Abstract
Recent research on a wide variety of systems has demonstrated that animals generally coevolve with their microbial symbionts. Although such relationships are most often established anew each generation, the partners associate with fidelity, i.e., they form exclusive alliances within the context of rich communities of non-symbiotic environmental microbes. The mechanisms by which this exclusivity is achieved and maintained remain largely unknown. Studies of the model symbiosis between the Hawaiian squid Euprymna scolopes and the marine luminous bacterium Vibrio fischeri provide evidence that the interplay between evolutionarily conserved features of the innate immune system, most notably MAMP/PRR interactions, and a specific feature of this association, i.e., luminescence, are critical for development and maintenance of this association. As such, in this partnership and perhaps others, symbiotic exclusivity is mediated by the synergism between a general animal-microbe ‘language’ and a ‘secret language’ that is decipherable only by the specific partners involved.
Keywords: Euprymna, Vibrio fischeri, MAMP, quorum sensing, symbiosis, bioluminescence
1. Introduction
Biologists have long known that the immune system manages a significant portion of an animal host’s response to the microbial world. The description of such responses has been largely focused upon the elements associated with pathogenesis. Decades of research in this area have identified a large number of molecules, pathways, and networks that are active when the animal host’s homeostasis is under pathogen-induced stress. Likewise, in our study of the microbes, research of the biology community has concentrated on the analysis of virulence factors that induce these host responses to pathogens. In the last 10 years, however, with the growing recognition of the prevalence of beneficial animal-microbe interactions, it has been necessary to modify the current views to incorporate this new knowledge into our concepts of the form and function of animal immune systems, as well as the activity of microbes [1,2].
One important discovery is that much of the cell and molecular biology of host-microbe pathogen interactions is shared with beneficial or benign interactions (see below). But, what might we be missing? Are there dedicated systems specific to the control of coevolved beneficial symbioses that work in concert with the immune system, as a sort of ‘secret’ language of any given partnership? Or, might such systems be considered a yet undiscovered arm of the immune system? If such systems do exist, one key role would be to ensure that the specific coevolved symbioses are established and maintained each generation. At this point in time, we have almost no understanding of the mechanisms underlying recognition and specificity in coevolved beneficial animal-microbe associations.
Relatively simple symbiotic relationships, such as those alliances characteristic of many invertebrates, can illustrate basic, conserved principles by which mutualisms are governed [3]. In several of these associations, the exact products that are exchanged between host and symbiont have been determined, i.e., the molecular basis of the mutualism is well characterized (see e.g., [4]). The present contribution explores the specific symbiotic language, i.e., luminescence, of one invertebrate-bacterial association, the squid-vibrio system. While luminescence is an unusual character in animal symbioses, it is easily measured and genetically manipulated and, thus, lends itself to experimental approaches. Studies of luminescence in this system have demonstrated that light production by the symbiont and its perception by the host are essential characters of the association, without which the symbiosis does not persist. We discuss here what is known about how symbiont light production works in concert with symbiont MAMPs to shape host development and homeostasis.
2. The common language – MAMPs and PRRs in animal-microbe interactions
‘Microbe-associated molecular patterns’ (MAMPs), a term coined by Koropatnick et al. [5] as a variation of ‘pathogen-associated molecular patterns’ (PAMPs), and host pattern-recognition receptors (PRRs) are well known, highly conserved mediators of animal-microbe interactions. Studies of mutualistic symbioses in recent years have demonstrated that these receptor-ligand dynamics can also be essential elements underlying the onset, maturation, and persistence of mutualistic animal-microbe partnerships. For example, in a benchmark 2004 contribution to Cell, Rakoff-Nahoum and coworkers [6] demonstrated that exposure to the MAMP lipopolysaccharide (LPS) of the microbiota is essential for gut homeostasis in mammals. Before this report, LPS (aka ‘endotoxin’) signaling to host cells was thought to be restricted to its role as a PAMP; PAMPs typically interact with host receptor molecules (e.g., Toll-like receptors, NOD (nucleotide-binding oligomerization domain) -like receptors, etc.) and activate pathways associated with response to microbial invasion of host tissues, such as the NF(nuclear-factor)-kappaB pathway (for reviews see [7,8]). Similarly, analyses by Bouskra and coworkers [9] showed that peptidoglycan (PGN), well studied for its role in pathogenesis, is critical for development of the gut-associated lymphoid tissue of mammals. PGN is also critical for the homeostasis of the gut of Drosophila melanogaster [10]. Taken together, these recent data indicate that it is the context of MAMP/PRR interactions, i.e., when, where and with what partnership, that determines the nature of the interaction (i.e., benign or pathogenic) and that the dynamic between these molecules is widespread across the animal kingdom.
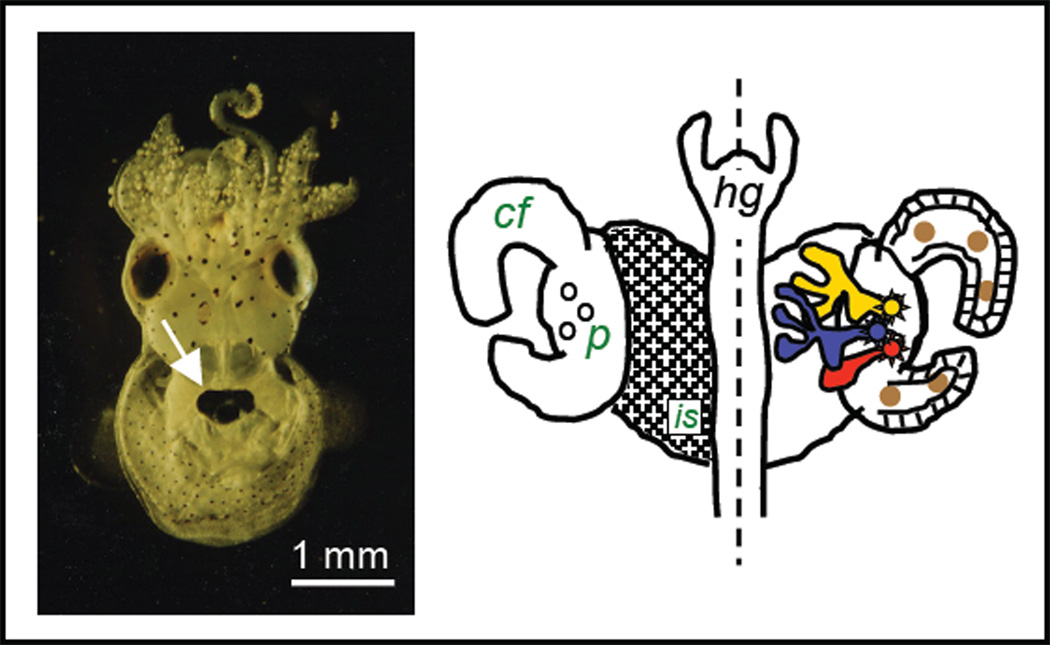
The receptor-ligand activities of MAMPs and their associated PRRs have been studied extensively in the squid-vibrio symbiosis [11,12,13,14]. In this association, the Hawaiian bobtail squid Euprymna scolopes harbors in its tissues luminous Vibrio fischeri cells and uses this luminescence as an antipredatory strategy in its nocturnal behavior. The symbiont cells occur extracellularly along the apical surfaces of epithelial cells in a complex structure, or light organ, in the center of the body cavity (Fig. 1) (for review see [15]). The epithelia-lined crypt spaces, which are the blind-ended regions of the light organ that house the symbionts, are not unlike those of the small intestine of mammals. This experimental model is exploited to provide insight into the mechanisms underlying the onset and maintenance of persistent colonization of epithelia by gram-negative bacteria, perhaps the most common type of animal-bacterial interaction that occurs in nature. The principles gained by the study of the squid-vibrio model are directly applicable to many invertebrate associations, where binary symbioses or symbioses with few microbial partners are common. In the vertebrates, where consortia of microbes are the ‘rule’, the model will not provide insight into how consortia interact, but rather provide a valuable comparison for studies involving the monoclonization of germ-free vertebrates.
Fig. 1.
The light organ system of Euprymna scolopes. Left, a ventral view of a juvenile animal showing the location of the light organ in the mantle cavity (white arrow). Symbiont-containing tissue is surrounded by the ink sac, diverticula of which serve to modulate symbiont light emission from the animal. Right, the external (left half) and internal (right half) features of the juvenile light organ. The surface of the organ is covered by a ciliated field (cf), which overlies the ink sac (is), at the base of which are three pores (p), the sites of symbiont entry into host tissues during initial inoculation of the organ. Right, small numbers of symbiont cells enter the three pores and travel into three independent blind-ended crypt spaces (red, blue, yellow), where they grow out and begin to luminesce. Association of the symbiont cells with host tissues induces hemocyte (brown spheres) trafficking into the blood sinus of the ciliated field. hg, hindgut.
Early studies of this symbiosis revealed that the symbiont V. fischeri is the exclusive partner of the host squid in the light organ; i.e., in the absence of the symbiont, none of the other hundreds of bacterial species in the surrounding seawater colonize host tissues [16]. In addition, the bacteria drive development of the tissues with which they associate [17]. Once the symbiosis is established, it is maintained in dynamic balance by a profound diel rhythm, an activity that involves an expulsion of much of the resident symbiont population followed by regrowth to fill the crypts [18]. The molecular dialog underlying these events is dominated by derivatives of the two specific MAMPs mentioned above, LPS and PGN [11]; recent data provide evidence that at least a portion of the host response is mediated by lipopolysaccharide-binding proteins (members of the LBP/BPI protein family) [19,14] and peptidoglycan-recognition proteins (PGRPs) [12,13].
3. The secret language - luminescence
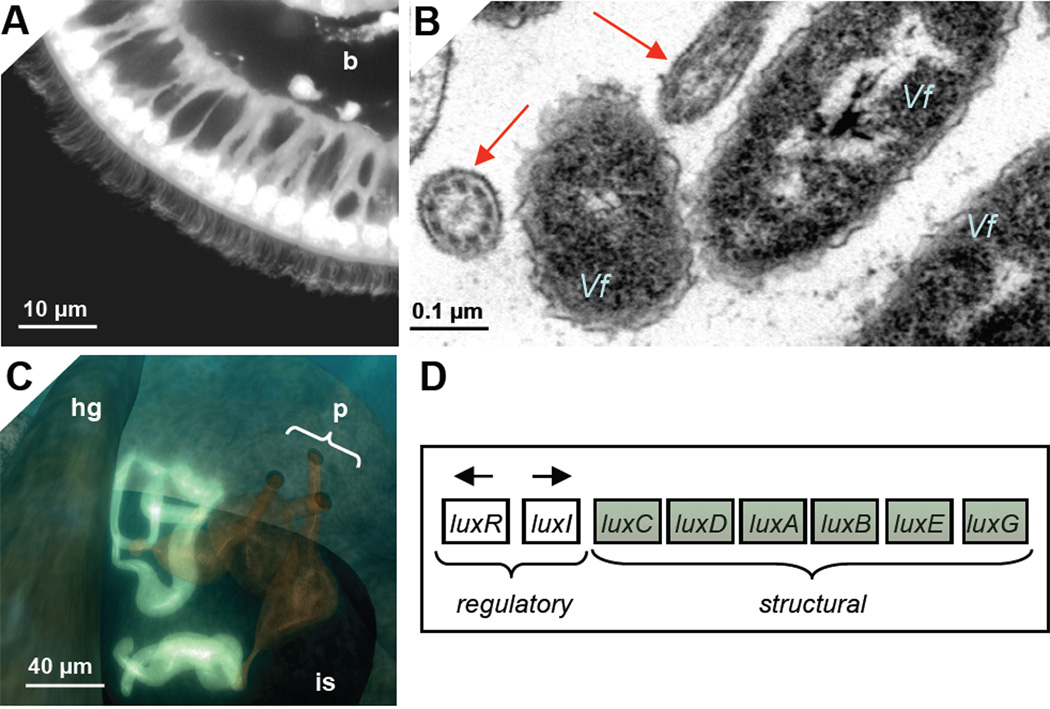
Luminescence is the principal product, or ‘currency’, provided by the bacterial symbionts to the host in the squid-vibrio symbiosis; i.e., it is unlike most symbiotic associations, where the microbial partner provides nutrients to the host. As discussed in detail below, we have evidence for host detection of symbiont light production in two regions of the host light organ: (1) in the cells of the superficial ciliated epithelium, where symbionts attach during initial stages of the colonization process (Fig. 2A/B); and, (2) in the crypt spaces, where, following colonization, the bacteria reside for the life of the host (Fig. 2C). The light produced by V. fischeri results from the activity of the genes in the lux locus (Fig. 2D). This bacterial species is one of the many that exhibit regulation through quorum sensing, i.e., expressing a specific phenotype only at high population density [20]. In this instance, the quorum sensing results in induction of luminescence. An autoinducer molecule, the synthesis of which is under the control of the luxI gene, is constitutively produced, so that the lux operon is transcribed at low levels even in solitary V. fischeri cells. This activity results in very low levels of light production by individual cells, such as those in the bacterioplankton that would encounter host cilia during initial interactions with host tissues. When V. fischeri achieves high population density in crypt spaces, the exported autoinducer molecule concentration builds up and positively regulates, through the luxR gene, the activity of the lux operon. As such, the bacteria become highly induced for luminescence, which the animal uses in its behavior. This first induction of luminescence in the crypts occurs at ~12 h following initial exposure to environmental V. fischeri. Thereafter the luminescence is always induced, although the intensity follows a pronounced diel rhythm [21].
Fig. 2.
Host tissues and bacterial light production. Light is produced at and perceived by two regions of the organ, the ciliated field and the crypt spaces. A. The ciliated epithelial field where symbiont cells first associate with host tissues. The epithelium is a single layer overlying a blood sinus (b). B. Symbiont cells (Vf) closely associate with host cilia (red arrows). C. Following migration from the cilia into the pores (p), the bacterial population grows out and luminesces in the crypts (bright blue). D. The lux operon. The regulatory genes operate to sense symbiont cell density in the phenomenon called quorum sensing. When the population grows to high enough density, the transcription of the structural genes, which encode substrates and enzymes of the light reaction, is induced. is, ink sac; hg, hindgut.
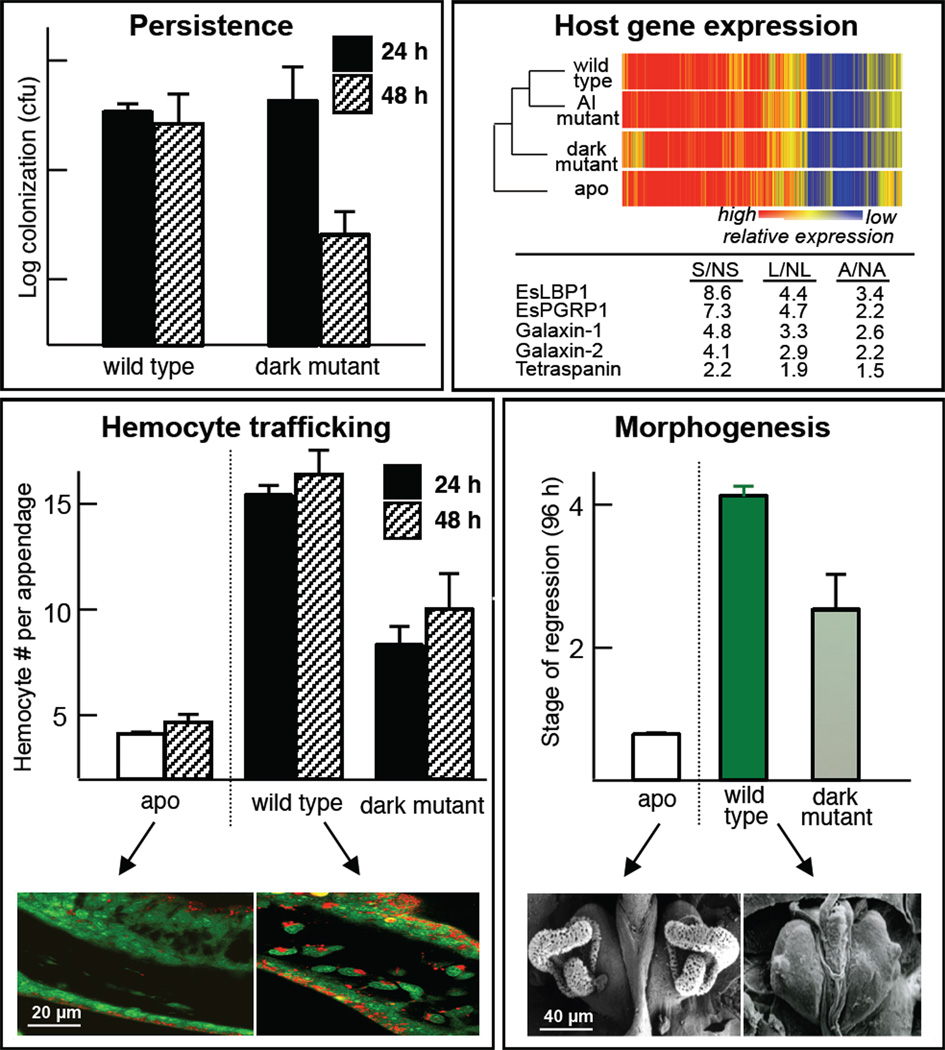
It is perhaps not surprising that the light, as the pivotal currency of the association, plays a central role in the squid-vibrio symbiosis. The first indication that host tissues could perceive the bacterial light came from studies of V. fischeri mutants defective in the luxA gene, which encodes for one of the subunits of the bacterial luciferase [22]. Such mutants, while capable of initial colonization, are sanctioned as ‘cheaters’, i.e., excluded beginning at about 48 h and eventually lost from the crypt spaces (Fig. 3, upper left). Similar results were subsequently obtained by a deletion of the entire lux operon [23]. Studies of the host have demonstrated that bacterial light production is essential for normal transcriptomic responses (Fig. 3, upper right), and normal behavior of host cells associated with bacteria-induced development (Fig 3; lower). Specifically, mutants defective in light production do not cause the swelling of the host crypt epithelial cells, which is typically induced by wild-type symbionts [22] (data not shown); and, they neither induce the hemocyte trafficking in (Fig. 3, lower left) nor signal the morphogenesis of the ciliated superficial epithelial tissue (Fig. 3, lower right).
Fig. 3.
The importance of light production to normal symbiosis. Upper left, mutants defective in light production (Δlux, ‘dark mutant’) fail to persist in the host organ. No defect occurs with colonization, as these mutants colonize to wild-type levels at 24 h, but by 48 h, the population has diminished several fold and the colonization of these mutants is entirely lost within days. Upper right, dark mutants are defective in inducing normal symbiosis-induced changes in the expression of several genes. A heat map (upper) analysis shows that the absence of symbiont luminescence dramatically affects the overall patterns of the transcriptome. Most notable is the defect in changes in expression of genes encoding the MAMPs-interacting proteins, EsLBP1 and EsPGRP1. Lower left, dark mutants are defective in inducing normal activities of host cells, including hemocyte trafficking into the blood sinus. Lower right, dark mutants are also defective in normal development, i.e., in the symbiosis-induced loss of the ciliated field.
These studies of the influence of symbiont luminescence on host development have provided evidence that light production acts in concert with V. fischeri MAMPs, specifically the lipid A component of LPS and the peptidoglycan monomer TCT (‘tracheal cytotoxin’), which are known morphogens in the system. Two of five annotated genes [19] with expression profiles at 18 h that are affected by defects in the lux operon (a lipopolysaccharide-binding protein, EsLBP1, and a peptidoglycan-recognition protein, EsPGRP1) are orthologs of evolutionarily conserved MAMP receptors [14,24]. The host cellular phenotypes of hemocyte trafficking and apoptosis-mediated morphogenesis, processes that are largely induced by symbiont lipid A and TCT, also do not occur at normal levels in animals colonized by symbionts defective in light production [25]. Taken together, the evidence thus far suggests that light works synergistically with bacterial surface molecules to trigger much of the bacteria-induced development of the light organ. We are currently studying the mechanisms underlying the synergy of MAMPs and luminescence in the induction of development.
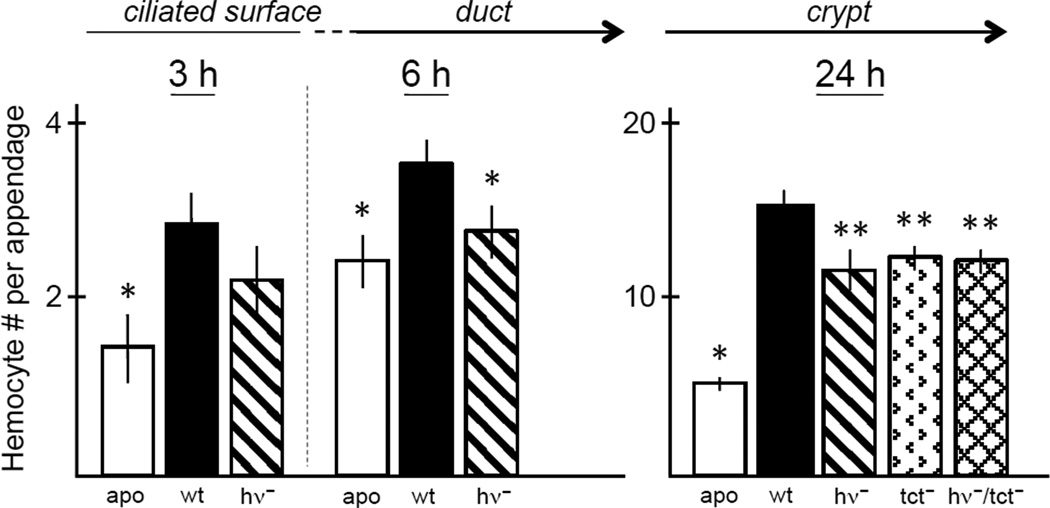
Symbiont-induced hemocyte trafficking into the tissues of the ciliated surface of the juvenile organ begins during harvesting of the symbionts from the environment, and can be induced by TCT presented as a pharmacological agent [25]. A recent study has demonstrated that, under these normal conditions of low symbiont inoculum (~5,000 symbiont cells/ml of seawater), hemocyte trafficking is induced by 3–5 V. fischeri cells attaching to the cilia of the epithelial surface of the organ (unpublished data). Although not yet tested, much of this host phenotype is likely due to the constitutive production of TCT by the few attaching symbiont cells. For the present contribution, we carried out experiments to determine whether the light produced by wild-type V. fischeri cells in the bacterioplankton might act synergistically with TCT to induce normal hemocyte trafficking during this period. We found that mutants in the lux operon are not capable of inducing normal hemocyte trafficking in an early stage of the symbiosis (Fig. 4, left). These data provide evidence that even low levels of symbiont light produced by the wild-type cells are critical for normal host responses and that the host is aware of interaction with a luminescence-defective mutant, or cheater, at the very earliest stages of the onset of the association. These data were surprising, although we already knew that the presentation of the V. fischeri MAMP TCT was important to induce hemocyte trafficking. Our finding that light also signals this cell phenotype prompted us to examine a double mutant, defective in both light production and TCT transport, to determine whether these two signals might work synergistically. When the tissues were examined at 24 h, a time when trafficking is at its peak, we found that the effect was not additive (Fig. 4B), which suggests that these two signals, although both active, likely operate through different mechanisms, or only one is necessary (i.e., they are redundant). What still remains to be determined is the connection between host receptors of symbiont MAMPs and the eventual behavior of hemocyte trafficking.
Fig. 4.
Host responses to V. fischeri cells uninduced and induced for light production, and the interplay with MAMPs. Animals are exposed to bacteria-rich seawater in the absence (apo) or presence of V. fischeri, either wild-type (wt) or mutant. Left graphs, perception of the small amount of light produced by uninduced V. fischeri cells. As early as 3 h, when bacteria are attaching to the cilia (left graph), a statistically significant (*) increase in hemocyte trafficking into the appendages of the ciliated fields can be observed in animals exposed to wild-type symbionts. Mutants defective in light production (hv-) show less, although not statistically significant, induced trafficking than wild-type (wt). By 6 h, when symbionts are moving through the ducts and into the crypts, both apo and mutants defective in light production are statistically significantly different (*) from wild-type. Right graph, responses at 24 h to V. fischeri cells in the crypts, which are induced for luminescence. Mutants defective in light continue to be defective in hemocyte trafficking. Mutants defective in transport of TCT (tct-), the principal inducer of this host phenotype, also show a defect, but the double mutant (hv-/tct-) does not show synergy of these two signals. N = 25–36 animals for each condition. Significance was determined by calculated Poissonian p-values for the comparisons examined.
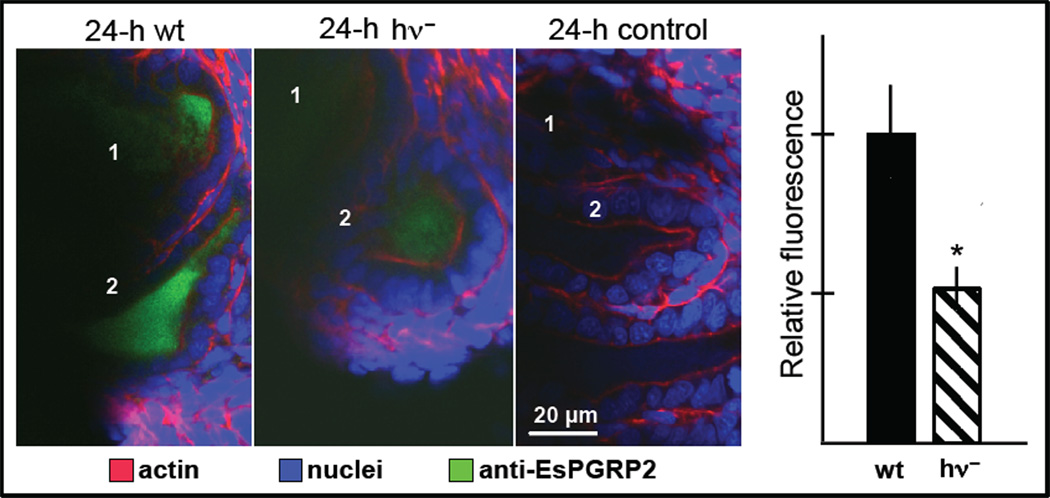
Finally, we also determined whether light production might affect the behavior of the host molecules that interact with the bacterial MAMPs. An easily quantifiable response is the symbiont-induced secretion of EsPGRP2 into the crypt space [12]. Mutants defective in light production are not capable of inducing normal levels of secretion of this molecule (Fig. 5). Biochemical studies of purified EsPGRP2 have shown that it is capable of breaking down TCT [12]. As V. fischeri cells export TCT constitutively, it is likely that this molecule is in very high concentration in crypt spaces where the dense populations of the symbiont reside. The export of EsPGRP2 into the crypt spaces is likely to ‘tame’ this perturbing MAMP [12]. The data on mutants in light production provide evidence that the dark bacterial phenotype compromises the breakdown of the TCT molecule, a process that may be critical for symbiont persistence. To determine whether this interpretation is valid will require further research.
Fig. 5.
The effect of normal light production on EsPGRP2 into the crypts. Confocal images of immunocytochemistry of the host light organ at 24 h: antiEsPGRP2 (rabbit polyclonal), green; nuclei (TOTO3; Invitrogen), blue; actin cytoskeleton (rhodamine phalloidin), red. Left, colonization by wild-type (wt) V. fischeri induces strong transport of EsPGRP2 into the crypt spaces, which is attenuated in colonization by mutants defective in light production (hν-); control, preimmune serum alone. Graph right, quantification of fluorescence (n = 11–14 crypts for each treatment) * = p < 0.05 by a Student’s t-test for unequal variance.
Thus, key findings from the squid-vibrio system include: (1) symbiont luminescence is required for normal host-tissue development, i.e., luminescence, in addition to conserved MAMPs, acts as a morphogen; and, (2) the symbiont must be luminous to persist in host tissues, i.e., if the symbiont is not ‘doing its job’, it is eliminated from the partnership. In addition, variants of V. fischeri that are dim or dark have not thus far been identified among the hundreds of strains isolated from the light organs. These findings suggest that mechanisms are present by which host tissues perceive bacterial luminescence, and this feature of V. fischeri is a sine qua non for its ability to be a symbiont.
4. Host sensing of symbiont luminescence – eyes, light organs, and immune privilege
How does the host know that the symbiont is making light? The squid light organ contains features, at multiple levels of biological organization, similar to those that are found in the eye. At the morphological level, both organs have a lens and reflective tissue [26,27], and at the molecular and biochemical levels, the light organ expresses the same genes and produces the same proteins involved in phototransduction [28]. Among these proteins are opsin, which activates phototransduction, and rhodopsin kinase and arrestin, which deactivate phototransduction. We are also finding that both organs contain cryptochromes, which are blue-light sensitive proteins known to set circadian rhythms (pers. obs). As described above (section 2), light-organ tissues were found to perceive light. Such physiological perception was validated with electroretinograms, which indicated a net hyperpolarization upon simulation of the tissues [28]. Most recently, we are finding that the eye and light organ are likely to develop under the same inductive signals, as both organs express genes that are critical for eye formation, such as pax6 (paired box gene 6), eya (eyes absent), six (sine oculis), and dac (dachshund).
Interestingly, the convergence of the eye and light organ may also extend beyond form, function, and development to a phenomenon referred to as immune privilege. The phrase, immune privilege, was first used in reference to the anterior segment of the vertebrate eye (i.e., cornea), as such tissues are among the few within the body to accept donor grafts [29,30]. In association with V. fischeri, light-organ tissues might be considered to have such 'privilege’ in that the bacterial symbionts are somehow protected from clearance by the squid immune system. However, that V. fischeri persists where no other bacterial species is able to do so would beg the question: Why is the light organ not open to other bacterial species? A key feature that renders vertebrate corneal tissues such immune protection is that they are largely non-vascularized [30,31]. As such, these tissues are not subject to immune system responses delivered through the circulatory system. As described above (section 3), portions of the light organ, similar to the retina, are highly vascularized and the squid circulatory system responds dramatically upon induction of symbiosis. However, other portions of the light organ that are analogous to the vertebrate cornea, including the tissues closely associated with the lens, are not well vascularized. Taken together, the dramatic convergence of eye and light organ appears to represent a striking example of ‘evolutionary tinkering’, i.e., the creation of novelty (the light organ) by the use of pre-existing characters (features that drive the form and function of the eye) [32,33]. In the case of the light organ, evolutionary tinkering was driven by animal-bacterial interaction.
5. Concluding remarks and future challenges
Further study of the dynamics of beneficial host-microbe interactions will certainly begin to reveal features that are unique to this type of symbiosis. While the squid-vibrio system offers a dramatic case, we are rapidly gaining insight into other systems. For example, recent research on Bacteroides fragilis, a constituent of the mammalian microbiota, has demonstrated that a single molecular species, a particular zwitterionic polysaccharide (PSA), dramatically impacts the development and function of the immune system [34].
In light of these new findings, biologists, particularly immunologists, may be expanding their view of the immune system under a larger umbrella. For example, as symbiont luminescence is critical to the host recognition of and response to the bacterial partner in the squid-vibrio system, it might be considered a MAMP. Similarly the light-responsive host elements, such as components of the visual transduction cascade or cryptochromes, might be considered PRRs (although this interpretation may broaden the definition of PRR beyond what is acceptable by most biologists). Might PSA of B. fragilis also be considered a MAMP?
Defining the ‘secret’ language of mutualistic symbioses, particularly those that involve multiple partners, such as in the vertebrate gut, will be challenging. The genomics age has allowed biologists a glimpse into the diversity of the microbiota of animals. Much insight into how the microbiota shapes the biology of a given animal is also likely to come from further host and symbiont genomic (e.g., the orphan genes) and transcriptomic (hypothetical genes whose expression is shared among mutualistic associations) signatures. As such, the study of mutualisms offers a remarkably fertile frontier for the field of biology.
Acknowledgements
We are grateful to AM Wier for TEM micrographs and EV Stabb for mutant strains of V. fischeri. This research was funded by NIH RO1-AI50661 to MMN, NSF IOS 0841507 to MMN and EG Ruby, NIH RR R01-12294 to EG Ruby, the WM Keck Foundation to MMN and EG Ruby.
Abbreviations
- LPS
lipopolysaccharide
- MAMP
microbe-associated molecular pattern
- PAMP
pathogen-associated molecular pattern
- PGN
peptidoglycan
- PGRP
peptidoglycan-recognition protein
- PRR
pattern-recognition receptor
- TCT
the peptidoglycan monomer, ‘tracheal cytotoxin’
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Margaret McFall-Ngai, Email: mjmcfallngai@wisc.edu.
Elizabeth A. C. Heath-Heckman, Email: heathheckman@wisc.edu.
Amani A. Gillette, Email: amani.gillette@yahoo.com.
Suzanne M. Peyer, Email: smpeyer@wisc.edu.
Elizabeth A. Harvie, Email: harvie@wisc.edu.
References
- 1.Eberl G. A new vision of immunity: homeostasis of the superorganism. Mucosal Immunol. 2010;3:450–460. doi: 10.1038/mi.2010.20. [DOI] [PubMed] [Google Scholar]
- 2.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruby EG. Symbiotic conversations are revealed under genetic interrogation. Nat Rev Microbiol. 2008;6:752–762. doi: 10.1038/nrmicro1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald SJ, Thomas GH, Douglas AE. Genetic and metabolic determinants of nutritional phenotype in an insect-bacterial symbiosis. Mol Ecol. 2011;20:2073–2084. doi: 10.1111/j.1365-294X.2011.05031.x. [DOI] [PubMed] [Google Scholar]
- 5.Koropatnick TA, Engle JT, Apicella MA, Stabb EV, Goldman WE, McFall-Ngai MJ. Microbial factor-mediated development in a host-bacterial mutualism. Science. 2004;306:1186–1188. doi: 10.1126/science.1102218. [DOI] [PubMed] [Google Scholar]
- 6.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 8.Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21:R488–R493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 10.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFall-Ngai M, Nyholm S, Castillo M. The role of the immune system in the initiation and persistence of the Euprymna scolopes-Vibrio fischeri symbiosis. Sem Immunol. 2010;22:48–53. doi: 10.1016/j.smim.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troll JV, Bent EH, Pacquette N, Wier AM, Goldman WE, Silverman N, McFall-Ngai MJ. Taming the symbiont for coexistence: A host PGRP neutralizes a bacterial symbiont toxin. Environ Microbiol. 2009 doi: 10.1111/j.1462-2920.2009.02121.x. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troll JV, Adin DM, Wier AM, Paquette N, Silverman N, Goldman WE, Stadermann FJ, Stabb EV, McFall-Ngai MJ. Peptidoglycan induces loss of a nuclear peptidoglycan recognition protein during host tissue development in a beneficial animal-bacterial symbiosis. Cellular Microbiol. 2009;11:1114–1127. doi: 10.1111/j.1462-5822.2009.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krasity BC, Troll JV, Weiss JP, McFall-Ngai MJ. LBP/BPI proteins and their relatives: Conservation over evolution and roles in mutualism. Biochem Soc Trans. 2011;39:1039–1044. doi: 10.1042/BST0391039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFall-Ngai MJ. The squid-vibrio association: A naturally occurring experimental model of animal-bacterial partnerships. Adv Exp Med Biol. 2008;635:102–112. doi: 10.1007/978-0-387-09550-9_9. [DOI] [PubMed] [Google Scholar]
- 16.McFall-Ngai MJ, Ruby EG. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science. 1991;254:1491–1494. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery MK, McFall-Ngai M. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development. 1994;120:1719–1729. doi: 10.1242/dev.120.7.1719. [DOI] [PubMed] [Google Scholar]
- 18.Wier AM, Nyholm SV, Mandel MJ, Massengo-Tiasse RP, Schaefer AL, Koroleva I, Splinter-BonDurant S, Brown B, Manzella L, Snir E, Almabrazi H, Scheetz TE, Bonaldo MD, Casavant TL, Soares MB, Cronan JE, Reed JL, Ruby EG, McFall-Ngai MJ. Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc Natl Acad Sci USA. 2010;107:2259–2264. doi: 10.1073/pnas.0909712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun CK, Troll JV, Koroleva I, Brown B, Manzella L, Snir E, Almabrazi H, Scheetz TE, Bonaldo M, Casavant TL, Soares MB, Ruby EG, McFall-Ngai MJ. Effects of colonization, luminescence and autoinducer on global host transcription in the developing squid-vibrio symbiosis. Proc Natl Acad Sci USA. 2008;105:11323–11328. doi: 10.1073/pnas.0802369105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boettcher KJ, Ruby EG, McFall-Ngai MJ. Bioluminescence in the symbiotic squid Euprymna scolopes is controlled by a daily biological rhythm. J Comp Physiol. 1996;179:65–73. [Google Scholar]
- 22.Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol. 2000;182:4578–4586. doi: 10.1128/jb.182.16.4578-4586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bose JL, Rosenberg CS, Stabb EV. Effects of luxCDABEG induction in Vibrio fischeri: enhancement of symbiotic colonization and conditional attenuation of growth in culture. Arch Microbiol. 2008;190:169–183. doi: 10.1007/s00203-008-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodson MS, Kojadinovic M, Troll JV, Scheetz TE, Casavant TL, Soares MB, McFall-Ngai MJ. Identifying components of the NF-kappaB pathway in the beneficial Euprymna scolopes-Vibrio fischeri light organ symbiosis. Appl Environ Microbiol. 2005;71:6934–6946. doi: 10.1128/AEM.71.11.6934-6946.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koropatnick TA, Kimbell JR, McFall-Ngai MJ. Responses of host hemocytes during the initiation of the squid-vibrio symbiosis. Biol Bull. 2007;212:29–39. doi: 10.2307/25066578. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery MK, McFall-Ngai MJ. The muscle-derived lens of a squid bioluminescent organ is biochemically convergent with the ocular lens. Evidence for recruitment of aldehyde dehydrogenase as a predominant structural protein. J Biol Chem. 1992;267:20999–21003. [PubMed] [Google Scholar]
- 27.Crookes WJ, Ding LL, Huang QL, Kimbell JR, Horwitz J, McFall-Ngai MJ. Reflectins: the unusual proteins of squid reflective tissues. Science. 2004;303:235–238. doi: 10.1126/science.1091288. [DOI] [PubMed] [Google Scholar]
- 28.Tong D, Rozas NS, Oakley TH, Mitchell J, Colley NJ, McFall-Ngai MJ. Evidence for light perception in a bioluminescent organ. Proc Natl Acad Sci USA. 2009;106:9836–9841. doi: 10.1073/pnas.0904571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billingham RE, Boswell T. Studies on the problem of corneal homografts. Proc R Soc Lond B Biol Sci. 1953;141:392–406. doi: 10.1098/rspb.1953.0049. [DOI] [PubMed] [Google Scholar]
- [19 to 30].Simpson E. A historical perspective on immunological privilege. Immunol Rev. 2006;213:12–22. doi: 10.1111/j.1600-065X.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- 31.Medwar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob F. Evolution and tinkering. Science. 1977;196:1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- 33.Jacob F. Complexity and tinkering. Ann N Y Acad Sci. 2001;929:71–73. doi: 10.1111/j.1749-6632.2001.tb05708.x. [DOI] [PubMed] [Google Scholar]
- 34.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]