Abstract
Aging represents a major risk factor for prostate cancer; however, mechanisms responsible for this relationship remain unclear. Preclinical and some clinical investigations support the protective role of selenium against prostate cancer possibly through the reduction of oxidative stress. While increased levels of oxidative stress together with decreases in selenium and the major cellular antioxidant glutathione (GSH) are common in tissues of old animals, there is little data available on these parameters in the prostate. In the present study we have compared the levels of selenium, GSH and protein-bound GSH (GSSP) in blood and prostate tissues in young (4-month), mature (12-month), old (18 month), and very old (24 month) male F344 rats. Each prostate lobe (dorsolateral, DL; anterior, AL; ventral, VL) was analyzed separately based upon their differing potential for prostate cancer development. At all ages, selenium levels were lowest in DL<VL<AL. After 12 mo, an 85% reduction in selenium in the DL was observed (P<0.05), while levels in other lobes were unchanged. In animals of all ages, levels of GSH were lowest in the VL<DL=AL and no significant changes were observed in GSH levels by 18 mo. However, GSSP, a marker of oxidative stress, was increased 90% after 18 mo in the DL only (P<0.01). These findings of age-related changes in GSSP and selenium in the DL prostate are consistent with the sensitivity of this lobe to carcinogenesis and, thus, may be playing a mechanistic role.
Keywords: aging, prostate, rats, selenium, glutathione, oxidative stress, cancer
1. Introduction
Prostate cancer (PC) is most commonly diagnosed cancer among elderly American men and second leading cause of cancer death in men (Jemal et al., 2009). While androgens and dietary factors play a critical role in the development of prostate cancer, aging represents the strongest risk factor for PC as well as benign prostatic hypertrophy. The chance of getting PC goes up dramatically by age 50, with over 80% of prostate cancer patients being over 65 years of age (Bracarda et al., 2005). Little is known about the nature of the link between the aging process and prostate cancer development. Numerous aging-related changes have been implicated in the pathogenesis of the disease including impairments in apoptotic pathways (Banerjee et al., 2000) and the accumulation of DNA damage and/or reduction in DNA repair mechanisms (Lockett et al., 2005). In particular, numerous studies have pointed to the importance of enhanced inflammatory processes (De Marzo et al., 1999) and increases in oxidative stress (Khandrika et al., 2009) as critical factors in prostate carcinogenesis (De Marzo et al., 1999; Hamid et al., ; Khandrika et al., 2009).
Aging is characterized by an increase in intracellular oxidative stress due, in part, to progressive decreases of intracellular antioxidant systems. The generation of reactive oxygen species (ROS) is thought to play an essential role in the aging process and lies at the heart of many of the most accepted theories of aging over the past 40 years (Harman, 1956; Muller et al., 2007; Sohal and Weindruch, 1996). Enhanced levels of oxidative stress have also been linked to the generation of most aging-related diseases including prostate cancer (Khandrika et al., 2009; Minelli et al., 2009). Increased oxidative damage to cellular macromolecules in the prostate has been observed in aging (Ghatak and Ho, 1996) as well as during the development prostatic malignancy (Bostwick et al., 2000; Oberley et al., 2000). In general, the prostate exhibits substantially higher levels of lipofuscin, a product of oxidative damage to cellular membranes, than other human tissues (Morrissey et al., 2002). Increases in oxidative damage to DNA, measured by the accumulation of nuclear 8-hydroxydeoxyguanosine (8-OHdG) have been observed in aging prostate tissues (Malins et al., 2001). Finally, a role for oxidative stress in prostate cancer is supported by observations foods high in antioxidants such as fruits and vegetables are protective (Donkena et al.).
Glutathione (GSH) is the most abundant antioxidant in cells and tissues, and plays a primary role in protection against oxidative stress (Sies, 1999). GSH is also an important substrate for the conjugation and detoxification of known toxins and carcinogens (Coles and Ketterer, 1990) and plays a critical role in the maintenance of immune function (Hamilos and Wedner, 1985). During aging, GSH levels are depleted, in part, due to a shift in redox state to more oxidized forms including glutathione disulfide (GSSG) and glutathione protein mixed disulfides (GSSP) (Richie, 1992; Thomas and Mallis, 2001). GSH depletion has been identified as an important factor in the aging process occurring as a general phenomenon in tissues and blood of aging organisms concurrently with enhancements in oxidative stress-related damage. Because of the many protective roles of GSH in carcinogenesis (Townsend et al., 2003) it has been suggested that GSH depletion during aging may be, in part, responsible for enhanced risk for cancer (Richie, 1992).
Numerous epidemiological and laboratory investigations showed that dietary selenium is protective against the development of cancer at many sites including prostate (El-Bayoumy, 2001; El-Bayoumy and Sinha, 2005). Furthermore, some clinical chemoprevention trials provide support of the protective role of selenium against cancer development including prostate cancer (Clark et al., 1996; Facompre and El-Bayoumy, 2009). While the mechanisms of cancer prevention by selenium remain unclear, a reduction in oxidative stress and its related damage may be involved (Brenneisen et al., 2005; Klaunig and Kamendulis, 2004). Selenium has numerous antioxidant-like activities in biological systems including the protection against oxidative stress through the selenoemzymes glutathione peroxidase, which is primarily responsible for the intracellular reduction of hydrogen peroxide and lipid peroxides, and thioredoxin reductase (Brigelius-Flohe and Flohe, 2003; Ganther, 1999). Both clinical and preclinical studies have indicated that depending on the form, selenium supplementation can enhance the levels of intracellular GSH. The organoselenium chemopreventive agent 1,4-phenylenebis(methylene)selenocyanate (p-XSC) enhanced GSH levels while inhibiting carcinogen-induced lung carcinogenesis in A/J mice (Richie et al., 2006). p-XSC also decreased levels of GSSP, consistent with a decrease in levels of oxidative stress (Giustarini et al., 2004; Muscat et al., 2004). Increased levels of GSH and decreased levels of GSSP were observed in healthy adults supplemented for 9 months with selenium-enriched yeast (247 μg/d) (El-Bayoumy et al., 2002) and were associated with higher selenium levels in un-supplemented adults (Richie et al., 2011). Finally, as with GSH, blood selenium levels have been observed to decrease during aging in a number of clinical investigations (Masse, 1995; Ray et al., 2006; Simonoff et al., 1992).
In the present study we hypothesized that an aging-related decline in selenium and alteration of GSH redox status in prostate tissues may be responsible, in part, for enhanced levels of oxidative stress and, thus, play at role in prostate cancer development. To study this, we have measured the levels of selenium, GSH and GSSP in different prostate lobes of the aging F-344 rat, a commonly used model for aging which develops spontaneous prostate carcinomas (Conn, 2006; Reznik et al., 1981).
2. Materials and Methods
2.1. Animals
Male F344 rats were obtained from Harlan at 4, 12, 18 and 24 months of age representing young, mature, old and very old age groups, respectively, based on survival characteristics for this strain of rat. All rats were housed in solid bottom polycarbonate cages with corn cob bedding under standardized conditions (22 + 2° C; 50 ± 10 % relative humidity; 12 h light-dark cycle). Animals were fed Harlan Teklad 2014 pellet diet with no additional selenium supplementation. The amount of selenium in this diet is 0.23 mg/kg.
2.2. Tissue harvesting
Six rats per age group were sacrificed by CO2 asphyxiation and cardiac exsanguination. Blood was collected by cardiac puncture into tubes containing EDTA as an anticoagulant. After removal of an aliquot (100 μl) of whole blood for analysis of GSH and GSSP, the remaining fraction was centrifuged at 2,000 g for 5 min and plasma and red cell fractions were aliquoted and stored at -80°C until analysis. Prostates were removed and dissected to obtain the major lobes as follows: dorsolateral lobe (DL), ventral lobe (VL) and anterior lobe (AL). Prostate tissues were rinsed in ice cold saline (0.9% NaCl), blotted dry and frozen in liquid nitrogen. All samples were kept at −80° C until analyzed.
2.3. Biochemical analyses
2.3.1. Determination of total selenium
Tissues were homogenized and the homogenates and blood serum were digested in a MARS Xpress microwave digestion system (CEM Corp., Mathews, NC) equipped with 55 ml Teflon PFA vessels and a turntable. The digestion was conducted in 50% nitric acid and was diluted to 20% before Se analysis by Atomic Absorption Spectroscopy (AAnalyst 600, PerkinElmer, Waltham, MA). Using a graphite furnace, total selenium was analyzed by measuring the absorbance peak area at 196 nm for each sample. Palladium matrix modifier was added along with each sample to the furnace. A reference standard solution of selenium dioxide at various concentrations range was used to construct standard curves for comparison with all assays. For each group at least three samples were analyzed and the results were expressed as mean + SE (n=3–6).
2.3.2. Glutathione analyses
For the analysis of GSH, tissues were weighed and the homogenates (10%, w/v) were prepared immediately in ice cold 5% (w/v) metaphosphoric acid (MPA) using a Ten-Broeck all glass homogenizer. Blood was processed by treatment with 5% MPA (1:5). All MPA-treated samples were then kept on ice for 5–10 minutes, and centrifuged at 10,000 × g for 5 minutes. The supernatant fractions were removed and stored at 80°C until analysis for GSH by HPLC with electrochemical detection (Kleinman and Richie, 2000).
Acid-insoluble pellets derived from MPA-extracts were used to determine GSSP levels measured as GSH released after reduction with potassium borohydride as described previously (Kleinman et al., 2003). In brief, after washing three times by re-suspension in 5% MPA and centrifugation, the pellets were re-suspended in 8 M urea/1 mM EDTA and incubated for 10 minutes at 40°C. Potassium borohydride was added to a final concentration of 0.38 M and the solution was incubated for 45 minutes at 40°C. A few drops of octanol were added prior to the addition of potassium borohydride to reduce foaming. The solution was precipitated by 20% MPA for 15 minutes at room temperature. The mixture was then centrifuged at 3,000 × g for 15 minutes and the supernatant was stored at 80°C. Released GSH was analyzed as described above.
2.4. Statistical analyses
Summary statistics are provided for outcome measurements for blood and different prostate lobes and age groups. Data are reported as mean ± standard deviation. ANOVA was used to assess for differences between age groups. Tests for linear trend were conducted using median values in each age group as a continuous variable. Differences between groups were considered statistically significant if p < 0.05.
3. Results
3.1. Effect of age on body and organ weights
Body and organ weights are provided for rats of each age group in Table 1. Total body weight increased with age during growth and maturation from 4 to 12 months of age as expected. Thereafter, a progressive decrease in body weight was observed during aging. Conversely, prostate weights continued to increase during aging in each of the lobes. Compared to 12 month mature adults, in 24 month old animals, increases of 20%, 124% and 69% were observed for the AL, VL and DL, respectively.
Table 1.
Total Body and Prostate Lobe Weights in Aging Rats
| Age (mo) | Weight (g)
|
|||
|---|---|---|---|---|
| Total Body | Prostate Lobe
|
|||
| Anterior | Ventral | Dorsolateral | ||
| 4 | 302 ± 5.2 | 0.086 ± 0.017 | 0.281 ± 0.024 | 0.173 ± 0.025 |
| 12 | 468 ± 7.6a | 0.143 ± 0.013 | 0.257± 0.046 | 0.296 ± 0.006 |
| 18 | 397 ± 19.3 a,b | 0.108 ± 0.006 | 0.320 ± 0.015 | 0.284 ± 0.039 |
| 24 | 403 ± 9.8 a,b | 0.171 ± 0.018 a | 0.576 ± 0.078 a,b | 0.501 ± 0.099 a |
Values are mean ± SE (n=3)
significantly different from 4 mo age group, P<0.05
significantly different from 12 mo age group, P<0.05
3.2. Effect of age on prostate and plasma selenium
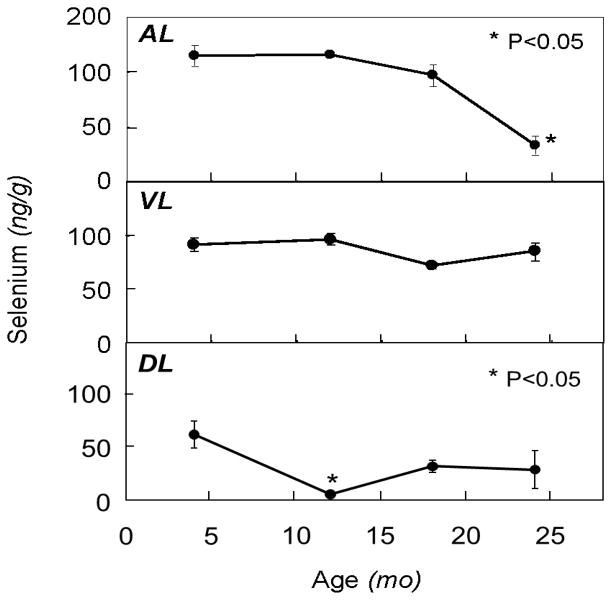
The effects of aging on levels of selenium in the different prostate lobes are summarized in Figure 1. Selenium levels were highest in the AL > VL > DL for all age groups except the very old (24 month). In the AL, selenium levels were decreased by 16% and 71% in the old and very old groups, respectively, compared with the young, with only the very old group being statistically significant (P<0.05). In the DL, selenium levels were decreased compared to the young rats by 87%, 48% and 46% in the mature, old and very old groups, respectively, with only the differences in the mature group being significant (P<0.05). No significant changes were observed in the VL.
Figure 1.
Effect of aging on levels of selenium in different prostate lobes of the rat. Rats were sacrificed at 4, 12, 18 and 24 months of age and prostate lobes (AL, anterior; VL, ventral; DL, dorsolateral) were removed and analyzed for selenium content as described in text. Points and error bars represent mean ± SEM (n=3–6). Asterisks reflect statistical significance compared to the young group (P<0.05).
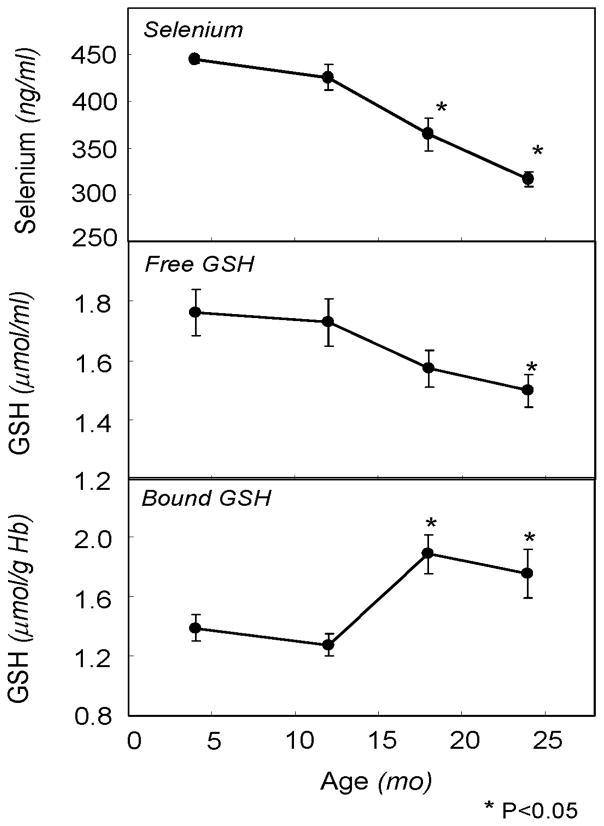
The effect of aging on plasma levels of selenium are shown in Figure 2 (top panel). A progressive decrease in selenium levels with increasing age was observed such that, compared to 4 month old rats, selenium levels were decreased 19% and 29% in 18 and 24 month old rats, respectively (P<0.05).
Figure 2.
Lifespan profiles of blood selenium and free and protein-bound glutathione levels in aging rats. Rats were sacrificed at 4, 12, 18 and 24 months of age. Plasma selenium levels and whole blood free and bound glutathione levels were measured as described in text. Points and error bars represent mean ± SEM (n=3–6). Asterisks reflect statistical significance compared to the young group (P<0.05).
3.3. Effect of age on blood and prostate glutathione
The effects of aging on free and protein-bound glutathione in blood are described in Figure 2. A progressive decrease in GSH levels was observed across age groups (Ptrend<0.05), with a 16% difference between the young to the very old age group being statistically significant (P<0.05). Levels of protein-bound GSH were increased from 29% to 44% in the two oldest age groups compared to both the young group (P<0.05).
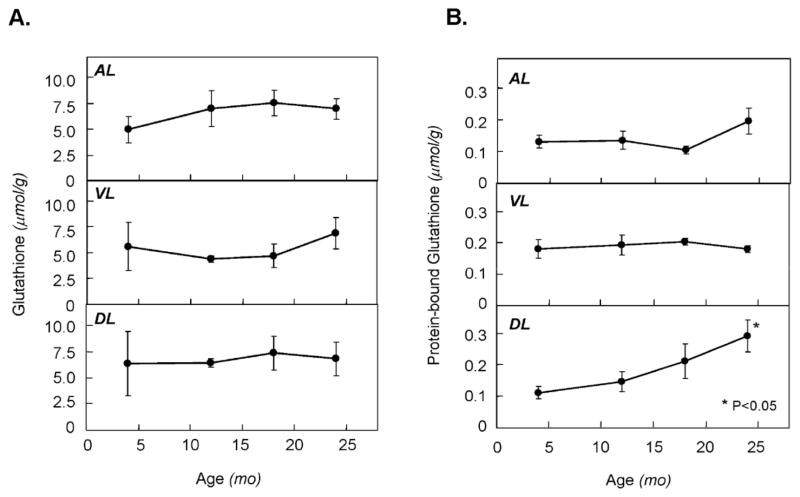
The effects of aging on levels of free glutathione in different lobes of the prostate are summarized in Figure 3A. Overall, GSH levels in the prostate ranged between 4 and 8μmol/g wet weight and, at each age were similar in AL and DL. While mean GSH levels tended to be 30–40% lower in the VL compared to the DL and AL for ages 4–18 months, these differences were not statistically significant. No significant aging differences for GSH were observed in any of the lobes examined. Protein-bound GSH represented about 1–5% of total GSH in all tissues with levels being similar across lobes (Figure 3B). A progressive and aging-specific increase in GSSP levels was observed in the DL (Ptrend<0.05) with the >2-fold increase at 24 months compared to young and mature groups being significant (P<0.05). No significant aging related changes were observed in either AL or VL.
Figure 3.
Effect of aging on levels of free and protein-bound glutathione in different prostate lobes of the rat. Rats were sacrificed at 4, 12, 18 and 24 months of age and prostate lobes (AL, anterior; VL, ventral; DL, dorsolateral) were removed and analyzed for free glutathione (A) and protein-bound glutathione (B) content as described in text. Points and error bars represent mean ± SEM (n=3). Asterisks reflect statistical significance compared to the young group (P<0.05).
4. Discussion
Our results indicate that aging is associated with a decrease in selenium content and increase in levels of GSSP specifically in the DL lobe of the rat. These observations are consistent with previous findings that biomarkers of oxidative stress were increased during aging specifically in the DL but not VL lobes (Ghatak and Ho, 1996). Aging represents a major risk factor for prostate cancer development and an aging-related enhancement in oxidative stress and its impact on pathways involved in cancer development may be an important mechanism involved. Previous studies have show an accumulation of lipid peroxidation and DNA oxidation markers including 8-OHdG in prosatic tissues during aging (Malins et al., 2001; Morrissey et al., 2002). Observed changes in gene expression in the rat prostate during aging are also consistent with enhanced oxidative stress and inflammation and associated changes in pathways involved in proliferation and apoptosis (Bethel et al., 2009; Reyes et al., 2005). Recent studies have shown that oxidative stress can induce DNA hypomethylation in the prostate (Bhusari et al.) suggesting that aging related inflammatory proliferative atrophy, linked with prostatic hypomethylation may be playing an important role in prostatic carcinogenesis (De Marzo et al., 1999).
GSH is the major endogenous antioxidant in cells and tissues and, consequently, plays a critical role in protecting against oxidative stress. After exposure to free radicals and reactive oxygen species, GSH can become oxidized to its disulfide GSSG. While, GSSG levels normally remain low due to its conversion back to GSH by glutathione reductase, transient high levels of GSSG can occur during periods of oxidative stress (Giustarini et al., 2003; Muscat et al., 2004). Another product of GSH oxidation, GSSP, forms from either the direct reaction of glutathionyl radicals with free Cys groups in proteins or through thiol disulfide exchange with GSSG (Dalle-Donne et al., 2009). Since GSSP levels are increased during periods of oxidative stress and appear to be fairly stable over time, they have been utilized as a sensitive indicator of redox status in blood and tissues (Klatt and Lamas, 2000; Muscat et al., 2004). Further, the formation of GSSP, also known as protein glutathionylation, has recently been recognized as an important redox sensitive posttranslational modification which is directly involved in the regulation of numerous cell functions including proliferation and apoptosis (Dalle-Donne et al., 2009; Mieyal et al., 2008; Tew et al.). Here we show, for the first time, that GSSP levels are elevated with aging in the DL prostate. This finding is not only consistent with previous findings of enhanced levels or oxidative stress in the aging prostate, but may also indicate the involvement of protein glutathionylation-regulated pathways in the mechanisms by which oxidative stress can impact carcinogenesis.
While the mechanisms of increased oxidative stress in the prostate during aging are not known, it does not appear to be a result of decreased levels of GSH. Regardless of lobe, no age related changes were observed in GSH levels. This is in contrast to levels in blood which were significantly decreased during aging as well as to previous results in other tissues, where aging decreases in GSH have been consistently observed (Bagchi et al., 1996; Richie et al., 1994). It is possible that deceases in other antioxidant pathways, e.g. superoxide dismutase, may be responsible. Alternatively, increased production or exposure to ROS and free radicals may also be involved. It was of interest, however, that GSH levels in all lobes of the prostate were high (4–8 mM) compared to most organs in the F-344 rat (which range from 1–3 mM) and were comparable to levels found in the liver (Richie et al., 2004). To our knowledge, there have been few studies reported to date on prostate GSH which could the light on the possible roles for high levels of GSH in this tissue.
Our current results of an aging-related decline in plasma selenium levels are consistent with previous findings in humans where plasma selenium levels are decreased in healthy elderly individuals (Bates et al., 2002; Ducros et al., 1997; Erden-Inal et al., 2002; Olivieri et al., 1994). In a longitudinal study of plasma selenium in elderly men and women, decreased levels during aging were associated with obesity and cardiovascular disease (Arnaud et al., 2007). Further, epidemiological studies have suggested that lower selenium levels are associated with decreased survival in elderly women (Ray et al., 2006). Decreased levels of plasma selenium during aging may be indicative of enhanced susceptibility for prostate cancer in light of studies which associate low blood selenium levels with increased prostate cancer risk (Platz and Helzlsouer, 2001; Pourmand et al., 2008; Vogt et al., 2003).
To our knowledge, this is the first report of the effects of aging on prostate selenium levels. While our results do not show a consistent trend across lobes and age groups, decreased levels were observed in the AL in the very old age group and a precipitous drop in the DL in 12 month old rats. Decreased selenium levels in the DL of 18 and 24 month old rats were not statistically significant compared to young rats. Selenium is thought to accumulate in prostate tissue, particularly after supplementation of selenium compounds, an effect that has been confirmed in clinical trials (Sabichi et al., 2006). While decreases in body weight in the old age groups may be indicative of a decrease in overall food intake, it is unlikely that these changes in dietary intake could account for the changes in prostate levels. In addition, the age pattern in body weight does not parallel DL selenium levels at the older age groups. The changes in selenium levels in the DL were not consistent with those of GSSP, as the selenium effects were observed in mature animals whereas the changes in GSSP occurred in the older age groups. However, the period of selenium depletion may represent a time point of specific sensitivity to prostate carcinogenesis.
The specificity of these age-related effects for the DL is of biological significance because, while in general the anatomy and physiology of the rodent prostate differs considerably from that of humans, it is the DL of the rat prostate that has been reported to be comparable to the peripheral zone of the human prostate (Celec and Conn, 2006). It is well demonstrated that VL and DL exhibit marked cytological, biochemical, and functional differences, and many normal and aberrant prostatic functions are regulated differentially among the various prostatic lobes (Jesik et al., 1982). In addition, the DL also appears to be particularly sensitive to cancer induction by some carcinogens. When induced with a combination of 3,2’-dimethyl-4-aminobiphenyl and testosterone propionate, F-344 rats commonly develop invasive adenocarcinomas predominantly in the DL (Shirai et al., 1993). In contrast, the carcinogen, 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP) induces tumors in the VL in F-344 rats [40]. While the development of spontaneous prostate tumors in rats tend to be rare, the nonmetastasizing localized intraductal carcinomas have been reported to spontaneously develop in aged F-344 rats (Reznik et al., 1981).
Overall, these findings point to prostate lobe and aging specific changes in selenium and protein glutathionylation which may impact carcinogenesis. Additional studies are warranted to clarify the potential involvement of these changes as mechanistic factors in the association of aging with prostate cancer risk.
Highlights.
We examined selenium and glutathione levels in blood and prostate of the aging rat.
Selenium levels in blood and dorsolateral prostate were decreased with age.
Protein bound glutathione in blood and dorsolateral prostate was increased with age.
Results support a potential role for oxidative stress in prostate cancer in aging.
Acknowledgments
This study was supported in part by National Cancer Institute grant CA127729 as well as by seed funds from the Penn State Hershey Cancer Institute.
Abbreviations
- GSH
glutathione
- GSSP
protein-bound glutathione
- DL
dorsolateral lobe
- AL
anterior lobe
- VL
ventral lobe
- PC
prostate cancer
- ROS
reactive oxygen species
- p-XSC
1,4-phenylenebis(methylene)selenocyanate
- MPA
metaphosphoric acid
- PhIP
2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Bracarda S, de Cobelli O, Greco C, Prayer-Galetti T, Valdagni R, Gatta G, de Braud F, Bartsch G. Cancer of the prostate. Crit Rev Oncol Hematol. 2005;56:379–96. doi: 10.1016/j.critrevonc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Banerjee PP, Brown TR. Castration-induced apoptotic cell death in the Brown Norway rat prostate decreases as a function of age. Endocrinology. 2000;141:821–32. doi: 10.1210/endo.141.2.7339. [DOI] [PubMed] [Google Scholar]
- Lockett KL, Snowhite IV, Hu JJ. Nucleotide-excision repair and prostate cancer risk. Cancer Lett. 2005;220:125–35. doi: 10.1016/j.canlet.2004.08.019. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–92. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–36. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AR, Umbas R, Mochtar CA. Recent role of inflammation in prostate diseases: chemoprevention development opportunity. Acta Med Indones. 43:59–65. [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Bellezza I, Conte C, Culig Z. Oxidative stress-related aging: A role for prostate cancer? Biochim Biophys Acta. 2009;1795:83–91. doi: 10.1016/j.bbcan.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Ghatak S, Ho SM. Age-related changes in the activities of antioxidant enzymes and lipid peroxidation status in ventral and dorsolateral prostate lobes of noble rats. Biochem Biophys Res Commun. 1996;222:362–7. doi: 10.1006/bbrc.1996.0749. [DOI] [PubMed] [Google Scholar]
- Bostwick DG, Alexander EE, Singh R, Shan A, Qian J, Santella RM, Oberley LW, Yan T, Zhong W, Jiang X, Oberley TD. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer. 2000;89:123–34. [PubMed] [Google Scholar]
- Oberley TD, Zhong W, Szweda LI, Oberley LW. Localization of antioxidant enzymes and oxidative damage products in normal and malignant prostate epithelium. Prostate. 2000;44:144–55. doi: 10.1002/1097-0045(20000701)44:2<144::aid-pros7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Morrissey C, Buser A, Scolaro J, O'Sullivan J, Moquin A, Tenniswood M. Changes in hormone sensitivity in the ventral prostate of aging Sprague-Dawley rats. J Androl. 2002;23:341–51. [PubMed] [Google Scholar]
- Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61:6025–8. [PubMed] [Google Scholar]
- Donkena KV, Young CY, Tindall DJ. Oxidative stress and DNA methylation in prostate cancer. Obstet Gynecol Int. 2010:302051. doi: 10.1155/2010/302051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–21. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Coles B, Ketterer B. The role of glutathione and glutathione transferases in chemical carcinogenesis. Crit Rev Biochem Mol Biol. 1990;25:47–70. doi: 10.3109/10409239009090605. [DOI] [PubMed] [Google Scholar]
- Hamilos DL, Wedner HJ. The role of glutathione in lymphocyte activation. I Comparison of inhibitory effects of buthionine sulfoximine and 2-cyclohexene-1-one by nuclear size transformation. J Immunol. 1985;135:2740–7. [PubMed] [Google Scholar]
- Richie JP., Jr The role of glutathione in aging and cancer. Exp Gerontol. 1992;27:615–26. doi: 10.1016/0531-5565(92)90015-r. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Mallis RJ. Aging and oxidation of reactive protein sulfhydryls. Exp Gerontol. 2001;36:1519–26. doi: 10.1016/s0531-5565(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–55. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bayoumy K. The protective role of selenium on genetic damage and on cancer. Mutat Res. 2001;475:123–39. doi: 10.1016/s0027-5107(01)00075-6. [DOI] [PubMed] [Google Scholar]
- El-Bayoumy K, Sinha R. Molecular chemoprevention by selenium: a genomic approach. Mutat Res. 2005;591:224–36. doi: 10.1016/j.mrfmmm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Jr, Park HK, Sanders BB, Jr, Smith CL, Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–63. [PubMed] [Google Scholar]
- Facompre N, El-Bayoumy K. Potential stages for prostate cancer prevention with selenium: implications for cancer survivors. Cancer Res. 2009;69:2699–703. doi: 10.1158/0008-5472.CAN-08-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneisen P, Steinbrenner H, Sies H. Selenium, oxidative stress, and health aspects. Mol Aspects Med. 2005;26:256–67. doi: 10.1016/j.mam.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Flohe L. Is there a role of glutathione peroxidases in signaling and differentiation? Biofactors. 2003;17:93–102. doi: 10.1002/biof.5520170110. [DOI] [PubMed] [Google Scholar]
- Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999;20:1657–66. doi: 10.1093/carcin/20.9.1657. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Kleinman W, Desai DH, Das A, Amin SG, Pinto JT, El-Bayoumy K. The organoselenium compound 1,4-phenylenebis(methylene)selenocyanate inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorgenesis and enhances glutathione-related antioxidant levels in A/J mouse lung. Chem Biol Interact. 2006;161:93–103. doi: 10.1016/j.cbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Giustarini D, Rossi R, Milzani A, Colombo R, Dalle-Donne I. S-glutathionylation: from redox regulation of protein functions to human diseases. J Cell Mol Med. 2004;8:201–12. doi: 10.1111/j.1582-4934.2004.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat JE, Kleinman W, Colosimo S, Muir A, Lazarus P, Park J, Richie JP., Jr Enhanced protein glutathiolation and oxidative stress in cigarette smokers. Free Radic Biol Med. 2004;36:464–70. doi: 10.1016/j.freeradbiomed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- El-Bayoumy K, Richie JP, Jr, Boyiri T, Komninou D, Prokopczyk B, Trushin N, Kleinman W, Cox J, Pittman B, Colosimo S. Influence of selenium-enriched yeast supplementation on biomarkers of oxidative damage and hormone status in healthy adult males: a clinical pilot study. Cancer Epidemiol Biomarkers Prev. 2002;11:1459–65. [PubMed] [Google Scholar]
- Richie JP, Muscat JE, IEACWK, El-Bayoumy K. Association of selenium status and blood glutathione concentrations in blacks and whites. Nutr Cancer. 2011 doi: 10.1080/01635581.2011.535967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse J. Selenium status in aging. Am J Clin Nutr. 1995;61:1172–3. doi: 10.1093/ajcn/61.4.1172. [DOI] [PubMed] [Google Scholar]
- Ray AL, Semba RD, Walston J, Ferrucci L, Cappola AR, Ricks MO, Xue QL, Fried LP. Low serum selenium and total carotenoids predict mortality among older women living in the community: the women's health and aging studies. J Nutr. 2006;136:172–6. doi: 10.1093/jn/136.1.172. [DOI] [PubMed] [Google Scholar]
- Simonoff M, Sergeant C, Garnier N, Moretto P, Llabador Y, Simonoff G, Conri C. Antioxidant status (selenium, vitamins A and E) and aging. Exs. 1992;62:368–97. doi: 10.1007/978-3-0348-7460-1_37. [DOI] [PubMed] [Google Scholar]
- Conn PM. Handbook of Models for Human Aging 2006 [Google Scholar]
- Reznik G, Hamlin MH, 2nd, Ward JM, Stinson SF. Prostatic hyperplasia and neoplasia in aging F344 rats. Prostate. 1981;2:261–8. doi: 10.1002/pros.2990020304. [DOI] [PubMed] [Google Scholar]
- Kleinman WA, Richie JP., Jr Status of glutathione and other thiols and disulfides in human plasma. Biochem Pharmacol. 2000;60:19–29. doi: 10.1016/s0006-2952(00)00293-8. [DOI] [PubMed] [Google Scholar]
- Kleinman WA, Komninou D, Leutzinger Y, Colosimo S, Cox J, Lang CA, Richie JP., Jr Protein glutathiolation in human blood. Biochem Pharmacol. 2003;65:741–6. doi: 10.1016/s0006-2952(02)01560-5. [DOI] [PubMed] [Google Scholar]
- Bethel CR, Chaudhary J, Anway MD, Brown TR. Gene expression changes are age-dependent and lobe-specific in the brown Norway rat model of prostatic hyperplasia. Prostate. 2009;69:838–50. doi: 10.1002/pros.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes I, Reyes N, Iatropoulos M, Mittelman A, Geliebter J. Aging-associated changes in gene expression in the ACI rat prostate: Implications for carcinogenesis. Prostate. 2005;63:169–86. doi: 10.1002/pros.20164. [DOI] [PubMed] [Google Scholar]
- Bhusari SS, Dobosy JR, Fu V, Almassi N, Oberley T, Jarrard DF. Superoxide dismutase 1 knockdown induces oxidative stress and DNA methylation loss in the prostate. Epigenetics. 5:402–9. doi: 10.4161/epi.5.5.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustarini D, Dalle-Donne I, Colombo R, Petralia S, Giampaoletti S, Milzani A, Rossi R. Protein glutathionylation in erythrocytes. Clin Chem. 2003;49:327–30. doi: 10.1373/49.2.327. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–44. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–88. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew KD, Manevich Y, Grek C, Xiong Y, Uys J, Townsend DM. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic Biol Med. 51:299–313. doi: 10.1016/j.freeradbiomed.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi M, Bagchi D, Patterson EB, Tang L, Stohs SJ. Age-related changes in lipid peroxidation and antioxidant defense in Fischer 344 rats. Ann N Y Acad Sci. 1996;793:449–52. doi: 10.1111/j.1749-6632.1996.tb33539.x. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994;8:1302–7. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Komninou D, Leutzinger Y, Kleinman W, Orentreich N, Malloy V, Zimmerman JA. Tissue glutathione and cysteine levels in methionine-restricted rats. Nutrition. 2004;20:800–5. doi: 10.1016/j.nut.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Bates CJ, Thane CW, Prentice A, Delves HT. Selenium status and its correlates in a British national diet and nutrition survey: people aged 65 years and over. J Trace Elem Med Biol. 2002;16:1–8. doi: 10.1016/s0946-672x(02)80002-5. [DOI] [PubMed] [Google Scholar]
- Ducros V, Faure P, Ferry M, Couzy F, Biajoux I, Favier A. The sizes of the exchangeable pools of selenium in elderly women and their relation to institutionalization. Br J Nutr. 1997;78:379–96. doi: 10.1079/bjn19970158. [DOI] [PubMed] [Google Scholar]
- Erden-Inal M, Sunal E, Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem Funct. 2002;20:61–6. doi: 10.1002/cbf.937. [DOI] [PubMed] [Google Scholar]
- Olivieri O, Stanzial AM, Girelli D, Trevisan MT, Guarini P, Terzi M, Caffi S, Fontana F, Casaril M, Ferrari S, et al. Selenium status, fatty acids, vitamins A and E, and aging: the Nove Study. Am J Clin Nutr. 1994;60:510–7. doi: 10.1093/ajcn/60.4.510. [DOI] [PubMed] [Google Scholar]
- Arnaud J, Akbaraly TN, Hininger I, Roussel AM, Berr C. Factors associated with longitudinal plasma selenium decline in the elderly: the EVA study. J Nutr. 2007;18:482–7. doi: 10.1016/j.jnutbio.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz EA, Helzlsouer KJ. Selenium, zinc, and prostate cancer. Epidemiol Rev. 2001;23:93–101. doi: 10.1093/oxfordjournals.epirev.a000801. [DOI] [PubMed] [Google Scholar]
- Pourmand G, Salem S, Moradi K, Nikoobakht MR, Tajik P, Mehrsai A. Serum selenium level and prostate cancer: a case-control study. Nutr Cancer. 2008;60:171–6. doi: 10.1080/01635580701627277. [DOI] [PubMed] [Google Scholar]
- Vogt TM, Ziegler RG, Graubard BI, Swanson CA, Greenberg RS, Schoenberg JB, Swanson GM, Hayes RB, Mayne ST. Serum selenium and risk of prostate cancer in U.S. blacks and whites. Int J Cancer. 2003;103:664–70. doi: 10.1002/ijc.10866. [DOI] [PubMed] [Google Scholar]
- Sabichi AL, Lee JJ, Taylor RJ, Thompson IM, Miles BJ, Tangen CM, Minasian LM, Pisters LL, Caton JR, Basler JW, Lerner SP, Menter DG, Marshall JR, Crawford ED, Lippman SM. Selenium accumulation in prostate tissue during a randomized, controlled short-term trial of l-selenomethionine: a Southwest Oncology Group Study. Clin Cancer Res. 2006;12:2178–84. doi: 10.1158/1078-0432.CCR-05-0937. [DOI] [PubMed] [Google Scholar]
- Celec P, Conn PM. Benign prostatic hyperplasia. Handbook of Models for Human Aging. 2006:641–649. [Google Scholar]
- Jesik CJ, Holland JM, Lee C. An anatomic and histologic study of the rat prostate. Prostate. 1982;3:81–97. doi: 10.1002/pros.2990030111. [DOI] [PubMed] [Google Scholar]
- Shirai T, Imaida K, Iwasaki S, Mori T, Tada M, Ito N. Sequential observation of rat prostate lesion development induced by 3,2'-dimethyl-4-aminobiphenyl and testosterone. Jpn J Cancer Res. 1993;84:20–5. doi: 10.1111/j.1349-7006.1993.tb02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]