Abstract
We have previously shown that immune tolerance induced by the anti-DNA Ig peptide pCons in (NZB × NZW)F1 (NZB/W) lupus mice prolonged survival of treated animals and delayed the appearance of autoantibodies and glomerulonephritis. Part of the protection conferred by pCons could be ascribed to the induction of regulatory T cells (TReg) that suppressed the production of anti-DNA antibodies in a p38 MAPK-dependent fashion. Here we show that another effect of pCons in the induction of immune tolerance in NZB/W lupus mice is the facilitation of effector T cell suppression by TReg. These new findings indicate that pCons exerts protective effects in NZB/W lupus mice by differentially modulating the activity of different T cell subsets, implying new considerations in the design of TReg–based approaches to modulate T cell autoreactivity in SLE.
Keywords: Systemic lupus erythematosus, immune tolerance, anti-DNA antibodies, regulatory T cells, effector T cells, tolerogenic peptide
1. Introduction
Systemic lupus erythematosus (SLE) is a multi-organ autoimmune disease characterized by the presence of dysregulated mechanisms of immune tolerance that include autoantibodies, autoreactive CD4+ T cells, and reduced numbers and/or function of regulatory T cells (TReg) that suppress CD4+CD25−Foxp3−effector T cells (TEff) [1]. Although it has long been held that the inability of TReg to control autoimmune reactivity in SLE could be either secondary to their decreased frequency or abnormal suppressive capacity [2], some studies have suggested that another possibility could be that TEff can become resistant to TReg-mediated suppression [3-5]. This phenomenon prompted our interest in revisiting the role of TReg in the mechanisms that control immune tolerance in SLE. In particular, we had previously shown that the treatment of (NZB × NZW)F1 (NZB/W) lupus-prone mice with the anti-DNA Ig peptide pCons effectively delayed lupus-like disease and prolonged mice survival [6]. We also showed that part of the protection had to be ascribed to pCons induced TReg that suppressed autoimmune responses in a p38-dependent fashion [7]. However, the effects of pCons on TEff remained elusive. Here we report that the threshold for TEff suppression by TReg in untreated NZB/W mice is lowered by pCons in a p38-independent fashion, and this favors the inhibition of autoimmune reactivity. These findings can have implications for the design of targeted interventions in the inhibition of autoimmune T cell reactivity in SLE.
2. Materials and Methods
2.1 Mice
Female NZB/W mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and treated according to the National Institutes of Health guidelines for the use of experimental animals, with the approval of the Institutional Animal Research Committee.
2.2 Peptides
pCons has T cell determinants from different J558 VH regions of NZB/W anti-dsDNA Ig, while the negative control peptide pNeg has similar MHC binding motifs but is does not activate CD4+ T cells [6]. Peptides were synthesized at Chiron Mimotopes (San Diego, CA), purified to a single peak by HPLC, and analyzed by mass spectroscopy for expected amino acid content before use.
2.3 Tolerance induction and cell preparation
For tolerance induction, 10- to 12-wk-old NZB/W mice received a single i.v. dose of 1 mg pCons dissolved in saline [6]. Control mice received either a similar amount of pNeg or saline. Ten days after treatment, single cell suspensions of splenocytes were prepared by passing cells through a sterile wire mesh. After lysis of red blood cells with ACK lysing buffer (Sigma-Aldrich, St. Louis, MO), cells were centrifuged, washed and resuspended in HL-1 medium (Lonza, Walkerville, MD). TReg or TEff were purified from splenocytes using the CD4+CD25+ Regulatory T cell isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions.
2.4 p38 inhibition
The p38 inhibitor 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole (SB203580) and negative control molecule 4-ethyl-2 (p-methoxyphenyl)-5-(4/ -pyridyl)-IH-imidazole (SB202474) were purchased from Calbiochem (San Diego, CA) and dissolved in saline. For p38 inhibition in vivo, mice were injected i.p. daily for 2 wk with 2 mg/kg SB203580 or SB202474 or equal volumes of saline [7].
2.5 Western blot
Total cell lysates from sorted TEff were obtained in 50 mM HEPES (pH 7.5), 250 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10 mM sodium fluoride, 1mM sodium orthovanadate, 2μg/ml aprotinin, 2μg/ml leupeptin, 2μg/ml pepstatin. Proteins were separated by SDS-PAGE and transferred onto PVDF membranes (BioRad Laboratories, Hercules, CA). Membranes were blocked in 5% nonfat milk/PBS, 0.5% Tween 20 (PBST) at 4°C for 2 h, and then incubated with anti-phospho Abs from Cell Signaling Technology (Danvers, MA) (anti-p-ZAP-70Tyr319, anti-p-ERK1/2Thr202/Tyr204, anti-p-STAT1Ser727, anti-p-STAT3Ser727, anti-p-STAT6Tyr641, anti-p-SAPKThr183/Tyr185, anti-p-p38Thr180/Tyr182) or Santa Cruz Biotechnology (Santa Cruz, CA) (anti-p-JNKThr183/Tyr185, anti-p-p38Tyr182). After wash in PBST, membranes were incubated with peroxidase-conjugated secondary Ab. After additional wash, peroxidase activity was detected with the ECL system (Amersham, Piscataway, NJ) or Femto system (Thermo Scientific, Rockford, IL). Membranes were subsequently stripped and reprobed with Ab for the corresponding non-phosphorylated protein purchased from Cell Signaling Technology: anti-ZAP-70, anti-p27kip1, anti-ERK1/2, anti-STAT1, anti-STAT3, anti-STAT6, anti-SAPK and anti-p38, or Santa Cruz Biotechnology: anti-JNK, anti-p38, before peroxidase detection using secondary Ab. Finally, membranes were stripped for final reprobing with anti-β-actin Ab (Cell Signaling) to determine protein equivalency. Exposed films were quantified by densitometric analysis using ScionImage program (Scion Corporation, Frederick, MD).
2.6 Suppression assay
After cell sorting, TEff were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA) by incubation in 0.5 μM CFSE at 37°C for 10 min, then placed in HL-1/5% FCS and incubated in ice for 5 minutes before extensive washes. Mixtures of CFSE-labeled TReg and TEff at ratios of 1:1 or 1:4 or TEff alone were plated in round-bottom 96-well plates and stimulated with Dynabeads mouse anti-CD3/CD28 Ab (Invitrogen) at a ratio of 0.5 bead/cell. Since TReg:TEff ratios of 1:1 or 1:4 ratios gave comparable results, the manuscript only reports data on 1:1 ratios. Flow cytometry was performed after three days using a FACSCalibur™ instrument (BD Biosciences, San Jose, CA) and analysis was done using FlowJo software (Tree Star Inc., Ashland, OR). Cells were analyzed for CFSE dilution and percent suppression was determined based on the percentage of dividing CFSE-labeled cells in the coculture as compared with CFSE-labeled cells cultured alone.
2.7 Statistical analyses
Statistical analyses were done using Prism 4 software (GraphPad, La Jolla, CA). Comparisons between two groups were done by Mann-Whitney U test, and ANOVA for more than two groups. P<0.05 was considered statistically significant.
3. Results
3.1 Effects of pCons on TEff pathways
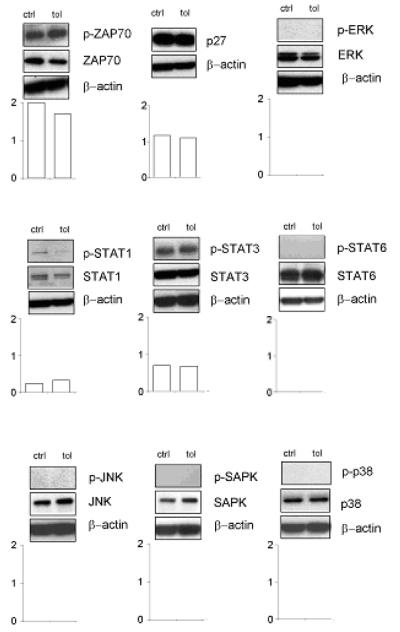
To study the effects of pCons on TEff, we analyzed molecular pathways related to cell cycle, anergy and T cell receptor signaling in sorted TEff from pCons-treated animals versus controls. No differences were observed in the activation of ZAP-70, p27, ERK, STAT1, STAT3, STAT6, JNK, SAPK and p38 in TEff from tolerized mice and controls (Fig.1).
Figure 1. Signaling pathways in TEff after tolerization with pCons.
Western blots for phosphorylated (p-) and non-phosphorylated ZAP-70, p27, ERK, STAT1, STAT3, STAT6, JNK, SAPK and p38 in sorted TEff from mice tolerized with pCons and control mice receiving pNeg (saline gave identical results, not shown). Graphs show the densitometric quantitation of each protein to its non-phosphorylated form. One representative experiment of four is shown.
3.2 pCons facilitates TEff suppression by TReg
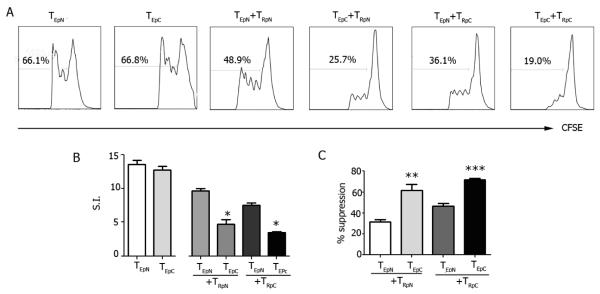
Although intracellular signaling in the pathways tested in TEff was not influenced by pCons, the suppression of TEff by TReg was more effective in pCons-tolerized mice as compared to mock-treated controls (Fig. 2). Since it has been shown that TEff can acquire resistance to Treg suppression in autoimmune conditions including SLE [2-4], we tested the possibility that pCons could modulate this aspect of the mechanisms of TReg-mediated suppression in NZB/W mice. In cocultures of CFSE-labeled TEff plus TReg from pCons-tolerized or control (pNeg-treated) mice, TReg more effectively suppressed TEff from pCons-tolerized than from control mock-treated mice, whether the Treg were derived from either tolerized or control NZB/W mice (Fig. 2). Conversely, TEff from tolerized mice were suppressed more than TEff from control mice independently of whether TReg were derived from pCons-treated or control mice (Fig. 2). Thus, pCons increased the sensitivity of TEff to TReg suppression in NZB/W mice. The observed effects were not due to altered TEff responsiveness after peptide treatment, since proliferative responses of TEff after polyclonal stimulation were similar between control and pCons-tolerized mice (Fig. S1).
Figure 2. pCons reduces TEff resistance to suppression by TReg in NZB/W lupus mice.
CFSE-labeled TEff (TE) were cocultured with TReg (TR) from pCons-tolerized (pC) or pNeg-treated control (pN) NZB/W mice in the presence of CD3/CD28 Ab for 3 days before flow cytometry. Representative (A) and cumulative (B) results including the percent of TEff suppression by TReg (C). *P<0.004; **P<0.009; ***P<0.007.
3.3 pCons effects on TEff resistance are p38-independent
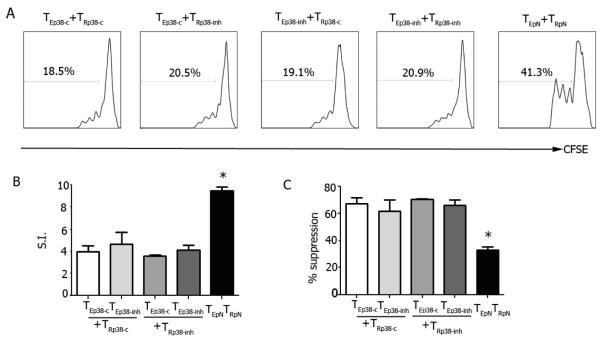
We previously showed that a modulation of p38 activity in TReg contributed to the protection induced by pCons in NZB/W mice [7]. Although here we did not find differences in major signaling events (Fig. 1) or TEff proliferation (Fig. S1) after pCons-induced tolerance, it could still be possible that p38 might influence TEff activity. To address this possibility, NZB/W mice were injected with p38 inhibitor SB203580 or with control SB202474 or saline for 14 days. On day 7, mice were tolerized with pCons or left untreated, and on day 15 TEff and TReg were isolated for functional studies. The proliferation of TEff from mice treated with SB203580 or SB202474 (or saline, not shown) was similar when TEff were suppressed by TReg from mice treated with SB203580 or SB202474 (Fig. 3), suggesting that the increased sensitivity of TEff to TReg suppression after pCons treatment was independent of the p38 pathway in TEff .
Figure 3. pCons reduces TEff suppression by TReg in a p38-independent fashion.
Groups of 7-8 NZB/W mice each were injected daily with the p38 inhibitor SB203580 (p38-inh) or control SB202474 (p38-c) for 14 days. On day 7, mice were tolerized with pCons or given control pNeg. After one week, ex vivo sorted TEff (TE) were CFSE-labeled and cocultured with TReg (TR) in the presence of CD3/CD28 Ab for 3 days before flow cytometry. Representative (A) and cumulative data of TEff suppression by TReg (B-C). *P<0.002 by ANOVA.
4. Discussion
In SLE, dysregulated T cell responses include an inadequate control of the activity of TEff by TReg [8]. Functional deficits and/or abnormal numbers of TEff and TReg have both been proposed as mechanisms that contribute to the loss peripheral immune tolerance in SLE [2]. For example, lupus TEff could differentiate into pathogenic T cell subsets and proliferate excessively [9], or TReg could be numerically reduced and/or be functionally deficient (and thus favor T cell autoreactivity) [10]. The findings reported in this manuscript might explain the conflicting data in the literature that reported abnormal or normal TReg number and/or function in SLE [2], since TEff resistance to suppression by TReg could influence outcomes. Importantly, our results also show that TEff resistance to TReg suppression can be modulated by the induction of immune tolerance. We acknowledge that additional factors that influence TReg suppression and TEff susceptibility, such distinct TCR ligands or cytokines or environmental factors, could all contribute to the TReg suppression of TEff [11-14]. Yet the finding that pCons operates at multiple levels that include a modulation of TEff resistance to suppression imply that a manipulation of TReg in therapeutic settings might be complicated by the responsiveness of target cells. For example, if TEff resistance occurred in the setting of a numeric increase of TReg., those TReg might not be clinically effective. Conversely, the induction of a reduced threshold of Teff suppression by TReg (such as after tolerance), could result in beneficial effects on the disease progression and outcome.
Supplementary Material
Highlights.
We studied the effects of the tolerogenic peptide pCons on TEff in NZB/W lupus mice
We found that pCons decreased the resistance of TEff to suppression by TReg
The findings are relevant in the design of TReg-based therapies in SLE
Acknowledgments
The work was supported in part by the National Institutes of Health (AI095921 to A. L. C. and AI083294 to F-D. S.) and the Muscular Dystrophy Association (to F-D. S.).
Abbreviations
- SLE
systemic lupus erythematosus
- TEff
T effector cells
- TEff
T regulatory cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hahn BH. An overview of the pathogenesis of systemic lupus erythematosus. In: Wallace DJ, Hahn BH, editors. Dubois’ Lupus Erythematosus. Lippincott Williams & Wilkins; Philadelphia: 2002. pp. 87–96. [Google Scholar]
- 2.La Cava A. T-regulatory cells in systemic lupus erythematosus. Lupus. 2008;17:421–425. doi: 10.1177/0961203308090028. [DOI] [PubMed] [Google Scholar]
- 3.Monk CR, Spachidou M, Rovis F, Leung E, Botto M, Lechler RI, Garden OA. MRL/Mp CD4+, CD25− T cells show reduced sensitivity to suppression by CD4+, CD25+ regulatory T cells in vitro: a novel defect of T cell regulation in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1180–1184. doi: 10.1002/art.20976. [DOI] [PubMed] [Google Scholar]
- 4.Venigalla RK, Tretter T, Krienke S, Max R, Eckstein V, Blank N, Fiehn C, Ho AD, Lorenz HM. Reduced CD4+, CD25− T cell sensitivity to the suppressive function of CD4+, CD25high, CD127−/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008;58:2120–2130. doi: 10.1002/art.23556. [DOI] [PubMed] [Google Scholar]
- 5.Vargas-Rojas MI, Crispin JC, Richaud-Patin Y, Alcocer-Varela J. Quantitative and qualitative normal regulatory T cells are not capable of inducing suppression in SLE patients due to T-cell resistance. Lupus. 2008;17:289–294. doi: 10.1177/0961203307088307. [DOI] [PubMed] [Google Scholar]
- 6.Hahn BH, Singh RR, Wong WK, Tsao BP, Bulpitt K, Ebling FM. Treatment with a consensus peptide based on amino acid sequences in autoantibodies prevents T cell activation by autoantigens and delays disease onset in murine lupus. Arthritis Rheum. 2001;44:432–441. doi: 10.1002/1529-0131(200102)44:2<432::AID-ANR62>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Lourenço EV, Procaccini C, Ferrera F, Iikuni N, Singh RP, Filaci G, Matarese G, Shi FD, Brahn E, Hahn BH, La Cava A. Modulation of p38 MAPK activity in regulatory T cells after tolerance with anti-DNA Ig peptide in (NZB × NZW)F1 lupus mice. J. Immunol. 2009;182:7415–7421. doi: 10.4049/jimmunol.0804214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black × New Zealand White)F1 mice suppress in vitro production of antibodies to DNA. J. Immunol. 2004;173:3542–3548. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- 9.Shin MS, Lee N, Kang I. Effector T-cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr. Opin. Rheumatol. 2011;23:444–448. doi: 10.1097/BOR.0b013e328349a255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scalapino KJ, Daikh DI. Suppression of glomerulonephritis in NZB/NZW lupus prone mice by adoptive transfer of ex vivo expanded regulatory T cells. PLoS One. 2009;4:e6031. doi: 10.1371/journal.pone.0006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 12.De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Yates J, Rovis F, Mitchell P, Afzali B, Tsang JY, Garin M, Lechler RI, Lombardi G, Garden OA. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int. Immunol. 2007;19:785–799. doi: 10.1093/intimm/dxm047. [DOI] [PubMed] [Google Scholar]
- 14.Hänig J, Lutz MB. Suppression of mature dendritic cell function by regulatory T cells in vivo is abrogated by CD40 licensing. J. Immunol. 2008;180:1405–1413. doi: 10.4049/jimmunol.180.3.1405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.