Abstract
Aim
People who quit smoking often gain 11–12 pounds, on average, which can frequently lead to a relapse to smoking. This study evaluated whether extended vs. standard duration treatment with nicotine patch helps those able to quit smoking to reduce cessation-induced weight gain and explored nicotine patch adherence as a mediator of treatment effects.
Design and Setting
We examined data from a completed randomized placebo-controlled clinical trial of extended (24 weeks) vs. standard (8 weeks plus 16 weeks of placebo) transdermal nicotine patch therapy. Changes in measured weight over 24 weeks were compared across the two treatment arms, controlling for gender, baseline smoking rate, and previous weight. Adherence to patch use was assessed using self-report of daily use over 24 weeks.
Participants
139 clinical trial participants who were confirmed to be abstinent at weeks 8 and 24.
Findings
Compared to participants who received 8 weeks of nicotine patch therapy, participants who received 24 weeks of treatment showed significantly less weight gain from pre-treatment to week 24 (β = −4.76, 95% CI: −7.68 to −1.84, p = .002) and significantly less weight gain from week 8 to week 24 (β = −2.31, 95% CI: −4.39 to −0.23, p = .03). Extended treatment increased patch adherence which, in turn, reduced weight gain; patch adherence accounted for 20% of the effect of treatment arm on weight gain.
Conclusion
Compared to 8 weeks of transdermal nicotine therapy, 24 weeks of patch treatment may help to reduce the weight gain that is typical among smokers who are able to achieve abstinence from tobacco use. Extended treatment increased nicotine patch adherence which, in turn, reduced weight gain.
1. Introduction
The relationship between tobacco use and weight is well documented. Many adolescents cite weight control as the reason for initiating smoking (Potter et al., 2004) and many adults report that they continue smoking to control their weight (Klesges & Klesges, 1988). Current smokers weigh less than non-smokers (Albanes et al., 1987) and many smokers report that worry about cessation-induced weight gain undermines attempts to quit smoking (Hall et al., 1986). Lastly, fear of weight gain predicts smoking relapse (Meyers et al., 1997) and, on average, smokers who successfully quit smoking gain 11–12 pounds over the 2 years following cessation (Fromm et al., 1998). It is critical to identify interventions that can prevent abstinence-induced weight gain.
Few interventions for nicotine dependence also limit weight gain. While the inclusion of a behavioral weight control component within a tobacco treatment program limits weight gain, the benefits are not sustainable (Spring et al., 2009). The inclusion of physical activity components within a smoking cessation program does not limit weight gain (Bize et al., 2010). There is no evidence that varenicline prevents weight gain (Gonzales et al., 2006) and, while bupropion may attenuate weight gain, the effects are not sustained (Hurt et al., 1997; Levine et al., 2010). Nicotine replacement therapy (NRT) may attenuate weight gain among those who quit smoking (Gross et al., 1989; Allen et al., 2005), but this effect may depend on level of treatment adherence (Ferguson et al., 2010). Lastly, while the use of adjunctive medications to prevent weight gain are being explored, such as naltrexone (O'Malley et al., 2006; King et al., 2006; Toll et al., 2008), sibutramine (Sood et al., 2009), and fluoxetine (Saules et al., 2004), a recent meta-analyses concluded that the existing literature is insufficient to offer clinical recommendations (Parsons et al., 2009).
A recently completed placebo-controlled randomized trial, comparing standard 8-weeks of transdermal nicotine treatment to 24-weeks of treatment, showed almost a 2-fold increase in abstinence with extended treatment (Schnoll et al., 2010)†. A previous clinical trial with nicotine gum suggested that the effect of NRT on weight gain attenuation may be due to the pharmacological effects of nicotine (Ferguson et al., 2010) and pre-clinical data suggest that, in the absence of nicotine, the reinforcing value of food increases (Donney et al., 2011). Thus, we hypothesized that extended treatment with nicotine patch could attenuate weight gain among abstinent smokers, versus 8-weeks of treatment, and that greater adherence with nicotine patch therapy could mediate the relationship between extended treatment and lower weight gain. The results of this study could show that attenuation of weight gain is an additional benefit of extended nicotine patch therapy and support the hypothesis that continuous exposure to nicotine serves as a plausible mechanism of NRT's effect on weight gain.
2. Methods
2.1. Study design
A complete description of the trial from which the present data were ascertained can be found elsewhere (Schnoll et al., 2010). Briefly, the trial included treatment-seeking smokers recruited through media ads. Eligible participants were randomized to 8 weeks of 21mg nicotine patches (Nicoderm CQ; GlaxoSmithKline) plus 16 weeks of placebo patches (standard) or 24 weeks of 21mg nicotine patches (extended). All participants received 7 standard behavioral counseling sessions (Lerman et al., 2004). At a pre-treatment session (week −2), participants completed measures including an assessment of weight using a standardized scale. Smoking behavior and patch adherence was assessed at weeks 0, 1, 4, 8, 12, 16, 20, and 24. Smoking cessation at weeks 8 and 24 was defined as 7-day point prevalence abstinence confirmed with a breath carbon monoxide (CO) sample since cotinine analysis could not be used in light of nicotine patch use (Hughes et al., 2003). Participants were assumed to be smoking if they were lost to follow-up, failed to provide a CO sample, or had CO levels > 10ppm (Benowitz et al., 2002). Measured weight was again assessed at weeks 8 and 24.
2.2. Participants
Participants were eligible if they were ages 18–65 and smoked ≥ 10 cigarettes/day for at least the past year. Participant exclusions consisted of: a medical condition or a medication which increased risk for adverse effects from nicotine patch, current drug or alcohol dependence, a history of an Axis I psychiatric disorder, or current treatment for nicotine dependence. In the original trial, the intent-to-treat sample was comprised of 286 participants in standard therapy and 282 in extended therapy. To control for the potential confounding from concurrent tobacco use, the present hypotheses were addressed using a sub-sample of 139 individuals who were confirmed to be abstinent from smoking at both weeks 8 and 24, as done previously (Ferguson et al., 2010).
2.3. Measures
2.3.1. Demographic variables
Socio-demographic characteristics were collected such as age, race, ethnicity, and level of education.
2.3.2. Smoking variables
Smoking characteristics were collected including cigarettes per day and level of nicotine dependence using the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991).
2.3.3. Adherence
Patch adherence was based on self-report during weeks 0–24. A total adherence score was computed by summing the number of days the patch was used across the 24 weeks of the trial.
2.3.4. Weight
Weight was assessed using a standard upright scale as in past studies (Ferguson et al., 2010) at pre-treatment (week −2) and then at weeks 8 and 24.
2.4. Statistical analysis
Our primary analyses examined the difference between treatment arms in terms of measured weight gain from week 8 to 24, corresponding to when the standard treatment participants used placebo but the extended treatment participants continued on NRT. Treatment arm effect on weight gain from pre-treatment to week 24 was examined as a secondary outcome. For both outcomes, we used mixed models linear regression to examine the incremental weight gain (in pounds), only admitting data for those abstinent at weeks 8 and 24. The models controlled for gender and baseline smoking as done previously (Schnoll et al., 2010). The regression for weight gain from week 8 to week 24 controlled for week 8 weight and the regression for weight gain from pre-treatment to week 24 controlled for pre-treatment weight. We analyzed mediation using a regression-based path model (MacKinnon, 2007) and weight gain between week 8 and 24 as the outcome variable, controlling for sex and baseline smoking. Standardized outcomes were modeled using standardized predictors and regressions were fitted jointly using the seemingly unrelated estimation approach (Beasley, 2008; Zellner, 1962). This method partitions the effect of treatment on outcome into direct (and unexplained) effects versus mediated effects; it tests the overall mediation hypothesis using the proportion of treatment effect explained (Lin et al., 1997; Vittinghoff, 2009) and the strength of each mediating pathway.
3. Results
3.1. Sample characteristics
There were no differences between the treatment arms at baseline in terms of gender, age, race, level of education, level of nicotine dependence, cigarettes per day, and weight. For the present sample, 49% of participants were female, 73% had at least some college education, 83% were of European ancestry, the mean age of the sample was 44 years (SD = 9.9), the mean level of nicotine dependence was 4.7 (SD = 2.1), the average number of cigarettes smoker per day at baseline was 19.1 (SD = 8.4), and the average measured weight was 186 pounds (SD = 41.5).
3.1. Treatment effect on weight
As shown in Table 1, we found a significant effect of treatment arm on measured weight gain between week 8 and week 24 (β = −2.31, 95% CI: −4.39 to −0.23, p = .03). Between week 8 and week 24, participants who received 8 weeks of transdermal nicotine therapy gained, on average, 4.69 pounds (SD = 5.44), whereas participants who received 24 weeks of transdermal nicotine therapy gained, on average, 2.52 pounds (SD = 6.72). As shown in Table 1, we also found a significant effect of treatment arm on measured weight gain between week −2 and week 24 (β = −4.76, 95%CI: −7.68 to −1.84, p = .002). Between week −2 and week 24, participants who received 8 weeks of transdermal nicotine therapy gained, on average, 10.37 pounds (SD = 8.86), whereas participants who received 24 weeks of transdermal nicotine therapy gained, on average, 5.83 pounds (SD = 9.11).
Table 1.
Measured Weight Gain Across Treatment Arms
| Variable | Standard (M, SD) | Extended (M, SD) | β | 95% CI | p |
|---|---|---|---|---|---|
| Measured Weight (pre-treatment to week 24) | 10.37 (8.86) | 5.83 (9.11) | −4.76 | −7.68 to −1.84 | 0.002 |
| Measured Weight (week 8 to week 24) | 4.69 (5.44) | 2.52 (6.72) | −2.31 | −4.39 to −0.23 | 0.03 |
Note. β = beta weight; CI = Confidence Interval; the models included main effects for the treatment arm and gender, baseline smoking rate, and previous weight as covariates.
3.2. Mediation analysis
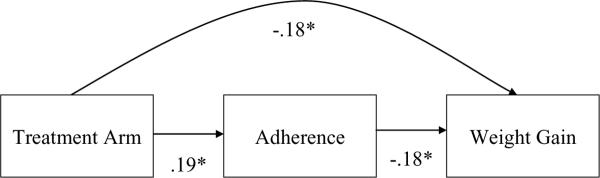
Figure 1 shows the mediational model for treatment arm, patch adherence, and weight gain between weeks 8 and 24. Participants in extended nicotine patch treatment showed significantly greater levels of patch adherence over 24 weeks, versus participants in standard treatment (β = .19, 95% CI: −.33 to −.03, p = .02). In turn, higher levels of patch adherence over 24 weeks predicted significantly less weight gain (β = −.18, 95% CI: −.34 to −.02, p = .02). The formal test of mediation was not statistically significant (p = .11) but the mediational pathway explained 20% of the effect of treatment arm on weight gain.
Figure 1.
Mediation model of treatment, adherence, and weight gain.
Note. Treatment is coded 0 = standard, 1 = extended; * p < .05.
4. Discussion
This study used data from a completed trial of extended duration nicotine patch treatment to evaluate if longer treatment with nicotine patches attenuates weight gain among individuals who are able to achieve abstinence from tobacco use. Additionally, the role of patch adherence was explored as a mediator of the relationship between extended treatment and weight gain. As hypothesized, extended treatment with nicotine patches led to lower weight gain over 24 weeks and between 8 and 24 weeks of the study, corresponding to when standard treatment participants were switched to placebo. In particular, controlling for sex, the number of cigarettes smoked at trial entry, and previous weight, individuals who were able to achieve abstinence gained about half as much weight if they received 24 weeks vs. 8 weeks of nicotine patches.
In addition, the present study suggests that level of patch adherence could explain part of the effect of extended nicotine patch treatment on limiting weight gain among abstainers. Extended treatment participants showed significantly greater levels of patch adherence which, in turn, predicted lower weight gain. Taken together, these results are consistent with previous studies that show that NRT can attenuate weight gain, particularly among individuals who show higher rates of treatment adherence (Ferguson et al., 2010). While the exact mechanism through which nicotine limits weight gain is not fully explicated, pre-clinical studies suggest that nicotine can effect the reinforcing value of food, with cessation enhancing food reinforcement (Donney et al., 2011). As such, the reduced weight gain observed in the present analysis from extended NRT may be a function, in part, of enhanced exposure to nicotine via longer duration and better adherence.
Concern over and actual weight gain among individuals who quit smoking frequently leads to smoking relapse (Meyers et al., 1997). Concern over weight gain also often prevents smokers from contemplating a quit attempt (Hall et al., 1986). To date, few interventions have shown efficacy at both promoting smoking cessation and minimizing weight gain. The present study indicates that the use of extended nicotine patch treatment, along with high levels of patch adherence, can offer some hope to smokers who worry about weight gain following a quit attempt. Smokers who express a fear of abstinence-induced weight gain could be encouraged to utilize extended nicotine patch treatment as a therapeutic option.
This study is limited by a relatively small sample of abstinent smokers from a larger trial and the consequent limited statistical power. In addition, treatment adherence was measured using self-report, which may over-represent actual use. Further, we did not track use of weight loss treatment programs or medications outside the context of the clinical trial.Lastly, the treatment effect on weight gain may not extend beyond when treatment is stopped but measured weight was not assessed beyond week 24 in this study. Nevertheless, the present study indicates that extended and compliant use of nicotine patches can minimize the weight gain that is typical among individuals who successfully quit smoking. Indeed, participants in this study exhibited, on average, about half the weight gain over the course of 24 weeks that is typical of individuals who successfully quit smoking (Fromm et al., 1998) and that was observed among participants in the present trial who received the standard 8 weeks of treatment. Given the lack of available treatments for nicotine dependence that also attenuate weight gain, the present results represent some hope for smokers that options are available to increase their ability to quit smoking without gaining substantial weight.
Research Highlights
Extended use of nicotine patches, compared to standard duration, attenuates weight gain among those who quit smoking.
2. Greater adherence to patch use may mediate the effect of treatment duration on weight gain.
3. The results may offer a novel treatment approach for smokers concerned about post-cessation weight gain.
4. The results may support the theory that nicotine has pharmacological effects that prevent weight gain.
Acknowledgements
The authors thank the following individuals who assisted with study implementation or with manuscript preparation: Dr. Freda Patterson, Dr. Janet Audrain-McGovern, Dr. Margaret Rukstalis, Angela Pinto, Susan Ware, Kia Kerrin, Janice Biddle, Sean Fleming, Paul Sanborn, and Susan Kerns.
Role of Funding Source This research was supported by National Institutes of Health grants P50 CA143187 and U01 DA020830.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Dr. Lerman has served as a consultant to GlaxoSmithKline, Astra Zeneca, Pfizer, and Novartis. Dr. Schnoll has served as a consultant for GlaxoSmithKline and received research support from Pfizer. Financial support for this study was not provided from an industry sponsor.
Contributors Dr. Lerman was responsible for study conception and design, analysis and interpretation of data, drafting of the manuscript, and obtaining funding. Drs. Schnoll and Wileyto were responsible for analysis and interpretation of data and drafting of the manuscript.
This trial also showed no safety concerns with extended use of the nicotine patch. Fifteen side effects were assessed and compared across treatment arms at week 1 and week 12. The only significant difference was for sleep problems at week 1, with extended treatment participants showing a higher frequency of sleep problems compared to standard treatment participants.
References
- Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. American Journal of Public Health. 1987;77:439–444. doi: 10.2105/ajph.77.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Hatsukami D, Brintnell DM, Bade T. Effect of nicotine replacement therapy on post-cessation weight gain and nutrient intake: a randomized controlled trial of postmenopausal female smokers. Addictive Behavior. 2005;30:1273–1280. doi: 10.1016/j.addbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Beasley TM. Seemingly unrelated regression (SUR) models as a solution to path analytic models with correlated errors. Multiple Linear Regression Viewpoints. 2008;34:1–7. [Google Scholar]
- Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Bize R, Willi C, Chiolero A, Stoianov R, Payot S, Locatelli I, et al. Participation in a population-based physical activity programme as an aid for smoking cessation: a randomized trial. Tobacco Control. 2010;19:488–494. doi: 10.1136/tc.2009.030288. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF. The reinforcement-enhancing effects of nicotine: Implications for the relationship between smoking, eating and weight. Physiology & Behavior. 2011;104:143–148. doi: 10.1016/j.physbeh.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Rohay JM, Gitchell JG, Garvey AJ. Effect of compliance with nicotine gum dosing on weight gained during a quit attempt. Addiction. 2010;106:651–656. doi: 10.1111/j.1360-0443.2010.03244.x. [DOI] [PubMed] [Google Scholar]
- Froom P, Melamed S, Benbassat JJ. Smoking cessation and weight gain. Journal of Family Practice. 1998;46:460–464. [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. sustained-release buprorion and placebo for smoking cessation: a randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gross J, Stitzer ML, Maldonado J. Nicotine replacement: effects on post-cessation weight gain. Journal of Clinical and Consulting Psychology. 1989;57:87–92. doi: 10.1037//0022-006x.57.1.87. [DOI] [PubMed] [Google Scholar]
- Hall SM, Ginsberg D, Jones RT. Smoking cessation and weight gain. Journal of Counseling and Clinical Psychology. 1986;54:342–346. doi: 10.1037//0022-006x.54.3.342. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. New England Journal of Medicine. 1997;23:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- King A, de Wit H, Riley RC, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine & Tobacco Research. 2006;8:671–682. doi: 10.1080/14622200600789767. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Klesges LM. Cigarette smoking as a weight loss strategy in a university population. International Journal of Eating Disorders. 1988;7:413–419. [Google Scholar]
- Klesges RC, Meyers AW, Klesges LM, La Vasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychological Bulletin. 1989;106:204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain-McGovern J. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Annals of Internal Medicine. 2004;140:426–433. doi: 10.7326/0003-4819-140-6-200403160-00009. [DOI] [PubMed] [Google Scholar]
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, et al. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Archives of Internal Medicine. 2010;170:543–550. doi: 10.1001/archinternmed.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Statistical Medicine. 1997;16:1515–1527. doi: 10.1002/(sici)1097-0258(19970715)16:13<1515::aid-sim572>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers AW, Kleges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. Journal of Consulting and Clinical Psychology. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, et al. A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Archives of Internal Medicine. 2006;166:667–674. doi: 10.1001/archinte.166.6.667. [DOI] [PubMed] [Google Scholar]
- Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database System of Reviews. 2009;21:CD006219. doi: 10.1002/14651858.CD006219.pub2. [DOI] [PubMed] [Google Scholar]
- Potter BK, Pederson LL, Chan SS, Aubut JA, Koval JJ. Does a relationship exist between body weight, concerns about weight, and smoking among adolescents? An integration of the literature with an emphasis on gender. Nicotine & Tobacco Research. 2004;6:397–425. doi: 10.1080/14622200410001696529. [DOI] [PubMed] [Google Scholar]
- Saules KK, Schuh LM, Arfken CL, Reed K, Kilbey MM, Schuster CR. Double-blind placebo-controlled trial of fluoxetine in smoking cessation treatment including nicotine patch and cognitive-behavioral group therapy. American Journal of Addiction. 2004;13:438–446. doi: 10.1080/10550490490512762. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan D, Shields A, Asch D, et al. Effectiveness of extended duration transdermal nicotine therapy: A randomized trial. Annals of Internal Medicine. 2010;152:144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A, Ebbert JO, Clark MM, Croghan IT, Schroeder DR, Hays JT. Sibutramine for weight gain attenuation during smoking cessation with varenicline: a pilot study. Nicotine & Tobacco Research. 2009;11:1479–1484. doi: 10.1093/ntr/ntp162. [DOI] [PubMed] [Google Scholar]
- Spring B, Howe D, Berendsen M, McFadden HG, Hitchcock K, Rademaker AW, et al. Behavioral intervention to promote smoking cessation and prevent weight gain: a systematic review. Addiction. 2009;104:1472–1486. doi: 10.1111/j.1360-0443.2009.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, Leary V, Wu R, Salovey P, Meandzija B, O'Malley SS. A preliminary investigation of naltrexone augmentation of bupropion to stop smoking with less weight gain. Addictive Behavior. 2008;33:173–179. doi: 10.1016/j.addbeh.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittinghoff E, Sen S, McCulloch CE. Sample size calculations for evaluating mediation. Statistical Medicine. 2009;28:541–557. doi: 10.1002/sim.3491. [DOI] [PubMed] [Google Scholar]
- Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. Journal of the American Statistical Association. 1962;57:348–368. [Google Scholar]