Abstract
The human glutaminyl-tRNA synthetase is able to bind to its own mRNA. The enzyme contains two binding regions. One is located in the central section of the enzyme which includes its most hydrophilic portion with ten lysine residues in a block of 20 amino acids. This part of the enzyme binds unspecifically to all RNA sequences tested. A second binding region is located in that part of the enzyme which shows high degrees of sequence similarities with the bacterial and yeast glutaminyl-tRNA synthetases, and which is most likely responsible for the charging of tRNA with glutamine. This second RNA binding region specifically interacts with a site in the 3' noncoding region of the synthetase's mRNA. The binding site in the mRNA is characterized by an extended secondary structure that includes elements of the 'identity set' of nucleotides recognized by the enzyme when interacting with tRNA. We discuss possible physiological implications of the interaction between glutaminyl-tRNA synthetase and its mRNA.
Full text
PDF
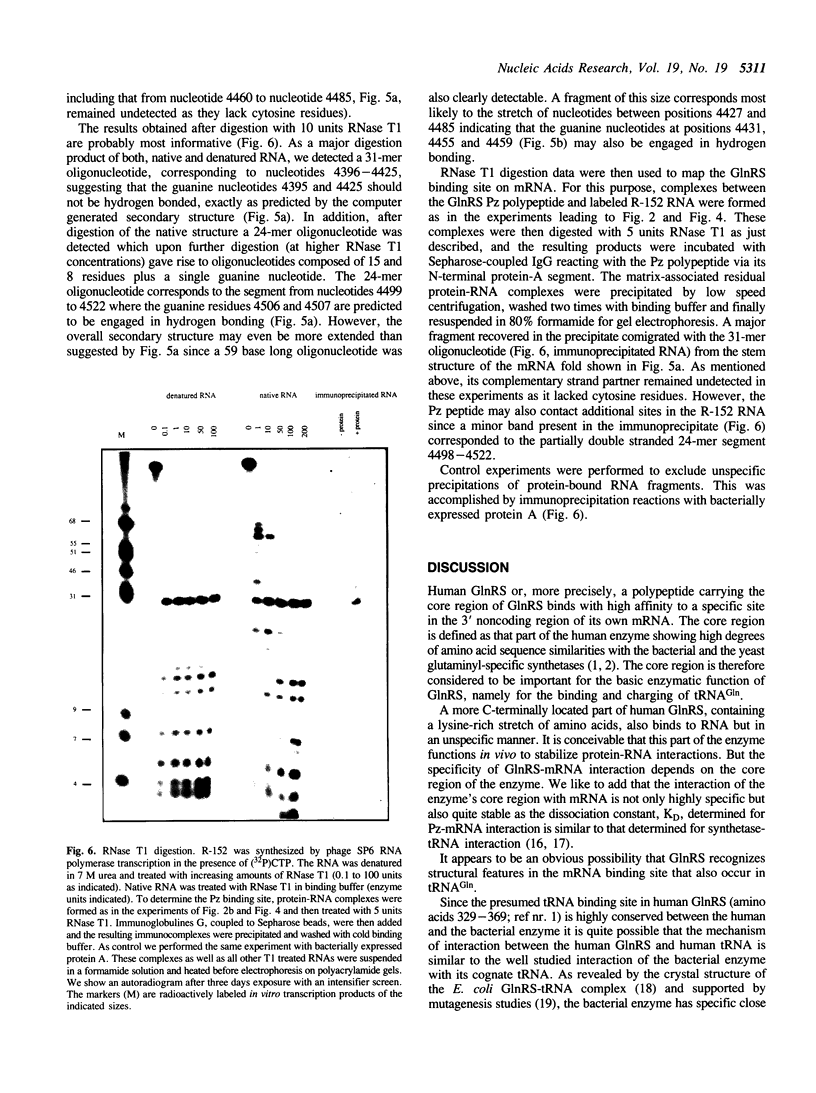

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fett R., Knippers R. The primary structure of human glutaminyl-tRNA synthetase. A highly conserved core, amino acid repeat regions, and homologies with translation elongation factors. J Biol Chem. 1991 Jan 25;266(3):1448–1455. [PubMed] [Google Scholar]
- Freist W., Sternbach H. Isoleucyl-tRNA synthetase from baker's yeast. Discrimination of 20 amino acids in aminoacylation of tRNA(Ile)-C-C-3'dA; role of terminal hydroxyl groups aminoacylation of tRNA(Ile)-C-C-A. Eur J Biochem. 1989 Jan 2;178(3):595–602. doi: 10.1111/j.1432-1033.1989.tb14487.x. [DOI] [PubMed] [Google Scholar]
- Godar D. E., Godar D. E., Garcia V., Jacobo A., Aebi U., Yang D. C. Structural organization of the multienzyme complex of mammalian aminoacyl-tRNA synthetases. Biochemistry. 1988 Sep 6;27(18):6921–6928. doi: 10.1021/bi00418a038. [DOI] [PubMed] [Google Scholar]
- Grossi de Sa M. F., Standart N., Martins de Sa C., Akhayat O., Huesca M., Scherrer K. The poly(A)-binding protein facilitates in vitro translation of poly(A)-rich mRNA. Eur J Biochem. 1988 Oct 1;176(3):521–526. doi: 10.1111/j.1432-1033.1988.tb14309.x. [DOI] [PubMed] [Google Scholar]
- Hoben P., Royal N., Cheung A., Yamao F., Biemann K., Söll D. Escherichia coli glutaminyl-tRNA synthetase. II. Characterization of the glnS gene product. J Biol Chem. 1982 Oct 10;257(19):11644–11650. [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Electrophoretic separation of complexes involved in the splicing of precursors to mRNAs. Cell. 1986 Sep 12;46(6):845–855. doi: 10.1016/0092-8674(86)90066-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. A profusion of controls. J Cell Biol. 1988 Jul;107(1):1–7. doi: 10.1083/jcb.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruys V., Marinx O., Shaw G., Deschamps J., Huez G. Translational blockade imposed by cytokine-derived UA-rich sequences. Science. 1989 Aug 25;245(4920):852–855. doi: 10.1126/science.2672333. [DOI] [PubMed] [Google Scholar]
- Ludmerer S. W., Schimmel P. Gene for yeast glutamine tRNA synthetase encodes a large amino-terminal extension and provides a strong confirmation of the signature sequence for a group of the aminoacyl-tRNA synthetases. J Biol Chem. 1987 Aug 5;262(22):10801–10806. [PubMed] [Google Scholar]
- Marzluff W. F., Pandey N. B. Multiple regulatory steps control histone mRNA concentrations. Trends Biochem Sci. 1988 Feb;13(2):49–52. doi: 10.1016/0968-0004(88)90027-8. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W. A binding consensus: RNA-protein interactions in splicing, snRNPs, and sex. Cell. 1989 Apr 7;57(1):1–3. doi: 10.1016/0092-8674(89)90164-5. [DOI] [PubMed] [Google Scholar]
- Norcum M. T. Isolation and electron microscopic characterization of the high molecular mass aminoacyl-tRNA synthetase complex from murine erythroleukemia cells. J Biol Chem. 1989 Sep 5;264(25):15043–15051. [PubMed] [Google Scholar]
- Oey J. L., Knippers R. Properties of the isolated gene 5 protein of bacteriophage fd. J Mol Biol. 1972 Jul 14;68(1):125–138. doi: 10.1016/0022-2836(72)90268-9. [DOI] [PubMed] [Google Scholar]
- Rogers M. J., Söll D. Discrimination between glutaminyl-tRNA synthetase and seryl-tRNA synthetase involves nucleotides in the acceptor helix of tRNA. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6627–6631. doi: 10.1073/pnas.85.18.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romby P., Moine H., Lesage P., Graffe M., Dondon J., Ebel J. P., Grunberg-Manago M., Ehresmann B., Ehresmann C., Springer M. The relation between catalytic activity and gene regulation in the case of E coli threonyl-tRNA synthetase. Biochimie. 1990 Jun-Jul;72(6-7):485–494. doi: 10.1016/0300-9084(90)90072-o. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Roy K. L., Cooke H., Buckland R. Nucleotide sequence of a segment of human DNA containing the three tRNA genes. Nucleic Acids Res. 1982 Nov 25;10(22):7313–7322. doi: 10.1093/nar/10.22.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A. B., Davis R. W. The poly(A) binding protein is required for poly(A) shortening and 60S ribosomal subunit-dependent translation initiation. Cell. 1989 Sep 8;58(5):857–867. doi: 10.1016/0092-8674(89)90938-0. [DOI] [PubMed] [Google Scholar]
- Schimmel P. Parameters for the molecular recognition of transfer RNAs. Biochemistry. 1989 Apr 4;28(7):2747–2759. doi: 10.1021/bi00433a001. [DOI] [PubMed] [Google Scholar]
- Schray B., Thömmes P., Knippers R. Glutaminyl-tRNA synthetase as a component of the high-molecular weight complex of human aminoacyl-tRNA synthetases. An immunological study. Biochim Biophys Acta. 1990 Oct 23;1087(2):226–234. doi: 10.1016/0167-4781(90)90209-k. [DOI] [PubMed] [Google Scholar]
- Schümperli D. Multilevel regulation of replication-dependent histone genes. Trends Genet. 1988 Jul;4(7):187–191. doi: 10.1016/0168-9525(88)90074-1. [DOI] [PubMed] [Google Scholar]
- Springer M., Graffe M., Dondon J., Grunberg-Manago M. tRNA-like structures and gene regulation at the translational level: a case of molecular mimicry in Escherichia coli. EMBO J. 1989 Aug;8(8):2417–2424. doi: 10.1002/j.1460-2075.1989.tb08372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Meissner F., Moll J., Vorderwülbecke T. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1987;15 (Suppl):r53–188. doi: 10.1093/nar/15.suppl.r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
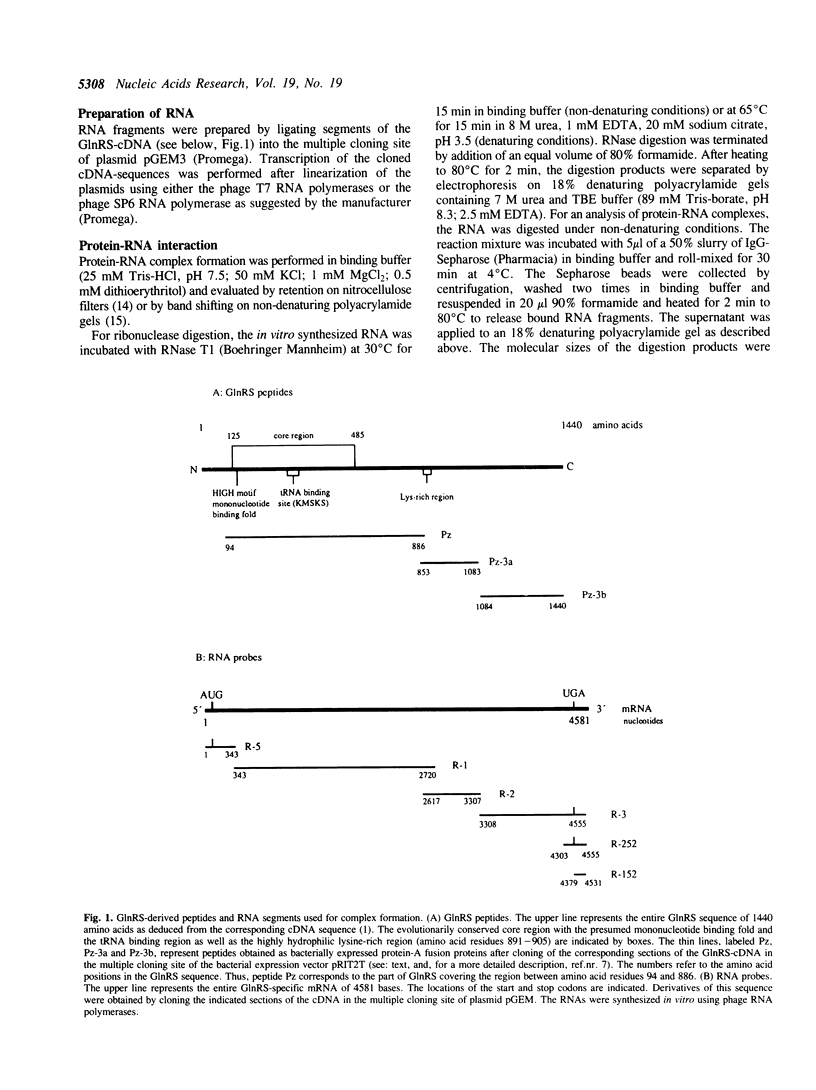
- Thömmes P., Fett R., Schray B., Kunze N., Knippers R. The core region of human glutaminyl-tRNA synthetase homologies with the Escherichia coli and yeast enzymes. Nucleic Acids Res. 1988 Jun 24;16(12):5391–5406. doi: 10.1093/nar/16.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. In the beginning is the end: regulation of poly(A) addition and removal during early development. Trends Biochem Sci. 1990 Aug;15(8):320–324. doi: 10.1016/0968-0004(90)90022-4. [DOI] [PubMed] [Google Scholar]
- Yarus M. tRNA identity: a hair of the dogma that bit us. Cell. 1988 Dec 2;55(5):739–741. doi: 10.1016/0092-8674(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Zamore P. D., Zapp M. L., Green M. R. Gene expression. RNA binding: beta s and basics. Nature. 1990 Dec 6;348(6301):485–486. doi: 10.1038/348485a0. [DOI] [PubMed] [Google Scholar]