76:S11–S22. doi: 10.1259/bjr/12913493. [DOI] [PubMed] [Google Scholar]
- 149.Wolf BB, Otto AMA, Brischwein MM, Kraus MM, Henning TT. Relevance of tumor microenvironment for progression, therapy and drug development. Anti-Cancer Drugs. 2004;15:7–14. doi: 10.1097/00001813-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 150.Gerweck LE, Seetharaman K. Cellular pH Gradient in Tumor versus Normal Tissue: Potential Exploitation for the Treatment of Cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 151.van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdan S, Galons JP, Gillies RJ. In vivo imaging of extracellular pH using H-1 MRSI. Magnetic Resonance In Medicine. 1999;41:743–750. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 152.Obata Y, Tajima S, Takeoka S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. Journal of Controlled Release. 2010;142:267–276. doi: 10.1016/j.jconrel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 153.Li S-D, Huang L. Stealth nanoparticles: High density but sheddable PEG is a key for tumor targeting. Journal of Controlled Release. 2010;145:178–181. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. "SMART" Drug Delivery Systems: Double-Targeted pH-Responsive Pharmaceutical Nanocarriers. Bioconjugate Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu R, Li D, He B, Xu X, Sheng M, Lai Y, Wang G, Gu Z. Anti-tumor drug delivery of pH-sensitive poly(ethylene glycol)-poly(L-histidine-)-poly(L-lactide) nanoparticles. Journal of Controlled Release. 2011;152:49–56. doi: 10.1016/j.jconrel.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 156.Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. Journal of Controlled Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 157.Kim D, Lee ES, Oh KT, Gao ZG, Bae YH. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small. 2008;4:2043–2050. doi: 10.1002/smll.200701275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim D, Gao ZG, Lee ES, Bae YH. In Vivo Evaluation of Doxorubicin-Loaded Polymeric Micelles Targeting Folate Receptors and Early Endosomal pH in Drug-Resistant Ovarian Cancer. Molecular Pharmaceutics. 2009;6:1353–1362. doi: 10.1021/mp900021q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annual Review of Biochemistry. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 160.Lee ES, Na K, Bae YH. Super pH-Sensitive Multifunctional Polymeric Micelle. Nano Letters. 2005;5:325–329. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- 161.Mohajer G, Lee ES, Bae YH. Enhanced Intercellular Retention Activity of Novel pH-sensitive Polymeric Micelles in Wild and Multidrug Resistant MCF-7 Cells. Pharmaceutical Research. 2007;24:1618–1627. doi: 10.1007/s11095-007-9277-5. [DOI] [PubMed] [Google Scholar]
- 162.Nie Y, Günther M, Gu Z, Wagner E. Pyridylhydrazone-based PEGylation for pH-reversible lipopolyplex shielding. Biomaterials. 2011;32:858–869. doi: 10.1016/j.biomaterials.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 163.Niidome T, Ohga A, Akiyama Y, Watanabe K, Niidome Y, Mori T, Katayama Y. Controlled release of PEG chain from gold nanorods: Targeted delivery to tumor. Bioorganic & Medicinal Chemistry. 2010;18:4453–4458. doi: 10.1016/j.bmc.2010.04.070. [DOI] [PubMed] [Google Scholar]
- 164.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 165.Terada T, Iwai M, Kawakami S, Yamashita F, Hashida M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. Journal of Controlled Release. 2006;111:333–342. doi: 10.1016/j.jconrel.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 166.Hatakeyama H, Akita H, Ito E, Hayashi Y, Oishi M, Nagasaki Y, Danev R, Nagayama K, Kaji N, Kikuchi H, et al. Systemic delivery of siRNA to tumors using a lipid nanoparticle containing a tumor-specific cleavable PEG-lipid. Biomaterials. 2011;32:4306–4316. doi: 10.1016/j.biomaterials.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 167.Hatakeyama H, Akita H, Kogure K, Oishi M, Nagasaki Y, Kihira Y, Ueno M, Kobayashi H, Kikuchi H, Harashima H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Therapy. 2007;14:68–77. doi: 10.1038/sj.gt.3302843. [DOI] [PubMed] [Google Scholar]
- 168.Mok H, Bae KH, Ahn C-H, Park TG. PEGylated and MMP-2 Specifically DePEGylated Quantum Dots: Comparative Evaluation of Cellular Uptake. Langmuir. 2009;25:1645–1650. doi: 10.1021/la803542v. [DOI] [PubMed] [Google Scholar]
- 169.Söderberg A, Sahaf B, Rosén A. Thioredoxin Reductase, a Redox-active Selenoprotein, Is Secreted by Normal and Neoplastic Cells: Presence in Human Plasma. Cancer Research. 2000;60:2281–2289. [PubMed] [Google Scholar]
- 170.Kaasgaard T, Andresen TL. Liposomal cancer therapy: exploiting tumor characteristics. Expert Opinion on Drug Delivery. 7:225–243. doi: 10.1517/17425240903427940. [DOI] [PubMed] [Google Scholar]
- 171.Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 172.Ren T-B, Xia W-J, Dong H-Q, Li Y-Y. Sheddable micelles based on disulfide-linked hybrid PEG-polypeptide copolymer for intracellular drug delivery. Polymer. 2011;52:3580–3586. [Google Scholar]
- 173.Oumzil K, Khiati S, Grinstaff MW, Barthélémy P. Reduction-triggered delivery using nucleoside-lipid based carriers possessing a cleavable PEG coating. Journal of Controlled Release. 2011;151:123–130. doi: 10.1016/j.jconrel.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 174.Takenaka H, Kawashima Y, Lin SY. Micromeritic properties of sulfamethoxazole microcapsules prepared by gelatin-acacia coacervation. J. Pharm. Sci. 1980;69:513–516. doi: 10.1002/jps.2600690509. [DOI] [PubMed] [Google Scholar]
- 175.Paoli EE, Kruse DE, Seo JW, Zhang H, Kheirolomoom A, Watson KD, Chiu P, Stahlberg H, Ferrara KW. An optical and microPET assessment of thermally-sensitive liposome biodistribution in the Met-1 tumor model: Importance of formulation. Journal of Controlled Release. 2011;143:13–22. doi: 10.1016/j.jconrel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nikolova AN, Jones MN. Effect of grafted PEG-2000 on the size and permeability of vesicles. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1996;1304:120–128. doi: 10.1016/s0005-2760(96)00112-9. [DOI] [PubMed] [Google Scholar]
- 177.Hossann M, Wang T, Wiggenhorn M, Schmidt R, Zengerle A, Winter G, Eibl H, Peller M, Reiser M, Issels RD, et al. Size of thermosensitive liposomes influences content release. Journal of Controlled Release. 2010;147:436–443. doi: 10.1016/j.jconrel.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 178.Nagle JF, Scott HL., Jr Lateral compressibility of lipid mono- and bilayers. Theory of membrane permeability. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1978;513:236–243. doi: 10.1016/0005-2736(78)90176-1. [DOI] [PubMed] [Google Scholar]
- 179.Paliwal S, Mitragotri S. Therapeutic opportunities in biological responses of ultrasound. Ultrasonics. 2008;48:271–278. doi: 10.1016/j.ultras.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 180.Huang S-L. Liposomes in ultrasonic drug and gene delivery. Advanced Drug Delivery Reviews. 2008;60:1167–1176. doi: 10.1016/j.addr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 181.Ibsen S, Benchimol M, Simberg D, Schutt C, Steiner J, Esener S. A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. Journal of Controlled Release. 2011 doi: 10.1016/j.jconrel.2011.06.032. In Press, Uncorrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam K-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. Journal of Controlled Release. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Geers B, Lentacker I, Sanders NN, Demeester J, Meairs S, De Smedt SC. Self-assembled liposome-loaded microbubbles: The missing link for safe and efficient ultrasound triggered drug-delivery. Journal of Controlled Release. 2011;152:249–256. doi: 10.1016/j.jconrel.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 184.Evjen TJ, Nilssen EA, Barnert S, Schubert R, Brandl M, Fossheim SL. Ultrasound-mediated destabilization and drug release from liposomes comprising dioleoylphosphatidylethanolamine. European Journal of Pharmaceutical Sciences. 2011;42:380–386. doi: 10.1016/j.ejps.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 185.Gao Z-G, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. Journal of Controlled Release. 2005;102:203–222. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 186.Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proceedings of the National Academy of Sciences. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]