Abstract
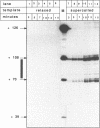
We investigated the effect of left-handed Z-DNA on transcription by bacteriophage T7 RNA polymerase in vitro and, surprisingly, found that the enzyme can efficiently utilize a template containing a stretch of left-handed DNA close to the promoter. Analysis of transcription products revealed that only a small fraction of elongating polymerases abort transcription either at the promoter proximal or at the distal B-to-Z junction and, even less frequently, within the stretch of left-handed DNA. Our results indicate that, unlike E. coli RNA polymerase, T7 RNA polymerase can utilize a template with a CG stretch in an alternate conformation. In contrast, polymerases are completely blocked at the promoter proximal junction by a monoclonal antibody directed against Z-DNA. This blockage remains stable over a remarkable time, even when negative supercoiling is released by linearization of the template. Together with our recent finding of transcription-induced formation of Z-DNA (3), our data provide an example for a possible auto-regulatory mechanism that employs a change in DNA conformation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azorin F., Hahn R., Rich A. Restriction endonucleases can be used to study B-Z junctions in supercoiled DNA. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5714–5718. doi: 10.1073/pnas.81.18.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Kornberg A. Transcriptional activation of initiation of replication from the E. coli chromosomal origin: an RNA-DNA hybrid near oriC. Cell. 1988 Oct 7;55(1):113–123. doi: 10.1016/0092-8674(88)90014-1. [DOI] [PubMed] [Google Scholar]
- Bliska J. B., Cozzarelli N. R. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J Mol Biol. 1987 Mar 20;194(2):205–218. doi: 10.1016/0022-2836(87)90369-x. [DOI] [PubMed] [Google Scholar]
- Brahms J. G., Dargouge O., Brahms S., Ohara Y., Vagner V. Activation and inhibition of transcription by supercoiling. J Mol Biol. 1985 Feb 20;181(4):455–465. doi: 10.1016/0022-2836(85)90419-x. [DOI] [PubMed] [Google Scholar]
- Brill S. J., Sternglanz R. Transcription-dependent DNA supercoiling in yeast DNA topoisomerase mutants. Cell. 1988 Jul 29;54(3):403–411. doi: 10.1016/0092-8674(88)90203-6. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge P., Nordheim A. Transcription-induced conformational change in a topologically closed DNA domain. Nucleic Acids Res. 1991 Jun 11;19(11):2941–2946. doi: 10.1093/nar/19.11.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge P., Sogo J. M., Stahl H. Inhibition of DNA synthesis by aphidicolin induces supercoiling in simian virus 40 replicative intermediates. EMBO J. 1985 Dec 1;4(12):3241–3246. doi: 10.1002/j.1460-2075.1985.tb04072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand R., Job C., Zarling D. A., Teissère M., Jovin T. M., Job D. Comparative transcription of right- and left-handed poly[d(G-C)] by wheat germ RNA polymerase II. EMBO J. 1983;2(10):1707–1714. doi: 10.1002/j.1460-2075.1983.tb01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G. N., Wang J. C. Supercoiling of intracellular DNA can occur in eukaryotic cells. Cell. 1988 Dec 2;55(5):849–856. doi: 10.1016/0092-8674(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Gunderson S. I., Chapman K. A., Burgess R. R. Interactions of T7 RNA polymerase with T7 late promoters measured by footprinting with methidiumpropyl-EDTA-iron(II). Biochemistry. 1987 Mar 24;26(6):1539–1546. doi: 10.1021/bi00380a007. [DOI] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Manes T., Kohwi Y. Unusual conformational effect exerted by Z-DNA upon its neighboring sequences. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2223–2227. doi: 10.1073/pnas.84.8.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Structure of RNAs replicated by the DNA-dependent T7 RNA polymerase. Cell. 1990 Nov 2;63(3):609–618. doi: 10.1016/0092-8674(90)90456-o. [DOI] [PubMed] [Google Scholar]
- Lilley D. M. DNA opens up--supercoiling and heavy breathing. Trends Genet. 1988 Apr;4(4):111–114. doi: 10.1016/0168-9525(88)90099-6. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D. K., Martin C. T., Coleman J. E. T7 RNA polymerase interacts with its promoter from one side of the DNA helix. Biochemistry. 1989 Apr 18;28(8):3306–3313. doi: 10.1021/bi00434a028. [DOI] [PubMed] [Google Scholar]
- Ostrander E. A., Benedetti P., Wang J. C. Template supercoiling by a chimera of yeast GAL4 protein and phage T7 RNA polymerase. Science. 1990 Sep 14;249(4974):1261–1265. doi: 10.1126/science.2399463. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C., Nordheim A., Rich A. Rate of B to Z structural transition of supercoiled DNA. J Mol Biol. 1986 Jul 5;190(1):125–127. doi: 10.1016/0022-2836(86)90082-3. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Transcriptional block caused by a negative supercoiling induced structural change in an alternating CG sequence. Cell. 1985 Jan;40(1):129–137. doi: 10.1016/0092-8674(85)90316-2. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Dynamics of the B-to-Z transition in supercoiled DNA. Proc Natl Acad Sci U S A. 1986 Jul;83(14):4983–4987. doi: 10.1073/pnas.83.14.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M. Hysteretic behaviour of a Z-DNA-antibody complex. Biophys Chem. 1987 May 9;26(2-3):385–390. doi: 10.1016/0301-4622(87)80038-8. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Rice G. A., Kane C. M., Chamberlin M. J. Footprinting analysis of mammalian RNA polymerase II along its transcript: an alternative view of transcription elongation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4245–4249. doi: 10.1073/pnas.88.10.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Romano L. J. Promoter and nonspecific DNA binding by the T7 RNA polymerase. Nucleic Acids Res. 1986 Mar 25;14(6):2811–2827. doi: 10.1093/nar/14.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H. Genetic and physical mapping of the late region of bacteriophage T7 DNA by use of cloned fragments of T7 DNA. J Mol Biol. 1981 Dec 15;153(3):503–525. doi: 10.1016/0022-2836(81)90405-8. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M., Murchie A. I., Lilley D. M. Long range structural communication between sequences in supercoiled DNA. Sequence dependence of contextual influence on cruciform extrusion mechanism. J Biol Chem. 1988 Sep 15;263(26):13074–13082. [PubMed] [Google Scholar]
- Thomae R., Beck S., Pohl F. M. Isolation of Z-DNA-containing plasmids. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5550–5553. doi: 10.1073/pnas.80.18.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao Y. P., Wu H. Y., Liu L. F. Transcription-driven supercoiling of DNA: direct biochemical evidence from in vitro studies. Cell. 1989 Jan 13;56(1):111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Giaever G. N. Action at a distance along a DNA. Science. 1988 Apr 15;240(4850):300–304. doi: 10.1126/science.3281259. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]