Abstract
Although infection of mouse embryofibroblasts (MEFs) with WNV Eg101 induced interferon (IFN) beta production and STAT1 and STAT2 phosphorylation, these transcription factors (TFs) were not detected in the nucleus or on the promoters of four IRF-3-independent interferon stimulated genes (ISGs): Oas1a and Irf7 (previously characterized as IFN/ISGF3-dependent), Oas1b and Irf1. These ISGs were upregulated in WNV Eg101-infected STAT1−/−, STAT2−/−, and IFN alpha/beta receptor −/− MEFs. Although either IRF-3 or IRF-7 could amplify/sustain Oas1a and Oas1b upregulation at later times after infection, these factors were not required for the initial gene activation. The lack of upregulation of these ISGs in WNV Eg101-infected IRF-3/9−/− MEFs suggested the involvement of IRF-9. Activation of Irf1 in infected MEFs did not depend on any of these IRFs. The data indicate that additional alternative activation mechanisms exist for subsets of ISGs when a virus infection has blocked ISG activation by the canonical IFN-mediated pathway.
Keywords: West Nile virus, type I interferon, interferon stimulated gene, Oas1a, Oas1b, Irf7, Ifr1
INTRODUCTION
The family Flaviviridae, genus Flavivirus, contains several human pathogens including West Nile virus (WNV), dengue virus, yellow fever virus (YFV), Japanese encephalitis virus (JEV) and tick borne encephalitis virus (TBEV). WNV is a mosquito-borne virus that is transmitted in a bird-mosquito cycle but occasionally mammals including humans and horses are infected. WNV infections in humans are usually asymptomatic but can induce a mild febrile illness; however, some patients develop encephalitis or a poliomyelitis-like disease. WNV particles contain a single-stranded, positive-sense RNA genome encoding a single polyprotein that is cleaved by viral and cellular proteases into three structural and seven nonstructural proteins (Brinton, 2002). WNV isolates have been divided into two main lineages. Lineage I strains are often associated with outbreaks of human disease, while the majority of lineage II strains are non-emerging and cause zoonotic infections in Africa (Brinton, 2002).
IFNs were first discovered as cytokines that inhibit viral replication (Isaacs and Lindenmann, 1957). Induction of type I IFNs is primarily triggered by pattern recognition receptors (PRRs) that recognize viral nucleic acids. Viral double stranded RNA (dsRNAs) or single stranded RNAs (ssRNAs) are recognized by cytoplasmic PRRs, such as retinoic acid inducible gene-I (RIG-I) and melanoma differentiation antigen 5 (MDA5), or by cell surface and endosomal PRRs, such as Toll-like receptor 3 (TLR-3), TLR7 and TLR-8 (Saito and Gale, 2007). Recognition of viral nucleic acids by these PRRs leads to activation of the TFs, IRF-3, NF-kappa B and ATF2/c-Jun (AP1), that assemble on the IFN-beta promoter and activate IFN-beta gene expression (Merika and Thanos, 2001). Secreted IFN beta binds to cell surface IFN alpha/beta receptor (IFN alpha/beta R) complexes inducing activation of receptor-associated Jak kinases followed by recruitment and phosphorylation of the TFs, STAT1 and STAT2. STAT1, STAT2 and IFN regulatory factor-9 (IRF-9) form a trimeric transcription factor complex referred to as IFN stimulated gene factor 3 (ISGF3) that translocates to the nucleus and binds to IFN-stimulated response elements (ISREs) in the promoters of IFN-stimulated genes (ISGs) (Stark et al., 1998). The induction of IFN-beta is biphasic due to the induction of ISGs, such as IRF-7, which enhance IFN beta gene expression and sustain ISG production. IRF-7 also induces the expression of IFN-alpha which amplifies the type I IFN response via a positive feedback loop (Marie et al., 1998; Sato et al., 2000).
The expression of IFN-beta and multiple interferon stimulated genes (ISGs), many of which have antiviral functions, represents the first line of defense against a viral infection. Many viruses suppress this innate immune response by blocking the Jak-STAT signaling pathway. Different flaviviruses were reported to block this pathway by either inhibiting Jak phosphorylation (Lin et al., 2004; Best et al., 2005; Guo et al., 2005; Ho et al., 2005), reducing the expression of STAT2 (Jones et al., 2005; Mazzon et al., 2009) or blocking STAT1 and STAT2 phosphorylation (Munoz-Jordan et al., 2003; Best et al., 2005; Liu et al., 2005; Munoz-Jordan et al., 2005). The lineage I WNV strains TX02 and NY99 were previously shown to effectively block STAT1 phosphorylation in primate cells (Laurent-Rolle et al.; Liu et al., 2005; Keller et al., 2006). In contrast, WNV Eg101, a lineage I stain closely related to NY99 but less neurounvasive (Beasley et al., 2002) was previously reported not to inhibit STAT1 phosphorylation in mouse cells. (Scherbik et al., 2006; Scherbik et al., 2007). However, the effects of WNV Eg101 infection on STAT2 phosphorylation and ISGF3 nuclear translocation in these cells were not investigated.
Inhibition of the Jak-STAT signaling pathway by a viral infection would be expected to suppress ISG expression which is required for the establishment of an effective cellular antiviral response. However, even though ISGs were first described as IFN-induced genes, some were subsequently shown to be upregulated by viral infections in an IFN-independent manner though recognition of viral components by PRRs and activation of constitutively expressed TFs such as IRF-3 and NF-kappa B (Nakaya et al., 2001; Grandvaux et al., 2002; Peters et al., 2002; Elco et al., 2005; Andersen et al., 2008; Basagoudanavar et al., 2011). Subsequent upregulation of additional TFs, such as IRF-7, was also shown to play a role in the expression of some ISGs in infected cells (Barnes et al., 2004). A previous report showed that IRF-7 and IRF-3 were important for IFN production and protection against WNV (Fredericksen et al., 2004; Daffis et al., 2007; Daffis et al., 2008).
Due to the high degree of homology between the ISRE and IRF binding element (IRF-E) consensus sequences, the ISREs of some ISGs can be directly induced by either IRF-3 or IRF-7 (Lin et al., 2000; Morin et al., 2002; Schmid et al., 2010). In MEFs, Irf7 gene expression must first be induced and then IRF-7 has to be activated before this TF can directly upregulate a subset of ISGs (Barnes et al., 2004). In contrast, IRF-3 is a constitutively expressed protein that is found in the cytoplasm of all cells in an inactive form. IRF-3 activation is mediated by phosphorylation at multiple C terminal serine and threonine residues. Phosphorylation-induced dimerization leads to nuclear translocation of IRF-3 in a complex with histone acetyltransferases p300 and CREB-binding protein (CBP) (Yoneyama et al., 1998; Suhara et al., 2002; Fitzgerald et al., 2003; Sharma et al., 2003). The results of an analysis of murine ISG expression during a Newcastle disease virus (NDV) infection in IRF-3−/− MEFs led to the classification of ISGs such as ISG15, ISG54, IP-10 and GBP as genes activated in an IRF-3-dependent manner and ISGs such as Oas1a and Irf7 as genes upregulated in an IRF-3-independent but IFN-dependent manner through activation of the ISGF3 transcription factor complex (Nakaya et al., 2001). A more recent analysis of ISG expression in IRF-3−/− MEFs infected with NDV confirmed that the expression of the Oas1a and Irf7 did not depend on IRF-3 and showed that this was also the case for the Oas1b and Irf1 genes (Andersen et al., 2008).
NF-kappa B exists in the cytoplasm in an inactive form in a complex with an inhibitory I kappa B protein (Whiteside and Israel, 1997). Activation of the NF-kappa B pathway by viral infection upregulates the expression of a specific subset of ISGs encoding cytokines and chemokines, such as RANTES, TNF alpha and others, regulators of apoptosis and TFs, including A20, Irf1 and Irf2 and others (Pahl, 1999; Elco et al., 2005).
The murine 2′-5′ oligoadenylate synthetase 1a (Oas1a) and Oas1b genes are ISGs that have antiviral functions. Oas1a is an enzymatically active synthetase that upon binding to viral dsRNA synthetizes 2′-5′-oligoadenylates (2–5A) from ATP which activate the latent endonuclease RNase L. Activated RNaseL degrades both cellular and viral single-stranded RNAs (Samuel, 2001). Oas1b is an inactive synthetase that mediates resistance to flavivirus-induced disease through an unknown RNase L independent mechanism (Scherbik et al., 2006). Oas1b was also reported to inhibit in vitro Oas1a synthetase activity in a dose-dependent manner (Elbahesh et al., 2011). IFN beta activation of Oas1a expression was shown to be STAT1- and STAT2-dependent while that of Oas1b was STAT1-independent and STAT2-dependent indicating that these two duplicated genes are differentially regulated by IFN beta (Pulit-Penaloza et al., 2012). WNV Eg101 infection in MEFs was previously reported to activate IFN-beta expression, induce STAT1 Tyr701 phosphorylation and upregulate the expression of Oas1a, Oas1b as well as other ISGs including Irf7 and Irf1(Scherbik et al., 2006; Scherbik et al., 2007). However, whether the upregulation of these IRF-3-independent ISGs in WNV-infected cells is mediated by IFN or by an alternative virus-activated pathway was not previously analyzed.
Although IFN beta expression is upregulated and STAT1 and STAT2 are phosphorylated in WNV Eg101 infected cells, the present study showed that nuclear translocation of these TFs was blocked. Consistent with this observation, no increase in the binding of either STAT1 or STAT2 to the Oas1a, Oas1b or Irf7 promoters or of STAT1 to the Irf1 promoter was observed. Each of these genes was also upregulated by WNV Eg101 infection in control, STAT1−/−, STAT2−/− and IFN alpha/beta R−/− MEFs indicating that these ISGs were not upregulated by the canonical type 1 IFN-mediated Jak-STAT pathway or by an alternative IFN alpha/beta R-mediated signaling pathway. Oas1a, Oas1b and Irf7 were also upregulated in infected IRF-3−/−, IRF-7−/− MEFs and initially in IRF-3/7−/− MEFs but not in infected IRF-3/9−/− MEFs suggesting the involvement of IRF-9. Either IRF-3 or IRF-7 could enhance Oas1a and Oas1b upregulation at later times after infection. Activation of Irf1 in infected MEFs did not depend on any of these IRFs. The data support the existence of alternative mechanisms of ISG upregulation when the canonical type I IFN pathway is blocked by a WNV infection.
RESULTS
The kinetics of IFN beta expression, secretion and signaling in WNV Eg101-infected MEFs
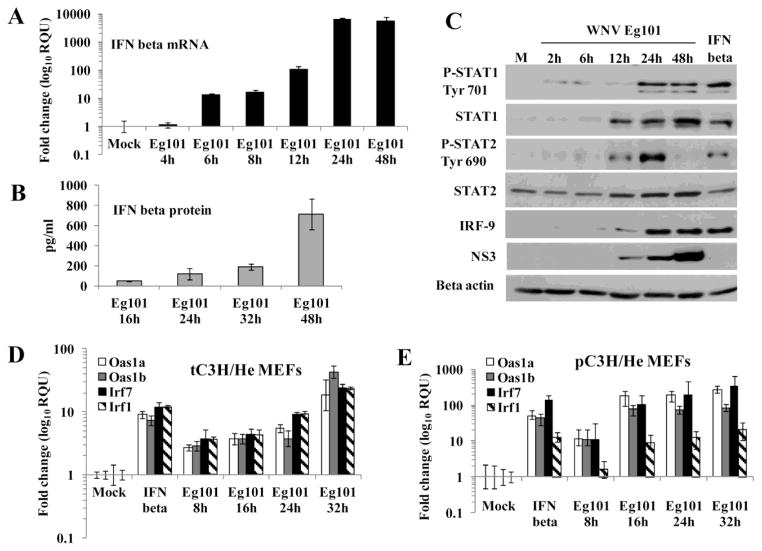
Previous studies reported increased STAT1 phosphorylation as well as increased expression of many ISGs, including Oas1a, Oas1b, Irf1 and Irf7 in WNV Eg101-infected MEFs (Scherbik et al., 2006; Scherbik et al., 2007). To determine whether ISG expression in WNV Eg101-infected MEFs is temporally related to the induction of IFN beta, the kinetics of IFN beta expression in WNV Eg101 infected [multiplicity of infection (MOI) of 5], transformed C3H/He (tC3H/He) MEFs were analyzed by real time qRT-PCR. IFN beta mRNA levels in tC3H/He MEFs were elevated by 10 fold at 6 h, by 100 fold at 12 h and by more than 5000 fold at 24 and 48 h after WNV Eg101 infection (Fig. 1A). The results obtained were similar to those previously reported for infected primary C3H/He (pC3H/He) MEFs (Scherbik et al., 2006). Analysis of extracellular IFN beta protein levels with an enzyme-linked immunosorbent assay (ELISA) detected low levels of IFN beta at 16 h after WNV Eg101 infection of tC3H/He MEFs that continued to increase through 48 h (Fig. 1B). At 48 h, 860 pg/ml of IFN beta were detected which corresponds to about 730 International units/ml based on ELISA data obtained with standard curves done on dilutions of an IFN beta sample of known unit concentration. Similar IFN levels were previously reported for primary MEFs (Daffis et al., 2009).
Figure 1. IFN beta is produced by WNV Eg101 infected MEFs and induces phosphorylation of STAT1 and STAT2.
MEFs were mock-infected or infected with WNV Eg101 at a MOI of 5 for the indicated times or treated with 1000 U/ml of murine IFN beta for 3h. (A) Total RNA from tC3H/He MEFs was extracted and IFN beta mRNA levels were measured by real-time qRT-PCR. (B) Extracellular IFN beta protein levels in tC3H/He MEF culture fluid was measured by ELISA. The values shown are averages from at least two independent experiments performed in triplicate. The error bars represent SDM. (C) tC3H/He cell lysates were analyzed by Western blotting using antibodies specific for the indicated proteins. Actin was used as the loading control. The blots shown are representative of results obtained from three independent experiments. (D) and (E) Comparison of ISG activation by IFN beta in primary and transformed MEFs. Total RNA was extracted from (D) tC3H/He or (E) pC3H/He MEFs infected with WNV Eg101 (MOI 5) for the indicated times, mock-infected or treated with murine IFN beta (1000 U/ml) for 3h. The changes in the levels of Oas1a and Oas1b mRNA were assessed by real-time qRT-PCR. Each experiment was repeated at least two times in triplicate. The mRNA level of each gene was normalized to the level of GAPDH mRNA in that sample and is shown as the fold change over the amount of mRNA in mock samples expressed in relative quantification units (RQU). The error bars represent the calculated SEM (n = 3) and are based on an RQMin/Max of the 95% confidence level.
The binding of IFN beta to its cell surface receptor results in activation of the Jak-STAT signaling pathway and phosphorylation of STAT1 and STAT2. A previous study showed that robust phosphorylation of STAT1 occurred in primary C3H/He MEFs after infection with WNV Eg101 (Scherbik et al., 2007). To determine whether this was also the case in transformed MEFs, activation of the Jak-STAT signaling pathway in tC3H/He MEFs by either IFN beta treatment or WNV Eg101-infection were analyzed by Western blotting. Increased levels of both total and phospho-STAT1 as well as of total and phospho-STAT2 were observed after a 3 h incubation of cells with murine IFN beta (Fig. 1C). Upregulation of both total STAT1 and STAT2 levels was also observed from 12 h through 48 h after WNV Eg101 infection. Low levels of phopshorylated STAT1 (Tyr 701) were detected at 2, 6 and 12 h after infection, while robust phosphorylation was seen at 24 and 48 h. Low levels of STAT2 phosphorylation (Tyr 690) were detected at 2 through 6 h and high levels were seen at 12 and 24 h after infection but not at 48 h. IRF-9 upregulation was detected as early as 6 h and was robust by 24 h after infection and also after a 3 h incubation with IFN beta. Viral NS3 protein levels were analyzed to estimate the efficiency of viral infection. NS3 was detected by 12 h after infection and the levels increased at both 24 and 48 h (Fig 1C). The results indicated that upregulation of IFN beta production and its activation of the Jak-STAT signaling pathway began early in WNV Eg101-infected MEFs and continued throughout the infection. Although WNV Eg101 infection induced STAT1 and STAT2 phosphorylation in murine cells, infection of A549 (human) or MA104 (African green monkey) cells with the this virus did not result in STAT phosphorylation (data not shown) similar to what was previously reported for other lineage I WNV strain infections in primate cells (Laurent-Rolle et al.; Liu et al., 2005; Keller et al., 2006).
Comparison of Oas1a, Oas1b, Irf7 and Irf1 expression levels in primary and transformed C3H/He MEFs infected with WNV Eg101
It was previously reported that transformed cells respond to IFN beta treatment but that the levels of ISG upregulation are lower in these cells compared to those in primary cells (Bartee and McFadden, 2009). Since both primary and transformed MEFs were used in this study, IFN-induced ISG upregulation in tC3H/He (Fig. 1D) and pC3H/He (Fig. 1E) MEFs was compared. The four ISGs analyzed, Oas1a, Oas1b, Irf7 and Irf1, were upregulated in both types of cells by IFN beta-treatment and WNV Eg101-infection. However, consistent with the previous report, higher levels of ISG induction by both IFN and virus were observed in infected primary MEFs than in transformed MEFs.
Association of STAT1 and STAT2 with ISG promoters in WNV Eg101-infected MEFs
The observation that STAT1 and STAT2 were phosphorylated in WNV Eg101-infected MEFs suggested that upregulation of the Oas1a, Oas1b, Irf7 and Irf1 genes might be mediated by the canonical type I IFN pathway. The Oas1a and Oas1b promoters each contain a canonical ISRE (Pulit-Penaloza et al., 2012), Irf7 contains an inverted ISRE (Ning et al., 2005; Schmid et al., 2010) and the Irf1 promoter has a GAS element instead of an ISRE which is upregulated in IFN-stimulated cells by a STAT1 dimer (Pine et al., 1994). STAT1 and STAT2 occupancy on the Oas1a, Oas1b and Irf7 promoters and STAT1 occupancy on the Irf1 promoter in vivo was analyzed with a chromatin immunoprecipitation (ChIP) assay done as described in Materials and Methods using tC3H/He MEFs that were mock-infected, infected with WNV Eg101 (MOI of 5) for 7, 16 or 24 h or treated with 1000 U/ml of murine IFN beta for 30 m. Briefly, in vivo crosslinked DNA-protein complexes were immunoprecipitated using anti-STAT1, anti-STAT2 or a nonspecific IgG antibody. After reversing the crosslinks, immunoprecipitated DNA was purified and quantified by real-time qPCR. An increase in STAT1 (Fig. 2A) and STAT2 (Fig. 2B) promoter binding compared to that in untreated cells was detected for Oas1a, Oas1b and Irf7 in cells treated with IFN beta. In contrast, no significant increase in either STAT1 or STAT2 promoter binding compared to mock infected cells was detected in cells infected with WNV Eg101 for 7, 16 or 24 h. An increase in STAT1 binding to the Irf1 promoter was observed in cells treated with IFN but not in cells infected with WNV Eg101. These results suggested that the nuclear translocation of phosphorylated STAT1 and STAT2 was blocked in the WNV Eg101-infected cells. To test this hypothesis, the kinetics of STAT1 and STAT2 nuclear translocation in tC3H/He MEFs infected with WNV Eg101 (MOI of 5) were analyzed by confocal microscopy. Robust STAT2 nuclear translocation, as indicated by STAT2 accumulation in the nucleus, was detected in IFN beta-treated cells (Fig. 2D top row) but not in mock-treated cells or cells infected with WNV Eg101 for 8, 16, 24, 32 and 48 h (Fig. 2C and E). Similar confocal microscopy results were also observed for STAT1 (data not shown). Western blotting of cytoplasmic and nuclear cell fractions from infected and IFN-treated t129 MEFs cells confirmed the lack of STAT1 and STAT2 nuclear accumulation in infected MEFs of a different genetic background (Fig. 2F). Similar Western results were obtained with tC3H/He MEFs (data not shown). To test whether a WNV Eg101 infection could block STAT2 nuclear translocation mediated by the addition of exogenous IFN beta, tC3H/He MEFs infected with WNV Eg101 for 8, 16, 24, 32 or 48 h were incubated with murine IFN beta (1000 U/ml) for 30 m before fixing the cells. In mock-infected cells and cells infected for 8 h (a time when viral dsRNA is not yet detectable by immunofluorescence; Fig. 2C second row), the addition of IFN beta resulted in detectable STAT2 nuclear translocation (Fig. 2D second row). In contrast, nuclear translocation of STAT2 was not observed when IFN beta was added to cells infected for 16, 24, 32 or 48 h (Fig. 2D). The results indicate that similar to other lineage I WNV strains studied (Keller et al., 2006; Laurent-Rolle et al., 2010), a WNV Eg101 infection can block STAT1 and STAT2 nuclear translocation.
Figure 2. STAT1 and STAT2 translocation to the nucleus is inhibited in WNV Eg101-infected MEFs.
A chromatin immunoprecipitation assay (ChIP) assessed binding (A) of STAT1 to the Oas1a, Oas1b, Irf7 and Irf1 promoters and (B) of STAT2 to the Oas1a, Oas1b and Irf7 promoters in vivo. The amount of precipitated Oas1a and Oas1b promoter DNA was quantified by real-time qPCR with promoter specific primers and fluorogenic TaqMan FAM/MGB probes. Nonspecific IgG antibody was used as a negative control and averaged values are shown. The averages from at least two independent experiments performed in triplicate were plotted and the error bars show SDM. (C) tC3H/He MEFs were infected with WNV at a MOI of 5 for the indicated times. (D) tC3H/He MEFs were incubated with 1000 U/ml of IFN beta for 30 m at different times after WNV Eg101 infection. Cellular localization was analyzed by laser scanning confocal microscopy. Nuclei were stained with Hoescht dye (blue), STAT2 was detected using anti-STAT2 antibody (green) and infected cells were detected with anti-dsRNA antibody (red). The results are representative of two independent experiments. (E) Quantification of the results in panels A and B. The pixel intensity in the representative area in nucleus was measured and divided by the pixel intensity of the same size area in the cytoplasm. The measurement was done for 20 cells at each time after infection and each treatment. The mean values were plotted and error bars indicate SDM (n = 20). (F) t129 MEFs were mock treated, treated with IFN beta (500 U/ml) for 30 m or infected with WNV Eg101 at a MOI of 5 for 16 or 24 h. Whole cell lysates were separated into cytoplasmic and nuclear fractions and analyzed by Western blotting using antibodies specific for STAT1, STAT2 or histone 3 (H3). H3 was used as a control nuclear protein. The blots shown are representative of the results obtained from two independent experiments.
Expression of ISGs in WNV Eg101-infected tSTAT1−/− and tSTAT2−/− MEFs
The ChIP and nuclear translocation assay results indicated that upregulation of Oas1a, Oas1b, Irf7 and Irf1 gene expression in WNV Eg101-infected MEFs was mediated in a STAT1- and STAT2-independent manner. As an additional means of confirming this, the expression of these four ISGs genes was analyzed by real time qRT-PCR in tSTAT1−/− and tSTAT2−/− MEFs and matched control wild type t129 MEFs that were mock-infected, infected with WNV Eg101 (MOI of 5) or treated with murine IFN beta (1000 U/ml) for 3 h. A 10-fold or greater increase in Oas1a, Oas1b and Irf7 mRNA levels was observed in wild type t129 MEFs stimulated with IFN beta for 3 h or infected with WNV Eg101 for 32 h (Fig. 3A, B and C). Consistent with previous observations (Ousman et al., 2005; Pulit-Penaloza et al., 2012), no upregulation of Oas1a after stimulation with IFN beta was observed in either tSTAT1−/− or tSTAT2−/− MEFs (Fig. 3A), while Oas1b (Fig. 3B) and Irf7 (Fig. 3C) upregulation was observed in tSTAT1−/− but not in tSTAT2−/− MEFs. In contrast to the tSTAT1- and tSTAT2-dependence of Oas1a upregulation by IFN beta, Oas1a mRNA was detected at 8 h and increased by more than 50 fold by 32 h after WNV infection in both tSTAT1−/− and tSTAT2−/− MEFs (Fig. 3A). Oas1b mRNA levels increased by about 3-fold at 24 h and by about 300-fold at 32 h after infection in tSTAT1−/− MEFs and by about 3-fold at 24 h and by about 20-fold at 32 h after infection in tSTAT2−/− MEFs. Although the fold increase observed for Oas1b expression in infected tSTAT2−/− MEFs was lower than that in tSTAT1−/− MEFs, it was still higher than the 10-fold increase observed in infected control t129 MEFs. In both tSTAT1−/− and tSTAT2−/− MEFs, Irf7 mRNA levels increased by about 2-fold by 16 h after infection. Although a 60-fold increase in Irf7 expression was observed in both wild type t129 and tSTAT1−/− MEFs by 32 h after infection, only a 7-fold increase in Irf7 mRNA levels was observed in infected tSTAT2−/− MEFs by this time. No upregulation of Irf-1 after stimulation with IFN beta was observed in either tSTAT1−/− or tSTAT2−/− MEFs similar to what was observed with Oas1a. A significant increase in Irf1 mRNA levels was observed in both tSTAT1−/− and tSTAT2−/− MEFs at 24 h after infection but these levels were lower compared to those in the control t129 MEFs. However, by 32 h, the levels of Irf1 mRNA were the same in the control and both types of knockout cells (Fig. 3D). Virus yields from tSTAT1−/− and tSTAT2−/− MEFs were initially higher than those from t129 control cells but all of these cell types produced a peak titer of about 107 PFU/ml by 32 h after infection (Fig. 3E).
Figure 3. Oas1a, Oas1b, Irf7 and Irf1 gene expression is induced in WNV Eg101-infected tSTAT1−/− and tSTAT2−/− MEFs.
Fold induction of (A) Oas1a, (B) Oas1b, (C) Irf7 and (D) Irf1 mRNA mRNA expression levels in t129, tSTAT1−/− and tSTAT2−/− MEF cell lines was measured by real-time qRT-PCR. Because the expression of Oas1a was not detected (ND) in the mock-treated tSTAT1−/− and tSTAT2−/− MEFs, the expression of Oas1a was normalized to the 8 h WNV Eg101 sample. Each experiment was repeated at least two times in triplicate. The mRNA level of each gene was normalized to the level of GAPDH mRNA in that sample and is shown as the fold change over the amount of mRNA in mock samples expressed in RQU. The error bars represent the calculated SEM (n = 3) and are based on an RQMin/Max of the 95% confidence level. (E) Culture fluid samples harvested at the indicated times after infection were titered for infectivity by plaque assay. Each data point is the average of duplicate titrations from at least two experiments. Error bars indicate SDM. (F) t129 and tSTAT2−/− MEF cell lysates were analyzed by Western blotting using antibodies specific for the indicated proteins. Actin was used as the loading control. The blots shown are representative of results obtained from two independent experiments.
IRF-3 was previously reported to be activated by 16 h after a WNV Eg101 infection in pC3H/He MEFs indicating virus activation of cytoplasmic PRRs (Scherbik et al., 2007). The activation of IRF-3 was compared in t129 and tSTAT2−/− MEFs by Western blotting using antibodies against total IRF-3 and IRF-3 phosphorylated on Ser396. Phosphorylated IRF-3 was detected at 24 and 32 h after WNV Eg101 infection in control t129 MEFs and at 16, 24 and 32 h in tSTAT2 −/− MEFs (Fig. 3F). Although earlier and more robust activation of IRF-3 was observed in tSTAT2−/− MEFs compared to wild type MEFs the level of ISG upregulation was lower in these cells.
Analysis of the dependence of Oas1a, Oas1b, Irf7 and Irf1 expression in WNV Eg101-infected MEFs on alternative signaling pathways activated by the IFN alpha/beta receptor
In addition to activation of the Jak-STAT pathway, type I IFN binding to the IFN alpha/beta R has also been reported to activate the PI3K (Kaur et al., 2005) and p38 mitogen-activated protein kinase (MAPK) signaling pathways (Katsoulidis et al., 2005). Although the results described above indicate that the Jak-STAT pathway is not involved in the upregulation of the ISGs analyzed in WNV Eg101-infected MEFs, the involvement of an alternative signaling cascade induced by type I IFN had not been ruled out. To determine whether the upregulation of the Oas1a, Oas1b, Irf7 and Irf1 genes in WNV Eg101-infected MEFs was mediated by type 1 IFN alpha/beta R signaling through an alternative pathway, the expression of these genes was analyzed in pIFN alpha/beta R −/− MEFs infected with WNV Eg101 (MOI of 5) by real time qRT-PCR. All four of the ISGs were upregulated in control p129 MEFs treated with either universal type I IFN (human IFN alpha) or murine IFN beta as well as in control MEFs infected with WNV Eg101 (Fig. 4A, B, C and D). Because primary 129 MEFs were used for these experiments, the level of gene upregulation was higher compared to that in the transformed 129 MEFs used for the experiments shown in Fig. 3. As expected, no increase in Oas1a, Oas1b, Irf7 or Irf1 expression was observed in pIFN alpha/beta R −/− MEFs after IFN alpha or IFN beta treatment. Consistent with the results obtained with tSTAT1−/− and tSTAT2−/− MEFs, basal Oas1a expression was also not detected in mock-infected pIFN alpha/beta R −/− MEFs. In contrast to the lack of upregulation by IFN beta, a significant increase in Oas1a, Oas1b, Irf7 and Irf1 gene expression was observed in pIFN alpha/beta R −/− MEFs infected with WNV Eg101 for 16, 24 or 32 h. The upregulation of the Oas1a and Oas1b genes in the infected pIFN alpha/beta R −/− MEFs was delayed compared to that in the control MEFs; however, by 24 h after infection the mRNA levels for both these genes were comparable to those in wild type MEFs. The activation of Irf7 was delayed and although Irf7 mRNA levels increased by about 50-fold by 32 h after infection in pIFN alpha/beta R −/− MEFs, the maximal upregulation was 20 times less efficient compared to that in the wild type cells (Fig. 4C). Irf1 expression in the IFN receptor knockout cells was also delayed and less efficient as compared to that observed in the wild type cells (Fig. 4D). Similar to what was previously reported for WNV NY 2000 (Daffis et al., 2009), WNV Eg101 replicated more efficiently in IFN alpha/beta R−/− MEFs than in the control MEFs (Fig. 4E).
Figure 4. Oas1a, Oas1b, Irf7 and Irf1 are induced in a type I IFN independent manner in MEFs infected with WNV Eg101.
Fold increase in (A) Oas1a, (B) Oas1b, (C) Irf7 and (D) Irf1 mRNA mRNA expression levels in p129 and pIFN alpha/beta R −/− MEFs was measured by real-time qRT-PCR. The mRNA level of each gene was normalized to the level of GAPDH mRNA in that sample and is shown as the fold change over the amount of mRNA in mock samples expressed in RQU. Because the expression of Oas1a was not detected (ND) in the mock-, IFN alpha- or IFN beta-treated pIFN alpha/beta R−/− cells, Oas1a expression in these cells was normalized to the 8 h WNV Eg101 sample. Each experiment was repeated at least two times in triplicate. The error bars represent the calculated SEM (n = 3) and are based on an RQMin/Max of the 95% confidence level. (E) Culture fluid harvested at the indicated times after infection was titered for infectivity by plaque assay. Each data point is the average of duplicate titrations from at least two experiments. Error bars indicate SDM.
Analysis of the signaling pathways and transcription factors regulating ISG expression during WNV Eg101 infection
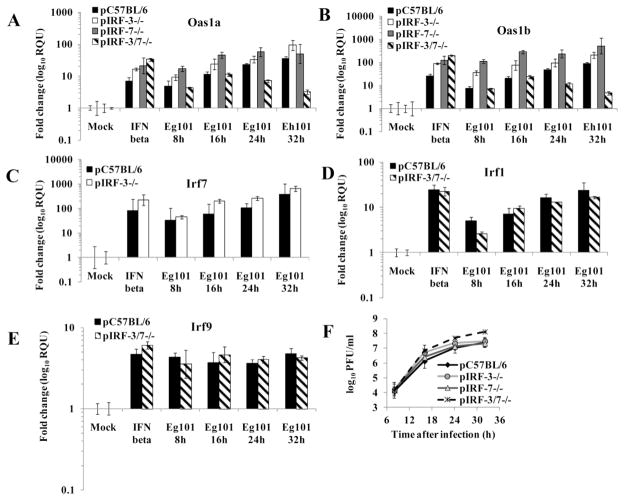
To further investigate the role of IRF-3 in upregulating the four ISGs analyzed in WNV Eg101-infected MEFs, pIRF-3−/− and matched wild type pC57/BL6 MEFs were infected with WNV Eg101 (MOI 5) and the Oas1a, Oas1b, Irf7 and Irf1 mRNA levels were measured by real time qRT-PCR. The Oas1a, Oas1b and Irf7 genes were efficiently upregulated in IRF-3−/− and control cells by both IFN beta and WNV infection (Fig. 5A, B and C). Similarly, the Oas1a and Oas1b genes were efficiently upregulated by IFN beta and WNV infection in pIRF-7−/− MEFs. These data suggest that neither IRF-3 nor IRF-7 alone is critical for the upregulation these ISGs in WNV Eg101-infected MEFs. The more efficient upregulation of the tested ISG observed in cells deficient in IRF-3 or IRF-7 compared to the wild type control cells suggested the possibility that these two transcription factors may be involved in a negative feedback mechanism of ISG expression. Since the combination of IRF-3 and IRF-7 was previously reported to be required for robust production of type I IFN as well as for the induction of ISGs such as ISG54, ISG49 and RIG-I in MEFs infected with WNV NY 2000 (Daffis et al., 2009), the induction of Oas1a and Oas1b expression was also analyzed in pIRF-3/7−/− MEFs. Although Oas1a and Oas1b expression was upregulated to similar levels in control and pIRF-3/7−/− MEFs at 8 and 16 h after WNV Eg101 infection, a decrease in Oas1a and Oas1b mRNA levels compared to the control cells was observed at 24 and 32 h after infection suggesting that at least one of these TFs is needed to enhance the upregulation of these ISGs in infected cells at later times of infection. Irf1 upregulation by WNV Eg101 infection was similar in pIRF-3/7−/− and control MEFs (Fig. 5D). Irf9 mRNA levels were also assessed in pIRF-3/7−/− MEFs. Irf9 was upregulated to similar levels in WNV Eg101-infected control and pIRF-3/7−/− MEFs (Fig. 5E). WNV Eg101 replicated as efficiently in pIRF-3−/− and pIRF-7−/− MEFs as in wild type cells but the pIRF-3/7−/− MEFs produced higher yields of virus (Fig. 5F).
Figure 5. The expression of Oas1a and Oas1b but not Ir1 and Irf9 is reduced in pIRF-3/7 −/− MEFs.
Fold increase in (A) Oas1a, (B) Oas1b, (C) Irf7 mRNA, (D) Irf9 and (E) Irf1 expression levels in pC57BL/6, pIRF-3−/−, pIRF-7−/− and pIRF-3/7−/− MEFs was measured by real-time qRT-PCR. Each experiment was repeated at least two times in triplicate. The mRNA level of each gene was normalized to the level of GAPDH mRNA in that sample and is shown as the fold change over the amount of mRNA in mock samples expressed in RQU. The error bars represent the calculated SEM (n = 3) and are based on an RQMin/Max of the 95% confidence level. (F) Culture fluid harvested at the indicated times after infection was titered for infectivity by plaque assay. Each data point is the average of duplicate titrations from at least two experiments. Error bars indicate SDM.
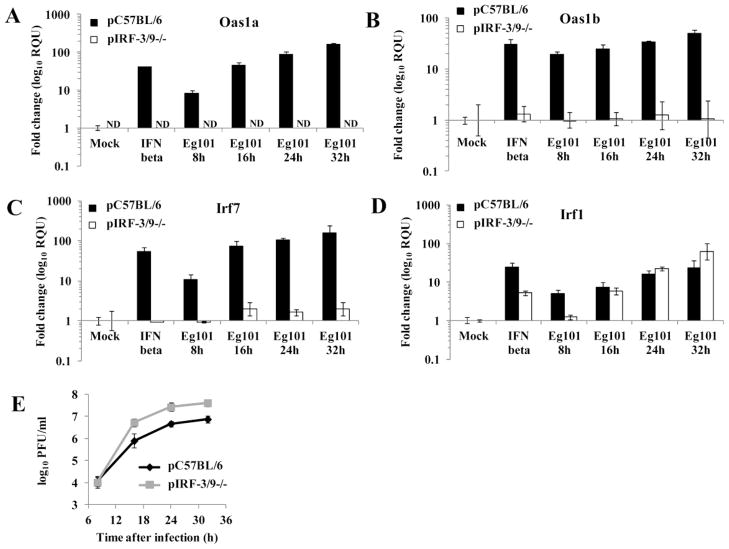
The induction of Irf7, Irf1 and Oas1a gene expression by poly(I:C) was previously shown to be blocked in IRF-3/9−/− MEFs (DeWitte-Orr et al., 2009). To test whether the expression of these ISGs is induced by WNV Eg101 infection in the absence of IRF-3 and IRF-9, pIRF-3/9−/− MEFs were infected with WNV Eg101 (MOI 5) for 8, 16, 24 or 32 h and Oas1a, Oas1b, Irf7 and Irf1 mRNA levels were measured by real time qRT-PCR. No significant upregulation of Oas1a, Oas1b or Irf7 expression was observed in these knockout cells (Fig. 6A, B and C). Since IRF-3 was shown not to be required for ISG upregulation in WNV infected MEFs, the data obtained with the pIRF-3/9−/− MEFs suggested that IRF-9 was required. Irf1 expression was upregulated in pIRF-3/-9−/− MEFs to the same or higher levels than in control MEFs (Fig. 6D) indicating that this gene is upregulated in infected cells by a different set of TFs than those regulating the Oas1a, Oas1b and Irf7 genes. Analysis of the WNV Eg101 yields produced by control and pIRF-3/9−/− MEFs indicated that virus replication was more efficient in the pIRF-3/9−/− MEFs (Fig. 6E).
Figure 6. Irf-1, but not Oas1a, Oas1b and Irf7, is upregulated in pIRF-3/9 −/− MEFs infected with WNV Eg101.
Fold increase in (A) Oas1a, (B) Oas1b, (C) Irf7 and (D) Irf1 mRNA expression levels in C57BL/6 and IRF-3/9/- MEFs was measured by real-time qRT-PCR. Each experiment was repeated at least two times in triplicate. The mRNA level of each gene was normalized to the level of GAPDH mRNA in that sample and is shown as the fold change over the amount of mRNA in mock samples expressed in RQUs. The error bars represent the calculated SEM (n = 3) and are based on an RQMin/Max of the 95% confidence level. (E) Culture fluid harvested at the indicated times after infection was titered for infectivity by plaque assay. Each data point is the average of duplicate titrations from at least two experiments. Error bars indicate SDM.
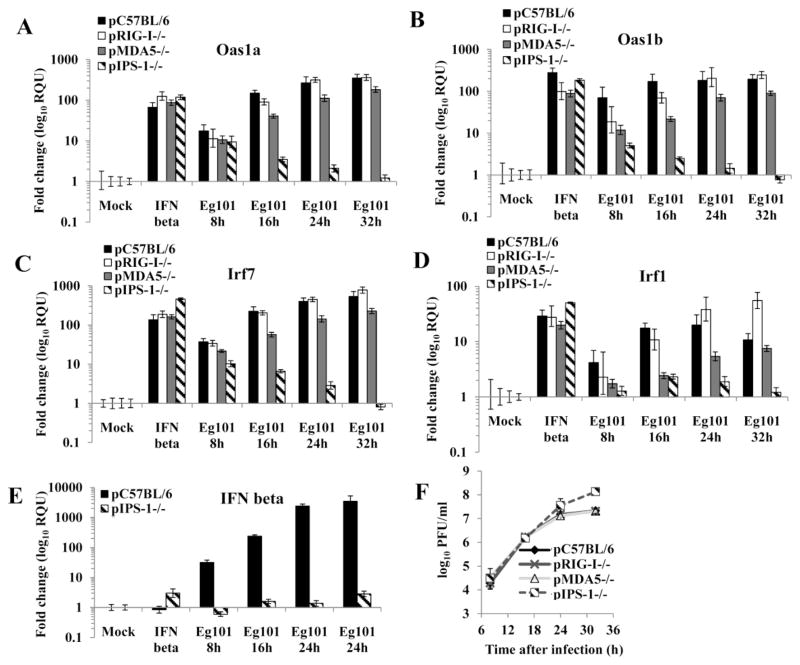
Previous studies showed that a WNV infection is sensed by the cytoplasmic RNA sensors RIG-I and MDA5 (Fredericksen and Gale, 2006; Fredericksen et al., 2008). Upon recognition of viral RNA RIG-I and MDA5 interact through their caspase activation and recruitment domains (CARDs) with adaptor molecule promoter-stimulating factor (IPS-I) (Kato et al., 2008; Takeuchi and Akira, 2008). A previous study showed that IPS-1 is required for the activation of IRF-3 and the IRF-3 target genes, ISG54 and ISG56, in WNV infected cells (Fredericksen et al., 2008; Suthar et al., 2010). To assess the roles of RIG-I, MDA5 and IPS-1 in the induction of IRF-3 independent ISG upregulation, pRIG-I−/−, pMDA5−/− and pIPS-1−/− MEFs were mock treated, treated with 1000 U/ml of IFN beta or infected with WNV Eg101 at a MOI of 5 and the Oas1a, Oas1b, Irf7 and Irf1 mRNA levels were measured by real time qRT-PCR (Fig. 7A, B, C and D). Although the level of Oas1a, Oas1b, Irf7 and Irf1 upregulation was similar in WNV infected RIG-I−/− and control MEFs, the expression of these ISGs in infected MDA5−/− MEFs was lower suggesting that MDA5 signaling plays a more important role during WNV infection. It was previously reported that RIG-I and MDA5 play different but redundant roles in the activation of IRF-3 and IRF-3 dependent genes in WNV infected cells (Fredericksen et al., 2008; Loo et al., 2008). At 8 h after infection, Oas1a was upregulated to similar levels in control and IPS-1−/− MEFs while the upregulation of Oas1b and Irf7 was lower in infected IPS-1−/− MEFs than in control cells. However, the mRNA levels of all three of these ISGs decreased progressively at later times after infection. No significant upregulation of Irf1 was observed in infected IPS-1−/− MEFs at 8h. A small increase in Irf1 upregulation was observed at 16 h but the mRNA levels decreased at later times. The data indicate that IPS-1 is only partially involved in the initial induction of IRF-3-independent ISGs in WNV infected cells but is required for gene expression at later times after infection. Only minimal upregulation of the IFN beta gene was observed in either IFN beta or WNV infected pIPS-1−/− MEFs (Fig. 7E). Higher WNV Eg101 yields were produced by IPS-1−/− MEFs than by RIG-I−/−, MDA5−/− or wild type MEFs (Fig. 7F).
Figure 7. IPS-1 is involved in the upregulation of Oas1a, Oas1b, Irf7 and Irf1 in MEFs infected with WNV Eg101.
Fold increase in (A) Oas1a, (B) Oas1b, (C) Irf7,(D) Irf1 and (E) IFN beta mRNA expression levels in pC57BL/6, pRIG-I−/−, pMDA5−/− and pIPS-1−/− MEFs was measured by real-time qRT-PCR. Each experiment was repeated at least twice in triplicate. The mRNA level of each gene was normalized to the level of GAPDH mRNA in that sample and is shown as the fold change over the amount of mRNA in mock samples expressed in RQUs. The error bars represent the calculated SEM (n = 3) and are based on an RQMin/Max of the 95% confidence level. (F) Culture fluid harvested at the indicated times after infection was titered for infectivity by plaque assay. Each data point is the average of duplicate titrations from at least two experiments. Error bars indicate SDM.
DISCUSSION
Activation of ISGs by type 1 IFN is mediated by the ISGF3 TF complex composed of STAT1, STAT2 and IRF-9. STAT1 and STAT2 proteins primarily reside in the cytoplasm and the formation and nuclear translocation of the ISGF3 complex depends on IFN-mediated phosphorylation of STAT1 on Tyr701 and STAT2 on Tyr690 (Schindler et al., 1992; Shuai et al., 1992; Qureshi et al., 1996). Most viruses, including flaviviruses, have evolved mechanisms to suppress the type 1 IFN pathway. The lineage I WNV strains TX02 and NY99 inhibit STAT1 and STAT2 nuclear translocation by blocking phosphorylation of these proteins in primate cells and the WNV NY99 NS5 protein was shown to mediate this suppression (Keller et al., 2006; Laurent-Rolle et al., 2010). In contrast, the attenuated lineage I strain, Kunjin, is a poor suppressor of STAT1 phosphorylation due to a single NS5 mutation, Phe653Ser (Laurent-Rolle et al., 2010). Eg101 and NY99 are closely related lineage I viruses but Eg101 it is less neuroinvasive (Beasley et al., 2002). The WNV Eg101 NS5 has a Phe at position 653 and blocks STAT phosphorylation in primate cells (Scherbik and Brinton, unpublished data). In contrast, WNV Eg101 infections did not block STAT1 phosphorylation at Tyr701 or STAT2 phosphorylation of Tyr690 in MEFs. A decrease in STAT2 phosphorylation was only observed at 48 h. These results indicate host species specific differences in the ability of WNV to suppress STAT phosphorylation. How a WNV Eg101 infection prevents nuclear translocation of phospho-STAT1 and phospho-STAT2 in MEFs is currently under investigation. The dengue virus NS5 protein was previously reported to bind to and target human STAT2 for degradation but was not able to mediate the degradation of murine STAT2 (Ashour et al., 2010). No evidence of either STAT1 or STAT2 degradation was observed in WNV Eg101-infected MEFs.
Activation of the Oas1a, Oas1b, Irf7 and Irf1 genes was previously reported to be IRF-3-independent (Nakaya et al., 2001; Andersen et al., 2008) and the Oas1a and Irf7 genes were reported to be activated only by canonical type I IFN signaling (Nakaya et al., 2001). Data from the present study indicated that upregulation of Oas1a, Oas1b, Irf7 and Irf1 occurred by a STAT1-, STAT2- and type I IFN-independent regulatory mechanism in WNV Eg101-infected MEFs. Although the activation of IRF factors, such as IRF-3 and IRF-7, by viral infections was previously reported to mediate IFN-independent upregulation of subsets of ISGs, the Oas1a, Oas1b, Irf7 and Irf1genes were efficiently upregulated by WNV Eg101 infection in IRF-3−/− MEFs and the Oas1a, Oas1b, and Irf1 genes were efficiently activated in IRF-7−/− MEFs indicating that neither IRF-3 nor IRF-7 alone was sufficient to induce upregulation of these genes in infected MEFs. A previous study done with WNV NY 2000-infected IRF-3/7 −/− MEFs reported that both IRF-3 and IRF-7 were necessary for production of type I IFN and induction of ISGs such as ISG54, ISG49 and RIG-I (Daffis et al., 2009). We also found only a very low level of IFN beta gene upregulation in WNV Eg101-infected IRF-3/7 −/− MEFs; at 32 h after infection, there was a 27 fold increase in IFN beta mRNA levels compared to an 8000 fold increase in wild type MEFs (data not shown). In contrast, efficient induction of the Oas1a, Oas1b, Irf7 and Irf1 genes was observed in IRF-3/7 −/− MEFs infected for 8 and 16 h with WNV Eg101. However, the absence of both IRF-3 and IRF-7 reduced the levels of Oas1a and Oas1b expression at later times of infection suggesting that at least one of these IRFs is needed to enhance and sustain the upregulation of these two ISGs. No Oas1a and Oas1b upregulation was observed in IRF-3/9−/− MEFs and the expression of Irf7 was also not upregulated in these cells. These observations together with the observation that Irf9 was upregulated in infected IRF-3/7−/− MEFs suggest the possibility that IRF-9 is involved in the initial upregulation of these ISGs in WNV Eg101-infected cells. Since IRF-9 cannot transactivate gene expression alone (Kraus et al., 2003), it must interact with additional partners to active ISG expression in MEFs infected with WNV Eg101. However, it is not currently known whether IRF-9 can form complexes with as yet unidentified TF(s) and whether IRF-9 can interact with IRF-3 and/or IRF-7 to upregulate a subset of ISGs in WNV Eg101-infected MEFs.
IRF-1 was originally described as a TF involved in the induction of the IFN-alpha and IFN-beta genes (Miyamoto et al., 1988). However, it can also upregulate the expression of some ISGs in an IFN-independent manner (Schoggins et al., 2011). Overexpression of Irf1 was recently shown to inhibit the replication of WNV, dengue virus, yellow fever virus, hepatitis C virus, Chikungunya virus, Venezuelan encephalitis virus and human immunodeficiency virus I (Schoggins et al., 2011). One of the IRF-1 target genes, viperin, (Stirnweiss et al., 2010) was reported to inhibit both WNV and dengue virus infections (Jiang et al., 2010). In a previous study from our lab, the kinetics of upregulation of multiple ISGs in primary C3H/He MEFs infected with WNV Eg101 was analyzed (Scherbik et al., 2007). ISGs such as Oas1a, Oas1b and Irf7 were efficiently upregulated by WNV Eg101 at early times after infection while the upregulation of a subset of ISGS including Irf1 was delayed. The Irf1 gene promoter contains a GAS element instead of an ISRE and upregulation of this gene by type I and type II IFNs is dependent on STAT1 dimers rather than the ISGF3 TF complex (Pine et al., 1994). The murine Irf1 gene was previously reported to be activated in an IRF-3-independent manner (Andersen et al., 2008). However, another study reported that Irf1 upregulation by poly(I:C) was blocked in IRF-3/9−/− MEFs (DeWitte-Orr et al., 2009). The results of the present study indicate that the upregulation of Irf1 gene expression in WNV Eg101-infected MEFs is mediated in an IRF-3-, IRF-9- and IRF-7-independent manner. An Irf1 promoter analysis predicted the presence of NF-kappa B binding sites (Harada et al., 1994; Pine et al., 1994) and Irf1 gene expression was previously reported to be induced by this TF (Pahl, 1999). NF-kappa B is activated by a WNV Eg101-infection in MEFs (Scherbik et al., unpublished results) and the involvement of NF-kappa B components in regulating Irf1 upregulation in infected cells is currently under investigation.
During a WNV infection, viral RNA is recognized by RIG-I and MDA5 cytoplasmic sensors (Fredericksen et al., 2008) which signal through the IPS-1 adaptor molecule to activate IRF-3 leading to the induction of IFN and IRF-3-dependent gene expression (Fredericksen et al., 2008; Daffis et al., 2009; Suthar et al., 2010). The results of the present study showed that lack of MDA5 but not of of RIG-I resulted in reduction in the upregulation of Oas1a, Oas1b, Irf7 and Irf1 suggesting that MDA5 may be functionally more important for the upregulation of these genes. In addition, the results of this study showed that the initial upregulation of Oas1a, Oas1b and Irf7 but not of Irf1 in WNV Eg101infected MEFs is only partially dependent on IPS-1 since the mRNA levels of these genes were upregulated in IPS-1 deficient cells at 8 h after infection. The lack of induction of IFN beta gene expression at 8 h after infection in IPS-1−/− MEFs confirmed that the upregulation of these genes was independent of IFN-beta. The mechanism by which the initial IFN- and IPS-1-independent upregulation of a subset of ISGs occurs is currently not known. Early induction of ISGs may be due to activation of signaling pathways activated by WNV entry. The existence of such a response to virus particle entry was previously reported and shown to be IFN-, TLR- and RIG-I independent (Paladino et al., 2006). The interaction of the fusion peptides of enveloped viruses with cellular membranes were also shown to activate several cell transduction pathways leading to early activation of ISGs by AP-1 and NF-kappa B (Vitiello et al., 2010). However, IPS-1 is required for ISG expression at later times of infection.
The higher viral loads in tissues and increased mortality observed with WNV NY99-infected IFN alpha/beta R−/− and IFN beta−/− mice indicate the importance of the type I IFN response for host protection (Samuel and Diamond, 2005; Lazear et al., 2011). Type I IFN secreted by infected cells induces an antiviral state in uninfected cells through the induction of ISGs and this reduces viral spread. The upregulation of ISGs by IFN-independent mechanisms in infected cells would be expected to reduce virus yields even though canonical IFN signaling was blocked by the infection in these cells. It was previously shown that IRF-3-dependent ISGs could be upregulated in WNV-infected cells and experiments in IRF-3−/− mice indicated that IRF-3 protected mice by both IFN-dependent and IFN-independent mechanisms (Fredericksen et al., 2004; Daffis et al., 2007). IRF-7 was shown to protect mice primarily through the induction of IFN alpha (Daffis et al., 2008). The data obtained in the present study indicate that there are additional mechanisms of IFN-independent ISG upregulation for subsets of ISGs in infected MEFs and that IFN-independent ISG upregulation is more complex than previously appreciated. The data also suggest that IRF-9 may also be involved in both IFN-dependent as well as IFN-independent activation complexes for some ISGs.
MATERIALS AND METHODS
Cells and viruses
SV40-T antigen transformed C3H/He (tC3H/He) MEFs were grown in minimal essential medium (MEM) supplemented with 5% fetal bovine serum (FBS) and 10 μg/ml gentamicin. Primary C3H/He MEFs (pC3H/He), pIRF-3−/−, pIRF-7−/− and pC57BL/6 MEFs (provided by Michael Diamond, Washington University School of Medicine, ST. Louis, Missouri) were grown in DMEM supplemented with 10% FBS and 10 μg/ml gentamicin. pIRF-3/7−/− MEFs (provided by Michael Diamond, Washington University School of Medicine, ST. Louis, Missouri) were grown DMEM supplemented with 15% FBS and 1% PenStrep (Gibco). t129/SvEv, tSTAT1−/− and tSTAT2−/− MEF lines (provided by Christian Schindler, Columbia University, New York, NY) were grown in MEM supplemented with 10% FBS and 1% PenStrep. p129 MEFs and pIFN alpha/beta R −/− MEFs (provided by Herbert Virgin, Washington University School of Medicine, St. Louis, MO) were grown in DMEM supplemented with 10% FBS and 1% PenStrep. IRF-3/9−/− MEFs (provided by Karen Mossman, McMaster University, Hamilton, Ontario, Canada) were grown in MEM alpha supplemented with 10% FBS and 1% PenStrep. All cell cultures were grown at 37°C in a 5% CO2 atmosphere.
A stock of lineage I WNV strain Eg101 was prepared by infecting BHK cells (Vaheri et al., 1965) grown in MEM supplemented with 5% FBS and 10 μg/ml gentamicin at a MOI of 0.1 and harvesting culture fluid 32 h after infection, a time when the infected cell monolayer was still intact. Clarified culture fluid was aliquoted and stored at –80°C. The titer of the stock virus was ~ 1 × 108 PFU/ml.
ELISA
Cell culture fluid was collected at various times after WNV Eg101 infection and the levels of secreted IFN beta protein in these samples were measured by a capture ELISA according to the manufacturer’s instructions (PBL Biomedical Laboratories, NJ) using a standard curve prepared by titrating a known concentration of recombinant murine IFN beta.
ChIP
Confluent tC3H/He monolayers were mock-infected, infected with WNV Eg101 at a MOI 5 for 7, 16 or 24 h or treated with 1000 U/ml of murine IFN beta for 3 h. The ChIP assay was done as previously described (Pulit-Penaloza et al., 2012). Isolated DNA was analyzed by real-time PCR using probes and primers designed to span the proximal Oas1a and Oas1b promoters. The sequences of the primers were: Oas1a forward primer 5′-GGATCCTAAGAAAGCTCAGACTTCA-3′, Oas1a reverse primer 5′-CCCGGCAGCCAATGG-3′, Oas1b forward primer 5′-GAAGCCCTAACGCCATTGG-3′, Oas1b reverse primer 5′-AGGGCGCGGATATGCA-3′, Irf7 forward primer 5′-CCTGCCTTGTCCCAATGTG-3′ and Irf7 reverse primer 5′-ACACCCGACCCTTACTCAGATC-3′ and Irf1 forward primer 5′-CCTTCGCCGCTTAGCTCTAC-3′ and Irf1 reverse primer 5′-CCCACTCGGCCTCATCATT-3′. The sequence of the FAM-MGB Oas1a probe was 5′-TGGAAGTGTGGGAAAGGTCTTT-3′, that of the Oas1b probe was 5′-CGGGCCTGGATGAT-3′ and that of the Irf7 probe was 5′-TTTCCTGAAGAGGTCCTG-3′ and that of Irf-1 probe was 5′-ACAGCCTGATTTCC-3′. To obtain standard curves, known amounts of Oas1a, Oas1b and Irf7 and Irf1 promoter DNA cloned into a TOPO-XL vector (Invitrogen) were titrated and assayed by real-time quantitative PCR (qPCR) using a FastStart Universal Probe Master (ROX) kit (Roche Applied Science) according to the manufacturer’s protocol. To quantify the immunoprecipitated DNA, standard curves were independently generated at the same time as the immunoprecipitated DNA samples by qPCR and the value of the fold change over the value obtained with a mock-infected sample was calculated. The average values from at least two independent experiments were plotted. Error bars represent SDM..
Quantification of mRNA levels
Real-time quantitative reverse transcription-PCR (qRT-PCR) analyses of mouse Oas1a and Oas1b gene expression were performed using a 50 μl reaction mixture ontaining 500 ng of total cellular RNA, the primer pair (1 μM), and the probe (0.2 μM) and an Applied Biosystems 7500 Sequence Detection System. Applied Biosystems Assays-on-Demand 20x primer and fluorogenic TaqMan FAM/MGB probe mixes Mn00836412_m1 for Oas1a, Mn00449297_m1 for Oas1b, Mn00516788_m1 for Ifr7 and Mn01288580_m1 for Irf1 were used. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an endogenous control for each sample and was detected using TaqMan mouse GAPDH primers and probe (Applied Biosystems). One-step RT-PCR was performed for each target gene and for the endogenous control in a singleplex format using 200 ng of RNA and the TaqMan One-Step RT-PCR Master Mix Reagent Kit (Applied Biosystems). The cycling parameters were as follows: reverse transcription at 48°C for 30 m, AmpliTaq activation at 95°C for 10 m, denaturation at 95°C for 15 s, and annealing/extension at 60°C for 1 m (cycle repeated 40 times). Each experiment was repeated at least two times in triplicate. Triplicate Ct values were analyzed with Microsoft Excel using the comparative Ct (Δ Δ Ct) method of the SDS Applied Biosystems software which also applied statistical analysis to the data (TINV test in Microsoft Excel). The values were normalized to those for GAPDH and presented as the relative fold change compared to the uninfected calibrator sample in relative quantification (RQ) units. Error bars represent the standard error of the mean (SEM) and indicate the calculated minimum (RQMin) and maximum (RQMax) of the mRNA expression levels based on a RQMin/Max of the 95% confidence level. The expression levels between two samples were considered statistically different (P value of <0.05) when the error bars did not overlap.
Confocal immunofluorescence microscopy
tC3H/He MEFs grown to 50% confluency on 15-mm glass coverslips in 12-well plates were infected with WNV Eg101 at a MOI of 5. The cells were fixed with 4% paraformaldehyde in PBS for 10 min at RT and then permeabilized with 0.1% Triton X-100 in PBS for 10 min. Coverslips were washed with PBS, blocked overnight in 5% heat-inactivated horse serum (Invitrogen) in PBS, and then incubated with mouse anti-dsRNA antibody (English and Scientific Consulting Bt, Hungary) diluted 1:500 in the blocking buffer or with rabbit anti-STAT2 antibody (generously provided by Christian Schindler, Columbia University, New York, NY) diluted 1:200 for 1h at room temperature. The coverslips were washed three times with PBS and incubated with donkey anti-mouse Alexa Fluor 594 or donkey anti-rabbit Alexa Fluor 488 secondary antibodies (Invitrogen) diluted 1:400 in blocking buffer containing 0.5 μg/ml of Hoescht 33342 dye (Invitrogen). The coverslips were washed with PBS and mounted on glass slides with Prolong Gold Antifade reagent (Invitrogen). Cells were visualized with a 40x oil-immersion objective on a LSM 700 laser confocal microscope (Zeiss, Oberkochen, Germany) using LSM 5 (version 4.2) software (Carl Zeiss Inc.). All of the images compared were obtained using the same instrument settings.
Western blotting
Whole tC3H/He cell extracts were prepared using radioimmunoprecipitation assay buffer [1x PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS containing Halt Protease and Phosphatase Inhibitor Single-Use Cocktail (Thermo Scientific). t129 MEF and STAT2−/− MEF nuclear and cytoplasmic extracts were prepared using Nuclear Extract kit (Active Motif) according to the manufacturer’s protocol. Following separation by SDS-polyacrylamide gel electrophoresis (PAGE), the proteins were electrophoretically transferred to a nitrocellulose membrane. The membrane was blocked with Tris-buffered saline pH 8, containing 5% dry milk (5% bovine serum albumin was substituted for milk when phosphorylated proteins were detected) and 0.1% Tween-20 for 1 h at 22°C and then incubated with a polyclonal primary antibody specific for: STAT1 (Cell Signaling), phospho-STAT1 (Tyr701) (Cell Signaling), STAT2 (generously provided by Christian Schindler, Columbia University, New York, NY), phospho-STAT2 (Tyr 690) (Abcam), IRF-9 (Santa Cruz Biotechnology), WNV NS3 (R&D Systems) or beta-actin (Abcam) overnight at 4°C in the presence of blocking buffer. The blots were then incubated with anti-rabbit antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology) or anti-mouse antibody conjugated with horseradish peroxidase (Cell Signaling) for 1 h at 22°C and processed for enhanced chemiluminescence using a Super-Signal West Pico detection kit (Thermo Scientific) according to the manufacturer’s instructions.
HIGHLIGHTS.
STAT1/2 were phosphorylated in infected MEFs but did not translocate to the nucleus.
STAT occupancy on Oas1a, Oas1b and Irf7 promoters did not increase after infection.
IRF-9 and IRF-3 or -7 are involved in IFN-independent ISG upregulation in infected cells.
Irf1 upregulation in WNV-infected MEFs is IRF-9, IRF-3 and IRF-7-independent.
Acknowledgments
This work was supported by Public Health Service research grant AI045135 to M.A.B. from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank B. Stockman for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen J, VanScoy S, Cheng TF, Gomez D, Reich NC. IRF-3-dependent and augmented target genes during viral infection. Genes Immun. 2008;9:168–175. doi: 10.1038/sj.gene.6364449. [DOI] [PubMed] [Google Scholar]
- Ashour J, Morrison J, Laurent-Rolle M, Belicha-Villanueva A, Plumlee CR, Bernal-Rubio D, Williams KL, Harris E, Fernandez-Sesma A, Schindler C, Garcia-Sastre A. Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe. 2010;8:410–421. doi: 10.1016/j.chom.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BJ, Richards J, Mancl M, Hanash S, Beretta L, Pitha PM. Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J Biol Chem. 2004;279:45194–45207. doi: 10.1074/jbc.M400726200. [DOI] [PubMed] [Google Scholar]
- Bartee E, McFadden G. Human cancer cells have specifically lost the ability to induce the synergistic state caused by tumor necrosis factor plus interferon-beta. Cytokine. 2009;47:199–205. doi: 10.1016/j.cyto.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basagoudanavar SH, Thapa RJ, Nogusa S, Wang J, Beg AA, Balachandran S. Distinct roles for the NF-kappa B RelA subunit during antiviral innate immune responses. J Virol. 2011;85:2599–2610. doi: 10.1128/JVI.02213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley DW, Li L, Suderman MT, Barrett AD. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology. 2002;296:17–23. doi: 10.1006/viro.2002.1372. [DOI] [PubMed] [Google Scholar]
- Best SM, Morris KL, Shannon JG, Robertson SJ, Mitzel DN, Park GS, Boer E, Wolfinbarger JB, Bloom ME. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J Virol. 2005;79:12828–12839. doi: 10.1128/JVI.79.20.12828-12839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. doi: 10.1146/annurev.micro.56.012302.160654. [DOI] [PubMed] [Google Scholar]
- Daffis S, Samuel MA, Keller BC, Gale M, JrDiamond MS. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and -independent mechanisms. PLoS Pathog. 2007;3:e106. doi: 10.1371/journal.ppat.0030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S, Samuel MA, Suthar MS, Keller BC, Gale M, JrDiamond MS. Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J Virol. 2008;82:8465–8475. doi: 10.1128/JVI.00918-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S, Suthar MS, Szretter KJ, Gale M, JrDiamond MS. Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS Pathog. 2009;5:e1000607. doi: 10.1371/journal.ppat.1000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitte-Orr SJ, Mehta DR, Collins SE, Suthar MS, Gale M, JrMossman KL. Long double-stranded RNA induces an antiviral response independent of IFN regulatory factor 3, IFN-beta promoter stimulator 1, and IFN. J Immunol. 2009;183:6545–6553. doi: 10.4049/jimmunol.0900867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbahesh H, Jha BK, Silverman RH, Scherbik SV, Brinton MA. The Flvr-encoded murine oligoadenylate synthetase 1b (Oas1b) suppresses 2–5A synthesis in intact cells. Virology. 2011;409:262–270. doi: 10.1016/j.virol.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elco CP, Guenther JM, Williams BR, Sen GC. Analysis of genes induced by Sendai virus infection of mutant cell lines reveals essential roles of interferon regulatory factor 3, NF-kappaB, and interferon but not toll-like receptor 3. J Virol. 2005;79:3920–3929. doi: 10.1128/JVI.79.7.3920-3929.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Fredericksen BL, Gale M., Jr West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J Virol. 2006;80:2913–2923. doi: 10.1128/JVI.80.6.2913-2923.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M., Jr Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol. 2008;82:609–616. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen BL, Smith M, Katze MG, Shi PY, Gale M., Jr The host response to West Nile Virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J Virol. 2004;78:7737–7747. doi: 10.1128/JVI.78.14.7737-7747.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandvaux N, Servant MJ, ten Oever B, Sen GC, Balachandran S, Barber GN, Lin R, Hiscott J. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J Virol. 2002;76:5532–5539. doi: 10.1128/JVI.76.11.5532-5539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JT, Hayashi J, Seeger C. West Nile virus inhibits the signal transduction pathway of alpha interferon. J Virol. 2005;79:1343–1350. doi: 10.1128/JVI.79.3.1343-1350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Takahashi E, Itoh S, Harada K, Hori TA, Taniguchi T. Structure and regulation of the human interferon regulatory factor 1 (IRF-1) and IRF-2 genes: implications for a gene network in the interferon system. Mol Cell Biol. 1994;14:1500–1509. doi: 10.1128/mcb.14.2.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LJ, Hung LF, Weng CY, Wu WL, Chou P, Lin YL, Chang DM, Tai TY, Lai JH. Dengue virus type 2 antagonizes IFN-alpha but not IFN-gamma antiviral effect via down-regulating Tyk2-STAT signaling in the human dendritic cell. J Immunol. 2005;174:8163–8172. doi: 10.4049/jimmunol.174.12.8163. [DOI] [PubMed] [Google Scholar]
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- Jiang D, Weidner JM, Qing M, Pan XB, Guo H, Xu C, Zhang X, Birk A, Chang J, Shi PY, Block TM, Guo JT. Identification of five interferon-induced cellular proteins that inhibit west nile virus and dengue virus infections. J Virol. 2010;84:8332–8341. doi: 10.1128/JVI.02199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Davidson A, Hibbert L, Gruenwald P, Schlaak J, Ball S, Foster GR, Jacobs M. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J Virol. 2005;79:5414–5420. doi: 10.1128/JVI.79.9.5414-5420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsoulidis E, Li Y, Mears H, Platanias LC. The p38 mitogen-activated protein kinase pathway in interferon signal transduction. J Interferon Cytokine Res. 2005;25:749–756. doi: 10.1089/jir.2005.25.749. [DOI] [PubMed] [Google Scholar]
- Kaur S, Uddin S, Platanias LC. The PI3′ kinase pathway in interferon signaling. J Interferon Cytokine Res. 2005;25:780–787. doi: 10.1089/jir.2005.25.780. [DOI] [PubMed] [Google Scholar]
- Keller BC, Fredericksen BL, Samuel MA, Mock RE, Mason PW, Diamond MS, Gale M., Jr Resistance to alpha/beta interferon is a determinant of West Nile virus replication fitness and virulence. J Virol. 2006;80:9424–9434. doi: 10.1128/JVI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus TA, Lau JF, Parisien JP, Horvath CM. A hybrid IRF9-STAT2 protein recapitulates interferon-stimulated gene expression and antiviral response. J Biol Chem. 2003;278:13033–13038. doi: 10.1074/jbc.M212972200. [DOI] [PubMed] [Google Scholar]
- Laurent-Rolle M, Boer EF, Lubick KJ, Wolfinbarger JB, Carmody AB, Rockx B, Liu W, Ashour J, Shupert WL, Holbrook MR, Barrett AD, Mason PW, Bloom ME, Garcia-Sastre A, Khromykh AA, Best SM. The NS5 protein of the virulent West Nile virus NY99 strain is a potent antagonist of type I interferon-mediated JAK-STAT signaling. J Virol. 84:3503–3515. doi: 10.1128/JVI.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent-Rolle M, Boer EF, Lubick KJ, Wolfinbarger JB, Carmody AB, Rockx B, Liu W, Ashour J, Shupert WL, Holbrook MR, Barrett AD, Mason PW, Bloom ME, Garcia-Sastre A, Khromykh AA, Best SM. The NS5 protein of the virulent West Nile virus NY99 strain is a potent antagonist of type I interferon-mediated JAK-STAT signaling. J Virol. 2010;84:3503–3515. doi: 10.1128/JVI.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Pinto AK, Vogt MR, Gale M, JrDiamond MS. Beta interferon controls west nile virus infection and pathogenesis in mice. J Virol. 2011;85:7186–7194. doi: 10.1128/JVI.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Genin P, Mamane Y, Hiscott J. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of alpha/beta interferon genes by interferon regulatory factors 3 and 7. Mol Cell Biol. 2000;20:6342–6353. doi: 10.1128/mcb.20.17.6342-6353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RJ, Liao CL, Lin E, Lin YL. Blocking of the alpha interferon-induced Jak-Stat signaling pathway by Japanese encephalitis virus infection. J Virol. 2004;78:9285–9294. doi: 10.1128/JVI.78.17.9285-9294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Wang XJ, Mokhonov VV, Shi PY, Randall R, Khromykh AA. Inhibition of interferon signaling by the New York 99 strain and Kunjin subtype of West Nile virus involves blockage of STAT1 and STAT2 activation by nonstructural proteins. J Virol. 2005;79:1934–1942. doi: 10.1128/JVI.79.3.1934-1942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, Garcia-Sastre A, Katze MG, Gale M., Jr Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzon M, Jones M, Davidson A, Chain B, Jacobs M. Dengue virus NS5 inhibits interferon-alpha signaling by blocking signal transducer and activator of transcription 2 phosphorylation. J Infect Dis. 2009;200:1261–1270. doi: 10.1086/605847. [DOI] [PubMed] [Google Scholar]
- Merika M, Thanos D. Enhanceosomes. Curr Opin Genet Dev. 2001;11:205–208. doi: 10.1016/s0959-437x(00)00180-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Morin P, Braganca J, Bandu MT, Lin R, Hiscott J, Doly J, Civas A. Preferential binding sites for interferon regulatory factors 3 and 7 involved in interferon-A gene transcription. J Mol Biol. 2002;316:1009–1022. doi: 10.1006/jmbi.2001.5401. [DOI] [PubMed] [Google Scholar]
- Munoz-Jordan JL, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin WI, Garcia-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005;79:8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Jordan JL, Sanchez-Burgos GG, Laurent-Rolle M, Garcia-Sastre A. Inhibition of interferon signaling by dengue virus. Proc Natl Acad Sci U S A. 2003;100:14333–14338. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya T, Sato M, Hata N, Asagiri M, Suemori H, Noguchi S, Tanaka N, Taniguchi T. Gene induction pathways mediated by distinct IRFs during viral infection. Biochem Biophys Res Commun. 2001;283:1150–1156. doi: 10.1006/bbrc.2001.4913. [DOI] [PubMed] [Google Scholar]
- Ning S, Huye LE, Pagano JS. Regulation of the transcriptional activity of the IRF7 promoter by a pathway independent of interferon signaling. J Biol Chem. 2005;280:12262–12270. doi: 10.1074/jbc.M404260200. [DOI] [PubMed] [Google Scholar]
- Ousman SS, Wang J, Campbell IL. Differential regulation of interferon regulatory factor (IRF)-7 and IRF-9 gene expression in the central nervous system during viral infection. J Virol. 2005;79:7514–7527. doi: 10.1128/JVI.79.12.7514-7527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Paladino P, Cummings DT, Noyce RS, Mossman KL. The IFN-independent response to virus particle entry provides a first line of antiviral defense that is independent of TLRs and retinoic acid-inducible gene I. J Immunol. 2006;177:8008–8016. doi: 10.4049/jimmunol.177.11.8008. [DOI] [PubMed] [Google Scholar]
- Peters KL, Smith HL, Stark GR, Sen GC. IRF-3-dependent, NFkappa B- and JNK-independent activation of the 561 and IFN-beta genes in response to double-stranded RNA. Proc Natl Acad Sci U S A. 2002;99:6322–6327. doi: 10.1073/pnas.092133199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine R, Canova A, Schindler C. Tyrosine phosphorylated p91 binds to a single element in the ISGF2/IRF-1 promoter to mediate induction by IFN alpha and IFN gamma, and is likely to autoregulate the p91 gene. EMBO J. 1994;13:158–167. doi: 10.1002/j.1460-2075.1994.tb06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulit-Penaloza JA, Scherbik SV, Brinton MA. Activation of Oas1a gene expression by type I IFN requires both STAT1 and STAT2 while only STAT2 is required for Oas1b activation. 2012 doi: 10.1016/j.virol.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi SA, Leung S, Kerr IM, Stark GR, Darnell JE., Jr Function of Stat2 protein in transcriptional activation by alpha interferon. Mol Cell Biol. 1996;16:288–293. doi: 10.1128/mcb.16.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Gale M., Jr Principles of intracellular viral recognition. Curr Opin Immunol. 2007;19:17–23. doi: 10.1016/j.coi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Scherbik SV, Paranjape JM, Stockman BM, Silverman RH, Brinton MA. RNase L plays a role in the antiviral response to West Nile virus. J Virol. 2006;80:2987–2999. doi: 10.1128/JVI.80.6.2987-2999.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbik SV, Stockman BM, Brinton MA. Differential expression of interferon (IFN) regulatory factors and IFN-stimulated genes at early times after West Nile virus infection of mouse embryo fibroblasts. J Virol. 2007;81:12005–12018. doi: 10.1128/JVI.01359-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Shuai K, Prezioso VR, Darnell JE., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992;257:809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Schmid S, Mordstein M, Kochs G, Garcia-Sastre A, Tenoever BR. Transcription factor redundancy ensures induction of the antiviral state. J Biol Chem. 2010;285:42013–42022. doi: 10.1074/jbc.M110.165936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, ten Oever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Shuai K, Schindler C, Prezioso VR, Darnell JE., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992;258:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Stirnweiss A, Ksienzyk A, Klages K, Rand U, Grashoff M, Hauser H, Kroger A. IFN regulatory factor-1 bypasses IFN-mediated antiviral effects through viperin gene induction. J Immunol. 2010;184:5179–5185. doi: 10.4049/jimmunol.0902264. [DOI] [PubMed] [Google Scholar]
- Suhara W, Yoneyama M, Kitabayashi I, Fujita T. Direct involvement of CREB-binding protein/p300 in sequence-specific DNA binding of virus-activated interferon regulatory factor-3 holocomplex. J Biol Chem. 2002;277:22304–22313. doi: 10.1074/jbc.M200192200. [DOI] [PubMed] [Google Scholar]
- Suthar MS, Ma DY, Thomas S, Lund JM, Zhang N, Daffis S, Rudensky AY, Bevan MJ, Clark EA, Kaja MK, Diamond MS, Gale M., Jr IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS Pathog. 2010;6:e1000757. doi: 10.1371/journal.ppat.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Vaheri A, Sedwick WD, Plotkin SA, Maes R. Cytopathic effect of rubella virus in RHK21 cells and growth to high titers in suspension culture. Virology. 1965;27:239–241. doi: 10.1016/0042-6822(65)90170-4. [DOI] [PubMed] [Google Scholar]
- Vitiello M, Finamore E, Falanga A, Raieta K, Cantisani M, Galdiero F, Pedone C, Galdiero M, Galdiero S. Viral fusion peptides induce several signal transduction pathway activations that are essential for interleukin-10 and beta-interferon production. Intervirology. 2010;53:381–389. doi: 10.1159/000317287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside ST, Israel A. I kappa B proteins: structure, function and regulation. Semin Cancer Biol. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]