Abstract
The rat possesses hemochorial placentation with deep intrauterine trophoblast cell invasion and trophoblast-directed uterine spiral artery remodeling; features shared with human placentation. Recognition of these similarities spurred the establishment of in vitro and in vivo research methods using the rat as an animal model to address mechanistic questions regarding development of the hemochorial placenta. The purpose of this review is to provide the requisite background to help move the rat to the forefront in placentation research.
Keywords: Hemochorial placentation, invasive trophoblast, metrial gland, uterine vascular remodeling
INTRODUCTION
Hemochorial placentation is a strategy involving modification of the maternal-fetal interface for the purpose of facilitating nutrient and waste exchange and development of healthy offspring. The hemochorial placenta possesses two elemental functions: i) ensuring the delivery of maternal nutrients to the placenta; ii) transferring nutrients from the placenta to the developing fetus. Accomplishment of each task requires execution of vital ancillary functions (e.g. immunoregulatory, endocrine, etc). A key feature of hemochorial placentation is the extensive vascular remodeling of maternal uterine spiral arteries, which facilitates nutrient flow and gas exchange to sustain the growing fetus [1–5]. This uteroplacental specialization is used by several species, including higher primates, rodents, lagomorphs, and others [6, 7].
Building a hemochorial placenta is a dynamic process involving the expansion of stem cell populations capable of differentiating into distinct trophoblast cell lineages. Mature trophoblast cells exhibit migratory and invasive properties and possess the capacity to recognize, modify, and emulate the behaviors of cells within their host environment. These developmental fates emerge under the direction of an intrinsic genetic program, the modulatory effects of the maternal environment, and the vitality of the fetus. Plasticity is the operative process in constructing a robust hemochorial placenta. A successful maternal-fetal interface is one that adapts most effectively to the stresses and challenges of pregnancy. Organization of the placentation site is sometimes flawed. Errors within regulatory pathways controlling placental development or maladaptive responses can negatively impact the health of the mother, the progression of fetal development, and can also have lasting effects on postnatal fitness. The intriguing biology and human health relevance make understanding the regulation of hemochorial placentation a compelling scientific pursuit.
Modern biomedical research has benefitted from the use of accessible model organisms (especially rodents) to study fundamental physiological and pathological processes. The benefits of such a research strategy for gaining insights into hemochorial placentation is not universally agreed upon. There are concerns about ‘evolutionary divergence’. Some differences in structure and function of rodent versus human hemochorial placentas and the biology of rodent versus human pregnancies exist [Table 1; 8]. However, there are also compelling similarities among species utilizing hemochorial placentation. Positive selection has resulted in the conservation of genes regulating mammalian placentation [9–13]. The mouse has been a valuable animal model for studying many aspects of placentation [13–15]; however, in contrast to the human, intrauterine trophoblast invasion is shallow and placentation superficial [16–18]. Organization of rat and human placentation sites exhibit striking similarities (Fig. 1; Table 1), especially regarding trophoblast-directed remodeling of the uterine spiral arteries [2, 17, 19–22]. Both species exhibit deep trophoblast invasion [19]. The rat has many of the advantages of the mouse, including the capacity for genetic manipulation [23]. Other rodents possess deep trophoblast invasion (e.g. guinea pig and hamster) [19]; however, experimental tools are not readily available for mechanistic analyses of placentation in these species. Nonhuman primates are advantageous in secondary evaluation of pathways established in other models but have practical limitations in most primary analyses.
Table 1.
Comparison of rat and human pregnancy and placentation
| Parameter | Rat | Human |
|---|---|---|
| 1. Uterine and vascular structure | Duplex uterus – bidirectional blood supply from aortic and internal iliac arteries; externally located arcuate and radial arteries | Simplex uterus-bidirectional blood supply from aortic and internal iliac arteries; internally located arcuate and radial arteries |
| 2. Maternal recognition of pregnancy | Mating-activated luteotropin secretion (pituitary prolactin)/placental-derived luteotropin (secondary) | Placental-derived luteotropin (chorionic gonadotropin) |
| 3. Embryo implantation: | Eccentric (secondarily interstitial); abembryonic | Interstitial; embryonic |
| i) Uterine modifications | Decidualization (hormonal dependence + implantation stimulus) | Decidualization (hormonal dependence) |
| ii) Immune cell trafficking | Abundance of NK cells associated with uterine spiral arteries | Abundance of NK cells associated with uterine spiral arteries |
| 4. Placental structure: | Hemochorial (discoid-chorioallantoic) | Hemochorial (discoid-chorioallantoic) |
| i) Placental-fetal interface | Labyrinth zone (hemotrichorial – syncytial barrier) | Villous trophoblast (hemomonochorial – syncytial barrier) |
| ii) Uterine-placental interface | Junctional zone and invasive trophoblast cells | Extravillous trophoblast cells |
| iii) Trophoblast invasion | Deep intrauterine – endovascular and interstitial | Deep intrauterine – endovascular and interstitial |
| iv) Spiral artery remodeling | Disappearance of smooth muscle, basement membrane restructuring, trophoblast cell replacement of endothelium and acquisition of a pseudo-endothelial phenotype | Disappearance of smooth muscle, basement membrane restructuring, trophoblast cell replacement of endothelium and acquisition of a pseudo-endothelial phenotype |
| 5. Hormonal maintenance of pregnancy: (source of sex steroid hormones) | Corpus luteum (primary)/placenta (secondary – progesterone and androgens) | Corpus luteum (1st trimester); placenta (remainder of pregnancy – progesterone and estrogens) |
| 6. Gestation length | Short (~21 days) | Long (~9 months) |
| 7. Number of offspring at birth | Polytocous | Monotocous/ditocous |
Fig. 1. Hemochorial placentation.
Schematic diagram showing homologous structures within human and rat hemochorial placentation sites.
The purpose of this review is to extol the merits of the rat as a model system for studying physiologically relevant mechanisms controlling hemochorial placentation. We describe aspects of the organization and physiology of the rat placenta and the unique features and tools available to generate mechanistic insights into the regulation of hemochorial placentation. The emphasis of the effort is on the invasive trophoblast cell lineage and uterine spiral artery remodeling.
I. DEVELOPMENT OF THE RAT HEMOCHORIAL PLACENTA
The parenchymal cell of the hemochorial placenta is the trophoblast cell. Trophoblast cells have a variety of phenotypes, which are generated from a multi-lineage differentiation pathway [24, 25]. Cellular specializations develop that facilitate trophoblast cell interactions with two vascular beds. Trophoblast cells associated with the maternal vasculature specialize in facilitating nutrient flow to the placenta (rat: junctional zone; human: extravillous trophoblast), whereas trophoblast cells developing in proximity to the fetal vasculature promote nutrient transfer to the fetus (rat: labyrinth zone; human: villous trophoblast).
The rat hemochorial placenta develops from stem cells arising from extraembryonic ectoderm, a derivative of the outer cellular layer of the blastocyst termed trophectoderm. Extraembryonic ectoderm differentiates into chorionic ectoderm and the ectoplacental cone, which subsequently contribute to the labyrinth zone and junctional zone, respectively [25, 26]. The labyrinth zone arises from the interaction of allantoic mesoderm with chorionic ectoderm, yielding trophoblast cell syncytialization and establishment of the barrier for maternal-fetal exchange [27]. The junctional zone borders the mesometrial uterine decidua. This region is also referred to as the ‘trophospongiosum’, ‘spongiotrophoblast’, and ‘spongy region’. Four trophoblast cell lineages differentiate from trophoblast progenitor cells within the junctional zone: i) trophoblast giant cells, ii) spongiotrophoblast cells, iii) glycogen cells, and iv) invasive trophoblast cells (Fig. 2). Trophoblast giant cells are the first trophoblast cell lineage to develop. They are the main endocrine cell of the placenta and possess some invasive abilities. Additionally there are subpopulations of trophoblast giant cells with specific intraplacental locations and functions [28]. Spongiotrophoblast cells are the main constituents of the junctional zone and also contributors to the endocrine function of the placenta. Glycogen cells first appear during midgestation, notably accumulate glycogen, and are probably progenitors for at least a subset of invasive trophoblast cells. Invasive trophoblast cells first appear at midgestation and consist of trophoblast cells that exit the junctional zone and enter the mesometrial uterine compartment, where they penetrate and surround the uterine spiral arteries. Endovascular invasive trophoblast cells replace the endothelium, while interstitial invasive trophoblast cells are situated between the vasculature [17, 20–22]. Invasive trophoblast cells are proposed to play key roles in uterine spiral artery remodeling [5, 29].
Fig. 2. Trophoblast stem cells and their differentiated lineages.
In the rat trophoblast stem cells can be directed toward distinct differentiated trophoblast cell lineages: trophoblast giant cells, spongiotrophoblast cells, glycogen cells, invasive trophoblast cells, and syncytial trophoblast.
Uterine spiral artery remodeling
Placentation sites are fed by uterine spiral arteries. These blood vessels undergo pregnancy-specific changes that facilitate delivery of nutrients. Specific structural modifications create conduits for high volume transfer free of maternal regulatory interference. This includes restructuring all components of the artery, including endothelium, basement membrane, and smooth muscle. Precise orchestration of this process is critical for ensuring appropriate nutrient delivery and preventing inappropriate exposure to deleterious reactive oxygen species [30]. Failures in uterine spiral artery remodeling are linked to pregnancy-associated diseases such as preeclampsia, intrauterine growth restriction, and premature pregnancy termination [1–5]. Mechanisms controlling uterine vascular remodeling are poorly understood. Putative regulators include natural killer (NK) cells and the specialized invasive/extravillous trophoblast cells [1, 5, 31, 32]. In the rat, NK cells and invasive trophoblast cells direct two distinct waves of uterine spiral artery remodeling (Fig. 3).
Fig. 3. Natural killer cell, uterine spiral artery, and trophoblast cell dynamics within rat placentation sites throughout gestation.
The schematic diagram highlights the two waves of uterine spiral artery remodeling executed through the actions of natural killer cells (first wave) and invasive trophoblast cells (second wave).
NK cells at the placentation site
Uterine adaptations to pregnancy include regulated intrauterine immune cell trafficking [31–35]. Following implantation, most maternal leukocytes are excluded from the implantation site except for uterine NK cells. NK cells are conspicuous cellular constituents of uteroplacental compartments in primates and rodents [31, 32, 35–37].
After implantation, in the mouse and rat, NK cells increase in number within the uterine mesometrial compartment [17, 32, 38–40]. This is the site where maternal blood vessels enter the uterus and is the region overlying the developing chorioallantoic placenta. By midgestation, NK cells migrate away from the placenta and establish more prominent relationships with the uterine spiral arteries. The mesometrial vasculature is situated between the mesometrial decidua and the mesometrial surface of the uterus. This morphologically distinct region is referred to as the ‘metrial gland’, ‘mesometrial triangle’, ‘mesometrial lymphoid aggregate of pregnancy’, or the ‘decidualized mesometrial triangle’ [41–45]. As gestation progresses NK cells disappear from the uterine mesometrial compartment. In the mouse, the disappearance of NK cells is caused by both necrotic and apoptotic mechanisms, and is possibly mediated by a Fas ligand-Fas-dependent signaling pathway [46, 47].
NK cells are linked to uterine spiral artery development. NK cell deficiency is associated with profound alterations in the uterine mesometrial vasculature [32, 48]. Genetic deficiency of uterine NK cells in the mouse leads to a lack of remodeling of uterine spiral arteries resulting in hypertrophied vascular media, swollen endothelial cells, and narrow vessel lumens [32, 49, 50]. NK cell depletion in the rat delays uterine spiral artery development resulting in a trophoblast cell-dependent compensatory response characterized by enhanced trophoblast-directed uterine spiral artery remodeling [48]. Experimentation with the mouse and rat as well as human tissues indicate that NK cell effects on the uterine vasculature may be achieved through the production of interferon γ (IFNγ) [50], nitric oxide [51], and/or an assortment of angiogenic growth factors, including vascular endothelial growth factors (VEGFs) [52–57].
Invasive trophoblast cell lineages
Specializations develop that facilitate trophoblast cell interactions with the maternal uterine vascular bed. Trophoblast cells connected to the maternal vasculature specialize in facilitating nutrient flow to the placenta. Intrauterine trophoblast cell invasion is a prominent feature of rat placentation. Rat invasive trophoblast cells have been characterized by their epithelial nature (expression of cytokeratin), polyploidy, accumulation of glycogen, expression of a unique subset of prolactin family cytokines, and their location at the uterine site of placental attachment [17, 20, 21, 58–61]. The first trophoblast cells penetrating into the uterine mesometrial compartment take an endovascular route and the depth of their invasion is generally limited to the mesometrial decidua compartment. Rat endovascular trophoblast cell invasion is a progressive process commencing at midgestation and migrating in a countercurrent fashion up the vessels. Endovascular trophoblast cells replace endothelial cells and become embedded in fibrin [19, 20]. Regulatory mechanisms controlling the propulsion of endovascular trophoblast cells along the inner lining of the uterine spiral arteries, their embedding in fibrin, and their replacement of the endothelium have not been elucidated. A second wave of trophoblast cell invasion begins after gestation day 13.5 and includes both interstitial and endovascular trophoblast cells [17, 20, 21]. Interstitial trophoblast cell migration extends throughout the metrial gland [17, 21]. Their expansion is linked to migration out of the junctional zone and is not associated with proliferation once they arrive in the metrial gland [21, 62]. Invasive trophoblast cells have been isolated from the metrial gland and analyzed by flow cytometry and determined to be primarily diploid [63]. Polyploid trophoblast giant cells arising from the ectoplacental cone do exhibit some limited penetration into the deciduum [21]. Interestingly, a recent report indicates that the depth of trophoblast cell invasion at the mouse placentation site is influenced by parity and extended in multiparous mice [66]. The impact of parity on placentation in the rat has not been reported.
Following parturition, evacuation of intrauterine trophoblast cells is associated with a return of uterine vasomotor control and restructuring of the uterus for the next pregnancy [65, 66]. Efficient postpartum demise of invasive trophoblast cells is critical to the health of the mother and success of subsequent pregnancies. Invasive trophoblast cells are initially retained in the rat uterus following parturition. Removal of intrauterine invasive trophoblast cells is complete within a few days following parturition, associated with an influx of macrophages into the uterine mesometrial compartment, and the return of smooth muscle cells to the uterine mesometrial vasculature [66]. The trigger for demise of retained intrauterine trophoblast cells is, at least in part, associated with their separation from the chorioallantoic placenta at parturition [66]. This observation implicates the placenta as a source of trophic factors, which sustain intrauterine invasive trophoblast cells.
NK cell – invasive trophoblast cell interactions
Although NK cells direct the initial wave of uterine spiral artery remodeling, their actions on the uterine mesometrial compartment are not required for the invasive trophoblast-directed second wave of uterine spiral artery remodeling [48]. This latter process is actually accelerated and more robust in the absence of NK cells [17, 48]. We hypothesize that by promoting spiral artery development, oxygen delivery, and establishing the hemochorial placenta, NK cells effectively delay endovascular trophoblast invasion. Delaying trophoblast entry into spiral arteries and the concomitant enhanced flow of maternal resources to the placenta minimizes the demands on the mother. Rat NK cells also appear to modulate the pseudo-endothelial phenotype of endovascular trophoblast cells [48]. NK cells from other species have been proposed to have direct interactions with trophoblast cells [67].
II. THE RAT AS AN EXPERIMENTAL SYSTEM FOR INVESTIGATING HEMOCHORIAL PLACENTATION
Experimental rat models have been established to investigate the impact of genetics and environmental challenges on placental development.
Genetics of placentation
The genetics of the rat offers benefits for investigating the biology of hemochorial placentation. There are striking strain differences in the organization of the rat placentation site. The Brown Norway (BN) inbred rat strain exhibits a subfertility phenotype and possesses growth-restricted placentation sites [22]. Litter size in the BN rat is limited to 3–5 pups versus 10–15 pups in more fertile inbred and outbred rat strains [22, 68–70]. Subfertility in the BN rat is associated with ovarian and uterine dysfunction [71]. BN rats ovulate fewer eggs and their ovaries show disruptions in their organization and in their abilities to produce steroid hormones. These ovarian anomalies contribute to uterine dysfunction. The BN rat uterus is less responsive to progesterone, which leads to an attenuated decidual reaction and a less than optimal maternal milieu for placental/fetal development [71]. Collectively, these maternal factors and potential intrinsic differences in BN rat trophoblast development contribute to growth-restricted placentas. The most prominent deficits in the BN rat placentation site are in the development of the junctional zone and in the depth of intrauterine invasion of trophoblast cells [22]. The junctional zone is thin and trophoblast invasion is shallow (Fig. 4). This relationship is probably causal and reflects the origin of invasive trophoblast within the junctional zone. The BN rat placental growth deficits are more striking in placentas associated with male fetuses than for placentas from female fetuses [72]. Whether this observation also extends to the unique features of BN rat junctional zone development and intrauterine trophoblast invasion remains to be determined. Some insights about the structural features of BN rat placentation sites have been obtained from gene expression profiling [72]. In comparison to late gestation Sprague Dawley rat placentas, BN rat placentas express higher levels of transcripts encoding proteins contributing to the renin-angiotensin system, and lesser amounts of transcripts encoding proteins regulating angiogenesis. These features may be responsible for the deficits observed in BN rat placentation or alternatively they may represent adaptive responses required for a successful BN rat pregnancy. Late gestation BN rat placentation sites are also unusual in that they possess abundant numbers of NK cells in close proximity to the mesometrial uterine vasculature [22]. The retention of NK cells may signify a BN rat adaptation to poor trophoblast invasion and a requirement to ensure sufficient uterine vascular delivery of nutrients to the placenta and fetus.
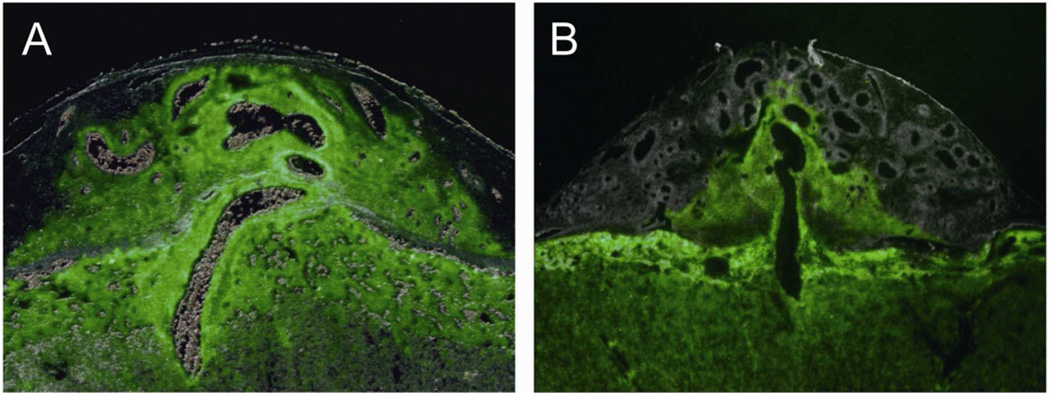
Fig. 4. Genetics of rat placentation.
Rat strains exhibit striking differences in the depth and extent of intrauterine trophoblast invasion. Trophoblast cell invasion can be tracked by fluorescence in transgenic placentas constitutively expressing enhanced green fluorescence protein in a wild type uterus. Panel A, Holtzman Sprague-Dawley rat control outbred placentation site exhibiting extensive trophoblast invasion (Adapted from Ref. 81). Panel B, Brown Norway rat placentation site showing limited intrauterine trophoblast invasion.
The quantifiable trait differences in placentation can be exploited to discover regulatory genes controlling placentation. Genetic control of physiological processes can be investigated in recombinant and in chromosome-substituted inbred rat strains [73, 74]. Both strategies have utilized the BN rat representing a normotensive rat and a variety of hypertensive inbred rat strains. Quantitative trait loci have been identified for litter size as well as placental and fetal weights using BN rat and Spontaneous Hypertensive rat recombinant inbred strains [69, 70]. A litter size locus was mapped to rat Chromosome 8 [69], whereas loci affecting placental and fetal weights were mapped to Chromosomes 15 and 1, respectively [70]). Chromosome-substituted rat strains have been generated with BN rat chromosomes introgressed into Dahl Salt Sensitive (DSS) or Fawn Hooded Hypertensive inbred rat strains [75, 76]. The strains have been used extensively to elucidate the genetic control of cardiovascular function [75, 76] and are also proving to be valuable for the characterization of the genetics of reproduction. These analyses are greatly facilitated because the BN rat was used for the first rat genome-sequencing project [77]. BN-DSS rat chromosome-substituted strains were used to identify specific chromosomes linked to placentation [78]. Chromosomes 14 and 17 were identified as possessing regulatory information controlling a quantitative trait associated with rat placentation. Once a quantitative trait is located to a specific chromosome then congenic rats can be generated to further define the locus. There are strategies for ascribing a gene or regulatory sequence to a trait, including comparative genomics, deep sequencing, and the use of bacterial artificial chromosome transgenics for phenotypic rescue [74].
Environmental manipulation of rat placentation
The organization of the rat placenta is sensitive to the maternal milieu. Several experimental manipulations have been performed that demonstrate the ability of the rat placentation site to adapt to an environmental challenge. These manipulations include alterations in maternal nutrition, oxygen delivery, and the induction of various disease states. In the next few paragraphs we provide some examples of environmental manipulations that impact rat placentation.
a) Nutritional modulation of the placentation site
Maternal nutrient availability affects the structure and development of the rat placenta. Maternal under nutrition impacts the entire placenta resulting in increased cell death in both the junctional and labyrinth compartments of the rat placenta [79]. Protein restriction leads to an expansion of the junctional zone, a decrease in the size of the labyrinth zone, and an increase in the surface area comprising the trophoblast-fetal interface [80]. In contrast, feeding pregnant rats a diet that promotes obesity is associated with decreased placental size, including reductions in both junctional and labyrinth zone volumes [81]. Similarly a high fat diet also decreases the size of the junctional zone but not the placental labyrinth zone [82]. The size of the junctional zone may be positively linked to circulating insulin-like growth factor (IGF) 2 levels [81]. In summary, it appears that within certain limits the developing placenta can respond to nutrient availability by adjusting allocation of trophoblast cells to the junctional zone compartment (uterine-trophoblast interface).
b) Hypoxia activation of the invasive trophoblast lineage
Oxygen is an essential cellular nutrient and a fundamental regulator of hemochorial placentation. During early pregnancy oxygen tensions tend to be low and then increase following the establishment of the hemochorial placenta [30, 83–86]. Insights about the role of oxygen supply as an intrinsic regulator of placentation have been derived from mutagenesis of genes in the mouse genome controlling cellular responses to oxygen deprivation [87]. The rat has proven to be an effective model for elucidating oxygen-dependent mechanisms controlling placentation. In vivo and in vitro experiments have demonstrated that development of the invasive/extravillous trophoblast lineage is activated by hypoxia [48, 64]. Low oxygen tension at the maternal-fetal interface alters cellular allocation, creating a preference for trophoblast lineages associated with junctional zone development, including the invasive trophoblast lineage. A critical period of sensitivity to hypoxia has been identified that coincides temporally with early stages of the placentation process [64]. In vivo responses to low oxygen are conserved in rodent and primate placentation [48, 88, 89].
c) Placentation responses to disease
Hypertension
Numerous rat models have been established to investigate pregnancy-associated hypertension and preeclampsia [90]. However, few of these experimental systems have examined the impact of the manipulation on placentation. The emphasis with these models has been examining perturbations in blood pressure regulation and kidney pathologies. An exception is the ‘renin-angiotensinogen transgenic rat model’. In this model system, dams possessing the human angiotensinogen transgene exhibit proteinuria, increased vascular resistance, and hypertension when mated to males expressing the human renin transgene [91, 92]. Endovascular invasive trophoblast cells invade more deeply into the uterine spiral arteries in this transgenic rat model [93, 94]. The increased vascular resistance observed in the renin-angiotensinogen rat transgenic model is linked to the presence of agonistic autoantibodies to the angiotensin II type 1 receptor and to increased uterine angiotensin II concentrations [95–97]. Maternal angiotensin II may also promote trophoblast invasion [98]. The vascular pathologies observed in this rat model may result in a hypoxia-driven activation of invasive trophoblast and thus an intact adaptive response, which is not observed in other pregnancy hypertension conditions such as human preeclampsia [1, 4, 5].
Diabetes
Glucose intolerance can be induced in the rat by treatment with various agents that destroy pancreatic islet cells (source of insulin) leading to a diabetic state [99]. In the pregnant rat, diabetes is associated with an increase in the glycogen cell contribution of the junctional zone and sustained trophoblast cell proliferation resulting in placentomegaly [100–103]. Maternal hyperinsulinemia decreases the number and depth of endovascular invasive trophoblast cells [104].
Maternal ethanol intake
Excess maternal ethanol consumption leads to a recognizable disease state referred as fetal alcohol syndrome [105]. This condition is associated with intrauterine growth restriction and postnatal deficits in brain function. Maternal ethanol intake also impacts rat placentation, including impairment of trophoblast-directed remodeling of uterine spiral arteries [106].
Inflammation
Inflammatory conditions are associated with a number of disease states, and are associated with the development of preeclampsia, intrauterine growth restriction, and spontaneous pregnancy termination. Experimentally, inflammation can be induced by systemic exposure to lipopolysaccharide (LPS). In rats, LPS treatment results in deficits in uteroplacental perfusion and trophoblast-directed uterine spiral artery remodeling [107]. This condition can be blocked by treatment with anti-inflammatory cytokines, such as interleukin 10, or by interfering with the action of tumor necrosis factor alpha.
Stress
Glucocorticoids are part of a response to physiological stressors that is associated with a number of disease states. Elevation of systemic glucocorticoids concentrations during pregnancy in the rat results in placental and fetal growth restriction [108–110]. Increases in apoptosis are observed in trophoblast cells throughout the placenta. These actions of excess glucocorticoids on placentation may be mediated through disruption of VEGF, IGF, peroxisome proliferator-activated receptor gamma, and/or Wnt signaling [110–113].
Rat experimental system overview
Insights gained from investigating the impact of genetics and environment on trophoblast cell invasion and spiral artery remodeling in the rat should have considerable relevance to other species, including the human. Human placental development and function are profoundly affected by the maternal environment [114]. An experimental animal model, such as the rat, offers the opportunity to investigate the progression of the diseased state and mechanisms underlying placental dysfunction. However, an important consideration in interpreting the impact of an experimental perturbation on pregnancy in the rat versus a disease state in the human is that the rat is a polytocous species. Consequently, the rat has an advantage in ensuring at least some offspring survive an environmental challenge or a disease state. They accomplish this fete by limiting the number of embryos implanting and by redirecting resources to fewer placentation sites, which are not routine options available to the human.
III. IN VITRO STRATEGIES FOR INVESTIGATING THE RAT TROPHOBLAST LINEAGE
Methodologies have been developed for in vitro investigation of rat trophoblast cells. These include the establishment of trophoblast stem (TS) cell lines, which have been shown to reflect the behavior of trophoblast cells developing in situ.
Rcho-1 TS cells
Almost three decades ago, Dr. Shinichi Teshima and colleagues at the National Cancer Institute (Tokyo, Japan) induced a transplantable rat choriocarcinoma with extraordinary attributes [115]. The tumor contained cells resembling trophoblast giant cells and they produced placental lactogens [115, 116]. Two research groups established cell lines from the rat choriocarcinoma termed RCHO [117] and Rcho-1 TS cells [118]. These cells are easy to expand and when factors that promote proliferation are removed the cells differentiate into mature trophoblast lineages [118]. Differentiation is directed primarily toward trophoblast giant cells; however, based on gene expression profiles it is evident that other differentiated trophoblast cell lineages are also present. Features distinguishing Rcho-1 TS cells from other established TS cells populations; include their independence of fibroblast growth factor-4 (FGF4) supplementation and their aneuploidy. These transformed rat TS cell lines have been used to investigate various aspects of trophoblast cell biology [119]. Most recently, the Rcho-1 TS cells have been utilized to elucidate the involvement of a phosphatidylinositol 3-kinase/AKT/FOS like antigen 1 signaling pathway controlling the invasive trophoblast phenotype [120–122].
Rat TS cells
A strategy for the ex vivo propagation of TS cells from mouse blastocysts was reported in 1998 [123]. The technique utilized FGF4, heparin, and mouse embryonic feeder layers as agents facilitating the establishment of the mouse TS cell cultures. FGF4-dependent rat TS cell lines have recently been described [124]. The cells were derived from rat blastocysts under conditions similar to those used for the establishment of mouse TS cells. Rat TS cells share many properties with mouse TS cells, including their ability to self renew and to differentiate into trophoblast cell lineages [124]. Similar to mouse TS cells and Rcho-1 TS cells, rat TS cells preferentially differentiate into trophoblast giant cells. They produce steroid hormones and a spectrum of peptide hormones, including members of the prolactin family. However, they also exhibit some differences [124]. Unlike mouse TS cells, rat TS cells do not express the pluripotency gene, Sox2 [125], in their stem state [124] and they do not readily contribute to the formation of a placenta following reintroduction into a blastocyst and transfer to a pseudopregnant rat [124]. Rat TS cells are also distinguished by their expression of Ascl2 [124], a gene implicated in regulating mouse placental morphogenesis [126]. The implication from these observations is that rat TS cells may have advanced beyond the developmental state of mouse TS cells [124]; potentially analogous to the relationship between mouse embryonic stem cells and mouse epiblast stem cells [127]. In vitro behavior of rat TS cells mimics trophoblast cell in vivo adaptive responses during rat placental morphogenesis. For example, low oxygen acutely induces rat TS cells to differentiate along an invasive lineage consistent with in vivo trophoblast cell responses to maternal hypoxia [48, 64].
TR-TBT cells
Nakashima and colleagues established immortalized rat labyrinth trophoblast cells (TR-TBT cells) from gestation day 18 pregnant transgenic rats possessing a temperature-sensitive SV40 large T antigen [128, 129]. The cells proliferate at 33°C. Growth is slowed at 37°C and differentiated functions are evident, such as expression of nutrient and waste transporters. TR-TBT cells have proven useful for studying drug metabolism and transport [128, 129].
Other rat trophoblast-related cell models
There is an assortment of additional cell models that have been developed from rat embryos and placentas that share features with trophoblast cells [130–134]. Some of these cell lines were byproducts of efforts to establish rat embryonic stem cells [132–134]. The mixed developmental phenotypes of the cultures and their limited capacity for differentiation toward trophoblast cells have restricted their usefulness for placental research. Pluripotent rat embryonic stem cells have also been established and could serve as a model for investigating early signals the derivation of the trophoblast cell lineage [135, 136]. Methodologies for establishing primary rat trophoblast cultures from the junctional and labyrinth zones have also been established, and have contributed to our understanding of placental biology [137, 138]. Finally, a precision-cut slice explant culture method has recently been introduced for ex vivo analysis of rat placental tissue [139].
IV. STRATEGIES FOR IN VIVO INVESTIGATION OF RAT PLACENTATION
Experimental approaches have been adapted for monitoring rat trophoblast cells in situ and for isolating and manipulating rat trophoblast cells situated at the rat placentation site.
Phenotypic analysis of the rat placenta
Protocols have been described for mating and gestational staging of rat pregnancy, dissection of placentation sites, and phenotypic characterization of trophoblast cell types [140, 141]. It is most meaningful to examine individual placentation sites in the context of the intact uterus and their associated fetuses. Unique gene and protein expression patterns have been used to distinguish specific rat trophoblast cell lineages developing in situ [140]. Imaging techniques have also been established for quantification of placental development, including the depth and extent of intrauterine trophoblast cell invasion [21, 22, 64].
Tracking invasive trophoblast cells
A couple of useful transgenic rat models are available for in situ monitoring of the invasive trophoblast cell lineage [142–144]. In each case, the transgene consists of a ‘constitutive’ promoter (ROSA 26 or chicken β actin, chβA) driving the expression of a reporter (human placental alkaline phosphatase, hAP or enhanced green fluorescent protein, EGFP). Intrauterine invasive trophoblast cells can be easily visualized on the wild-type uterine background by histochemical detection of heat stable alkaline phosphatase [145] or by fluorescence [66; Fig. 4]. ROSA 26-hAP invasive trophoblast can be quantified in the uterine mesometrial compartment by alkaline phosphatase enzymatic activity measurements [145], while chβA-EGFP positive invasive trophoblast can be recovered, analyzed, and sorted by flow cytometry [64]. There is an interesting caveat. The so-called ‘constitutive’ promoters are not equally expressed in all differentiated trophoblast lineages [64, 145, 146]; thus necessitating parallel experiments monitoring cell-specific reporter activity using histological techniques.
Manipulation of the trophoblast lineage
The true value of an animal model is its use for addressing in vivo mechanistic questions. There are several strategies for manipulating the rat genome [23, 147, 148]. A variety of approaches have been used to generate ‘gain-of-function’ mutations in the rat, including pronuclear injection and viral delivery [149–153]. There is also a growing list of techniques for producing ‘loss-of-function’ mutations, including the use of zinc finger nuclease and TALEN mutagenesis [154, 155, 156], chemical and transposon-based mutagenesis [157–159], and gene targeting using rat embryonic stem cells [160–162]. At this juncture, zinc finger mutagenesis is the most efficient of the techniques for producing knock-out rats [23]. Jacob and colleagues have recently generated ~100 strains of rats with specific mutations in genes implicated in the regulation of the cardiovascular system (http://rgd.mcw.edu/wg/physgenknockouts). Some of these mutant rat strains may be of interest to investigators studying placentation. The limitation with this strategy is that the generated mutations cannot be conditionally regulated in specific cell types. Gene targeting with rat embryonic stem cells represents a potential strategy for generating rats possessing cell-type specific mutations [162]. The latter will also require co-development of suitable mutant rats with trophoblast cell-specific manipulations capable of driving excision of relevant DNA sequences. Such tools are not currently available.
Another strategy of considerable promise is trophectoderm-specific lentiviral gene delivery, which was first developed in the mouse [163, 164]. In this technique, blastocysts are infected with lentiviral particles containing a gene construct of interest and then transferred into the uteri of pseudopregnant females. Gene delivery and activation is restricted to trophectoderm and its derivatives. Placentas can be harvested at various times during gestation and analyzed. The strategy has been successfully adapted for the rat and allows for the generation of both ‘gain-of-function’ and ‘loss-of-function’ [146; Fig. 5]. This technique has been used to investigate regulatory pathways controlling the invasive rat trophoblast cell lineage [122].
Fig. 5. Genetic manipulation through trophoblast-specific lentiviral delivery.
Panel A) Schematic diagram of the procedure. Lentiviral gene constructs are delivered to zona pellucida free blastocysts, which are then transferred to pseudopregnant recipients, and placentation sites analyzed during various stages of gestation. Panel B) Rat blastocysts examined under phase or fluorescence microscopy following incubation with lentiviral constructs expressing EGFP under the control of the phosphoglycerate kinase promoter. Panel C) Trophoblast-specific lentiviral gene delivery assessed at gestation d13.5. Transduced blastocysts were transferred into uteri of pseudopregnant rats and harvested at gestation d13.5 and examined under bright field or fluorescence microscopy. (Adapted from Ref. 135).
V. CONCLUDING COMMENTS
The use of animal model systems provides an essential tool for dissecting molecular mechanisms controlling cellular development. The premise of employing any animal model system is that if the process being studied is fundamental it will likely demonstrate conservation across species. Historically, the rat has been a valuable model for studying most aspects of reproduction and in many areas it still remains the preferred model system. The rat has been the preferred animal model for physiologists because of its size, which makes it more amenable to experimental manipulation, resulting in considerable understanding of rat physiology and its relationship to the human [165]. This led to the rat being the dominant pre-clinical model system used by the pharmaceutical and agro-chemical industries. Although there are some differences in the organization of the rat versus the human maternal-fetal interface, as indicated above similarities in the lineages of cells comprising the placentation site and their function exist. Paramount among the similarities between these two species is the process of trophoblast cell invasion and uterine spiral artery remodeling. If we understand biological processes at the maternal-fetal interface in species that can be experimentally manipulated, such as the rat, then we can more intelligently study the development of the human maternal-fetal interface and identify pivotal junctures of cellular control, facilitating diagnosis and therapeutic intervention. In some instances, cross species similarities may prevail, while in other cases the differences may be most compelling. Nonetheless, our appreciation for the biology of pregnancy and hemochorial placentation increases.
ACKNOWLEDGEMENTS
We would like to thank past and current members of our laboratory for contributing to the development of the rat as an animal model for placentation research. This work was supported by grants from the National Institutes of Health (HD20676, HD049503, HD055523, HD060115).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supported by a predoctoral fellowship from the American Heart Association.
Supported by postdoctoral fellowships from the Lalor Foundation and the Canadian Institute for Health Research.
REFERENCES
- 1.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pijnenborg R, Robertson WB, Brosens I, Dixon G. Trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2:71–91. doi: 10.1016/s0143-4004(81)80042-2. [DOI] [PubMed] [Google Scholar]
- 3.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placenta. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 5.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Enders AC, Welsh AO. Structural interactions of trophoblast and uterus during hemochorial placenta formation. J Exp Zool. 1993;266:578–587. doi: 10.1002/jez.1402660608. [DOI] [PubMed] [Google Scholar]
- 7.Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2:46. doi: 10.1186/1477-7827-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malassine A, Frendo J-L, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Human Reprod Update. 2003;9:531–539. doi: 10.1093/humupd/dmg043. [DOI] [PubMed] [Google Scholar]
- 9.Hou Z, Romero R, Uddin M, Than NG, Wildman DE. Adaptive history of single copy genes highly expressed in term placenta. Genomics. 2009;93:33–41. doi: 10.1016/j.ygeno.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wildman DE. Toward an integrated evolutionary understanding of the mammalian placenta. Placenta. 2011;32 Suppl:S142–S145. doi: 10.1016/j.placenta.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox B, Kotlyar M, Evangelou AI, Ignatchenko V, Ignatchenko A, Whitely K, Jurisica I, Adamson SL, Rossant J, Kislinger T. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol Syst Biol. 2009;5:279. doi: 10.1038/msb.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemberger M, Udayashankar R, Tesar P, Moore H, Burton GJ. ELF5-enforced transcriptional networks define an epigenetically regulated trophoblast stem cell compartment in the human placenta. Hum Mol Genet. 2010;15:2456–2467. doi: 10.1093/hmg/ddq128. [DOI] [PubMed] [Google Scholar]
- 13.Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossant J, Cross JC. Extraembryonic lineages. In: Rossan J, Tam PPL, editors. Mouse Development. New York: Academic Press; 2002. pp. 155–180. [Google Scholar]
- 15.El-Hashash AHK, Warburton D, Kimber SJ. Genes and signals regulating murine trophoblast cell development. Mech Dev. 2010:1–20. doi: 10.1016/j.mod.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- 17.Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 2003;260:176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 18.Coan PM, Conroy N, Burton GJ, Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn. 2006;235:3280–3294. doi: 10.1002/dvdy.20981. [DOI] [PubMed] [Google Scholar]
- 19.Pijnenborg R, Vercruysse L. Animal models of deep trophoblast invasion. In: Pijnenborg R, Brosens I, Romero R, editors. Placental Bed Disorders. Cambridge: Cambridge University Press; 2010. pp. 127–139. [Google Scholar]
- 20.Caluwaerts S, Vercruysse L, Luyten C, Pijnenborg R. Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta. 2005;26:574–584. doi: 10.1016/j.placenta.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Vercruysse L, Caluwaerts S, Luyten C, Pijnenborg R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta. 2006;27:22–33. doi: 10.1016/j.placenta.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Konno T, Rempel LA, Arroyo JA, Soares MJ. Pregnancy in the Brown Norway rat: a model for investigating the genetics of placentation. Biol Reprod. 2007;76:709–718. doi: 10.1095/biolreprod.106.056481. [DOI] [PubMed] [Google Scholar]
- 23.Jacob HJ, Lazar J, Dwinell MR, Moreno C, Geurts AM. Gene targeting in the rat: advances and opportunities. Trends Genet. 2010;26:510–518. doi: 10.1016/j.tig.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies J, Glasser SR. Histological and fine structural observations on the placenta of the rat. Acta Anat (Basel) 1968;69:542–608. doi: 10.1159/000143100. [DOI] [PubMed] [Google Scholar]
- 25.Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the placenta in the rat. Methods Mol Med. 2006;121:295–313. doi: 10.1385/1-59259-983-4:293. [DOI] [PubMed] [Google Scholar]
- 26.Soares MJ, Chapman BM, Rasmussen CA, Dai G, Kamei T, Orwig KE. Differentiation of trophoblast endocrine cells. Placenta. 1996;17:277–289. doi: 10.1016/s0143-4004(96)90051-x. [DOI] [PubMed] [Google Scholar]
- 27.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- 28.Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Verlohren S, Geusens N, Morton J, Verhaegen I, Hering L, Herse F, Dudenhausen JW, Muller DN, Luft FC, Cartwright JE, Davidge ST, Pijnenborg R, Dechend R. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension. 2010;56:304–310. doi: 10.1161/HYPERTENSIONAHA.110.153163. [DOI] [PubMed] [Google Scholar]
- 30.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215:27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lash GE, Robson SC, Bulmer JN. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31 Suppl:S87–S92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 2011;8:1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt JS, Petroff MG, Burnett TG. Uterine leukocytes: key players in pregnancy. Semin Cell Dev Biol. 2000;11:127–137. doi: 10.1006/scdb.2000.0158. [DOI] [PubMed] [Google Scholar]
- 34.Kruse A, Martens N, Fernekorn U, Hallmann R, Butcher EC. Alterations in the expression of homing-associated molecules at the maternal/fetal interface during the course of pregnancy. Biol Reprod. 2002;66:333–345. doi: 10.1095/biolreprod66.2.333. [DOI] [PubMed] [Google Scholar]
- 35.Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu CC, Young JD. Uterine natural killer cells in the pregnant uterus. Adv Immunol. 2001;79:297–329. doi: 10.1016/s0065-2776(01)79007-4. [DOI] [PubMed] [Google Scholar]
- 37.Slukvin II, Breburda EE, Golos TG. Dynamic changes in primate endometrial leukocyte populations: differential distribution of macrophages and natural killer cells at the rhesus monkey implantation site and in early pregnancy. Placenta. 2004;25:297–307. doi: 10.1016/j.placenta.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Head JR. Uterine natural killer cells during pregnancy in rodents. Nat Immun. 1996–1997;15:7–21. [PubMed] [Google Scholar]
- 39.Müller H, Liu B, Croy BA, Head JR, Hunt JS, Dai G, Soares MJ. Uterine natural killer cells are targets for a trophoblast cell-specific cytokine, prolactin-like protein A. Endocrinology. 1999;140:2711–2720. doi: 10.1210/endo.140.6.6828. [DOI] [PubMed] [Google Scholar]
- 40.Ain R, Tash JS, Soares MJ. Prolactin-like protein-A is a functional modulator of natural killer cells at the maternal-fetal interface. Mol Cell Endocrinol. 2003;204:65–74. doi: 10.1016/s0303-7207(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 41.Selye H, McKeown T. Studies on the physiology of the maternal placenta in the rat. Proc R Soc Lond Biol. 1935;119:1–31. [Google Scholar]
- 42.Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- 43.Croy BA. Hasn’t the time come to replace the term metrial gland? J Reprod Immunol. 1999;42:127–129. doi: 10.1016/s0165-0378(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 44.Pijnenborg R. The metrial gland is more than a mesometrial lymphoid aggregate of pregnancy. J Reprod Immunol. 2000;46:17–19. doi: 10.1016/s0165-0378(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 45.Ain R, Soares MJ. Is the metrial gland really a gland? J Reprod Immunol. 2004;61:129–131. doi: 10.1016/j.jri.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Kusakabe K, Okada T, Sasaki F, Kiso Y. Cell death of uterine natural killer cells in murine placenta during placentation and preterm periods. J Vet Med Sci. 1999;61:1093–1100. doi: 10.1292/jvms.61.1093. [DOI] [PubMed] [Google Scholar]
- 47.Kusakabe K, Otsuki Y, Kiso Y. Involvement of the fas ligand and fas system in apoptosis induction of mouse uterine natural killer cells. J Reprod Dev. 2005;51:333–340. doi: 10.1262/jrd.16086. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty D, Rumi MAK, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci USA. 2011;108:16295–16300. doi: 10.1073/pnas.1109478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 1997;56:169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- 50.Ashkar AA, Croy BA. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol. 2001;13:235–241. doi: 10.1006/smim.2000.0319. [DOI] [PubMed] [Google Scholar]
- 51.Burnett TG, Tash JS, Hunt JS. Investigation of the role of nitric oxide synthase 2 in pregnancy using mutant mice. Reproduction. 2002;124(1):49–57. doi: 10.1530/rep.0.1240049. [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Tanaka T, Nakamura H, Umesaki N, Hirai K, Ishiko O, Ogita S, Kaneda K. Granulated metrial gland cells in the murine uterus: localization, kinetics, and the functional role in angiogenesis during pregnancy. Microsc Res Tech. 2003;60:420–429. doi: 10.1002/jemt.10280. [DOI] [PubMed] [Google Scholar]
- 53.Wang C, Umesaki N, Nakamura H, Tanaka T, Nakatani K, Sakaguchi I, Ogita S, Kaneda K. Expression of vascular endothelial growth factor by granulated metrial gland cells in pregnant murine uteri. Cell Tissue Res. 2000;300:285–293. doi: 10.1007/s004410000198. [DOI] [PubMed] [Google Scholar]
- 54.Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik S, Day K, Licence D, Bowen JM, Gardner L, King A, Loke YW, Smith SK. Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J Clin Endocrinol Metab. 2001;86:1823–1834. doi: 10.1210/jcem.86.4.7418. [DOI] [PubMed] [Google Scholar]
- 55.Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, Robson SC, Bulmer JN. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- 56.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 57.Kaloglu C, Bulut HE. Vascular endothelial growth factor production by rat granulated metrial gland cells and their morphological features in normal and pathological conditions. Reprod Fertil Dev. 2007;19:341–350. doi: 10.1071/rd05143. [DOI] [PubMed] [Google Scholar]
- 58.Bridgman J. A morphological study of the development of the placenta of the rat. II. An histological and cytological study of the development of the chorioallantoic placenta of the white rat. J Morphol. 1949;83:195–224. doi: 10.1002/jmor.1050830204. [DOI] [PubMed] [Google Scholar]
- 59.Correia-da-Silva G, Bell SC, Pringel JH, Teixeira N. Expression of mRNA encoding insulin-like growth factors I and II by uterine tissues and placenta during pregnancy in the rat. Mol Reprod Dev. 1999;53:294–305. doi: 10.1002/(SICI)1098-2795(199907)53:3<294::AID-MRD5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 60.Zybina TG, Zybina EV. Genome multiplication in the tertiary giant trophoblast cells in the course of their endovascular and interstitial invasion into the rat placenta decidua basalis. Early Pregnancy. 2000;4:99–109. [PubMed] [Google Scholar]
- 61.Wiemers DO, Ain R, Ohboshi S, Soares MJ. Migratory trophoblast cells express a newly identified member of the prolactin gene family. J Endocrinol. 2003;179:335–346. doi: 10.1677/joe.0.1790335. [DOI] [PubMed] [Google Scholar]
- 62.Zybina TG, Zybina EV. Cell reproduction and genome multiplication in the proliferative and invasive trophoblast cell populations of mammalian placenta. Cell Biol Int. 2005;29:1071–1083. doi: 10.1016/j.cellbi.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 63.Litwin S, Cortina ME, Barrientos GL, Prados MB, Roux ME, Miranda SE. Multiparity increases trophoblast invasion and vascular endothelial growth factor expression at the maternal-fetal interface in mice. J Reprod Immunol. 2010;85:161–167. doi: 10.1016/j.jri.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nanaev AK, Kosanke G, Reister F, Kemp B, Frank HG, Kaufmann P. Pregnancy induced de-differentiation of media smooth muscle cells in uteroplacental arteries of the guinea pig is reversible after delivery. Placenta. 2000;21:306–312. doi: 10.1053/plac.1999.0490. [DOI] [PubMed] [Google Scholar]
- 66.Rosario GX, Ain R, Konno T, Soares MJ. Intrauterine fate of invasive trophoblast cells. Placenta. 2009;30:457–463. doi: 10.1016/j.placenta.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chazara O, Xiong S, Moffett A. Maternal KIR and fetal HLA-C: a fine balance. J Leukoc Biol. 2011;90:703–716. doi: 10.1189/jlb.0511227. [DOI] [PubMed] [Google Scholar]
- 68.Gill TJ, Kunz HW, Hansen CT. Litter sizes in inbred strains of rats (Rattus norvegicus) J Immunogenet. 1979;6:461–463. doi: 10.1111/j.1744-313x.1979.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 69.Zidek V, Pintir J, Musilova A, Bila V, Kren V, Pravenec M. Mapping of quantitative trait loci for seminal vesicle mass and litter size to rat chromosome 8. J Reprod Fertil. 1999;116:329–333. doi: 10.1530/jrf.0.1160329. [DOI] [PubMed] [Google Scholar]
- 70.Buresova M, Zidek V, Musilova A, Simakova M, Fucikova A, Bila V, Kren V, Kazdova L, Di Nicolantonio R, Pravenec M. Genetic relationship between placental and fetal weights and markers of the metabolic syndrome in rat recombinant inbred strains. Physiol Genomics. 2006;26:226–231. doi: 10.1152/physiolgenomics.00056.2006. [DOI] [PubMed] [Google Scholar]
- 71.Konno T, Graham AR, Rempel LA, Ho-Chen JK, Alam SM, Bu P, Rumi MA, Soares MJ. Subfertility linked to combined luteal insufficiency and uterine progesterone resistance. Endocrinology. 2010;151:4537–4550. doi: 10.1210/en.2010-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goyal R, Yellon SM, Longo LD, Mata-Greenwood E. Placental gene expression in a rat ‘ model’ of placental insufficiency. Placenta. 2010;31:568–575. doi: 10.1016/j.placenta.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Cowley AW, Roman RJ, Jacob HJ. Application of chromosomal substitution techniques in gene-function discovery. J Physiol. 2004;554:46–55. doi: 10.1113/jphysiol.2003.052613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lazar J, Moreno C, Jacob HJ, Kwitek AE. Impact of genomics on research in the rat. Genome Res. 2005;15:1717–1728. doi: 10.1101/gr.3744005. [DOI] [PubMed] [Google Scholar]
- 75.Kunert MP, Drenjancevic-Peric I, Dwinell MR, Lombard JH, Cowley AW, Jr, Greene AS, Kwitek AE, Jacob HJ. Consomic strategies to localize genomic regions related to vascular reactivity in the Dahl salt-sensitive rat. Physiol Genomics. 2006;26:218–225. doi: 10.1152/physiolgenomics.00004.2006. [DOI] [PubMed] [Google Scholar]
- 76.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Cowley AW, Jr, Jacob HJ. Chromosomal mapping of the genetic basis of hypertension and renal disease in FHH rats. Am J Physiol Renal Physiol. 2007;293:F1905–F1914. doi: 10.1152/ajprenal.00012.2007. [DOI] [PubMed] [Google Scholar]
- 77.Rat Genome Sequencing Project Consortium. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- 78.Konno T, Rempel LA, Rumi MA, Graham AR, Asanoma K, Renaud SJ, Soares MJ. Chromosome-substituted rat strains provide insights into the genetics of placentation. Physiol Genomics. 2011;43:930–941. doi: 10.1152/physiolgenomics.00069.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belkacemi L, Chen CH, Ross MG, Desai M. Increased placental apoptosis in maternal food restricted gestations: role of the Fas pathway. Placenta. 2009;30:739–751. doi: 10.1016/j.placenta.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 80.Doherty CB, Lewis RM, Sharkey A, Burton GJ. Placental composition and surface area but not vascularization are altered by maternal protein restriction in the rat. Placenta. 2003;24:34–38. doi: 10.1053/plac.2002.0858. [DOI] [PubMed] [Google Scholar]
- 81.Van Mieghem T, van Bree R, Van Herck E, Deprest J, Verhaeghe J. Insulin-like growth factor-II regulates maternal hemodynamic adaptation to pregnancy in rats. Am J Physiol. 2009;297:R1615–R1621. doi: 10.1152/ajpregu.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mark PJ, Sisala C, Connor K, Patel R, Lewis JL, Vickers MH, Waddell BJ, Sloboda DM. A maternal high-fat diet in rat pregnancy reduces growth of the fetus and the placental junctional zone, but not placental labyrinth zone growth. J Dev Origins Health Dis. 2011;2:63–70. [Google Scholar]
- 83.Mitchell JA, Hammer RE. Serotonin-induced disruption of implantation in the rat: I. Serum progesterone, implantation site blood flow, and intrauterine pO2. Biol Reprod. 1983;28:830–835. doi: 10.1095/biolreprod28.4.830. [DOI] [PubMed] [Google Scholar]
- 84.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;227:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 85.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- 86.Zamudio S. The placenta at high altitude. High Altitude Med Biol. 2003;4:171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- 87.Fryer BH, Simon MC. Hypoxia, HIF and the placenta. Cell Cycle. 2006;5:495–498. doi: 10.4161/cc.5.5.2497. [DOI] [PubMed] [Google Scholar]
- 88.Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. doi: 10.1053/plac.2002.0946. [DOI] [PubMed] [Google Scholar]
- 89.Zhou Y, Chiu K, Brescia RJ, Combs A, Katz MA, Kitzmiller JL, Heilbron DC, Fisher SJ. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. Am J Obstet Gynecol. 1993;169:224–229. doi: 10.1016/0002-9378(93)90172-f. [DOI] [PubMed] [Google Scholar]
- 90.McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. Animal models of preeclampsia; uses and limitations. Placenta. 2011;32:413–419. doi: 10.1016/j.placenta.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 91.Bohlender J, Ganten D, Luft FC. Rats transgenic for human renin and human angiotensinogen as a model for gestational hypertension. J Am Soc Nephrol. 2000 Nov;11(11):2056–2061. doi: 10.1681/ASN.V11112056. [DOI] [PubMed] [Google Scholar]
- 92.Verlohren S, Niehoff M, Hering L, Geusens N, Herse F, Tintu AN, Plagemann A, LeNoble F, Pijnenborg R, Muller DN, Luft FC, Dudenhausen JW, Gollasch M, Dechend R. Uterine vascular function in a transgenic preeclampsia rat model. Hypertension. 2008;51:547–553. doi: 10.1161/HYPERTENSIONAHA.107.103176. [DOI] [PubMed] [Google Scholar]
- 93.Geusens N, Verlohren S, Luyten C, Taube M, Hering L, Vercruysse L, Hanssens M, Dudenhausen JW, Dechend R, Pijnenborg R. Endovascular trophoblast invasion, spiral artery remodelling and uteroplacental haemodynamics in a transgenic rat model of pre-eclampsia. Placenta. 2008;29:614–623. doi: 10.1016/j.placenta.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Geusens N, Hering L, Verlohren S, Luyten C, Drijkoningen K, Taube M, Vercruysse L, Hanssens M, Dechend R, Pijnenborg R. Changes in endovascular trophoblast invasion and spiral artery remodelling at term in a transgenic preeclamptic rat model. Placenta. 2010;31:320–326. doi: 10.1016/j.placenta.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 95.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Bräsen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Müller DN. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 96.Herse F, Staff AC, Hering L, Müller DN, Luft FC, Dechend R. AT1-receptor autoantibodies and uteroplacental RAS in pregnancy and pre-eclampsia. J Mol Med (Berl) 2008;86:697–703. doi: 10.1007/s00109-008-0332-4. [DOI] [PubMed] [Google Scholar]
- 97.Brosnihan KB, Hering L, Dechend R, Chappell MC, Herse F. Increased angiotensin II in the mesometrial triangle of a transgenic rat model of preeclampsia. Hypertension. 2010;55:562–566. doi: 10.1161/HYPERTENSIONAHA.109.145656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hering L, Herse F, Geusens N, Verlohren S, Wenzel K, Staff AC, Brosnihan KB, Huppertz B, Luft FC, Muller DN, Pijnenborg R, Cartwright JE, Dechend R. Effects of circulating and local uteroplacental angiotensin II in rat pregnancy. Hypertension. 2010;56:311–318. doi: 10.1161/HYPERTENSIONAHA.110.150961. [DOI] [PubMed] [Google Scholar]
- 99.Jawerbaum A, White V. Animal models in diabetes and pregnancy. Endocr Rev. 2010;31:680–701. doi: 10.1210/er.2009-0038. [DOI] [PubMed] [Google Scholar]
- 100.Gewolb IH, Merdian W, Warshaw JB, Enders AC. Fine structural abnormalities of the placenta in diabetic rats. Diabetes. 1986;35:1254–1261. doi: 10.2337/diab.35.11.1254. [DOI] [PubMed] [Google Scholar]
- 101.Robinson J, Canavan JP, El Haj AJ, Goldspink DF. Maternal diabetes in rats. I. Effects on placental growth and protein turnover. Diabetes. 1988;37:1665–1670. doi: 10.2337/diab.37.12.1665. [DOI] [PubMed] [Google Scholar]
- 102.Husain SM, Frost R, Mughal ZM. Effect of diabetes mellitus on rat placenta cellularity. Early Hum Dev. 2001;3:207–214. doi: 10.1016/s0378-3782(00)00119-5. [DOI] [PubMed] [Google Scholar]
- 103.Zorn TMT, Zuniga M, Madrid E, Tostes R, Fortes Z, Giachini F, San Martin S. Maternal diabetes affects cell proliferation in developing rat placenta. Histol Histopathol. 2011;26:1049–1056. doi: 10.14670/HH-26.1049. [DOI] [PubMed] [Google Scholar]
- 104.Skarzinski G, Khamaisi M, Bursztyn M, Mekler J, Lan D, Evdokimov P, Ariel I. Intrauterine growth restriction and shallower implantation site in rats with maternal hyperinsulinemia are associated with altered NOS expression. Placenta. 2009;30:898–906. doi: 10.1016/j.placenta.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 105.Henderson GI, Chen JJ, Schenker S. Ethanol, oxidative stress, reactive aldehydes, and the fetus. Front Biosci. 1999;4:D541–D550. doi: 10.2741/henderson. [DOI] [PubMed] [Google Scholar]
- 106.Gundogan F, Elwood G, Longato L, Tong M, Feijoo A, Carlson RI, Wands JR, de la Monte SM. Impaired placentation in fetal alcohol syndrome. Placenta. 2008;29:148–157. doi: 10.1016/j.placenta.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Renaud SJ, Cotechini T, Quirt JS, Macdonald-Goodfellow SK, Othman M, Graham CH. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol. 2011;186:1799–1808. doi: 10.4049/jimmunol.1002679. [DOI] [PubMed] [Google Scholar]
- 108.Waddell BJ, Hisheh S, Dharmarajan AM, Burton PJ. Apoptosis in rat placenta is zone-dependent and stimulated by glucocorticoids. Biol Reprod. 2000;63:1913–1917. doi: 10.1095/biolreprod63.6.1913. [DOI] [PubMed] [Google Scholar]
- 109.Sugden MC, Langdown ML. Possible involvement of PKC isoforms in signaling placental apoptosis in intrauterine growth retardation. Mol Cell Endocrinol. 2001;185:119–126. doi: 10.1016/s0303-7207(01)00630-x. [DOI] [PubMed] [Google Scholar]
- 110.Ain R, Canham LN, Soares MJ. Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the AKT signaling pathway. J Endocrinol. 2005;185:253–263. doi: 10.1677/joe.1.06039. [DOI] [PubMed] [Google Scholar]
- 111.Hewitt DP, Mark PJ, Waddell BJ. Placental expression of peroxisome proliferator-activated receptors in rat pregnancy and the effect of increased glucocorticoids exposure. Biol Reprod. 2006;74:23–28. doi: 10.1095/biolreprod.105.045914. [DOI] [PubMed] [Google Scholar]
- 112.Hewitt DP, Mark PJ, Dharmarajan AM, Waddell BJ. Placental expression of secreted frizzled related protein-4 in the rat and the impact of glucocorticoids-induced fetal and placental growth restriction. Biol Reprod. 75:75–81. doi: 10.1095/biolreprod.105.047647. 2206. [DOI] [PubMed] [Google Scholar]
- 113.Hewitt DP, Mark PJ, Waddell BJ. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology. 2006;147:5568–5574. doi: 10.1210/en.2006-0825. [DOI] [PubMed] [Google Scholar]
- 114.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Teshima S, Shimosato Y, Koide T, Kuroki M, Kikuchi Y, Aizawa M. Transplantable choriocarcinoma of rats induced by fetectomy and its biological activities. Gann. 1983;74:205–212. [PubMed] [Google Scholar]
- 116.Faria TN, Deb S, Kwok SC, Vandeputte M, Talamantes F, Soares MJ. Transplantable rat choriocarcinoma cells express placental lactogen: identification of placental lactogen-I immunoreactive protein and messenger ribonucleic acid. Endocrinology. 1990;127:3131–3137. doi: 10.1210/endo-127-6-3131. [DOI] [PubMed] [Google Scholar]
- 117.Verstuyf A, Sobis H, Goebels J, Fonteyn E, Cassiman JJ, Vandeputte M. Establishment and characterization of a continuous in vitro line from a rat choriocarcinoma. Int J Cancer. 1990;45:752–756. doi: 10.1002/ijc.2910450430. [DOI] [PubMed] [Google Scholar]
- 118.Faria TN, Soares MJ. Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expressing embers of the placental prolactin family. Endocrinology. 1991;129:2895–2906. doi: 10.1210/endo-129-6-2895. [DOI] [PubMed] [Google Scholar]
- 119.Sahgal N, Canham LN, Canham B, Soares MJ. Rcho-1 trophoblast stem cells: a model system for studying trophoblast cell differentiation. Methods Mol Med. 2006;121:159–178. [PubMed] [Google Scholar]
- 120.Kamei T, Jones SR, Chapman BM, McGonigle K, Dai G, Soares MJ. Activation and involvement of the phosphatidylinositol 3-kinase/akt-signaling pathway in the endocrine differentiation of trophoblast cells. Mol Endocrinol. 2002;16:1469–1481. doi: 10.1210/mend.16.7.0878. [DOI] [PubMed] [Google Scholar]
- 121.Kent LN, Konno T, Soares MJ. Phosphatidylinositol 3-kinase modulation of trophoblast cell differentiation. BMC Dev Biol. 2010;10:97. doi: 10.1186/1471-213X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kent LN, Rumi MAK, Kubota K, Lee D-S, Soares MJ. FOSL1 is integral to establishing the maternal-fetal interface. Mol Cell Biol. 2011;31:4801–4813. doi: 10.1128/MCB.05780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 124.Asanoma K, Rumi MAK, Kent LN, Chakraborty D, Renaud SJ, Wake N, Lee D-S, Kubota K, Soares MJ. FGF4-dependent stem cells derived from rat blastocysts differentiate along the trophoblast lineage. Dev Biol. 2011;351:110–119. doi: 10.1016/j.ydbio.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Avilion AA, Nicolis SK, Pevny LH, Perez L, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371:333–336. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- 127.Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. J Clin Invest. 2010;120:995–1003. doi: 10.1172/JCI41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kitano T, Iizasa H, Terasaki T, Asashima T, Matsunaga N, Utoguchi N, Watanabe Y, Obinata M, Ueda M, Nakashima E. Polarized glucose transporters and mRNA expression properties in newly developed rat syncytiotrophoblast cell lines, TR-TBTs. J Cell Physiol. 2002;193:208–218. doi: 10.1002/jcp.10165. [DOI] [PubMed] [Google Scholar]
- 129.Kitano T, Iizasa H, Hwang IW, Hirose Y, Morita T, Maeda T, Nakashima E. Conditionally immortalized syncytiotrophoblast cell lines as new tools for study of the blood-placenta barrier. Biol Pharm Bull. 2004;27:753–759. doi: 10.1248/bpb.27.753. [DOI] [PubMed] [Google Scholar]
- 130.Soares MJ, Schaberg KD, Pinal CS, De SK, Bhatia P, Andrews GK. Establishment of a rat placental cell line expressing characteristics of extraembryonic membranes. Dev Biol. 1987;124:134–144. doi: 10.1016/0012-1606(87)90466-0. [DOI] [PubMed] [Google Scholar]
- 131.Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 132.Chuykin I, Lapidus I, Popova E, Vilianovich L, Mosienko V, Alenina N, Binas B, Chai G, Bader M, Krivokharchenko A. Characterization of trophoblast and extraembryonic endoderm cell lineages derived from rat preimplantation embryos. PLoS One. 2010;5:e9794. doi: 10.1371/journal.pone.0009794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galat V, Binas B, Iannaccone S, Postovit LM, Debeb BG, Iannaccone P. Developmental potential of rat extraembryonic stem cells. Stem Cells Dev. 2009;18:1309–1318. doi: 10.1089/scd.2009.0115. [DOI] [PubMed] [Google Scholar]
- 134.Demers SP, Desmarais JA, Vincent P, Smith LC. Rat blastocyst-derived stem cells are precursors of embryonic and extraembryonic lineages. Biol Reprod. 2011;84:1128–1138. doi: 10.1095/biolreprod.109.082792. [DOI] [PubMed] [Google Scholar]
- 135.Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying QL, Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 136.Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh CL, Pera MF, Ying QL. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu XJ, Deb S, Soares MJ. Spontaneous differentiation of trophoblast cells along the spongiotrophoblast cell pathway: expression of members of the placental prolactin gene family and modulation by retinoic acid. Dev Biol. 1994;163:86–97. doi: 10.1006/dbio.1994.1125. [DOI] [PubMed] [Google Scholar]
- 138.Beghin D, Delongeas JL, Claude N, Forestier F, Farinotti R, Gil S. Development and characterisation of a new model of rat trophoblasts. Toxicol In Vitro. 2009;23:141–147. doi: 10.1016/j.tiv.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 139.Gilligan J, Tong M, Longato L, de la Monte SM, Gundogan F. Precision-cut slice culture method for rat placenta. Placenta. 2011 doi: 10.1016/j.placenta.2011.10.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the rat placenta. Methods Mol Med. 2006;121:295–313. doi: 10.1385/1-59259-983-4:293. [DOI] [PubMed] [Google Scholar]
- 141.De Rijk EPCT, van Esch E, Flik G. Pregnancy dating in the rat: placental morphology and maternal blood parameters. Toxicol Pathol. 2002;30:271–282. doi: 10.1080/019262302753559614. [DOI] [PubMed] [Google Scholar]
- 142.Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- 143.Hasuwa H, Kaseda K, Einarsdottir T, Okabe M. Small interfering RNA and gene silencing in transgenic mice and rats. FEBS Lett. 2002;532:227–230. doi: 10.1016/s0014-5793(02)03680-3. [DOI] [PubMed] [Google Scholar]
- 144.Ikawa M, Yamada S, Nakanishi T, Okabe M. ‘Green mice’ and their potential usage in biological research. FEBS Lett. 1998;430:83–87. doi: 10.1016/s0014-5793(98)00593-6. [DOI] [PubMed] [Google Scholar]
- 145.Arroyo JA, Konno T, Khalili DC, Soares MJ. A simple in vivo approach to investigate invasive trophoblast cells. Int J Dev Biol. 2005;49:977–980. doi: 10.1387/ijdb.051993ja. [DOI] [PubMed] [Google Scholar]
- 146.Lee D-S, Rumi MAK, Konno T, Soares MJ. In vivo genetic manipulation of the rat trophoblast cell lineage using lentiviral vector delivery. Genesis. 2009;47:433–439. doi: 10.1002/dvg.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Voigt B, Serikawa T. Pluripotent stem cells and other technologies will eventually open the door for straightforward gene targeting in the rat. Dis Model Mech. 2009;2:341–343. doi: 10.1242/dmm.002824. [DOI] [PubMed] [Google Scholar]
- 148.Dwinell MR, Lazar J, Geurts AM. The emerging role for rat models in gene discovery. Mamm Genome. 2011;22:466–475. doi: 10.1007/s00335-011-9346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 150.Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- 151.Hamra FK, Gatlin J, Chapman KM, Grellhesi DM, Garcia JV, Hammer RE, Garbers DL. Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proc Natl Acad Sci USA. 2002;99:14931–14936. doi: 10.1073/pnas.222561399. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 153.Orwig KE, Avarbock MR, Brinster RL. Retrovirus-mediated modification of male germline stem cells in rats. Biol Reprod. 2002;67:874–879. doi: 10.1095/biolreprod.102.005538. [DOI] [PubMed] [Google Scholar]
- 154.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Geurts AM, Moreno C. Zinc-finger nucleases: new strategies to target the rat genome. Clin Sci (Lond) 2010;119:303–311. doi: 10.1042/CS20100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tesson L, Usal C, Menoret S, Leung E, Niles BJ, Remy S, Santiago Y, Vincent AI, Meng X, Zhang L, Gregory PD, Anegon I, Cost GJ. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol. 2011;29:695–696. doi: 10.1038/nbt.1940. [DOI] [PubMed] [Google Scholar]
- 157.Zan Y, Haag JD, Chen K-S, Shepel LA, Wigington D, Wang Y-R, Hu R, Lopez-Guajardo CC, Brose HL, Porter KI, Leonard RA, Hitt AA, Schommer SL, Elegbede AF, Gould MN. Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat Biotechnol. 2003;21:645–651. doi: 10.1038/nbt830. [DOI] [PubMed] [Google Scholar]
- 158.Lu B, Geurts AM, Poirier C, Petit DC, Harrison W, Overbeek PA, Bishop CE. Generation of rat mutants using a coat color-tagged Sleeping Beauty transposon system. Mamm Genome. 2007;18:338–346. doi: 10.1007/s00335-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 159.Izsvák Z, Fröhlich J, Grabundzija I, Shirley JR, Powell HM, Chapman KM, Ivics Z, Hamra FK. Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nat Methods. 2010;7:443–445. doi: 10.1038/nmeth.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tong C, Li P, Wu NL, Yan Y, Ying QL. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211–213. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tong C, Huang G, Ashton C, Li P, Ying QL. Generating gene knockout rats by homologous recombination in embryonic stem cells. Nat Protoc. 2011;6:827–844. doi: 10.1038/nprot.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang G, Tong C, Kumbhani DS, Ashton C, Yan H, Ying QL. Beyond knockout rats: new insights into finer genome manipulation in rats. Cell Cycle. 2011;10:1059–1066. doi: 10.4161/cc.10.7.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Georgiades P, Cox B, Gertsenstein M, Chawengsakophak K, Rossant J. Trophoblast-specific gene manipulation using lentivirus-based vectors. BioTechniques. 2007;42:317–325. doi: 10.2144/000112341. [DOI] [PubMed] [Google Scholar]
- 164.Okada Y, Ueshin Y, Isotani A, Saito-Fujita T, Nakashima H, Kimura K, Mizoguchi A, Oh-Hora M, Mori Y, Ogata M, Oshima RG, Okabe M, Ikawa M. Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat Biotechnol. 2007;25:233–237. doi: 10.1038/nbt1280. [DOI] [PubMed] [Google Scholar]
- 165.Jacob HJ, Kwitek AE. Rat genetics: attaching physiology and pharmacology to the genome. Nat Rev Genet. 2002;3:33–42. doi: 10.1038/nrg702. [DOI] [PubMed] [Google Scholar]