Abstract
Mouse blood contains four esterases that detoxify organophosphorus compounds: carboxylesterase, butyrylcholinesterase, acetylcholinesterase, and paraoxonase-1. In contrast human blood contains the latter three enzymes but not carboxylesterase. Organophosphorus compound toxicity is due to inhibition of acetylcholinesterase. Symptoms of intoxication appear after approximately 50% of the acetylcholinesterase is inhibited. However, complete inhibition of carboxylesterase and butyrylcholinesterase has no known effect on an animal’s well being. Paraoxonase hydrolyzes organophosphorus compounds and is not inhibited by them. Our goal was to determine the effect of plasma carboxylesterase deficiency on response to sublethal doses of 10 organophosphorus toxicants and one carbamate pesticide. Homozygous plasma carboxylesterase deficient ES1−/− mice and wild-type littermates were observed for toxic signs and changes in body temperature after treatment with a single sublethal dose of toxicant. Inhibition of plasma acetylcholinesterase, butyrylcholinesterase, and plasma carboxylesterase was measured. It was found that wild-type mice were protected from the toxicity of 12.5 mg/kg parathion applied subcutaneously. However, both genotypes responded similarly to paraoxon, cresyl saligenin phosphate, diisopropylfluorophosphate, diazinon, dichlorvos, cyclosarin thiocholine, tabun thiocholine, and carbofuran. An unexpected result was the finding that transdermal application of chlorpyrifos at 100 mg/kg and chlorpyrifos oxon at 14 mg/kg was lethal to wild-type but not to ES1−/− mice, showing that with this organochlorine, the presence of carboxylesterase was harmful rather than protective. It was concluded that carboxylesterase in mouse plasma protects from high toxicity agents, but the amount of carboxylesterase in plasma is too low to protect from low toxicity compounds that require high doses to inhibit acetylcholinesterase.
Keywords: organochlorine, organophosphates, pesticides, carboxylesterase knockout mouse
1. Introduction
The acute toxicity of organophosphorus poisons is due to inhibition of acetylcholinesterase. Carboxylesterase acts as a bioscavenger by stoichiometrically binding and inactivating organophosphorus poisons (OP), thereby reducing the number of OP molecules available for inhibiting AChE [1]. Studies in rats have concluded that plasma carboxylesterase plays a major role in protecting rats from the toxicity of parathion, paraoxon, chlorpyrifos, soman, sarin, and tabun, but not dichlorvos, diisopropylfluorophosphate, and VX [1–4].
Carboxylesterase in mouse plasma is a 70 kDa glycoprotein product of the ES1 gene on mouse chromosome 8, where it is one of 16 homologous carboxylesterase genes and pseudogenes. Humans have no carboxylesterase in plasma, whereas laboratory animals including mice, rats, guinea pigs, and rabbits have abundant carboxylesterase in plasma [5]. The presence of carboxylesterase in the plasma of mice makes them poor models for studies of organophosphorus intoxication in humans.
ES1 carboxylesterase in plasma is distinct from the carboxylesterases in liver and intestine, which are transcribed from the CES1 and CES2 gene clusters on chromosome 8 and not from the ES1 gene. Humans and all mammals have CES1 and CES2 carboxylesterases in liver, intestine, and other organs [6]. Carboxylesterase in rat plasma, but not in liver, intestine, and other organs is inhibited by toxic doses of soman, suggesting that the carboxylesterase in plasma is the most important OP detoxifying carboxylesterase [7,8].
In a previous report we described the plasma carboxylesterase knockout mouse (ES1−/−), created as a small animal model for studies that mirror human response to organophosphorus agents [9]. These mice have no detectable carboxylesterase activity in plasma, but have normal carboxylesterase activity in liver, intestine, and other organs. Our goal in the present report was to compare the response of ES1−/− and ES1+/+ wild-type mice to various organophosphorus toxicants and one carbamate pesticide.
Materials and methods
2.1. Materials
The following were from Chem Service Inc. (West Chester, PA): chlorpyrifos (PS-674); chlorpyrifos oxon (Met-674B); trichloropyridinol (Met-674A); parathion (PS-95); paraoxon (PS610); and dichlorvos (PS-89).
The following were from Sigma-Aldrich (St. Louis, MO): carbofuran (# 426008); DFP (# D0879); Cremophor EL (C5135); and Polyethylene glycol average Mn 400 (#202398).
Diazinon was from Ciba-Geigy Corp. (Switzerland). CBDP was a gift from Dr. Wolf-Dietrich Dettbarn (Vanderbilt University, Nashville, TN) and Dr. David Lenz (US Army Institute of Chemical Defense, Aberdeen Proving Gd, MD). It was custom synthesized by Starks Associates, Buffalo, NY and was 99.5% pure. The nerve agent model compounds, cyclosarin thiocholine S(p) and tabun thiocholine S(p) were custom synthesized in the laboratory of Dr. John R. Cashman (Human BioMolecular Research Institute, San Diego, CA) as described [10]. The stereoisomers had the S(p) configuration, which is more reactive with acetylcholinesterase than the R(p) configuration [10].
2.2. ES1 knockout mouse
Animal work was conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the U.S. National Institutes of Health. Formal approval to conduct animal experiments was obtained from the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center. C57BL/6 mice completely deficient in plasma carboxylesterase activity were created by homologous recombination of the ES1 gene on mouse chromosome 8 [9]. The ES1 gene was inactivated by deleting exon 5 and introducing a frame shift for amino acids translated from exons 6 to 13, thus deleting the catalytic triad residues Ser, Glu, His. The ES1−/− mice are healthy and fertile. They have normal acetylcholinesterase, butyrylcholinesterase, and paraoxonase-1 activity in plasma and organs. ES1+/− mice were bred to create ES1+/+, ES1+/− and ES1−/− mice. This breeding protocol allowed siblings to be utilized in experiments, reducing the possibility of effects from genetic drift when comparing the response of wild-type and knockout mice to intoxication.
The ES1 knockout mouse in strain C57BL/6 is available from The Jackson Laboratory Repository (Bar Harbor, ME) http://jaxmice.jax.org/query where it is listed as JAX Stock No. 014096 C57BL/6-Ces1ctm1.1Loc/J. An alternative name for the ES1 gene is Ces1c. The Jackson Laboratory provides heterozygous animals that must be bred to produce animals completely deficient in plasma carboxylesterase.
2.3. Determination of ES1 phenotype
Pups were phenotyped by assaying plasma carboxylesterase activity of blood collected from the saphenous vein. Carboxylesterase activity was measured with alpha-naphthyl acetate [4,11] in the presence of eserine to inhibit AChE and BChE and in the presence of EDTA to inhibit paraoxonase-1. Measured activity was 9–13 u/ml in ES1+/+, 4–7 u/ml in ES1+/− and 0.5–0.7 u/ml in ES1−/− plasma of adult mice. The residual activity in ES1−/− plasma was due to hydrolysis of alpha-naphthyl acetate by albumin [12,13].
2.4. Challenge with organophosphorus compounds and a carbamate pesticide
Adult male ES1+/+ and ES1−/− mice (n=3–6/genotype) were challenged with organophosphorus compounds or a carbamate pesticide to determine differences in toxicity and in effects on plasma BChE, AChE and carboxylesterase activities. Dose finding experiments were conducted in ES1+/+ mice to find non-lethal doses that would produce toxic signs or significantly decrease esterase activity. Challenge compounds were delivered subcutaneously (sc) with the exception of chlorpyrifos and chlorpyrifos oxon, which were delivered transdermally. Challenge compounds, doses, and delivery solvents were as follows: chlorpyrifos (CF) 100 mg/kg in acetone; chlorpyrifos oxon (CPO) 14 mg/kg in acetone; trichloropyridinol 100 mg/kg in acetone; parathion 15 mg/kg in ethanol; paraoxon 0.2 mg/kg in 50% ethanol; carbofuran 1.0 mg/kg in 5% dimethylsulfoxide; CBDP 20 mg/kg in polyethylene glycol 400; DFP 4 mg/kg in 2% ethanol/saline solution; diazinon 50 mg/kg in 7% Cremophor EL/10% ethanol/saline solution; dichlorvos 7.5 mg/kg in saline; cyclosarin thiocholine 0.05 mg/kg in ethanol; tabun thiocholine 6.0 mg/kg in ethanol. Toxicant solutions were freshly prepared immediately before use.
2.5. Functional observational battery
Mice were observed for the behavioral toxic signs described by McDaniel and Moser [14] including posture, involuntary motor movements, tremors, seizures, convulsions, palpebral closure, reactivity to being handled, lacrimation, salivation, piloerection, gait, mobility, arousal, stereotypy, straub tail, vocalization, righting reflex, and temperature. Mice challenged with a single sublethal dose displayed many of these toxic signs, but none had convulsions or stereotypical behavior.
2.6. Temperature
Axial body temperature was measured with a digital thermometer, Thermalert model TH-5, attached to a surface Microprobe MT-D, type T thermocouple (Physitemp Instruments, Clifton, NJ). Temperature was recorded prior to challenge, at 5 minute intervals for the first hour post-challenge, hourly through 8 hours, and finally at 24, 48, and 96 hours post dosing.
2.7. Determination of carboxylesterase, AChE, and BChE activity
Blood was collected prior to challenge and at various times post dosing via the saphenous vein (50 μl) into heparinized collection tubes. Plasma carboxylesterase, AChE, and BChE activities were determined at each time point. Paraoxonase-1 activity was not measured because paraoxonase is not inhibited by organophosphorus compounds. Mice have a significant amount of soluble AChE in plasma [15], whereas humans have negligible AChE in plasma, but have membrane bound AChE on red blood cells where AChE constitutes the Yt blood group antigen [16].
All three enzyme activities were measured at 25°C in a 2-ml reaction volume using a Gilford spectrophotometer interfaced with a MacLab data recorder (ADInstruments Pty Ltd, Castle Hill, Australia).
Carboxylesterase activity was determined by monitoring the hydrolysis rate of alpha-naphthyl acetate [4,11]. Three microliters of plasma were added to 1.9 ml of 0.1 M potassium phosphate buffer pH 7.0 in the presence of 0.01 mM eserine (to inhibit AChE and BChE) and 1.3 mM EDTA (to inhibit paraoxonase-1). The samples were incubated at 25°C for 15 minutes to allow complete inhibition of AChE, BChE, and paraoxonase. After incubation, 100 μl of 0.1 M alpha-naphthyl acetate in ethanol was added to the cuvette and the rate of hydrolysis was followed at 321 nm. The extinction coefficient for the reaction product was 2220 M−1cm−1.
AChE and BChE activities were assayed at 412 nm using an extinction coefficient of 13,600 M−1cm−1 [17]. AChE activity was assayed by adding 3 μl of plasma to 2 ml of 0.1 M potassium phosphate pH 7.0, containing 1 mM acetylthiocholine and 0.5 mM dithiobisnitrobenzoic acid, at 25°C in the presence of 0.01 mM ethopropazine to inhibit BChE. BChE activity was assayed by adding 3 μl of plasma to 2.0 ml of 0.1 M potassium phosphate pH 7.0, containing 1 mM butyrylthiocholine and 0.5 mM dithiobisnitrobenzoic acid at 25°C.
A unit of AChE, BChE, or carboxylesterase activity was defined as one micromole of substrate hydrolyzed per minute. The concentrations of AChE, BChE, and carboxylesterase in mouse plasma are 0.2 mg/L, 2.6 mg/L, and 80 mg/L respectively [5]. The subunit molecular weights are 70 kDa for AChE, 85 kDa for BChE, and 70 kDa for carboxylesterase. These molecular weights are heavier than the protein molecular weights calculated for the primary amino acid sequences because they include the mass of N-linked glycans. Database codes for these mouse enzymes are: AChE (GenBank ID gi:49845; UniProt ID P21836), BChE (GenBank ID gi:191580; UniProt ID M99492), and ES1 carboxylesterase (GenBank ID gi:247269929; UniProt ID P23953; previously gi:6679689, which is 97% identical to the updated version gi:247269929).
2.8. Statistics
Comparison of means was by paired samples t-test with 95% confidence interval (± standard deviation). Analysis was conducted using SPSS software (IBM Corporation Chicago, IL).
3. Results
3.1. Plasma carboxylesterase protects against parathion challenge
3.1.1. Parathion
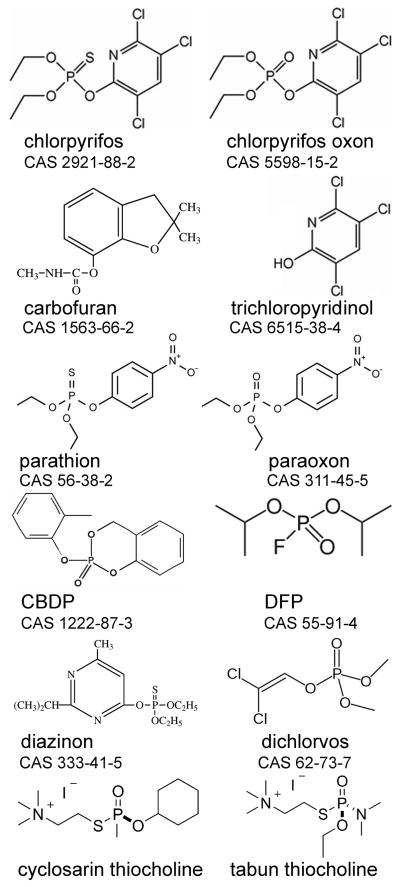
Parathion is a phosphorothioate pesticide (Figure 1). Its toxicity is mediated through its oxon metabolite, paraoxon, which is generated by cytochrome P450 enzymes in the liver [18,19]. ES1−/− and ES1+/+ mice (n=3 per genotype) were treated sc with 12.5 mg/kg parathion. Inhibition of plasma AChE, BChE, and ES1 carboxylesterase activities began to be apparent 0.5 hours after treatment. Inhibition levels increased with time reaching maximum levels at 6 to 24 hours (Figure 2A, 2B, 2C). The lag between exposure to parathion and inhibition of plasma esterases reflects the time to induce the cytochrome P450 enzymes that activate parathion to paraoxon. At 6 hours post dosing, plasma AChE was significantly more inhibited in ES1−/− than in ES1+/+ mice (73.1±4.6% versus 56±2.3%) (Table 1). At the same time, BChE was equally inhibited in both genotypes (71–76%) and ES1 in wild-type mice was inhibited 85%. One ES1−/− mouse was dead at 24 hours post dosing. Surface body temperature of ES1−/− mice dropped from 38 to 33 °C at 3 hours, and to 30 °C at 6 hours (Figure 2D). Behavioral toxic signs observed in ES1−/− mice at 6 hours post-dosing included muscle fasciculation, flattened posture, heavy mucus covering the eyes, palpebral closure, decreased handling reaction, ataxic gait, reduced mobility, decreased arousal and piloerection. Surface body temperature of ES1+/+ mice dropped 2 °C at 6 hours, but mice displayed no behavioral toxic signs. By 48 hours post dosing, body temperatures had recovered to baseline in both genotypes and all mice were free of behavioral toxic signs. These results indicate that plasma carboxylesterase plays a role in reducing the toxicity of parathion.
Figure 1.
Structures of chemicals used for challenge trials. The Chemical Abstracts Service (CAS) registry number is given for each compound.
Figure 2.
Parathion challenge. A) plasma AChE activity; B) plasma BChE activity; C) plasma carboxylesterase activity; D) surface body temperature. Mice were challenged with 12.5 mg/kg parathion sc (n=3 per genotype). Points are shown ± std dev. Wild-type mice, ES1+/+, are represented by white bars and white squares. ES1−/− mice are represented by black bars and black squares. ES1−/− mice had background plasma carboxylesterase activity of 0.6 u/ml due to hydrolysis of alpha-naphthyl acetate by albumin [12]. In the body temperature panel, measurements marked with an “a” are significantly different from those marked with a “b” based on a two tailed t-test p≤0.05.
Table 1.
Average percent inhibition of BChE, AChE and carboxylesterase ES1 activity in plasma of ES1−/− and +/+ mice after challenge with carbamate and OP at 0.5, 2, 4, 6, or 48 hours after treatment. (± std dev)
| Compound | Dose (mg/kg) | Time Post-dose (hours) | ES1 genotype | BChE % inhibition | AChE % inhibition | ES1 % inhibition | Behavioral Toxic signs |
|---|---|---|---|---|---|---|---|
| Carbofuran | 1.0 | 0.5 | −/− | 28.9±2.1 | 52.4±4.1 | ND | yes |
| Carbofuran | 1.0 | 0.5 | +/+ | 25.8±2.9 | 52.0±5.6 | 94.2±1.4 | yes |
| CBDP | 20.0 | 0.5 | −/− | 98.1±0.7 | 39.7±2.8 | ND | no |
| CBDP | 20.0 | 0.5 | +/+ | 97.8±0.2 | 32.2±5.4 | 99.2±0.6 | no |
| Chlorpyrifos | 100.0 | 6.0 | −/− | 75.4±3.3 | 70.1±15.1 | ND | yes |
| Chlorpyrifos | 100.0 | 6.0 | +/+ | 76.8±4.8 | 70.4±6.6 | 99.2±0.8 | yes |
| Chlorpyrifos oxon | 14.0 | 0.5 | −/− | 91.2±1.5 | 58.8±1.2 | ND | yes |
| Chlorpyrifos oxon | 14.0 | 0.5 | +/+ | 91.5±0.4 | 62.8±9.9 | 100±0.0 | yes |
| DFP | 4.0 | 0.5 | −/− | 55.2±2.7 | 59.9±4.8 | ND | yes |
| DFP | 4.0 | 0.5 | +/+ | 55.3±1.8 | 71.4±8.6 | 84.3±2.3 | yes |
| Diazinon | 50.0 | 2.0 | −/− | 81.7±1.1 | 93.5±0.5 | ND | yes |
| Diazinon | 50.0 | 2.0 | +/+ | 82.0±3.1 | 88.2±2.6 | 100±0.0 | yes |
| Dichlorvos | 7.5 | 0.5 | −/− | 25.5±3.6 | 53.7±4.8a | ND | yes |
| Dichlorvos | 7.5 | 0.5 | +/+ | 20.4±1.8 | 33.4±3.9b | 79.4±2.7 | yes |
| Paraoxon | 0.2 | 0.5 | −/− | 61.0±5.5 | 61.4±2.2 | ND | yes |
| Paraoxon | 0.2 | 0.5 | +/+ | 70.5±1.8 | 57.5±1.4 | 90.9±2.3 | yes |
| Parathion | 12.5 | 6.0 | −/− | 76.0±4.4 | 73.1±4.6c | ND | yes |
| Parathion | 12.5 | 6.0 | +/+ | 71.5±3.9 | 56.0±2.3d | 85.5±3.4 | no |
| Cyclosarin thiocholine | 0.05 | 0.5 | −/− | 61.2±0.8 | 67.5±1.7c | ND | yes |
| Cyclosarin thiocholine | 0.05 | 0.5 | +/+ | 49.9±2.8 | 32.2±4.6d | 8.6±3.4 | yes |
| Tabun thiocholine | 6.0 | 0.5 | −/− | 97.1±2.3 | 78.0±17.8 | ND | yes |
| Tabun thiocholine | 6.0 | 0.5 | +/+ | 97.5±1.7 | 83.3±11.6 | 24.1±0.9 | yes |
| Soman coumarine | 3.0 | 4.0 | −/− | 63.3±11.5 | 97.1±1.9 | ND | yes |
| Soman coumarin | 3.0 | 4.0 | +/+ | 63.0±11.2 | 83.6±15.4 | 98.6±1.1 | yes |
| Soman coumarin | 3.0 | 48.0 | −/− | 89.1 | 72.5 | ND | yes |
| Soman coumarin | 3.0 | 48.0 | +/+ | 10.3±12.1 | 0 | 18.0±3.0 | yes |
ND- no detectable activity in ES1−/− plasma. n = 3–6 per genotype.
is significantly different than
(p= 0.02); by Paired Samples T-test
is significantly different than
(p<0.01) by Paired Samples T-test.
Soman coumarin studies are from [13].
3.1.2. Paraoxon
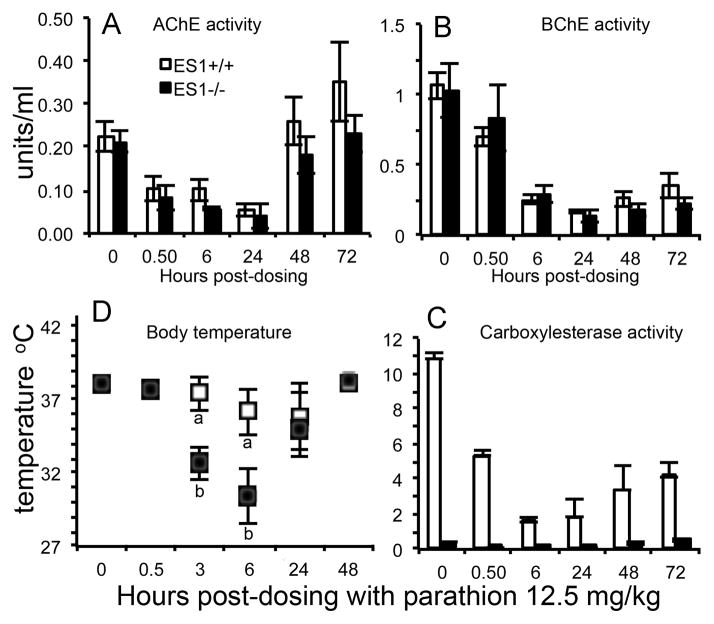
Paraoxon is the active metabolite of parathion (Figure 1). Mice (n=3 per genotype) were challenged sc with 0.2 mg/kg paraoxon. There was no lag time between administration of paraoxon and the appearance of behavioral toxic signs. AChE and BChE inhibition at 0.5 hours post dosing (Figure 3A, 3B) was equivalent between the genotypes (61–71% for BChE and 57–61% for AChE). There was 91% inhibition of ES1 in the wild-type mice (Figure 3C). Behavioral toxic signs and changes in body temperature through 4 hours were similar for both the ES1+/+ and ES1−/− mice. Behavioral toxic signs were the same as in the parathion treated animals. Body temperature dropped from 38 to 28 °C at 1 hour post-dosing (Figure 3D). Both AChE and BChE activities recovered to baseline by 24 hours in both genotypes while carboxylesterase ES1 activity in the wild-type mice was 60% of baseline by this time point. By 24 hours body temperature had returned to normal and all behavioral signs of toxicity were gone in both genotypes. We conclude that although mouse plasma carboxylesterase protected against parathion toxicity it played no significant role in protection against the active metabolite paraoxon.
Figure 3.
Paraoxon challenge. A) plasma AChE activity; B) plasma BChE activity; C) plasma carboxylesterase activity; D) body temperature. Mice were challenged with 0.2 mg/kg paraoxon sc (n=3 per genotype). Points are shown ± std dev. Wild-type mice, ES1+/+, are represented by white bars and white squares. ES1−/− mice are represented by black bars and black squares.
3.2. Wild-type mice die, but ES1 knockouts survive challenge with chlorpyrifos and chlorpyrifos oxon
3.2.1. Chlorpyrifos (CF)
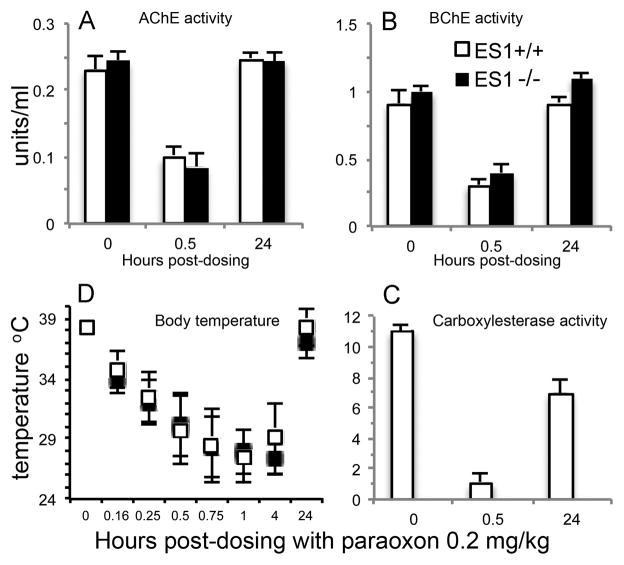
Chlorpyrifos is a phosphorothioate, organochlorine pesticide (Figure 1). Chlorpyrifos is metabolically activated by oxidative desulfuration to the oxon. The oxon, but not the parent compound, is toxic. Mice (n=6 per genotype) were treated by transdermal application with 100 mg/kg CF. No toxic signs were evident until 6 h post dosing when wild-type ES1+/+ mice, but not ES1−/− mice, started to display decreased surface body temperature (Figure 4D) and decreased motor activity. At 6 hours post dosing, AChE activity was inhibited 70% in both genotypes, BChE activity was inhibited 75–77% in both genotypes, and there was complete inhibition of ES1 carboxylesterase activity in the wild-type mice (Table 1). At 24 hours post-dosing, the ES1−/− mice had no behavioral toxic signs, but 4 of the 6 ES1+/+ wild-type mice were moribund with behavioral signs of cholinergic toxicity including mucus covered eyes, flattened posture, piloerection, and whole body tremor. There was also a drop in body temperature from 38 to 32 °C in the ES1+/+ mice (Figure 4D). At 48 h post-dosing, all wild-type mice were moribund or dead, whereas the ES1−/− mice displayed no signs of toxicity. These observations are precisely opposite to expectation.
Figure 4.
Chlorpyrifos challenge. A) plasma AChE activity; B) plasma BChE activity; C) plasma carboxylesterase activity; D) surface body temperature. Mice were challenged with 100 mg/kg chlorpyrifos (CF) transdermally (n=6 per genotype). Points are shown ± std dev. Wild-type mice, ES1+/+, are represented by white bars and white squares. ES1−/− mice are represented by black bars and black squares. At 24 h, 4 of the 6 ES1+/+ mice were moribund. At 48 h all wild-type mice (ES1+/+) were moribund or dead, whereas ES1−/− mice had no signs of toxicity. In the body temperature panel, measurements marked with an “a” are significantly different from those marked with a “b” based on a two tailed t-test p≤0.05.
Additional observations include the following. At 24 hours post-dosing with chlorpyrifos, plasma AChE activity in ES1−/− mice had partially recovered to 53% inhibition (Figure 4A). In contrast, plasma AChE activity in wild-type mice had not recovered, with the inhibition level remaining at 70%. At 24 h post-dosing, BChE activity in both genotypes (Figure 4B) was more inhibited than at 6 h (BChE was 92% inhibited in ES1−/− mice and 99% inhibited in ES1+/+ mice). At 24 h, carboxylesterase activity in wild-type plasma was still 99% inhibited (Figure 4C). Surface body temperatures of CF-treated mice were normal up to 6 hours. At 24 and 48 hours, there was a significant drop in the body temperature of ES1+/+ mice correlating to their moribund status (Figure 4D). Baseline AChE activity was recovered in the ES1−/− mice by 9 days while BChE activity had not recovered by 25 days post-dosing. We conclude that plasma carboxylesterase has an effect opposite to that expected. Rather than protecting from chlorpyrifos toxicity, plasma carboxylesterase increased the toxicity of chlorpyrifos. This observation in mice differs from the reported protective effect of carboxylesterase in chlorpyrifos treated rats [1,2].
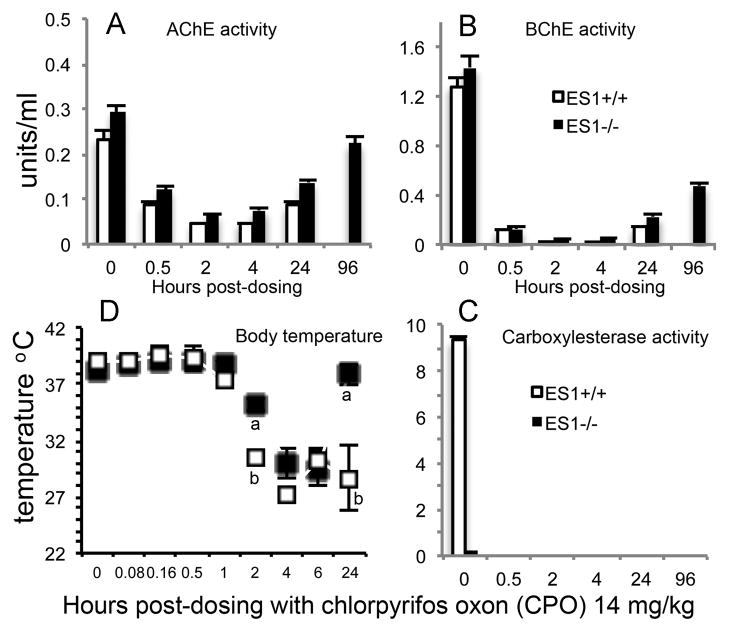
3.2.2. Chlorpyrifos oxon (CPO)
Chlorpyrifos oxon (Figure 1) is produced by oxidative desulfuration of chlorpyrifos through the action of cytochrome P450 enzymes [20]. The oxon is very toxic. Mice (n=6 per genotype) were treated transdermally with 14 mg/kg CPO. At 0.5 h post dosing, plasma AChE activity was inhibited 59–63% in both genotypes, plasma BChE was inhibited 91% in both genotypes and plasma ES1 carboxylesterase activity was completely inhibited in the wild-type ES1+/+ mice (Figure 5A, 5B, 5C). Between 2 and 6 hours post-dosing, mice of both genotypes had flattened posture, abnormal gait, decreased response to handling, and a drop in body temperature. At 24 h, ES1+/+ mice were either moribund (n=4) or dead (n=2) while the ES1−/− mice appeared to have recovered. At 24 h, the body temperature of ES1−/− mice was back to normal (Figure 5D) and they were free of behavioral toxic signs. At 24 h, plasma AChE activity in ES1−/− mice had partially recovered to an average inhibition of 53% compared to 63% inhibition in the moribund ES1+/+ mice. At 24 h, BChE activity was inhibited 84% in the ES1−/− mice and 89% in the moribund ES1+/+ mice. Baseline AChE activity was recovered in the ES1−/− mice by 6 days while BChE activity was recovered by 15 days post-dosing. Carboxylesterase activity was still completely inhibited in ES1+/+ mice at 24 h and could not be measured at later times because all ES1+/+ mice were dead. We conclude that chlorpyrifos oxon, like its parent compound chlorpyrifos, is more toxic to wild-type mice than to mice deficient in plasma carboxylesterase.
Figure 5.
Chlorpyrifos oxon challenge. A) plasma AChE activity; B) plasma BChE activity; C) plasma carboxylesterase activity; D) body temperature. Mice were challenged with 14 mg/kg chlorpyrifos oxon (CPO) transdermally (n=6 per genotype). Points are shown ± std dev. Wild-type mice, ES1+/+, are represented by white bars and white squares. ES1−/− mice are represented by black bars and black squares. At 24 h the wild-type mice (ES1+/+) were either moribund (n=4) or dead (n=2) while the ES1−/− mice had no toxic signs. In the body temperature panel, measurements marked with an “a” are significantly different from those marked with a “b” based on a two tailed t-test p≤0.05.
3.2.3. Trichloropyridinol
3,5,6-trichloro-2-pyridinol is released from both chlorpyrifos and chlorpyrifos oxon by hydrolysis of the ester bond (Figure 1). Wild-type mice would be expected to produce more of this metabolite than ES1−/− mice, because wild-type mice have more carboxylesterase. The possibility was tested that the higher levels of trichloropyridinol in wild-type mice might explain their greater susceptibility to poisoning by chlorpyrifos and chlorpyrifos oxon compared to ES1−/− mice. Mice (n= 3 per genotype) were treated transdermally with 100 mg/kg trichloropyridinol dissolved in acetone. The dose and route of exposure were identical to those for chlorpyrifos. Mice showed no toxic signs at any time up to 48 hours. Hanley et al. have also shown that trichloropyridinol is nontoxic [21]. It was concluded that poisoning by trichloropyridinol did not explain why wild-type mice, but not carboxylesterase deficient mice, died after treatment with chlorpyrifos or chlorpyrifos oxon.
3.3. Response of wild-type and ES1−/− mice to carbofuran, CBDP, DFP, diazinon, dichlorvos, cyclosarin thiocholine, and tabun thiocholine suggests a minor protective role of plasma carboxylesterase
3.3.1. Carbofuran
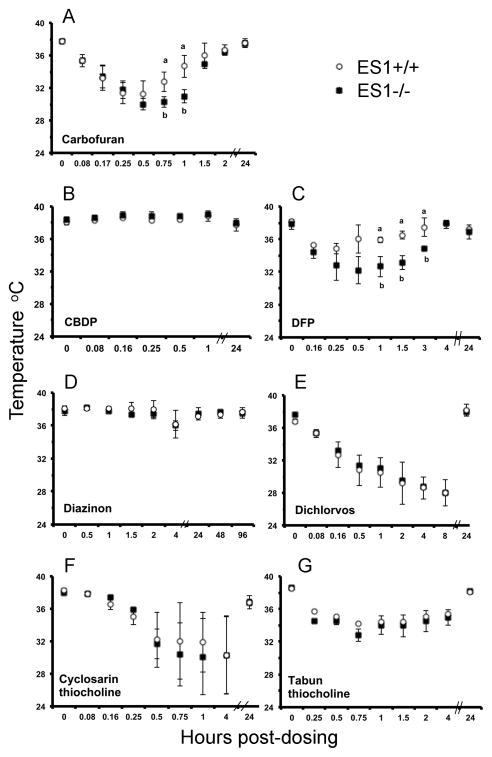
Carbofuran is a carbamate pesticide (Figure 1) that inhibits rat carboxylesterase at doses that do not significantly inhibit AChE [22], suggesting that carboxylesterase could protect against acute toxicity from carbofuran. To test the contribution of plasma carboxylesterase to protection against carbofuran toxicity we compared the effect of carbofuran on wild-type and plasma carboxylesterase knockout mice. ES1+/+ and ES1−/− mice (n=3 per genotype) were treated sc with 1 mg/kg carbofuran. Carbofuran-inhibited cholinesterases spontaneously reactivate with a half-life of about 2 h [23]. Therefore plasma samples were analyzed for activity immediately after collection to limit the degree of enzyme reactivation. AChE in both genotypes was inhibited to a greater extent (52%) than was BChE (26–29%) (Table 1). Plasma carboxylesterase activity was 94% inhibited in wild-type mice, confirming that carboxylesterase is more sensitive than AChE to inhibition by carbofuran. Behavioral signs of toxicity were similar for both genotypes including straub tail, ataxic gait, muscle fasciculations, clonic tremor, flattened posture, bugged eyes, salivation, lacrimation, arched back, and decreased arousal. The surface body temperature of ES1−/− mice dropped to 30–31°C by 0.5 h and remained at that low temperature for 1 h before beginning to rise (Figure 6A). The body temperature of ES1+/+ mice dropped to 31.5°C for only 15 min. After 2 hours, both genotypes had completely recovered normal body temperature and showed no behavioral signs of toxicity. By 24 h post dosing, the activity of plasma AChE, BChE and ES1 had returned to normal (data not shown). We conclude that plasma carboxylesterase has only a minor role in protection from carbofuran toxicity.
Figure 6.
Average surface body temperatures of mice after challenge with a single sublethal dose of a variety of toxicants. Points are shown ± std dev. Panel A, carbofuran (1 mg/kg); panel B, CBDP (20 mg/kg); panel C, DFP (4 mg/kg); panel D, diazinon (50 mg/kg); panel E, dichlorvos (7.5 mg/kg); panel F, cyclosarin thiocholine (0.05 mg/kg); and panel G, tabun thiocholine (6 mg/kg). The organophosphorus compounds were applied subcutaneously (n= 3 per genotype). Wild-type mice, ES1+/+, are represented by white circles. ES1−/− mice are represented by black squares. Measurements marked with an “a” are significantly different from those marked with a “b” based on a two tailed t-test p≤0.05.
3.3.2. CBDP
CBDP is a cyclic organophosphorus compound (Figure 1) that is used to inhibit rodent carboxylesterase in studies designed to equalize species responses to nerve agents [24]. CBDP is the active metabolite of tri-o-cresyl phosphate, a chemical implicated in aerotoxic syndrome [25]. The rate constant for inhibition of human BChE by CBDP is 100-fold faster than for inhibition of AChE [25]. It was of interest to determine the contribution of plasma carboxylesterase to protection from CBDP toxicity. Mice (n=3 per genotype) were treated sc with 20 mg/kg CBDP. At 30 min post dosing, AChE was inhibited 32% in wild-type and 40% in ES1−/− plasma, while BChE and ES1 carboxylesterase activity were inhibited 98 to 99%, respectively (Table 1). The nearly complete loss of plasma BChE and ES1 activity was not accompanied by behavioral signs of toxicity. The only notable sign of intoxication was a 1 degree increase in surface body temperature in both genotypes (Figure 6B). Body temperature returned to normal by 24 h post dosing. Although AChE activity was 20% less inhibited in wild-type than in ES1−/− plasma, the levels were not significantly different. We conclude that plasma carboxylesterase may afford only minor protection against AChE inhibition by CBDP.
3.3.3. Diisopropylfluorophosphate (DFP)
DFP is a research chemical (Figure 1) that is sometimes used as a surrogate for the nerve agent sarin. At one time DFP was used for the treatment of glaucoma [26]. Mice (n=3 per genotype) were challenged sc with 4.0 mg/kg DFP. AChE and BChE plasma activities were not significantly different between the genotypes at 30 minutes post-dosing (60–70% inhibited AChE and 55% inhibited BChE; Table 1). ES1 carboxylesterase activity was inhibited 84% in wild-type mice at 30 min post dosing. ES1−/− mice had significantly lower surface body temperatures than wild-type mice, from 1–3 hours post dosing with a recovery of body temperature by 4 hours post-dosing (Figure 6C). Both genotypes displayed similar behavioral toxic signs including mild muscle fasciculation, decreased handling response, decreased arousal and hunched posture through 4 hours post-dosing. We conclude that plasma carboxylesterase has a minor protective role in mice against DFP toxicity.
3.3.4. Diazinon
Diazinon is an OP pesticide (Figure 1) whose toxicity is mediated through its metabolite diazoxon. The rate constant for inhibition of human BChE by diazoxon is 100-fold faster than for inhibition of AChE [27]. Mice (n=3 per genotype) were challenged sc with 50 mg/kg diazinon. At 2 h post-dosing, plasma AChE was inhibited 88% in wild-type and 94% in ES1−/− mice, BChE was inhibited 82% in both genotypes, and ES1 was inhibited 100% in wild-type (Table 1). At 2–4 hours, all mice developed mild behavioral toxic signs including mucus covered eyes, palpebral closure, hunched posture, reduced handling reaction and reduced arousal. No behavioral toxic signs were observed at 24 hours post dosing. AChE activity had recovered to baseline levels in all animals by 48 hours while BChE activity was still inhibited 40% in both genotypes at 11 days post dosing (data not shown). Body temperatures in both genotypes dropped 2°C at 4 h post-dosing but returned to normal by 24 h (Figure 6D). We conclude that mouse plasma carboxylesterase does not have a significant role in protection from the toxicity of diazinon.
3.3.5. Dichlorvos
Dichlorvos is an active OP pesticide (Figure 1) that does not require activation to become a cholinesterase inhibitor. Dichlorvos is inactivated by paraoxonase-1 (“arylesterase” or “A-esterase” in the older literature) and is considered relatively nontoxic [28,29]. Carboxylesterases have been reported to be unimportant in detoxication of dichlorvos in mice [29]. Mice (n=3 per genotype) were challenged sc with 7.5 mg/kg dichlorvos. ES1−/− mice had significantly greater inhibition of plasma AChE at 30 minutes post dosing (54% inhibition) compared to ES1+/+ mice (33% inhibition) while BChE inhibition was equivalent between the genotypes. ES1 carboxylesterase was inhibited 80% in wild-type mice (Table 1). Behavioral toxic signs were similar for both genotypes. These signs were severe including muscle fasciculation, ataxic gait, splayed hindlimbs, straub tail, decreased arousal, mobility and handling reaction. AChE and BChE activities had recovered by 48 hours post dosing in both genotypes. Decreases in surface body temperature post-dosing were equivalent between the genotypes, dropping to 27.5°C at 8 h and returning to normal by 24 h (Figure 6E). We conclude that plasma carboxylesterase in mice plays a minor role in protection from dichlorvos toxicity.
3.3.6. Cyclosarin thiocholine
Cyclosarin thiocholine is a nerve agent model compound that differs from authentic cyclosarin by the presence of a thiocholine group in place of a fluoride ion (Figure 1). Thiocholine is a poorer leaving group than fluoride, making the model compound less reactive and less toxic than authentic cyclosarin [10]. The thiocholine nerve agent model compounds are quaternary amines and positively charged but nevertheless appear to get into the brain (unpublished observations). Because carboxylesterase is resistant to inhibition by positively charged inhibitors [30] it was expected that carboxylesterase would have no significant role in protection from the toxicity of this model compound. Mice (n=3 per genotype) were challenged sc with 0.05 mg/kg cyclosarin thiocholine. Behavioral toxic signs were similar between the genotypes including hunched posture, palpebral closure, decreased handling reaction and arousal, decreased body tone, lacrimation, salivation, reddened paws and nose, ataxic gait, and straub tail. Plasma AChE was significantly more inhibited at 30 minutes post dosing in the ES1−/− mice (68%) than in +/+ mice (32%) (p<0.02) while 9% of the plasma carboxylesterase was inhibited in the wild-type mice at this time point (Table 1). The concentration of carboxylesterase in plasma is about 400-fold greater than the concentration of AChE, suggesting that carboxylesterase scavenged more of the cyclosarin thiocholine than was able to react with AChE, even though the carboxylesterase inhibition level was only 9%. By 1 hour post dosing, BChE was inhibited about 70% in both genotypes, whereas AChE activity had returned to normal. BChE activity returned to normal after 5 days. ES1 carboxylesterase inhibition remained constant at 9%, through 24 hours. Surface body temperature in both genotypes dropped to an average of 30°C at 1 h post-dosing and returned to normal by 24 h (Figure 6F). We conclude that plasma carboxylesterase has a minor protective role against the toxicity of cyclosarin thiocholine.
3.3.7. Tabun thiocholine
Tabun thiocholine is a nerve agent model compound (Figure 1) that differs from authentic tabun by the presence of a thiocholine group in place of cyanide. Thiocholine is a poorer leaving group than cyanide, making tabun thiocholine less reactive and less toxic [10]. Mice (n=3 per genotype) were challenged sc with 6.0 mg/kg tabun thiocholine. Plasma AChE inhibition at 30 minutes post dosing was about 80% for both genotypes. Almost complete inhibition of BChE was found for both genotypes. Plasma carboxylesterase was inhibited 24% in the wild-type mice (Table 1). At 24% inhibition, carboxylesterase scavenged about 100 times more of the OP than was scavenged by AChE, and about 10 times more than was scavenged by BChE. Behavioral toxic signs and surface body temperature (Figure 6G) were similar between the genotypes. Behavioral toxic signs included hunched flattened posture, myoclonic jerks, palpebral closure, decreased handling reactivity, ataxic gait, decreased mobility and arousal, straub tail, vocalization when held, and decreased body temperature through 4 hours. At 24 hours mice had no behavioral toxic signs; their body temperature and plasma AChE activity had returned to normal, though BChE activity was still inhibited 85%. In conclusion, plasma carboxylesterase has a minor role in protection against the toxicity of tabun thiocholine.
4. Discussion
4.1. Carboxylesterase as a bioscavenger of OP
In agreement with Karanth and Pope (2001) we found that plasma carboxylesterase contributed to protection from the toxicity of parathion [1]. However, plasma carboxylesterase had only a minor role in protection from the toxicity for most of the compounds tested. An explanation for the latter finding takes into account the relative amounts of plasma carboxylesterase and toxicant in a 25 g mouse. The total amount of ES1 carboxylesterase in the wild-type mouse is estimated to be a minimum of 2 nmole. The OP compounds used in our study were administered in amounts slightly less than lethal. Table 1 shows the doses of OP. Conversion of mg/kg into nmoles/kg (based on a 25 g mouse) indicates that OP amounts between 5 nmoles (for cyclosarin- thiocholine) and 4100 nmoles (for diazinon) were administered per mouse. After cyclosarin- thiocholine, the next lowest doses were for paraoxon (18 nmoles) and DFP (540 nmoles). It is clear that stoichiometric scavenging by carboxylesterase could provide significant protection against only cyclosarin-thiocholine and paraoxon. Cyclosarin-thiocholine is cationic and therefore does not react well with carboxylesterase [30]. Nevertheless, plasma AChE was less inhibited in wild-type than in ES1−/− mice, suggesting some protection by plasma carboxylesterase. In rats the toxicity from paraoxon was barely enhanced by depletion of carboxylesterase through reaction with CBDP [4], making the absence of a difference between wild-type and ES1−/− mice for paraoxon in our study within experimental error of the result with rats.
The dose of parathion that we used was about 60-fold higher than the dose of paraoxon, yet plasma carboxylesterase protected mice from parathion toxicity. This observation can be partly explained by the fact that parathion is itself not toxic, and that it must be metabolically activated to the toxic oxon. The lag time observed between administration of parathion and appearance of toxic signs suggests that induction of cytochrome P450 desulfuration enzymes is required before activation can occur. Therefore, activation would be expected to take time. Consequently, the toxic oxon would be slowly produced and slowly released into the circulation [31] where it could be scavenged by carboxylesterase. We have no explanation for the observation that AChE activity is restored to normal 72 h after parathion at a time when BChE activity is still inhibited. If BChE were more sensitive than AChE to inhibition by paraoxon, or if paraoxon-inhibited BChE aged more rapidly than paraoxon-inhibited AChE, then BChE should have remained inhibited at 24 h in Figure 3B. However, both AChE and BChE activities returned to normal in 24 h, suggesting that there are no significant differences in the rates of inhibition and aging.
We agree with Maxwell that plasma carboxylesterase has no effect on the toxicity of DFP [4]. Maxwell used CBDP to deplete carboxylesterase in rats, whereas our study used mice genetically engineered to have no carboxylesterase in plasma. The lack of protection from DFP toxicity is explained as follows. DFP reacts poorly with AChE (2.9×104 M−1min−1) [30] and therefore causes toxicity only at high doses, i.e. doses that are much higher than the concentration of carboxylesterase in the subject. Even though DFP reacts readily with carboxylesterase (3.5×106 M−1min−1) [30], the carboxylesterase is used up before it can absorb a significant fraction of DFP, thereby affording no protection.
The time post-dosing when body temperature dropped correlated well with behavioral toxic signs and with reduced plasma AChE activity. However, the level of inhibition did not predict body temperature. For example at 6 h the parathion-treated ES1−/− mice had 73% of their plasma AChE activity inhibited and a drop in body temperature from 38 to 30 °C. In contrast the diazinon-treated ES1−/− mice had 93% of their plasma AChE activity inhibited at 2 h, and a drop in body temperature of 2 °C. Hypothermia lasting up to 24 h is a characteristic response of rodents to anticholinesterases [32].
4.2. Unexpected result: ES1+/+ mice are more sensitive than ES1−/− mice to chlorpyrifos and chlorpyrifos oxon
ES1+/+ mice became severely intoxicated by 6 hours after treatment with chlorpyrifos or chlorpyrifos oxon and were moribund or dead by 24–48 h. In contrast, ES1−/− mice showed the same behavioral signs of intoxication as the wild-type at 6 h, but recovered from the toxic effects by 24–48 h. This observation is contrary to the expectation that plasma carboxylesterase protects from the toxicity of OP. It was a surprise to find that mice with no plasma carboxylesterase, and therefore with reduced OP scavenging capacity, fared better than wild-type mice with the higher OP scavenging capacity afforded by the presence of carboxylesterase.
CPO and CF were applied transdermally, whereas all other toxicants were applied subcutaneously. The route of administration does not explain why wild-type mice are more sensitive to chlorpyrifos and chlorpyrifos oxon than carboxylesterase deficient mice because the route of administration was the same for wild-type and ES1−/− mice.
A possible explanation for this unexpected result is based on literature reports that organochlorines including chlorpyrifos induce the expression of cytochrome P450 isomers [33–35] and that cytochrome P450 isomers are capable of both dearylation (inactivation) and oxidative desulfuration (activation) of phosphorothioates. The dearylation step detoxifies chlorpyrifos by removing trichloropyridinol, whereas oxidative desulfuration activates the phosphorothioate by replacing the phosphoryl thiol with oxygen [36]. Some cytochrome P450 isomers are more proficient at dearylation than at oxidative desulfuration [37,38]. Chlorpyrifos induces the expression of isomers that are more active at dearylation (inactivation) than at desulfuration (activation) [37,39]. The levels of induced cytochrome P450 correlate with the amount of chlorpyrifos in circulation, so that higher levels of the detoxifying isomer are expected in response to higher levels of chlorpyrifos [33].
We hypothesize that in ES1−/− mice, which lack plasma carboxylesterase, higher levels of chlorpyrifos reached the liver microsomes, causing more cytochrome P450 to be induced. We further hypothesize that more of the detoxifying isomers than of the activating isomers were induced. Consequently, CPO and CF were more quickly inactivated in the ES1−/− mice than in the ES1+/+ mice. Support for this hypothesis comes from a study in rats which found that treatment with chlorpyrifos induced 4-fold more dearylation than desulfuration, whereas treatment with parathion induced 4 to 8-fold more desulfuration than dearylation [39]. This hypothesis is expected to apply only to a limited range of chlorpyrifos or chlorpyrifos oxon doses. We found that higher doses of either compound were lethal to animals of both genotypes, while lower doses caused no differences in toxic effects.
This hypothesis does not apply to chlorpyrifos oxon because the oxon is not a major substrate for any P450 isomer. Furthermore, the oxon does not need to be metabolized to be toxic, so the likelihood of induction of differential P450 gene expression is much smaller for the oxon than for chlorpyrifos.
Not all phosphorothioates showed this paradoxical effect. Parathion and diazinon caused greater toxicity in the ES1−/− mice. The most obvious difference between these OP and chlorpyrifos (or chlorpyrifos oxon) is the organochlorine nature of the latter. This suggests that the protective CYP orthologs are induced specifically by organochlorines and not by phosphorothioates in general. In support of this suggestion, a study in rats found that treatment with chlorpyrifos induced 4-fold more dearylation than desulfuration, whereas treatment with parathion induced 4 to 8-fold more desulfuration than dearylation [39].
Induction of new, detoxifying cytochrome P450 enzymes takes time. A time-dependent induction of protective cytochrome P450 is consistent with the observation that both wild-type and ES−/− mice displayed the same toxic signs over the first 6 hours, but that the ES−/− mice had a better long term outcome. Dearylation inactivates chlorpyrifos and chlorpyrifos oxon by removing the trichloropyridinol group, but trichloropyridinol by itself is nontoxic [21] (and the results from this paper).
4.3. AChE activity returns to normal within 48 hours
We observed return of plasma AChE activity to baseline levels within 48 hours after treatment with a variety of OP. Gupta et al. found that AChE activity in brain and muscle recovers by 48–72 h after treatment of rats with soman [8]. It is generally accepted that AChE is irreversibly inhibited by OP, although limited spontaneous reactivation has been reported. Examples of spontaneous reactivation include 1) an in vitro study of human erythrocyte dimethylphosphoryl-AChE which spontaneously regained 75% active enzyme within 2 h [40]; and 2) a study on homogenates of mouse brain inhibited with dichlorvos which showed that AChE regained 50% of its original activity in 2 h [41]. In contrast homogenates of mouse brain inhibited with paraoxon did not regain AChE activity [41]. In neither of these examples did reactivation restore full activity. It follows that the return to normal plasma AChE activity cannot be due simply to reactivation, but most likely involves the synthesis of new molecules of AChE as well. This would be especially true for the regain of AChE activity in our in vivo mouse studies with paraoxon where no reactivation would be predicted based on the example above. Synthesis of new AChE after treatment of an organism with OP is supported by gene expression studies that have shown that mRNA for AChE in brain increases when mice are treated with sarin [42]. New synthesis is also supported by the observation that AChE levels can rise above baseline after treatment with OP. For example, cultured chick myoblasts treated briefly with paraoxon had 2-fold more AChE activity compared to control cells [43]. Treatment of mice with a variety of OP increased plasma AChE activity 2.5-fold, a result attributed to induction of AChE synthesis [44].
5. Conclusion
Plasma carboxylesterase had only a minor role in protection from sublethal doses of the majority of toxicants tested. This result agrees with the finding of Maxwell [4] that plasma carboxylesterase is protective only against OP that are effective AChE inhibitors at low doses, notably the OP nerve agents. Less reactive OP, such as those used in the present report, must be administered in high doses to achieve AChE inhibition and toxicity. High doses far exceed the binding capacity of endogenous plasma carboxylesterase, thus explaining the lack of protection in mice against less potent OP. Thus, the wild-type mouse is as good a model as the ES1 knockout mouse for studies with OP pesticides. We predict that the ES1−/− mice will be more sensitive than ES1+/+ mice to highly reactive nerve agents such as soman and sarin.
Highlights.
Plasma carboxylesterase does not protect mice from most OP pesticides
High pesticide doses exceed the binding capacity of plasma carboxylesterase
Chlorpyrifos at 100 mg/kg is lethal to wild-type but not ES1 knockout mice
Acknowledgments
Part of the work was funded by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke (Award # U01 NS058038) to JRC, and the Direction Générale de l’Armement (Contract 08ca501) to FN.
Abbreviations
- AChE
acetylcholinesterase
- BChE
butyrylcholinesterase
- CBDP
cresylbenzodioxaphosphorin
- chlorpyrifos or CF
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate
- chlorpyrifos oxon or CPO
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphate
- diazinon
O,O-diethyl-O-(2-isopropyl-6-methyl-pyrimidine-4-yl)phosphorothioate
- cyclosarin thiocholine
2-[(dimethylamino)(ethoxy)phosphorylthio]-N,N,N-trimethylethanaminium iodide (Sp)
- dichlorvos
O,O-dimethyl 2,2-dichlorovinyl phosphate
- DFP
O,O-diisopropyl fluorophosphate
- ES1
plasma carboxylesterase
- ES1−/−
homozygous carboxylesterase knockout mouse
- ES1+/−
heterozygous carboxylesterase knockout mouse
- ES1+/+
wild-type mouse
- NCBI
National Center for Biotechnology Information
- OP
organophosphorus compound
- paraoxon
O,O-diethyl O-p-nitrophenyl phosphate
- parathion
O,O-diethyl O-p-nitrophenyl phosphorothioate
- sc
subcutaneously
- tabun thiocholine
2-[(dimethylamino)(ethoxy)phosphorylthio]-N,N,N-trimethylethanaminium iodide (Sp)
Footnotes
Conflict of interest statement. The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ellen G. Duysen, Email: ellenduysen@hotmail.com.
John R. Cashman, Email: jcashman@hbri.org.
Lawrence M. Schopfer, Email: lmschopf@unmc.edu.
Florian Nachon, Email: fnachon@nachon.net.
Patrick Masson, Email: pmasson@unmc.edu.
Oksana Lockridge, Email: olockrid@unmc.edu.
References
- 1.Karanth S, Pope C. Carboxylesterase and A-esterase activities during maturation and aging: relationship to the toxicity of chlorpyrifos and parathion in rats. Toxicol Sci. 2000;58:282–289. doi: 10.1093/toxsci/58.2.282. [DOI] [PubMed] [Google Scholar]
- 2.Moser VC, Chanda SM, Mortensen SR, Padilla S. Age- and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities. Toxicol Sci. 1998;46:211–222. doi: 10.1006/toxs.1998.2526. [DOI] [PubMed] [Google Scholar]
- 3.Dettbarn WD, Yang ZP, Milatovic D. Different role of carboxylesterases in toxicity and tolerance to paraoxon and DFP. Chem Biol Interact. 1999;119–120:445–454. doi: 10.1016/s0009-2797(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell DM. The specificity of carboxylesterase protection against the toxicity of organophosphorus compounds. Toxicol Appl Pharmacol. 1992;114:306–312. doi: 10.1016/0041-008x(92)90082-4. [DOI] [PubMed] [Google Scholar]
- 5.Li B, Sedlacek M, Manoharan I, Boopathy R, Duysen EG, Masson P, Lockridge O. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol. 2005;70:1673–1684. doi: 10.1016/j.bcp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Imai T, Taketani M, Shii M, Hosokawa M, Chiba K. Substrate specificity of carboxylesterase isozymes and their contribution to hydrolase activity in human liver and small intestine. Drug Metab Dispos. 2006;34:1734–1741. doi: 10.1124/dmd.106.009381. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell DM, Lenz DE, Groff WA, Kaminskis A, Froehlich HL. The effects of blood flow and detoxification on in vivo cholinesterase inhibition by soman in rats. Toxicol Appl Pharmacol. 1987;88:66–76. doi: 10.1016/0041-008x(87)90270-5. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RC, Patterson GT, Dettbarn WD. Biochemical and histochemical alterations following acute soman intoxication in the rat. Toxicol Appl Pharmacol. 1987;87:393–402. doi: 10.1016/0041-008x(87)90244-4. [DOI] [PubMed] [Google Scholar]
- 9.Duysen EG, Koentgen F, Williams GR, Timperley CM, Schopfer LM, Cerasoli DM, Lockridge O. Production of ES1 Plasma Carboxylesterase Knockout Mice for Toxicity Studies. Chem Res Toxicol. 2011;24:1891–1898. doi: 10.1021/tx200237a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barakat NH, Zheng X, Gilley CB, MacDonald M, Okolotowicz K, Cashman JR, Vyas S, Beck JM, Hadad CM, Zhang J. Chemical synthesis of two series of nerve agent model compounds and their stereoselective interaction with human acetylcholinesterase and human butyrylcholinesterase. Chem Res Toxicol. 2009;22:1669–1679. doi: 10.1021/tx900096j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zapf PW, Coghlan CM. A kinetic method for the estimation of pseudocholine esterase using naphthyl acetate substrate. Clin Chim Acta. 1973;44:237–242. doi: 10.1016/0009-8981(73)90386-0. [DOI] [PubMed] [Google Scholar]
- 12.Lockridge O, Xue W, Gaydess A, Grigoryan H, Ding SJ, Schopfer LM, Hinrichs SH, Masson P. Pseudo-esterase activity of human albumin: slow turnover on tyrosine 411 and stable acetylation of 82 residues including 59 lysines. J Biol Chem. 2008;283:22582–22590. doi: 10.1074/jbc.M802555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duysen EG, Koentgen F, Williams GR, Timperley CM, Schopfer LM, Cerasoli DM, Lockridge O. Production of ES1 plasma carboxylesterase knockout mice for toxicity studies. Chem Res Toxicol. 2011 Sep 7; doi: 10.1021/tx200237a. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDaniel KL, Moser VC. Utility of a neurobehavioral screening battery for differentiating the effects of two pyrethroids, permethrin and cypermethrin. Neurotoxicol Teratol. 1993;15:71–83. doi: 10.1016/0892-0362(93)90065-v. [DOI] [PubMed] [Google Scholar]
- 15.Skau KA. Acetylcholinesterase molecular forms in serum and erythrocytes of laboratory animals. Comp Biochem Physiol C. 1985;80:207–210. doi: 10.1016/0742-8413(85)90157-4. [DOI] [PubMed] [Google Scholar]
- 16.Bartels CF, Zelinski T, Lockridge O. Mutation at codon 322 in the human acetylcholinesterase (ACHE) gene accounts for YT blood group polymorphism. Am J Hum Genet. 1993;52:928–936. [PMC free article] [PubMed] [Google Scholar]
- 17.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 18.Davison AN. The conversion of schra dan (OMPA) and parathion into inhibitors of cholinesterase by mammalian liver. Biochem J. 1955;61:203–209. doi: 10.1042/bj0610203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gage JC. A cholinesterase inhibitor derived from OO-diethyl O-p-nitrophenyl thiophosphate in vivo. Biochem J. 1953;54:426–430. doi: 10.1042/bj0540426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sultatos LG, Murphy SD. Kinetic analyses of the microsomal biotransformation of the phosphorothioate insecticides chlorpyrifos and parathion. Fundam Appl Toxicol. 1983;3:16–21. doi: 10.1016/s0272-0590(83)80167-5. [DOI] [PubMed] [Google Scholar]
- 21.Hanley TR, Jr, Carney EW, Johnson EM. Developmental toxicity studies in rats and rabbits with 3,5,6-trichloro-2-pyridinol, the major metabolite of chlorpyrifos. Toxicol Sci. 2000;53:100–108. doi: 10.1093/toxsci/53.1.100. [DOI] [PubMed] [Google Scholar]
- 22.Gupta RC, Kadel WL. Concerted role of carboxylesterases in the potentiation of carbofuran toxicity by iso-OMPA pretreatment. J Toxicol Environ Health. 1989;26:447–457. doi: 10.1080/15287398909531268. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Ricordel I, Tong L, Schopfer LM, Baud F, Megarbane B, Maury E, Masson P, Lockridge O. Carbofuran poisoning detected by mass spectrometry of butyrylcholinesterase adduct in human serum. J Appl Toxicol. 2009;29:149–155. doi: 10.1002/jat.1392. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell DM, Brecht KM, O’Neill BL. The effect of carboxylesterase inhibition on interspecies differences in soman toxicity. Toxicol Lett. 1987;39:35–42. doi: 10.1016/0378-4274(87)90254-2. [DOI] [PubMed] [Google Scholar]
- 25.Carletti E, Schopfer LM, Colletier JP, Froment MT, Nachon F, Weik M, Lockridge O, Masson P. Reaction of Cresyl Saligenin Phosphate, the Organophosphorus Agent Implicated in Aerotoxic Syndrome, with Human Cholinesterases: Mechanistic Studies Employing Kinetics, Mass Spectrometry, and X-ray Structure Analysis. Chem Res Toxicol. 2011;24:797–808. doi: 10.1021/tx100447k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leopold IH, McDonald PR. Di-isopropyl fluorophosphate in treatment of glaucoma; further observations. Arch Ophthal. 1948;40:176–188. doi: 10.1001/archopht.1948.00900030181009. [DOI] [PubMed] [Google Scholar]
- 27.Schopfer LM, Voelker T, Bartels CF, Thompson CM, Lockridge O. Reaction kinetics of biotinylated organophosphorus toxicant, FP-biotin, with human acetylcholinesterase and human butyrylcholinesterase. Chem Res Toxicol. 2005;18:747–754. doi: 10.1021/tx049672j. [DOI] [PubMed] [Google Scholar]
- 28.Reiner E, Simeon V, Skrinjaric-Spoljar M. Hydrolysis of O,O-dimethyl-2,2-dichlorovinyl phosphate (DDVP) by esterases in parasitic helminths, and in vertebrate plasma and erythrocytes. Comp Biochem Physiol C. 1980;66:149–152. doi: 10.1016/0306-4492(80)90116-1. [DOI] [PubMed] [Google Scholar]
- 29.Cohen SD, Ehrich M. Cholinesterase and carboxylesterase inhibition by dichlorvos and interactions with malathion and triorthotolyl phosphate. Toxicol Appl Pharmacol. 1976;37:39–48. doi: 10.1016/s0041-008x(76)80006-3. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell DM, Brecht KM. Carboxylesterase: specificity and spontaneous reactivation of an endogenous scavenger for organophosphorus compounds. J Appl Toxicol. 2001;21(Suppl 1):S103–107. doi: 10.1002/jat.833. [DOI] [PubMed] [Google Scholar]
- 31.Sultatos LG, Minor LD, Murphy SD. Metabolic activation of phosphorothioate pesticides: role of the liver. J Pharmacol Exp Ther. 1985;232:624–628. [PubMed] [Google Scholar]
- 32.Gordon CJ. Thermoregulatory aspects of environmental exposure to anticholinesterase agents. Rev Environ Health. 1996;11:101–117. doi: 10.1515/reveh.1996.11.3.101. [DOI] [PubMed] [Google Scholar]
- 33.Verma RS, Mehta A, Srivastava N. Effect of phenobarbitone on cytochrome P450 activity and chlorpyrifos and 3,5,6-trichloropyridinol levels in liver and serum in rat. Indian J Biochem Biophys. 2005;42:254–257. [PubMed] [Google Scholar]
- 34.Campbell MA, Gyorkos J, Leece B, Homonko K, Safe S. The effects of twenty-two organochlorine pesticides as inducers of the hepatic drug-metabolizing enzymes. Gen Pharmacol. 1983;14:445–454. doi: 10.1016/0306-3623(83)90028-9. [DOI] [PubMed] [Google Scholar]
- 35.Das PC, Cao Y, Rose RL, Cherrington N, Hodgson E. Enzyme induction and cytotoxicity in human hepatocytes by chlorpyrifos and N,N-diethyl-m-toluamide (DEET) Drug Metabol Drug Interact. 2008;23:237–260. doi: 10.1515/dmdi.2008.23.3-4.237. [DOI] [PubMed] [Google Scholar]
- 36.Choi K, Joo H, Rose RL, Hodgson E. Metabolism of chlorpyrifos and chlorpyrifos oxon by human hepatocytes. J Biochem Mol Toxicol. 2006;20:279–291. doi: 10.1002/jbt.20145. [DOI] [PubMed] [Google Scholar]
- 37.Tang J, Cao Y, Rose RL, Brimfield AA, Dai D, Goldstein JA, Hodgson E. Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes. Drug Metab Dispos. 2001;29:1201–1204. [PubMed] [Google Scholar]
- 38.Mutch E, Williams FM. Diazinon, chlorpyrifos and parathion are metabolised by multiple cytochromes P450 in human liver. Toxicology. 2006;224:22–32. doi: 10.1016/j.tox.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Chambers JE, Ma T, Boone JS, Chambers HW. Role of detoxication pathways in acute toxicity levels of phosphorothionate insecticides in the rat. Life Sci. 1994;54:1357–1364. doi: 10.1016/0024-3205(94)00515-x. [DOI] [PubMed] [Google Scholar]
- 40.Worek F, Diepold C, Eyer P. Dimethylphosphoryl-inhibited human cholinesterases: inhibition, reactivation, and aging kinetics. Arch Toxicol. 1999;73:7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]
- 41.Van Asperen K, Dekhuijzen HM. A quantitative analysis of the kinetics of cholinesterase inhibition in tissue homogenates of mice and houseflies. Biochim Biophys Acta. 1958;28:603–613. doi: 10.1016/0006-3002(58)90527-4. [DOI] [PubMed] [Google Scholar]
- 42.Damodaran TV, Jones KH, Patel AG, Abou-Donia MB. Sarin (nerve agent GB)-induced differential expression of mRNA coding for the acetylcholinesterase gene in the rat central nervous system. Biochem Pharmacol. 2003;65:2041–2047. doi: 10.1016/s0006-2952(03)00160-6. [DOI] [PubMed] [Google Scholar]
- 43.Cisson CM, Wilson BW. Paraoxon increases the rate of synthesis of acetylcholinesterase in cultured muscle. Toxicol Lett. 1981;9:131–135. doi: 10.1016/0378-4274(81)90029-1. [DOI] [PubMed] [Google Scholar]
- 44.Duysen EG, Lockridge O. Induction of plasma acetylcholinesterase activity in mice challenged with organophosphorus poisons. Toxicol Appl Pharmacol. 2011;255:214–220. doi: 10.1016/j.taap.2011.06.021. [DOI] [PubMed] [Google Scholar]