Abstract
Great strides have been made regarding our understanding of the processes and signaling events influenced by Eph/ephrin signaling that play a role in cell adhesion and cell movement. However, the precise mechanisms by which these signaling events regulate cell and tissue architecture still need further resolution. The Eph/ephrin signaling pathways and the ability to regulate cell-cell adhesion and motility constitutes an impressive system for regulating tissue separation and morphogenesis [1, 2]. Moreover, the de-regulation of this signaling system is linked to the promotion of aggressive and metastatic tumors in humans [2]. In the following section, we discuss some of the interesting mechanisms by which ephrins can signal through their own intracellular domains (reverse signaling) either independent of forward signaling or in addition to forward signaling through a cognate receptor. In this review we discuss how ephrins (Eph ligands) “reverse signal” through their intracellular domains to affect cell adhesion and movement, but the focus is on modes of action that are independent of SH2 and PDZ interactions.
Keywords: ephrin, Eph, cell adhesion, reverse signaling, cell movement
1. Introduction
Members of the Eph/ephrin family have been implicated in regulating numerous morphogenetic processes such as axon outgrowth, neural crest and retinal progenitor cell migration, hindbrain segmentation, skeletal patterning, and angiogenesis [2, 3]. Interactions between the Eph receptor tyrosine kinases residing on one cell with their membrane bound ligands (ephrins) on another cell results in bi-directional signaling, in which both molecules transmit intracellular signals upon cell-cell contact. Although there is evidence supporting an ultimate role for both Eph receptors and ligands in affecting small GTPases such as Rho, numerous signaling molecules and pathways intersect with Eph receptor or ligand signaling. Additional studies are needed to define the Eph/ephrin signal transduction systems in various cellular contexts. Cell-cell contact events during development can initiate Eph/ephrin signaling that leads to cell sorting and boundary formation between receptor and ligand bearing cells [4]. When motile ligand or receptor-bearing cells come in contact with cells expressing the cognate receptor or ligand, the response is often adhesion or repulsion. Alternative growth factors and signaling pathways can mediate or regulate Eph/ephrin signaling to cooperatively regulate the movement and positioning of the cognate receptor or ligand-bearing cells [2]. These ligands and receptors play important roles in several morphogenetic events during development, but when de-regulated can lead to cancer invasion and metastasis [5, 6]. Recent data also show that members of the Eph/ephrin family mediate cell-cell interactions both in tumor cells and in the tumor microenvironment (ie. stroma and vasculature) [7, 8].
2. Ephrin-A and –B Ligands
Eph receptors are transmembrane receptor tyrosine kinases possessing an extracellular domain that includes an N-terminal ligand-binding domain, a cysteine-rich EGF-like domain, and two fibronectin type III motifs. These receptors are divided into two subclasses (A & B) by sequence similarities and binding specificity towards two subclasses of ligands (A & B) known as ephrins. The ephrins are all membrane-bound proteins with the A subclass consisting of glycosylphosphatidylinositol (GPI)-linked to the membrane, and the B subclass being transmembrane proteins with a short cytoplasmic domain. Generally, the A-type receptors have specificity toward A-type ligands, while B-types bind to their cognate receptors. The exceptions to this rule are EphA4 and EphB2 which can also bind all ephrin-Bs and ephrin-A5, respectively [4, 9]. Due to their role in cell adhesion, repulsion and boundary formation, loss of forward and/or reverse signaling in the Eph/ephrin system can lead to severe congenital malformations [2].
2.1 Ephrin-A Ligands and Reverse Signaling in Cell Adhesion
In this section we will focus on reverse signaling through the A-type ephrins, which are GPI-linked to the membrane. Despite this source of tethering, there are data suggesting that the A-type ephrins are capable of signaling within their host cell (Figure 1). Evidence indicating that A-type ephrins can regulate adhesion by reverse signaling comes from several studies. For example, loss of ephrin-A5 in mice leads to midline fusion in the neural tube. It has been proposed that cell adhesion may result from weakening the activation of the Eph/ephrin signaling that would normally promote cell repulsion [10]. In a study of ephrin-A5 null mice, an inverse correlation between ephrin-A5 gene dosage and adhesion was observed, and a decrease in adhesion of ephrin-A5 mutant cells as compared with wild-type counterparts [10]. This concept is supported by the observation that in tissues highly expressing spliced forms of EphA7 lacking kinase activity, such as the neural tube, the cells are redirected from a repulsive to an adhesive response upon ligand/receptor contact [10]. Forward signaling might also be abrogated via phosphatase function [11] or through cleavage and proteolysis [12], and therefore lead to increased adhesion.
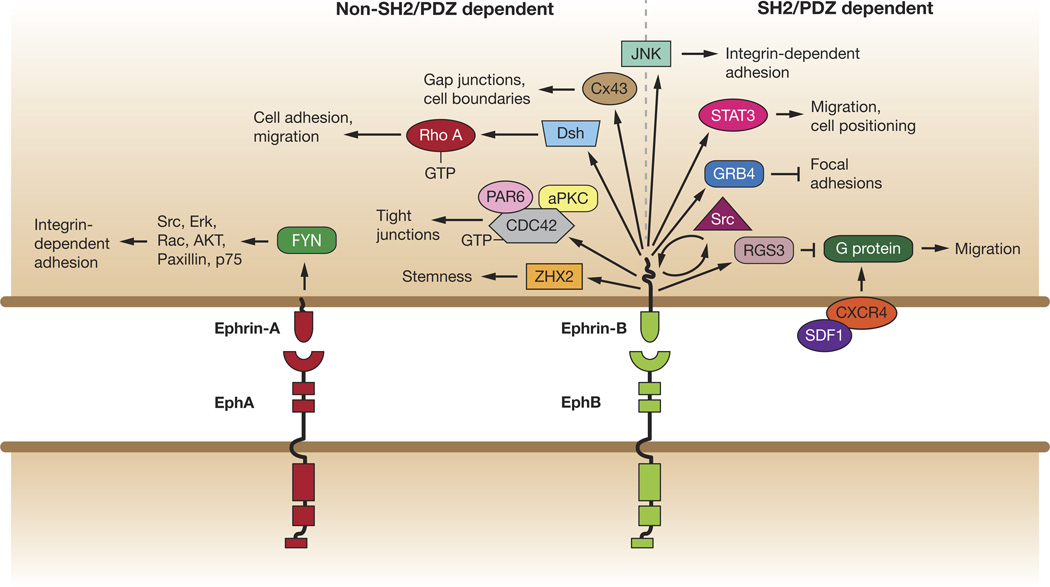
Figure 1.
SH2/PDZ -dependent and -independent reverse signaling pathways of ephrin-A and ephrin-B in cell adhesion and movement. Ephrin-A signaling via Fyn to affect downstream players in integrin-dependent adhesion. Ephrin-B reverse signaling pathways that are non-SH2/PDZ dependent are located to left of the dotted line and SH2/PDZ–dependent signaling is located to the right, such as regulation Src. Ephrin-B1 disrupts focal adhesions through GRB4 to affect focal adhesions. RGS3 is a PDZ domain interaction that inhibits signaling by CXCR4, a G protein-coupled chemokine receptor, to affect migration. STAT3 is an SH2 interaction affecting endothelial cell and mural cell migration and positioning. JNK (center) is likely to be a non SH2/PDZ interaction and influences integrin-dependent adhesion. Non-SH2/PDZ ephrin-B1 interactions include PAR6 to inhibit atypical protein kinase C (aPKC), and upon phosphorylation inhibits ephrin-B1 binding to PAR6, allowing PAR6 to bind GTP-bound CDC42 and activate aPKC. Ephrin-B also complexes with Dsh to affect RhoA and PCP signaling. Ephrin-B1 may regulate cell boundaries through Connexin 43 (Cx43).
Although affecting cell adhesion through a reduction of forward signaling may be a significant strategy for morphogenesis, another useful strategy is to employ reverse signals through the ephrin-As that promote attractive effects [13]. For example, in the Hek 293 system, it was shown that activation of ephrin-A2 or ephrin-A5 by EphA3 resulted in a beta1-integrin-dependent increase in adhesion to laminin by ephrin-A-expressing cells [13]. An independent study showed that in response to contact with its cognate Eph receptor, ephrin-A5 signals within caveola-like domains of the plasma membrane leading to increased adhesion of the cells to fibronectin [14]. In addition, ephrin-A5 induced an initial change in cell adhesion, leading to changes in cell morphology, and these effects were dependent on beta1 integrin activation [15]. Thus, class A ephrins may contribute to controlling the affinity of certain cells toward specific extracellular substrates by regulating integrin function [15]. Support for this concept can be found in other studies, for example, EphA3-/ephrin interactions increased adhesion of hematopoetic progenitor cells to fibronectin and VCAM-1, while a soluble EphA3-Fc antagonist increased progenitor cell and colony-forming unit-spleen cells in the peripheral blood relative to controls [16]. Another study provides support for the ideal that the magnitude of ephrin-A4 reverse signaling in lymphocytes determines the adhesion and transendothelial migration of B cells [17].
There are various molecules known to be regulated downstream of ephrin-A reverse signaling (ie. Src family members, ERK1/2, Rac, AKT, integrin, paxillin, and p75NTR), but the mechanism of activation and the pathways employed still need clarification [13–15, 18–21]. Moreover, the down-regulation of ephrinA signaling is also of great importance for regulating reverse signaling. One mechanism is through a ephrin cleavage involving the Kuzbanian metalloprotease (ADAM10) (104). ADAM10 constitutively associates with EphA3, and activation of the Eph receptor results in the kinase domain swinging away from the plasma membrane (Figure 2). This repositioning of the kinase domain abrogates its ability to sterically hinder ADAM10 from cleaving the ligand [22]. Cleavage of ephrin-A occurs when ADAM10 and its substrate are present on the membranes of opposing cells [23], thus allowing for a switch from adhesion to repulsion (Figure 2). Although this mechanism explains a passive mechanism for reduction of forward and reverse signaling, a thorough understanding of the active signaling that directs repulsion and adhesion is still under investigation.
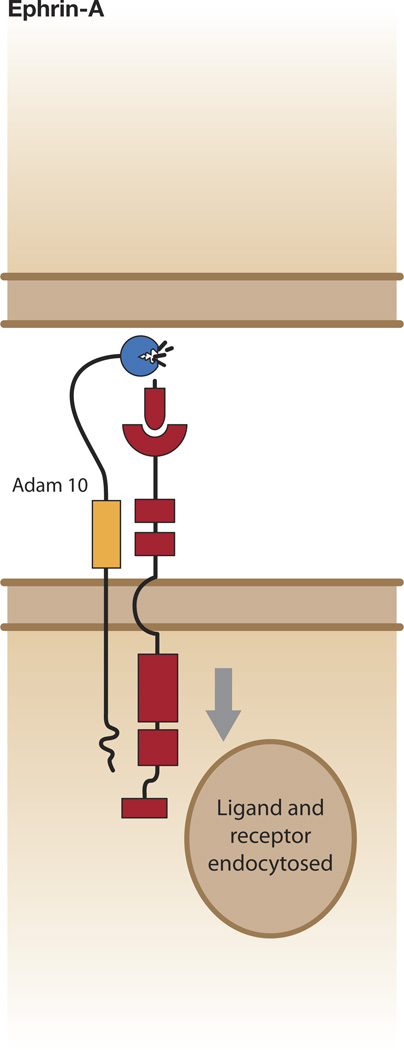
Figure 2.
Ephrin-A cleavage from the cell surface. ADAM10 is constitutively associated with the receptor. Ephrin-A binds to EphA, which clusters (not shown) and is activated, and results in extension of the kinase domain. ADAM10 now recognizes the substrate motif in ephrin-A and cleaves it. One possible result is ligand/receptor endocytosis into receptor bearing cell.
2. 2 Ephrin-B Ligands and Reverse Signaling
The B-type transmembrane ephrin ligands do not possess any intrinsic catalytic activity for signaling, but rely upon a scaffolding activity that recruits signaling molecules to transmit functional effects within the cell. It has been shown that ephrin-Bs utilize both phosphorylation-dependent and -independent signaling pathways, which may be viewed as different modes of reverse signaling: 1) one mode where tyrosine phosphorylation of the intracellular domain of ephrin-B leads to recruitment of signaling molecules that exert a functional effect; 2) another mode where unphosphorylated ephrin-B associates with a protein complex that transduces a signal, but upon tyrosine phosphosphorylation, the interaction of ephrin-B with the signaling complex is disrupted or modulated [24]; 3) a possible third mode where tyrosine phosphorylation may occur but is not required for specific signaling events [25].
Phosphorylation of ephrin-Bs occurs in response to binding and clustering of Eph receptors, leading to activation of a Src family kinase that phosphorylates the intracellular domain of B-type ephrins [26, 27]. Alternatively, specific growth factor receptors (ie. FGFR, PDGFR, TIE-2) or cell surface molecules (Claudins) induce this phosphorylation event in cis [26–30]. There are phosphorylation-dependent and - independent signaling molecules and pathways for both ephrin receptors and ligands [24]. A limited number interaction partners have been identified for ephrin-Bs that mediate a functional effect (Figure 1). Several of these partners require SH2 or PDZ interactions, while others do not use these modules for an interaction with ephrin-Bs (Figure 1). For example, an ephrin-B interaction with PDZ-RGS3, a GTP exchange factor, regulates the migration of cerebellar granule cells [31], and is critical for the maintenance of the neural progenitor cell state [32]. Another interacting partner is ZHX2 (a zinc finger homeodomain protein) that also regulates neural progenitor maintenance in the developing murine cerebral cortex [33]. In this case, a non-SH2/PDZ interaction is most likely, where the suggested binding domain of ephrin-B1 is located within the region adjacent to the transmembrane domain and is conserved between ephrin-B1 and B2 [33]. Both ephrin-B1 and ephrin-B2 interact with syntenin through their C-terminal PDZ-binding motif and have been shown to function with EphB to mediate presynaptic development [34–36]. Grb4, an adaptor protein with one SH2 and three SH3 domains, has been shown to associate with ephrin-B1 in a phosphorylation-dependent manner and mediate functional effects on cell morphology [37, 38]. These effects may be mediated through an association of Grb4 with other proteins implicated in cytoskeletal regulation (Figure 1), including Cbl-associated protein (CAP/ponsin), the Abl-interacting protein-1 (Abi-1), dynamin, p21-activated kinase (PAK 1), and axin [37]. Ephrin-B1 has also been shown to regulate dendritic spine morphogenesis through Grb4 and the G protein-coupled receptor kinase-interacting protein (GIT) [39]. STAT3 has recently been identified as a new member of this group of SH2 and phosphorylation-dependent ephrin-B-associated signaling molecules [40, 41] (Figure 1). The recruitment of STAT3 to ephrin-B1, and its resulting Jak2-dependent activation and transcription of reporter targets, may reveal a signaling pathway from ephrin-B1 to the nucleus [40, 41]. The in vivo relevance and function of the ephrin-B/STAT3 association is still unclear, however, evidence from a more recent study shows that the STAT3-dependent association is important for ephrin-B2 to contribute to endothelial and mural cell assembly into vascular structures [42]. In this study, it is postulated that STAT3 is unlikely to contribute to endothelial/pericyte assembly by regulating gene transcription due to the rapidity of the effect in a 3D co-culture system. One possible alternative is that STAT3 may work through its ability to regulate microtubule stability via an interaction with stathmin, a tubulin depolymerizing molecule [43], but further studies will be needed to sort out the mechanism.
2.3. Ephrin-B Ligands and non-SH2/PDZ Reverse Signaling in Cell Adhesion
In this section, we have chosen to focus on signaling by the transmembrane ephrin-B ligand through proteins that do not directly interact with ephrin-Bs via their PDZ and/or SH2 domains. Early evidence that ephrin-Bs may send signals affecting cell-cell adhesion in the absence of tyrosine phosphorylation came from Xenopus embryos, where the over-expression of ephrin-B1 caused the blastomeres of ectodermal tissue to dissociate [44]. This de-adhesion phenotype was also observed with the over-expression of ephrin-B1 lacking the receptor binding domain, indicating that these adhesive properties are independent of the Eph receptor/ephrin interaction [44]. Genetic evidence demonstrates that the intracellular domain of ephrin-Bs is critical for neural crest movement, vascular morphogenesis, and septation events, consistent with a signaling function for this domain [25, 45–48]. A role for ephrin-B reverse signaling cell-cell boundaries is beginning to emerge, and is consistent with ephrins regulating cell-cell adhesion [25, 49].
Both forward and reverse signaling through ephrin-B2 and its receptors, EphB2 and EphB3, also play a critical role in cell-cell adhesion events. One such event is the tubularization of the urethra and partitioning of the urinary and alimentary tracts. Generation of a mouse mutant harboring a mutation in the murine ephrin-B2 gene which specifically disrupts reverse signaling (cytoplasmic domain replaced with lacZ) leads to severe hypospadias and incomplete cloacal septation [50]. This study indicates a major contribution of reverse signaling in midline fusion of the urethra and cloaca.
A more recent study using phospho-specific antibodies shows ephrin-B tyrosine phosphorylation localizes to midline septation in the foregut. Of particular interest, closure of the ventral abdominal wall does not require forward signaling through the receptors (EphB2/B3), but ephrin-B reverse signaling is necessary [25]. Moreover, mice lacking all the tyrosine phosphorylation sites plus the PDZ binding motif do not phenocopy ephrin-B2lacZ/lacZ mice, indicating that these two signaling modes are not necessary in the midline septation events. In contrast, mice harboring one mutant allele for both tyrosine phosphorylation and the PDZ binding motif and another allele lacking the full cytoplasmic domain displayed increased hypospadias. Thus, the PDZ binding motif interactions of ephrin-B2 do contribute at least to certain specific midline events. Collectively, the data indicate that the ephrin-B2 reverse signal may have distinct tyrosine phosphorylation-independent pathways. One involves PDZ binding domain interactions, and the other is proposed to involve an interaction with claudin molecules, a major component of tight junctions, which establish paracellular barriers between epithelial cells [25].
Elucidation of some of the mechanisms of how ephrin-Bs can signal through their intracelleular domain via non-PDZ or SH2 interactions are beginning to come to light. For example, it has been shown that ephrin-B1 signaling may regulate cell-cell junctions through a cell polarity complex in vivo [49]. This study used the Xenopus system to assess whether ephrin-B1 mediated or regulated signaling affecting cell-cell junctions in epithelial cells. Ephrin-B1 was shown to associate with Par-6, a major scaffold protein required for establishing tight junctions, in both a human colon carcinoma cell line and in Xenopus embryos [49]. Par-6 is known to constitutively bind aPKC, and upon binding an active Cdc42-GTP undergoes a conformational change that leads to aPKC activation [51]. The Par-6/aPKC/Cdc42-GTP complex localizes to the apical cell junctions and regulates tight junction formation. The tight junction complexes are believed to associate with the actin cytoskeleton and assist in its reorganization in the formation and maintenance of cell-cell contacts [51].
Expression and immunoprecipitation analysis in Xenopus oocytes demonstrated that ephrin-B1 competes with the small GTPase Cdc42 for association with the Par-6 protein. When ephrin-B1 is over-expressed in embryonic ectoderm, it causes the loss of tight junctions and mis-localization of tight junction proteins (ZO-1 and Cingulin), suggesting a possible model where ephrin-B1 may compete with Par-6 and affect the Par polarity complex activities. This competition model (Figure 3) was supported by in vivo experiments showing that tight junction formation in ectoderm over-expressing ephrin-B1 can be rescued by appropriate levels of activated Cdc42 expression [49].
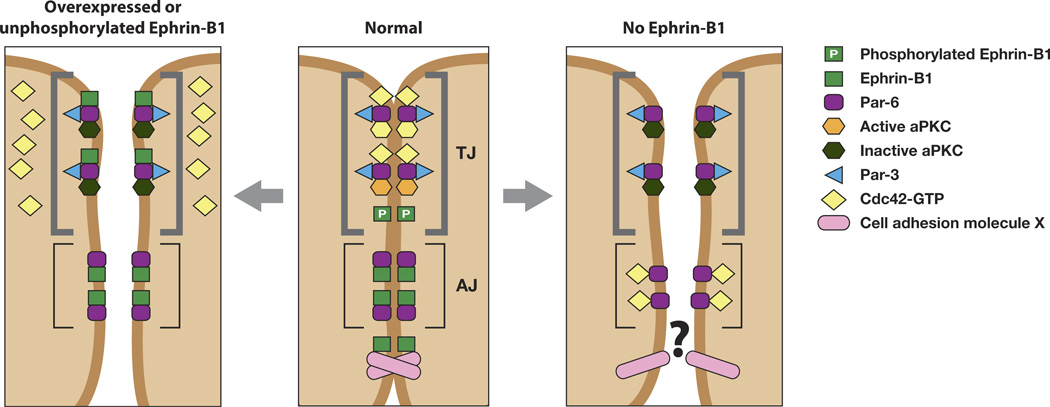
Figure 3.
Ephrin-B1 regulates tight junction formation through an interaction with Par-6. Unphosphorylated ephrin-B1 may compete with activated Cdc42 for binding to Par-6. Lack of a Par-6/Cdc42 interaction inhibits aPKC activation, leading to tight junction disruption (left panel). Tyrosine phosphorylation of ephrin-B1 precludes an interaction with Par-6, leaving it available to interact with Cdc42-GTP and establish tight junctions (middle panel). In the absence of ephrin-B1, Par-6 that is localized to adherens junctions and lateral cell borders may compete with tight junction localized Par-6 for active Cdc42. This may effectively reduce available Cdc42 at the apical border, resulting in a reduction of aPKC activity and disrupt tight junctions (right panel). Alternatively, an as yet unknown cell adhesion molecule that normally associates with ephrin-B1 may no longer function in the absence of ephrin-B1, resulting in loss of TJs and AJs. (Figure adapted from [24]).
Ephrin-B1 is phosphorylated on tyrosine residues in response to binding the extracellular domain of its cognate EphB receptor, and this is accomplished through a Src family kinase. Phosphorylation can also occur in cis by an active FGF receptor or by an interaction with claudins, which are small transmembrane proteins that are important components of tight junctions [26, 27, 30, 52]. Immunoprecipitation analysis of ephrin-B1 (endogenous or exogenous) demonstrates that tyrosine phosphorylation of ephrin-B1 abrogates the interaction with Par-6, and allows active Cdc42 and Par-6 to interact. This data is consistent with a model where unphosphorylated ephrin-B1 and active Cdc42 compete for Par-6 binding, while ephrin phosphorylation disrupts the ephrin-B1/Par-6 complex (Figure 3). Further support for the model is observed in vivo, where translation of endogenous ephrin-B1 is blocked in embryos by ephrin-B1 antisense morpholino oligonucleotides (MOs). In these embryos, the endogenous ephrin-B1 was replaced by the introduction of MO resistant RNAs encoding wild type ephrin-B1 or a tyrosine 310 mutant ephrin-B1 that fails to disengage from Par-6 upon phosphorylation [49]. Expression of the ephrin-B1Y310F mutant in the presence of ephrin-B1 MO failed to restore appropriate localization of the tight junction protein ZO-1, while wild-type ephrin-B1 rescued apical lateral positioning of ZO-1 [49]. These experiments provide in vitro and in vivo evidence for a mechanism where unphosphorylated ephrin-B1 possesses a competitive advantage for binding to Par-6, thus preventing Cdc42-GTP from interacting with Par-6 at apical lateral borders (Figure 3). Blocking the Cdc42/Par-6 interaction leads to reduced aPKC activity and tight junction disruption. In contrast, during cell-cell contact, ephrin-B1 can be phosphorylated at the apical junctions in response to a cognate Eph receptor (or an active FGF receptor or Claudin), causing dissociation of ephrin-B1 from Par-6 (Figure 3). Thus, Cdc42-GTP is free from competition with ephrin-B1 and is able to bind to Par-6, inducing aPKC activation and establishing tight junctions [49].
Intriguingly, Eph/ephrin signaling has been linked to regulation of cell-cell junctions through a role affecting Gap junctions. Gap junctions are specialized intercellular connections between various cell-types that directly connect the cytoplasm of two cells, and allow various molecules and ions to pass freely between cells. In zebrafish, expression of Eph receptors and ephrins in ectodermal explant cells were able to block Gap junction communication (GJC) at the border between two adjacent cell populations [53]. The effect on GJC was determined in zebrafish by injection of embryos with one of two lineage tracers. Ectodermal explants were dissected from the embryos, juxtaposed for adherence, and the cell aggregates were cultured and examined using confocal microscopy. This analysis revealed intermingling between cells from uninjected control explants, but not when one explant expresses ephrin-B and the other EphB. The authors tested GJC by examining whether a fluorescent dye could freely flow between two explants, one expressing ephrin-B and another expressing a cognate EphB. Lack of free flow of dye between these two explants indicated GJC was inhibited [53]. In a mammalian study using mice that are mosaic for the loss of ephrin-B1, GJC was inhibited at ectopic ephrin boundaries [54]. In addition, these authors tested whether Eph/ephrin signaling could regulate GJC using calcein-AM as a marker in vitro. Ephrin-B1 expressing NIH 3T3 cells were plated on cells expressing Eph-B2, resulting in inhibition of GJC, as evidenced by reduced transfer of calcein-AM, when compared to control cells. Similar results were obtained using primary neural crest cells that express both ephrin-B1 and Eph-B2 at low levels [54].
Upon further investigation, Connexin 43, a major gap junction protein, was found in the immune complexes from cells expressing wild-type ephrin-B1, however, no interaction with Connexin was observed with the extracellular domain of ephrin-B1 fused to Fc (Figure 1). Since this data indicated that the intracellular domain of ephrin-B1 was important for an interaction with Connexin 43, co-immunoprecipitations were performed with cells expressing a mutant ephrin-B1 lacking the PDZ binding motif. It was observed that both wild-type and the mutant ephrin-B1 interacted with Connexin, although wild-type ephrin-B1 displayed an affinity preference for phosphorylated Connexin and the ephrin-B1 mutant preferred unphosphorylated connexin [54]. Moreover, using chimeric mouse embryos, the authors show that the mutation in the PDZ binding motif of ephrin-B1 does not induce calvarial defects, which are attributed to GJC inhibition. These data suggest that ephrinB1 can interact with a major gap junction protein through its intracellular domain and regulates gap junction communication [54].
As mentioned previously, it was shown that ephrin-B1 interacts with claudins on the same cell surface in cis using MDCK cells [30]. Claudins are important components of tight junctions and establish the paracellular barrier that controls the flow of molecules in the intercellular space between the cells of an epithelium. Claudins contain four transmembrane domains, but the N-terminus and the C-terminus are located in the cytoplasm. It was observed that tyrosine phosphorylation of the intracellular domain of ephrin-B1 was significantly increased by cell–cell contacts in a claudin-dependent manner. The phosphorylated ephrin-B1 (probably the result of a Src family member) stimulated the paracellular permeability in MDCK cells, providing further evidence that ephrin-B1 is able to regulate tight junctions [30].
3 Ephrin-Bs and Reverse Signaling in Tissue Boundaries
Ephrin-Bs and reverse signaling have been shown to play a role in tissue boundary formation that is dependent upon cell-cell adhesive interactions [4, 55]. One example of this process is hindbrain segmentation (Figure 4), where Eph receptors and ephrins are expressed in alternating rhombomeres and are required for the proper sorting of cells at rhombomere boundaries [56, 57]. Using ectodermal explants from zebrafish, it was shown that bidirectional (but not unidirectional) signaling between EphA4 expressing cells and ephrin-B2a expressing cells restricted mixing between adjacent cell populations, leading to the proposal that bi-directional signaling is responsible for cell repulsion (Figure 4) [53]. However, an additional twist to this concept is a report indicating that the ephrin-B2 ligand in rhombomere 4, as well as EphA4 in its respective rhombomeres (r3 and r5), are required for normal cell affinity, and that EphA4 and EfnB2a regulate cell affinity independently within their respective rhombomeres to maintain rhombomere integrity [58]. Additionally, this study indicates that EphA4 and ephrin-B2a are specifically and individually required to facilitate normal integration of newborn progenitors during the cross-midline cell division that occurs in the neural keel, as part of the process maintaining rhombomere coherence and bilateral symmetry [58].
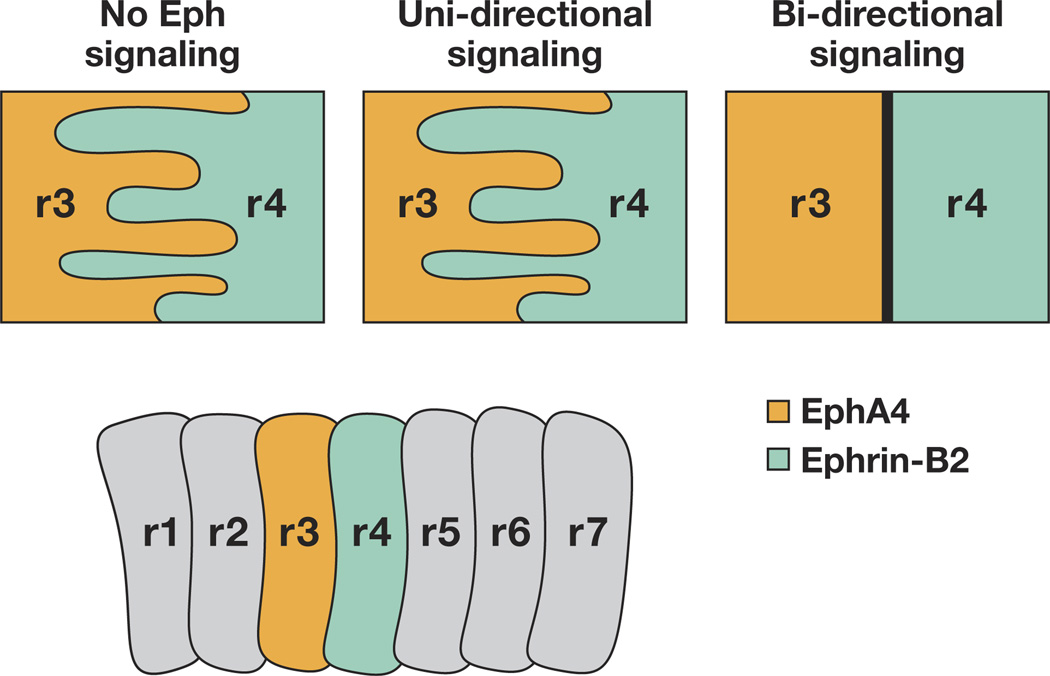
Figure 4.
Boundary formation in the vertebrate hindbrain in response to Eph and ephrin bidirectional signaling. Cartoon depiction of the rhombomeres in a developing vertebrate hindbrain where boundaries are maintained only when bidirectional signaling from the Eph-receptor–expressing cells (r3) contact ephrin-B-expressing cells (r4). r, rhombomere
Human mutations within ephrin-B1 lead to a morphogenetic disorder called craniofrontonasal syndrome (CFNS), which can cause frontonasal dysplasia and fusion of the coronal sutures [59]. The study suggests that, in heterozygous females, the mosaic loss of ephrinB1 (due to X-inactivation) disturbs tissue boundary formation at the developing coronal suture [59]. Davy and colleagues have shown that ephrinB1+/− mice exhibit calvarial defects, a phenotype that is very similar to CFNS and correlates with cell sorting defects in neural crest cells [54]. As mentioned previously, these authors went on to show that gap junction communication was affected in the mice [54].
A subsequent study in the Soriano laboratory demonstrated that ephrin-B1 forward signaling plays an intrinsic role in palatal shelf outgrowth in the mouse by regulating cell proliferation in the anterior shelf mesenchyme [60]. In contrast, a recent study revealed a role for ephrin-B reverse signaling in secondary palate fusion. This morphogenetic event requires adhesion and an epithelial-to-mesenchymal transition of the epithelial layers on opposing palatal shelves, and when this process fails, a cleft palate results. It was found that activation of ephrin-B reverse signaling induced fusion of chicken palates ex vivo (even in the absence of the normal inducer TGFbeta3), and that PI3K inhibition blocked this event [61]. Furthermore, abrogating ephrin-B reverse signaling with an unclustered Eph-Fc fusion protein inhibited TGFbeta3- induced fusion in the ex vivo chicken system and normal fusion in a mouse palate culture. Thus, ephrin reverse signaling appears to be necessary and sufficient to induce palate fusion in these systems [61].
Somite formation is another process where Eph/ephrin signaling is involved in cell-cell adhesive regulation. In this process, Eph-expressing cells that are adjacent to ephrin-expressing cells display repulsive interactions, leading to tissue segregation and the establishment of boundaries [4, 62]. During normal segmentation of somites, boundary cells undergo a mesenchymal-to-epithelial transition, and the border separation relies upon an intracellular signal from ephrin-B2 (‘reverse signaling’) in anterior cells adjacent to EphA4 expressing cells [63–67]. Mechanistic insight into the signaling pathway comes from a study that showed ephrin-B2 transduced an intracellular signal that suppressed Cdc42 activity, leading to formation of an intersomitic boundary that is rapidly followed by cell epithelialization at this border [67]. This finding in somites, where a mesenchymal-to-epithelial transition occurs, is in contrast to studies in epithelial cells (in vitro or in vivo) that demonstrate a requirement for Cdc42 activation for epithelial contacts and TJ formation [49, 68–70].
There is also evidence during zebrafish somite segmentation that ephrin-B2 reverse signaling plays a role in cell-substrate adhesion via Integrins [65, 71]. Cytoplasmic signals can initiate `inside-out' signaling that results in the clustering of Integrins and leads to increased avidity of Integrins for extracellular matrix proteins. Integrin α5β1 is a major receptor for fibronectin and is required for fibronectin matrix assembly. In vivo, tension within tissues mediated by Cadherin based cell-cell adhesion promotes fibronectin matrix assembly [72], and this matrix is found along the somitic boundaries [64]. During somite border morphogenesis, fibronectin matrix assembly is initially inhibited, but Integrin α5 activation by inside-out signaling frees the inhibition [65]. It was discovered that knock-down of ephrin-B2 does not cause a somite defect in wild-type embryos, but intensifies the somite defect in integrin α5 null embryos, and blocks EphA4 activation as well as Itegrin α5 clustering [71]. To assess the role of reverse signaling genetic mosaic experiments were performed in fused somites genetic mutant embryos that lack somite borders and are deficient in EphA4 expression, but display ubiquitous expression of ephrin-B2 [71]. Expression of a C-terminally truncated form of EphA4 (that lacks forward signaling) in these embryos induced somitic borders, Integrin clustering, and fibronectin matrix assembly. These data indicate that ephrin-B2 reverse signaling is sufficient to cause Integrin α5 clustering and FN matrix assembly [71].
4 Reverse Signaling Affecting Cell-Cell Contact and Movement
Recent work indicates that ephrin-B2 over-expression in endothelial cells, increases motility and triggers repeated cycles of actomyosin-dependent cell contraction as well as cell spreading in the absence of receptor (101). In response to soluble recombinant EphB4, cell shape changes were observed, but in a non-repetitive fashion that ceased with ligand internalization [73]. The C-terminal PDZ binding motif of ephrin-B is required for the morphological alterations within the cell, but the downstream and interacting signaling partners are unclear, save ROCK (Rho-associated protein kinase). Cell retraction and membrane blebbing induced by reverse signaling through Ephrin-B2 requires ROCK activation [73]. Support for a role of ROCK downstream of eprhin-B signaling comes from work in Xenopus where the Rho/ROCK pathway is involved in the sorting of EphB2- and ephrin-B1-expressing cell populations in a re-aggregation cell assay in vitro [74]. Rho activity has been shown to be critical for retinal progenitor cell movement in vivo [24, 75] and both these cell adhesion and movement events may be mediated by the scaffold protein Dishevelled (Dsh) (Figure 1). Knock-down of Dsh in retinal progenitor cells inhibited the normal dispersal of these cells away from the midline, resulting in a decreased contribution to the dorsal eye field. Knock-down of Dsh in these progenitor cells produced a similar result, and in non-retinal progenitors prevented ectopic ephrin-B1 expression from dispersing cells to the eye field [75]. Various deletion mutants of Dsh rescued the effects of a Dsh knockdown, but the DEP domain was essential for normal movement of ephrin-B1 expressing cells. Moreover, the DEP domain was critical for the interaction with the ephrin-B1 C-terminus, leading to localization of Dsh at the cell membrane, a hallmark of the activated planar cell polarity (PCP) pathway. Using knock-down and epistasis-like experiments, it was revealed that downstream PCP components contribute to ephrinB1-mediated cell dispersal into the eye field [75]. It had been shown previously that in early embryogenesis an activated FGF receptor represses cell movement into the eye field by restricting cells near the midline [29]. Using similar knock-down and rescue experiments in retinal progenitor cells, the FGF receptor-induced repression of cell movement was observed to be dependent upon phosphorylation within the intracellular domain of ephrinB1 [24]. Using phospho- specific antibodies and tyrosine mutants in ephrin-B1, it was determined that specific tyrosines within the C-terminus ephrin-B1 disrupt the ephrinB1/Dsh interaction, thus modulating retinal progenitor movement that is dependent on the PCP pathway [24].
One process that mechanistically remains elusive is how EphB/ephrin-B interactions cause a switch between adhesion and repulsion. During cell contact and subsequent retraction of cells and neuronal growth cones, EphB–ephrin-B complexes are endocytosed, which is sufficient to promote cell detachment [76]. This event is bi-directional, and in non-neuronal cells shown to be dependent on actin polymerization, which in turn is dependent on Rac signaling within the receptor-expressing cells [77]. Truncated EphB2, lacking the cytoplasmic region, leads to internalization of the EphB2/ephrin-B1 complex into the adjacent ephrin-B1-expressing cell. The reverse direction of trans-endocytosis is observed with a wild-type receptor and truncated ligand. Truncated versions of both EphB2 and ephrin-B1 precludes internalization and enhances cell adhesion [76]. It also appears that endocytosis of ephrin-Bs can regulate other receptor signaling pathways. For example, endothelial ephrin-B2 can serve as a regulator of VEGFR2 and VEGFR3 endocytosis in cultured cells or mutant mice, and the PDZ binding motif of ephrin-B is critical in this process [78, 79]. Although endocytosis of both receptor and ligand can affect cell adhesion and repulsion as well as other signaling pathways that might also affect cell segregation, the mechanism that links ephrin-B endocytosis to the regulation of the downstream signaling pathways, such as actin remodeling and Rac activity will require further studies.
Ephrin-B reverse signaling also affects cell-substrate adhesion (Figure 1), as observed when EphB1/Fc induces ephrin-B1 tyrosine phosphorylation and migration of endothelial cells. EphB1/Fc promotes integrin-mediated attachment and neovascularization in a mouse corneal micropocket assay, ex vivo [80]. A recent study examined the role of EphB/ephrinBs in mesenchymal stem cells (MSC) that are central to skeletal tissue homeostasis and represent a major source of osteogenic progenitor cells. Ex vivo human MSC populations express EphBs and ephrin-Bs, and reverse ephrin-B signaling was shown to inhibit MSC attachment and spreading. This inhibition occurred in part, by activating Src-, PI3 Kinase- and JNK-dependent signaling pathways [81]. It was previously shown that over-expression of ephrin-B1 in Hek 293 cells resulted in JNK activation and cell rounding, but did not require the C-terminal 33 amino acids of ephrin-B1 nor tyrosine phosphorylation [82].
Although JNK activation is a downstream event in ephrin-B reverse signaling [80], its precise role in cell-cell and cell-substrate modulation is not yet clear.
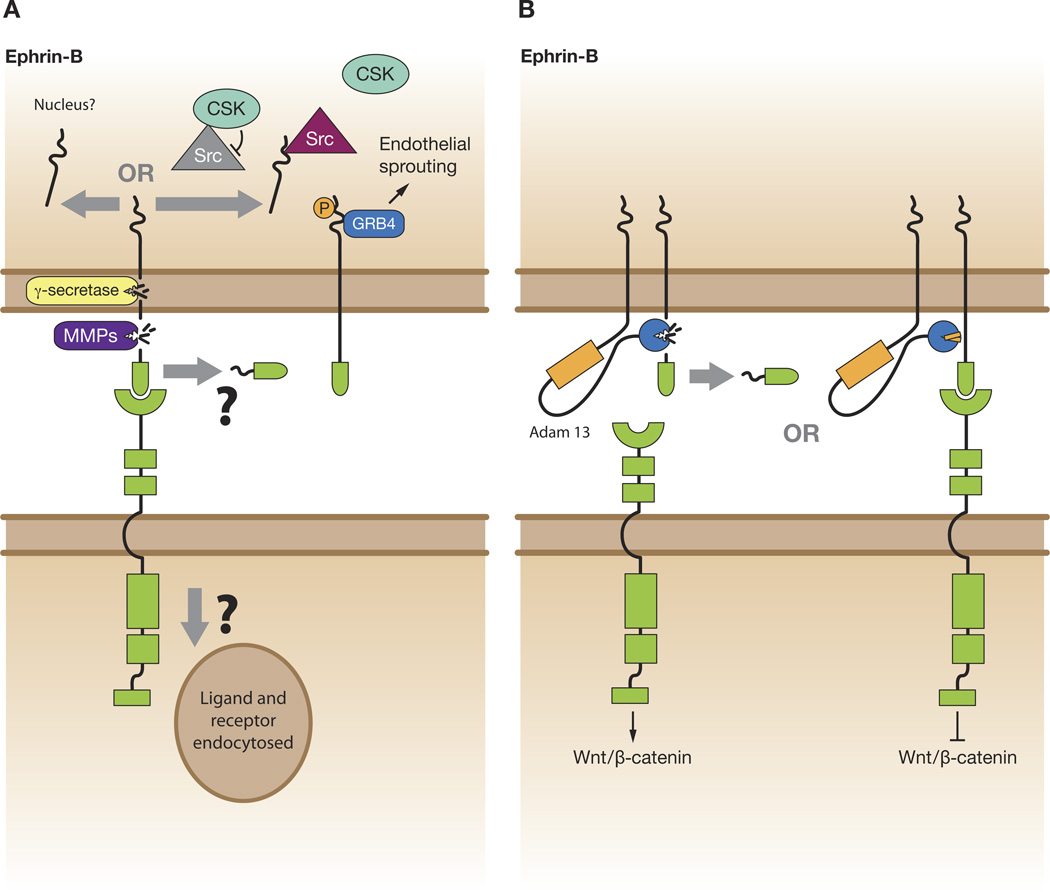
An alternative strategy to accomplish ephrin-B reverse signaling may be through cleavage and release of the ligand intracellular domain (Figure 5). It has been reported that ephrin-B1 can be cleaved by matrix metalloproteases (MMPs) and gamma-secretase in a sequential manner. The free C-terminal fragment can then localize to the nucleus under conditions where the proteasome system is inhibited [83], however, the functional significance of this event remains to be determined (Figure 5A). Ephrin-B2 has also been shown to be proteolytically processed by MMPs and presenilin 1/-gamma secretase to release a C-terminal fragment. The free fragment then binds to the Src kinase and inhibits its association with inhibitory kinase Csk, and allows Src autophosphorylation. Src, in turn, phosphorylates ephrin-B2 and inhibits its cleavage by gamma-secretase (Figure 5A). Thus, this may represent a regulatory system where gamma-secretase is required for EphB-induced reverse signaling through ephrin-B2 that regulates endothelial cell sprouting [84]. An intriguing study in Xenopus provides in vivo evidence for ephrin-B1 cleavage during development [85]. In this study, ephrin-B1 and ephrin-B2 are cleaved by ADAM13, which is expressed in the mesoderm during gastrulation (Figure 5B). The cleavage of ephrin-Bs leads to a marked increase in canonical Wnt signaling (Figure 5B) and the expression of the early neural crest marker snail2 [85]. It was shown in other studies that ROCK and the Wnt/PCP pathway are activated through Dishevelled upon engagement of forward signaling of the EphB receptors [74], and that in the absence of receptor binding, ephrin-B1 can also activate Wnt/PCP signaling cell autonomously by recruiting Dishevelled to the plasma membrane [24]. Thus, it may be possible that the processing of ephrin-Bs by ADAM13 shifts Wnt signaling towards the canonical Wnt/Beta-catenin pathway at the expense of the Wnt/PCP pathway, since the canonical and non-canonical Wnt pathways are known to antagonize one another [86].
Figure 5.
Ephrin-B cleavage and signaling. A) Upon bind the EphB receptor, ephrin-B may be proteolytically processed by MMPs and presenilin 1/-gamma secretase to release a C-terminal fragment. The free fragment may translocate to the nucleus to perform a yet unknown function, or possibly the fragment binds to the Src kinase and inhibits its association with inhibitory kinase Csk, and allows Src autophosphorylation. Src, in turn, phosphorylates ephrin-B2, which can recruit certain signaling partners, such as Grb4, leading to functional effects. B) Unliganded ephrin-Bs may be cleaved by ADAM13 leading to an increase in canonical Wnt signaling through the cognate EphB receptor. Upon binding to the EphB receptor, ephrin-Bs may be resistant to ADAM13 cleavage, thus activating non-canonical Wnt/PCP pathway (not pictured) at the expense of Wnt/beta-catenin signaling.
5 Outstanding Issues
Significant gains have been made in our understanding of the mechanisms of action and regulation of ephrin-A and -B reverse signaling in cell adhesive events, but further studies are required. For A-type reverse signaling, it is still unclear how a GPI-linked ligand tranduces a signal, and what mechanism and molecules are engaged proximally that lead to the reported downstream effects. Although ephrin-Bs are more thoroughly studied in this regard, many interesting questions remain. How does loss of ephrin-B1 disrupt tight junction formation? Knock-down of ephrin-B1 Xenopus embryonic ectoderm, or loss of ephrin-B1 in intestinal epithelia of the mouse shows a substantial reduction of tight junction assembly [49, 87]. We hypothesized a model (Figure 2) where loss of ephrin-B1 may result in more accessible Par-6 along the baso-lateral borders of the cell, which may allow Par-6 at these locations to compete for an interaction with Cdc42-GTP [24]. Thus, effectively displacing a portion of the Cdc42-GTP from the apical junction region where an interaction with aPKC is required for tight junction formation (Figure 2). Alternatively, ephrinB1 may regulate cell-cell adhesion independently from the Par complex, and its loss prevents an interaction with another unknown partner protein that is critical for adherens junction formation or maintenance. Consequently, loss of ephrin-B1 may affect adherens junction or even gap junction formation, leading to disruption of cell-cell adhesion (Figure 2).
Ephrin-B1 may regulate adherens junctions (AJs) to affect cell-cell adhesion, but by what mechanism? AJs are multi-protein complexes that mediate homotypic cell adhesion and Eph signaling has recently been shown to regulate AJ complexes [88], and Cdc42 has been linked to AJ stability. Cdc42 has been reported to function with Par-6 and aPKC to regulate E-Cadherin endocytosis through the Arp2/3 complex to maintain AJ stability [89, 90]. The Par complex has also been identified as a Cdc42 effector that regulates endocytosis of apical molecules, such as Crumbs, that play a crucial role in stabilizing AJs located along the basolateral surface of the cell [91]. In contrast to the ephrin-B1 directed regulation of the activated Cdc42 interaction with Par-6 observed in epithelia TJs, it is ephrin-B2-induced suppression of Cdc42 activity that drives somitic epithelialization [67]. Significantly more work is necessary to begin to understand the cell contexts, parameters, and mechanisms that are responsible for differential signaling from B-type ligands.
There is also the uncertainty of how the switch mechanism between adhesion and repulsion can actually initiate an instructive repulsive signal? Endocytosis of ligands and receptors can either send signals by clustering within vesicles or by termination of forward and reverse signals through disengagement of ligand/receptor signaling? What is the mechanism and parameters that determine these effects?
Does contact of ephrin-B1 with the EphB receptor activate the PCP pathway by clustering Dsh to promote the directed migration of ephrin-B-expressing cells entering Eph-expressing territory? Alternatively, does an ephrin B1/Eph interaction block the PCP pathway merely by preventing Dsh binding or clustering ? A recent report [93] supporting this concept shows phosphorylation of ephrin-B1 disengages Dsh from ephrin-B1 and restricts the movement of cells into the eye field [29, 93]. Is it also possible that the Eph receptor blocks ephrin-B1-induced PCP activation by causing the Eph/ephrin complex along with Dsh to be endocytosed? Therefore, inactivation of the PCP pathway at interfaces of Eph and ephrin expression may abrogate Wnt/PCP directed cell migration, and assist in the restriction of cell intermingling between tissues [94]. What protein (s) mediates the interaction with Dsh? Is Grb4 involved, or perhaps Par-6, which was recently demonstrated to recruit Smurf via Dvl to regulate the PCP pathway [92]?
Although much of the emphasis has been placed upon understanding the signaling events that occur when an Eph receptor-bearing cell contacts a ligand-bearing cell, remarkably little information is known about the inhibition or modulation of signaling that occurs when both ligand and receptor interact in cis. Clearly, there is still much to consider and many more studies to perform.
Acknowledgement
I wish to apologize to all of my colleagues whose work was not cited in this review. Many have contributed greatly to our understanding of the role of the Eph–ephrin system in biology, but space considerations prevented the inclusion of their work.
Abbreviations used in this paper
- Eph
Erythropoietin producing hepatoma
- ephrin
erythropoietin producing hepatoma interactor
- FGF
fibroblast growth factor
- Par
Partitioning defective
- PDZ
PSD-95/Dlg/ZO-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 2.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irie F, Okuno M, Pasquale EB, Yamaguchi Y. EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nat Cell Biol. 2005;7:501–509. doi: 10.1038/ncb1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–650. doi: 10.1016/j.yexcr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Merlos-Suarez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol. 2008;20:194–200. doi: 10.1016/j.ceb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Castano J, Davalos V, Schwartz S, Jr, Arango D. EPH receptors in cancer. Histol Histopathol. 2008;23:1011–1023. doi: 10.14670/HH-23.1011. [DOI] [PubMed] [Google Scholar]
- 8.Vaught D, Chen J, Brantley-Sieders DM. Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol Biol Cell. 2009;20:2572–2581. doi: 10.1091/mbc.E08-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Holmberg J, Clarke DL, Frisen J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–206. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- 11.Kikawa KD, Vidale DR, Van Etten RL, Kinch MS. Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J Biol Chem. 2002;277:39274–3929. doi: 10.1074/jbc.M207127200. [DOI] [PubMed] [Google Scholar]
- 12.Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- 13.Huai J, Drescher U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J Biol Chem. 2001;276:6689–6694. doi: 10.1074/jbc.M008127200. [DOI] [PubMed] [Google Scholar]
- 14.Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, et al. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davy A, Robbins SM. Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J. 2000;19:5396–5405. doi: 10.1093/emboj/19.20.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ting MJ, Day BW, Spanevello MD, Boyd AW. Activation of ephrin A proteins influences hematopoietic stem cell adhesion and trafficking patterns. Exp Hematol. 38:1087–1098. doi: 10.1016/j.exphem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Trinidad EM, Ballesteros M, Zuloaga J, Zapata A, Alonso-Colmenar LM. An impaired transendothelial migration potential of chronic lymphocytic leukemia (CLL) cells can be linked to ephrin-A4 expression. Blood. 2009;114:5081–5090. doi: 10.1182/blood-2009-03-210617. [DOI] [PubMed] [Google Scholar]
- 18.Ting MC, Wu NL, Roybal PG, Sun J, Liu L, Yen Y, et al. EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development. 2009;136:855–864. doi: 10.1242/dev.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, et al. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell. 2007;129:359–370. doi: 10.1016/j.cell.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Holen HL, Shadidi M, Narvhus K, Kjosnes O, Tierens A, Aasheim HC. Signaling through ephrin-A ligand leads to activation of Src-family kinases, Akt phosphorylation, and inhibition of antigen receptor-induced apoptosis. J Leukoc Biol. 2008;84:1183–1191. doi: 10.1189/jlb.1207829. [DOI] [PubMed] [Google Scholar]
- 21.Lim BK, Matsuda N, Poo MM. Ephrin-B reverse signaling promotes structural and functional synaptic maturation in vivo. Nat Neurosci. 2008;11:160–169. doi: 10.1038/nn2033. [DOI] [PubMed] [Google Scholar]
- 22.Janes PW, Wimmer-Kleikamp SH, Frangakis AS, Treble K, Griesshaber B, Sabet O, et al. Cytoplasmic relaxation of active Eph controls ephrin shedding by ADAM10. PLoS Biol. 2009;7:e1000215. doi: 10.1371/journal.pbio.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, et al. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Lee HS, Daar IO. EphrinB reverse signaling in cell-cell adhesion: is it just par for the course? Cell Adh Migr. 2009;3:250–255. doi: 10.4161/cam.3.3.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dravis C, Henkemeyer M. Ephrin-B reverse signaling controls septation events at the embryonic midline through separate tyrosine phosphorylation-independent signaling avenues. Dev Biol. doi: 10.1016/j.ydbio.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 27.Bruckner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- 28.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore KB, Mood K, Daar IO, Moody SA. Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev Cell. 2004;6:55–67. doi: 10.1016/s1534-5807(03)00395-2. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Kamata R, Sakai R. Phosphorylation of ephrin-B1 via the interaction with claudin following cell-cell contact formation. EMBO J. 2005;24:3700–3711. doi: 10.1038/sj.emboj.7600831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Q, Sun EE, Klein RS, Flanagan JG. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 32.Qiu R, Wang X, Davy A, Wu C, Murai K, Zhang H, et al. Regulation of neural progenitor cell state by ephrin-B. J Cell Biol. 2008;181:973–983. doi: 10.1083/jcb.200708091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C, Qiu R, Wang J, Zhang H, Murai K, Lu Q. ZHX2 Interacts with Ephrin-B and regulates neural progenitor maintenance in the developing cerebral cortex. J Neurosci. 2009;29:7404–7412. doi: 10.1523/JNEUROSCI.5841-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClelland AC, Sheffler-Collins SI, Kayser MS, Dalva MB. Ephrin-B1 and ephrin-B2 mediate EphB-dependent presynaptic development via syntenin-1. Proc Natl Acad Sci U S A. 2009;106:20487–20492. doi: 10.1073/pnas.0811862106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin D, Gish GD, Songyang Z, Pawson T. The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J Biol Chem. 1999;274:3726–3733. doi: 10.1074/jbc.274.6.3726. [DOI] [PubMed] [Google Scholar]
- 36.Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 37.Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–179. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- 38.Bong YS, Park YH, Lee HS, Mood K, Ishimura A, Daar IO. Tyr-298 in ephrinB1 is critical for an interaction with the Grb4 adaptor protein. Biochem J. 2004;377:499–507. doi: 10.1042/BJ20031449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segura I, Essmann CL, Weinges S, Acker-Palmer A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci. 2007;10:301–310. doi: 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- 40.Bong YS, Lee HS, Carim-Todd L, Mood K, Nishanian TG, Tessarollo L, et al. ephrinB1 signals from the cell surface to the nucleus by recruitment of STAT3. Proc Natl Acad Sci U S A. 2007;104:17305–17310. doi: 10.1073/pnas.0702337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jorgensen C, Sherman A, Chen GI, Pasculescu A, Poliakov A, Hsiung M, et al. Cell-specific information processing in segregating populations of Eph receptor ephrin-expressing cells. Science. 2009;326:1502–1509. doi: 10.1126/science.1176615. [DOI] [PubMed] [Google Scholar]
- 42.Salvucci O, Maric D, Economopoulou M, Sakakibara S, Merlin S, Follenzi A, et al. EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood. 2009;114:1707–1716. doi: 10.1182/blood-2008-12-192294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng DC, Lin BH, Lim CP, Huang G, Zhang T, Poli V, et al. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol. 2006;172:245–257. doi: 10.1083/jcb.200503021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones TL, Chong LD, Kim J, Xu RH, Kung HF, Daar IO. Loss of cell adhesion in Xenopus laevis embryos mediated by the cytoplasmic domain of XLerk, an erythropoietin-producing hepatocellular ligand. Proc Natl Acad Sci U S A. 1998;95:576–581. doi: 10.1073/pnas.95.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 48.Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee HS, Nishanian TG, Mood K, Bong YS, Daar IO. EphrinB1 controls cell-cell junctions through the Par polarity complex. Nat Cell Biol. 2008;10:979–986. doi: 10.1038/ncb1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, et al. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 51.Wang Q, Margolis B. Apical junctional complexes and cell polarity. Kidney Int. 2007;72:1448–1458. doi: 10.1038/sj.ki.5002579. [DOI] [PubMed] [Google Scholar]
- 52.Chong LD, Park EK, Latimer E, Friesel R, Daar IO. Fibroblast growth factor receptor-mediated rescue of x-ephrin B1-induced cell dissociation in Xenopus embryos. Mol Cell Biol. 2000;20:724–734. doi: 10.1128/mcb.20.2.724-734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mellitzer G, Xu Q, Wilkinson DG. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- 54.Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rohani N, Canty L, Luu O, Fagotto F, Winklbauer R. EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 9:e1000597. doi: 10.1371/journal.pbio.1000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Q, Alldus G, Holder N, Wilkinson DG. Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hindbrain. Development. 1995;121:4005–4016. doi: 10.1242/dev.121.12.4005. [DOI] [PubMed] [Google Scholar]
- 57.Xu Q, Alldus G, Macdonald R, Wilkinson DG, Holder N. Function of the Eph-related kinase rtk1 in patterning of the zebrafish forebrain. Nature. 1996;381:319–322. doi: 10.1038/381319a0. [DOI] [PubMed] [Google Scholar]
- 58.Kemp HA, Cooke JE, Moens CB. EphA4 and EfnB2a maintain rhombomere coherence by independently regulating intercalation of progenitor cells in the zebrafish neural keel. Dev Biol. 2009;327:313–326. doi: 10.1016/j.ydbio.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Twigg SR, Kan R, Babbs C, Bochukova EG, Robertson SP, Wall SA, et al. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc Natl Acad Sci U S A. 2004;101:8652–8657. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bush JO, Soriano P. Ephrin-B1 forward signaling regulates craniofacial morphogenesis by controlling cell proliferation across Eph-ephrin boundaries. Genes Dev. 24:2068–2080. doi: 10.1101/gad.1963210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.San Miguel S, Serrano MJ, Sachar A, Henkemeyer M, Svoboda KK, Benson MD. Ephrin reverse signaling controls palate fusion via a PI3 kinase-dependent mechanism. Dev Dyn. 240:357–364. doi: 10.1002/dvdy.22546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tepass U, Godt D, Winklbauer R. Cell sorting in animal development: signalling and adhesive mechanisms in the formation of tissue boundaries. Curr Opin Genet Dev. 2002;12:572–582. doi: 10.1016/s0959-437x(02)00342-8. [DOI] [PubMed] [Google Scholar]
- 63.Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell. 2003;14:3065–3081. doi: 10.1091/mbc.E02-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holley SA. The genetics and embryology of zebrafish metamerism. Dev Dyn. 2007;236:1422–1449. doi: 10.1002/dvdy.21162. [DOI] [PubMed] [Google Scholar]
- 65.Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, et al. Integrinalpha5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev Cell. 2005;8:587–598. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 66.Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol. 2003;13:1571–1582. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe T, Sato Y, Saito D, Tadokoro R, Takahashi Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc Natl Acad Sci U S A. 2009;106:7467–7472. doi: 10.1073/pnas.0902859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu X, Li S, Chrostek-Grashoff A, Czuchra A, Meyer H, Yurchenco PD, et al. Cdc42 is crucial for the establishment of epithelial polarity during early mammalian development. Dev Dyn. 2007;236:2767–2778. doi: 10.1002/dvdy.21309. [DOI] [PubMed] [Google Scholar]
- 69.Samarin S, Nusrat A. Regulation of epithelial apical junctional complex by Rho family GTPases. Front Biosci. 2009;14:1129–1142. doi: 10.2741/3298. [DOI] [PubMed] [Google Scholar]
- 70.Warner SJ, Longmore GD. Distinct functions for Rho1 in maintaining adherens junctions and apical tension in remodeling epithelia. J Cell Biol. 2009;185:1111–1125. doi: 10.1083/jcb.200901029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Julich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- 72.Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev Cell. 2009;16:421–432. doi: 10.1016/j.devcel.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bochenek ML, Dickinson S, Astin JW, Adams RH, Nobes CD. Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. J Cell Sci. 123:1235–1246. doi: 10.1242/jcs.061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanaka M, Kamo T, Ota S, Sugimura H. Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J. 2003;22:847–858. doi: 10.1093/emboj/cdg088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee HS, Bong YS, Moore KB, Soria K, Moody SA, Daar IO. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat Cell Biol. 2006;8:55–63. doi: 10.1038/ncb1344. [DOI] [PubMed] [Google Scholar]
- 76.Zimmer M, Palmer A, Kohler J, Klein R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- 77.Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 78.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 80.Huynh-Do U, Vindis C, Liu H, Cerretti DP, McGrew JT, Enriquez M, et al. Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J Cell Sci. 2002;115:3073–3081. doi: 10.1242/jcs.115.15.3073. [DOI] [PubMed] [Google Scholar]
- 81.Arthur A, Zannettino A, Panagopoulos R, Koblar SA, Sims NA, Stylianou C, et al. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 48:533–542. doi: 10.1016/j.bone.2010.10.180. [DOI] [PubMed] [Google Scholar]
- 82.Xu Z, Lai KO, Zhou HM, Lin SC, Ip NY. Ephrin-B1 reverse signaling activates JNK through a novel mechanism that is independent of tyrosine phosphorylation. J Biol Chem. 2003;278:24767–24775. doi: 10.1074/jbc.M302454200. [DOI] [PubMed] [Google Scholar]
- 83.Tomita T, Tanaka S, Morohashi Y, Iwatsubo T. Presenilin-dependent intramembrane cleavage of ephrin-B1. Mol Neurodegener. 2006;1:2. doi: 10.1186/1750-1326-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, et al. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei S, Xu G, Bridges LC, Williams P, White JM, DeSimone DW. ADAM13 induces cranial neural crest by cleaving class B Ephrins and regulating Wnt signaling. Dev Cell. 19:345–352. doi: 10.1016/j.devcel.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weidinger G, Moon RT. When Wnts antagonize Wnts. J Cell Biol. 2003;162:753–755. doi: 10.1083/jcb.200307181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cortina C, Palomo-Ponce S, Iglesias M, Fernandez-Masip JL, Vivancos A, Whissell G, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- 88.Cooper MA, Son AI, Komlos D, Sun Y, Kleiman NJ, Zhou R. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc Natl Acad Sci U S A. 2008;105:16620–16665. doi: 10.1073/pnas.0808987105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 90.Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol. 2008;18:1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 91.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 93.Lee HS, Mood K, Battu G, Ji YJ, Singh A, Daar IO. Fibroblast growth factor receptor-induced phosphorylation of ephrinB1 modulates its interaction with Dishevelled. Mol Biol Cell. 2009;20:124–133. doi: 10.1091/mbc.E08-06-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Poliakov A, Wilkinson DG. Ephrins make eyes with planar cell polarity. Nat Cell Biol. 2006;8:7–8. doi: 10.1038/ncb0106-7. [DOI] [PubMed] [Google Scholar]