Abstract
Pituitary tumor transforming gene (PTTG) is a well-studied oncogene for its role in tumorigenesis and serves as a marker of malignancy in several cancer types including lung. In the present study, we defined the role of PTTG in actin cytoskeleton remodeling, cell migration and induction of epithelial mesenchymal transition (EMT) through the regulation of integrin αVβ3-FAK signaling pathway. Overexpression of PTTG through an adenovirus vector resulted in a significant increase in the expression of integrins αV and β3, a process that was reversed with the down-regulation of PTTG expression through the use of an adenovirus expressing PTTG-specific siRNA. Western blot analysis of cells infected with adenovirus PTTG cDNA resulted in increased FAK and enhanced expression of adhesion complex molecules paxillin, metavincullin, and talin. Furthermore, downstream signaling genes Rac1, RhoA, Cdc42, and DOCK180 showed up-regulation upon PTTG overexpression. This process was dependent on integrin αV as blockage by antagonist echistatin (RGD peptide) or αV-specific siRNA resulted in a decrease in FAK and subsequent adhesion molecules. Actin cytoskeleton disruption was detected as a result of integrin-FAK signaling by PTTG as well as enhanced cell motility. Taken together our results suggest for the first time an important role of PTTG in regulation of integrins αV and β3 and adhesion complex proteins leading to induction of EMT.
Introduction
Integrins are a super family of heterodimeric transmembrane receptors responsible for cellular adhesion to extracellular matrix (ECM) proteins. A total of 18 α and 8 β subunits of integrins have been identified, which non-covalently bind to form 24 distinct transmembrane heterodimers, each with a specific, non-redundant function (Hynes, 2002). Specificity of an integrin in interacting with an extracellular ligand is determined by heterodimer composition of α and β subunits. The integrin αVβ3 binds to arginine-glycine-aspartic acid (RGD) containing compounds of the ECM such as vitronectin and fibronectin (Orlando and Cheresh, 1991), as well as blood and cell surface proteins (Ruoslahti, 1996). Integrins not only can trigger cytoskeletal rearrangements within the ECM but also connects to the cellular cytoskeleton through the actin-based microfilament system to mediate signals for the control of diverse cellular functions including survival, proliferation, differentiation, adhesion, and migration leading to changes in gene expression through outside-in signal transduction (Giancotti and Tarone, 2003; Hynes, 2002). This is accomplished with the aid of scaffolding proteins such as talin, vinculin, paxillin, and α-actinin as well as kinases (Berrier and Yamada, 2007). At least three kinases are activated through integrin-mediated cell attachment: focal adhesion kinase (FAK), protein kinase C (PKC), and Src (Berrier and Yamada, 2007; Ruoslahti, 1994), which modifies downstream signaling. FAK is a non-receptor protein tyrosine kinase (Parsons, 2003) that binds to the cytoplasmic tail of the integrin β-subunit via its SH3 domain located on the N-terminal tail (Huveneers et al., 2008; Schaller et al., 1995). The C-terminal tail of FAK contains protein-protein interaction sites that span ~100 amino acids (Martin et al., 2002). NMR and x-ray crystallography analysis show that this region contains a four-helix bundle that can be found on other adhesion proteins (Arold et al., 2002; Hayashi et al., 2002; Liu et al., 2002). Paxillin is activated by tyrosine phosphorylation and has been shown to be associated with the regulation of cytoskeletal organization, focal adhesion formation, cell migration, and cell motility (Petit et al., 2000; Turner, 2000). In addition to binding to integrins, vinculin also binds to the N-terminus of paxillin as well as actin. Binding of vinculin to actin is believed to contribute to the formation of structural links between integrin proteins and the actin cytoskeleton (Ezzell et al., 1997).
Metastasis is a physiological process in which tumor cells detach from the primary tumor, travel via the bloodstream to distance sites, invade, and form secondary tumors. This is accomplished through changes in cell polarity, which causes the tumor cells to lose cell-to-cell contact and exchange their epithelial markers for mesenchymal (Thiery, 2003), a process known as the epithelial to mesenchymal transition (EMT),which is a key program during the embryonic development but has more recently been implicated in cancer invasion and metastasis (Firrincieli et al., 2010; Thiery, 2003). Changes in cell polarity is accomplished through the loss of E-cadherin expression (Christiansen and Rajasekaran, 2006; Schmalhofer et al., 2009) and cell junction proteins, and coupled to the gain of mesenchymal markers such as vimentin and N-cadherin (Agiostratidou et al., 2007; Blanco et al., 2004). Changes in the expression and function of certain integrins, such as β1, has been implicated in cancer (Zuk and Hay, 1994). These polarity changes and resulting cellular focal adhesion changes enhance the ability of cells to migrate and invade the ECM, which is considered a functional hallmark of the EMT process (Christiansen and Rajasekaran, 2006; Danen et al., 2002).
Pituitary tumor transforming gene (PTTG) also known as securin regulates chromosomal segregation under normal physiological conditions (Kumada et al., 1998; Zou et al., 1999). PTTG was initially cloned from rat pituitary tumor cells as a 974 bp mRNA that encodes a 199 amino acid protein, which is not expressed in the normal pituitary (Pei and Melmed, 1997). Subsequently, PTTG was cloned from the adult testes and embryonic liver (Kakar and Jennes, 1999; Lee et al., 1999). PTTG is overexpressed in a variety of solid tumors including lung, ovary, uterine, pituitary, thyroid, liver, brain, and renal clear cell carcinoma (Chamaon et al., 2005; Cho-Rok et al., 2006; El-Naggar et al., 2007; Heaney and Melmed, 1999; Honda et al., 2003; Tang et al.; Tsai et al., 2005; Zhang et al., 1999). Furthermore, cloning and sequencing of PTTG isolated from tumors shares sequence homology with that isolated from human testes cDNA, suggesting that it is overexpression and not mutation that contributes to its oncogenic properties (Zou et al., 1999). Overexpression of PTTG is capable of stimulating cell proliferation in HEK293 and inducing cellular transformation in vitro using NIH3T3 and HEK293 cells as well as promotes tumor development in nude mice showing its tumorigenic potential without necessitating a partner oncogene (Hamid et al., 2005; Kakar and Jennes, 1999; Pei and Melmed, 1997). A 60% reduction of PTTG protein using siRNA in the lung cancer cell line H1299 showed inhibited colony formation using a soft agar assay and reduced xenograft tumor formation in nude mice (Kakar and Malik, 2006), furthermore demonstrating the role of PTTG in tumor growth and progression. In addition, a relationship between PTTG levels and cancer metastasis have been reported in several cancer types (Liang et al.; Shibata et al., 2002; Solbach et al., 2004; Yan et al., 2009). PTTG overexpression has also been shown to increase matrix-metalloproteinase 2 (MMP-2) expression and secretion resulting in increased cell migration and invasion to promote metastasis (Malik and Kakar, 2006). However, the relationship between PTTG and integrins αV and β3 in the promotion of actin cytoskeleton alternations through focal adhesion complex formation has not been explored. In the present study, we investigated the role of PTTG in regulation of integrins αV and β3 expression and the adhesion complex assembly to trigger alteration of the actin cytoskeleton and promote EMT in lung cancer cells.
Results
Overexpression of PTTG results in increased expression of integrins αVβ3, FAK, p-FAK and associated adhesion complex proteins paxillin, metavincullin, talin, Rac1, RhoA, Cdc42, and DOCK180
Adhesion complex formation is essential for rearrangement of the actin cytoskeleton for enhanced cell motility. In the present study, we used in vitro experiments to understand the molecular mechanisms involved in the formation of the focal adhesion complex by PTTG through the activation of integrins αVβ3 and subsequent activation of the FAK signaling pathway. For this purpose we generated an adenovirus expression system to over express PTTG cDNA (Ad-PTTG cDNA) and an adenovirus expressing PTTG siRNA (Ad-PTTG siRNA) to down-regulate the expression of PTTG. Human non-small cell lung carcinoma cell line H1299 and adenocarcinomic human alveolar basal epithelial cancer cell line A549 were selected to determine if these changes in expression were localized to a particular cell type or represented lung cancer in a broader sense. Quantitative real-time PCR (qPCR) analysis of PTTG mRNA showed a significant increase in expression upon infection of both A549 (Fig. 1A) and H1299 (Fig. 1C) cell lines with Ad-PTTG cDNA as compared to uninfected cells or cells infected with control Ad-GFP. Overexpression of PTTG was further confirmed by performing immunofluorescence analysis of both A549 and H1299 cells, which showed a significant increase in immunoreactive protein in Ad-PTTG cDNA infected cells compared to uninfected or cells infected with the control vector Ad-GFP (Fig. 1B, D).
Figure 1.
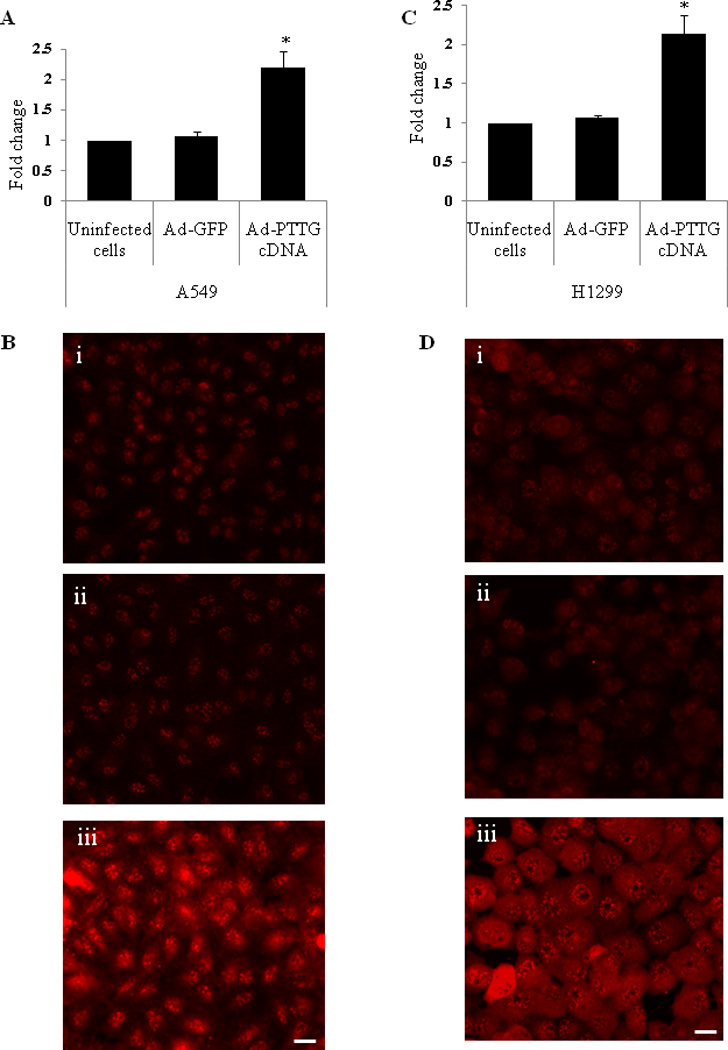
mRNA and protein expression of PTTG in A549 and H1299 cells. (A) mRNA expression in A549 uninfected cells, cells infected with Ad-GFP, or infected Ad-PTTG cDNA using qPCR. (B) PTTG protein expression in A549, i: uninfected cells, ii: Ad-GFP infected cells, iii: Ad-PTTG cDNA infected cells. (C) mRNA expression of PTTG in H1299 uninfected cells, cells infected with Ad-GFP, or infected with Ad-PTTG cDNA using qPCR. (D) PTTG protein expression in H1299, i: uninfected cells, ii: cells infected with Ad-GFP vector, iii: cells infected with Ad-PTTG cDNA. White bar shown in the right panels is 20 µm. qPCR values were normalized with GAPDH used as an internal control. Columns indicated the mean (n = 3); error bars represent SEM. *p < 0.05.
Integrins are the family of heterodimeric transmembrane adhesion receptors shown to be overexpressed in different tumors and tumor cell lines including lung cancer (Chen et al., 2005). To determine if PTTG regulates the expression of commonly expressed integrins αV and β3 in cancer, we overexpressed PTTG in A549 and H1299 cells by infecting the cells with Ad-PTTG cDNA and analyzed the expression of αV and β3 integrins using qPCR, which showed a significant increase in mRNA expression of both integrins αV and β3 (Fig. 2A, B). In addition, FACS analysis using the specific antibody for αVβ3 showed a significant increase in αVβ3 in cells infected with Ad-PTTG cDNA compared to uninfected cells or cells infected with Ad-GFP (Fig. 2C, D). In contrast, down regulation of PTTG by using Ad-PTTG siRNA resulted in a significant decrease in the levels of expression of integrins αV and β3 (Fig. 2A, B), suggesting regulation of expression of αV and β3 integrins by PTTG. Consistent with these findings, immunofluorescence analysis of A549 and H1299 cells for integrin αVβ3 revealed a significantly higher level of protein expression for αVβ3 in both cell lines infected with Ad-PTTG cDNA compared to uninfected cells or cells infected with Ad-GFP (Fig. 2E, F).
Figure 2.
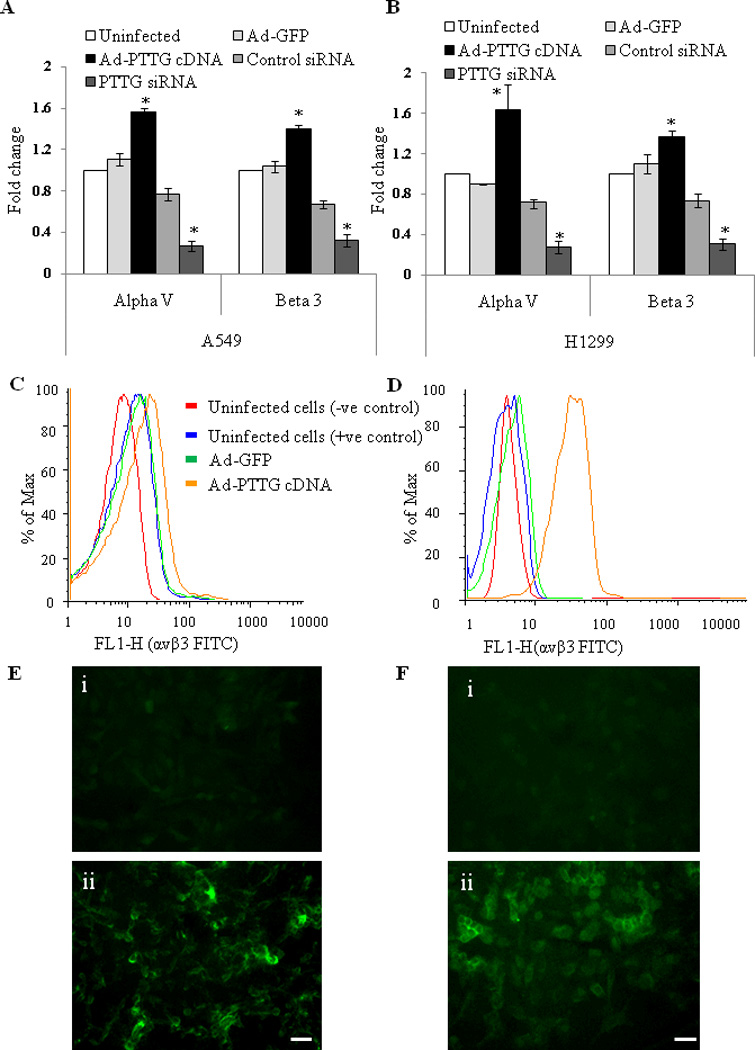
Expression of integrins αV and β3. (A) A549 and (B) H1299 cells infected with Ad-GFP or Ad-PTTG cDNA using qPCR. Values were normalized with GAPDH used as an internal control. Columns indicate the mean (n = 3); error bars represent SEM. *p < 0.05. (C–D) FACS analysis of cell stained with integrin αVβ3 Alexa Fluor 488 antibody: (C) FACS sorting in A549, (D) FACS sorting in H1299 cells. (E & F) Fluorescence staining for integrin αVβ3 in (E) A549 and (F) H1299 cells, i: uninfected cells, ii: Ad-PTTG cDNA infected. White bar shown in the right panels is 20 µm.
Tumor cell adhesion and migration, mediated by members of the integrin family, are linked to tumor cell growth and malignancy. Once ligated, integrins have several adhesion complex associated signaling molecules that mediate their biological function, such as FAK, leading to anchorage-independent survival and proliferation (Reddig and Juliano, 2005; Westhoff et al., 2004). As PTTG influences expression of integrins αVβ3, we investigated the effect of overexpression of PTTG on the phosphorylation and activation of FAK. Interestingly, we found that overexpression of PTTG in both A549 and H1299 cells resulted in an increase in the mRNA expression of total FAK compared to uninfected cells or cells infected with Ad-GFP (Fig. 3A, B). Increased expression of FAK was again confirmed by Western blotting. Both A549 (Fig. 3C,) and H1299 cells (Fig. 3D) showed a significantly higher level of FAK protein expression when infected with Ad-PTTG cDNA compared to uninfected cells and cells infected with Ad-GFP. As FAK is a non-receptor protein tyrosine kinase that becomes tyrosine phosphorylated and subsequently activated when integrins adhere to various matrix proteins, leading to increased cell migration (Zheng et al., 1999),we therefore investigated if PTTG-induced αVβ3 resulted in increased phosphorylation of FAK in lung cancer. Western blot analysis of phosphorylated FAK (p-FAK) showed an increase in level of p-FAK in response to increased total FAK in both A549 and H1299 cell lines upon Ad-PTTG cDNA infection compared to uninfected cells or cells infected with Ad-GFP (Fig. 3C, D). In response to phosphorylation, immunofluorescence of p-FAK showed localization to the cellular membrane upon treatment with Ad-PTTG cDNA (Fig. 3Eii), indicative of increased focal adhesion complex assembly.
Figure 3.
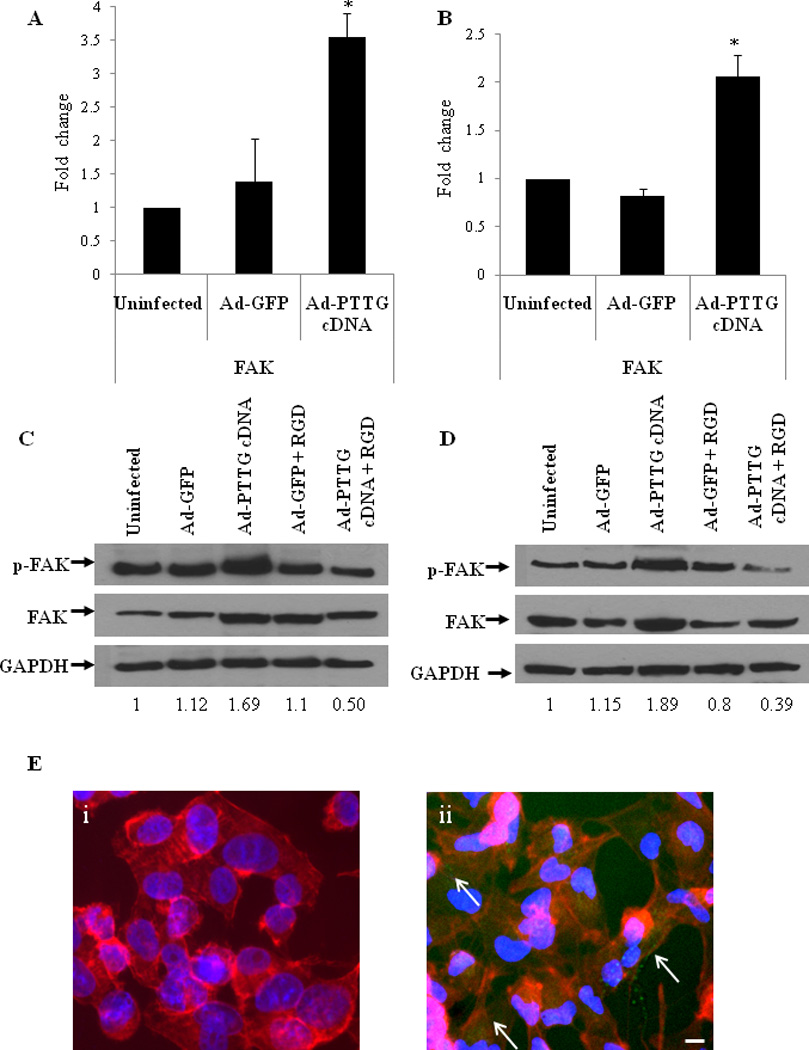
mRNA expression of FAK and protein expression of p-FAK and FAK. (A, B) mRNA expression of FAK using qPCR in A549 and H1299, respectively. Values were normalized with GAPDH. Column indicate the mean (n = 3); error bars represent SEM. *p < 0.05. (C–D) Western blot analysis for p-FAK, FAK, and GAPDH in A549 and H1299 cells, respectively. Cells were infected with Ad-GFP or Ad-PTTG cDNA for 48 hr. (E, F) Immunofluoroscence staining for p-FAK in A549 cells. i: uninfected cells, ii: cells infected with Ad-PTTG cDNA. p-FAK was detected by using Alexa Fluor 548 anti-mouse secondary antibody (green). Actin filaments were stained by phalloidin (red) and DAPI (blue) for nuclei. Bar shown in the right panels is 20 µm. Arrows indicate localization of p-FAK at the cellular membrane.
Additional adhesion complex molecules include talin, paxillin, and metavincullin (Ross, 2004). These signaling molecules interact with each other to produce adhesion dependent responses (Nishiya et al., 2001). In the case of lung cancer using A549 cells, PTTG overexpression resulted in a significantly higher level of expression of paxillin, metavincullin, and talin (Fig. 4A). Paxillin itself has several downstream signal transduction molecules including Rac1, RhoA, Cdc42, and DOCK180 (Valles et al., 2004). These molecules play important roles in migration and cell matrix adhesion (Danen et al., 2002). To fully understand their roles in mediating the function of integrins αVβ3 in response to overexpression by PTTG, we studied the expression of these molecules by qPCR. A549 cells infected with Ad-PTTG cDNA showed a higher level of mRNA expression for each Rac1, RhoA, Cdc42, and DOCK180, compared to uninfected cells or cells infected with control Ad-GFP (Fig. 4B).
Figure 4.
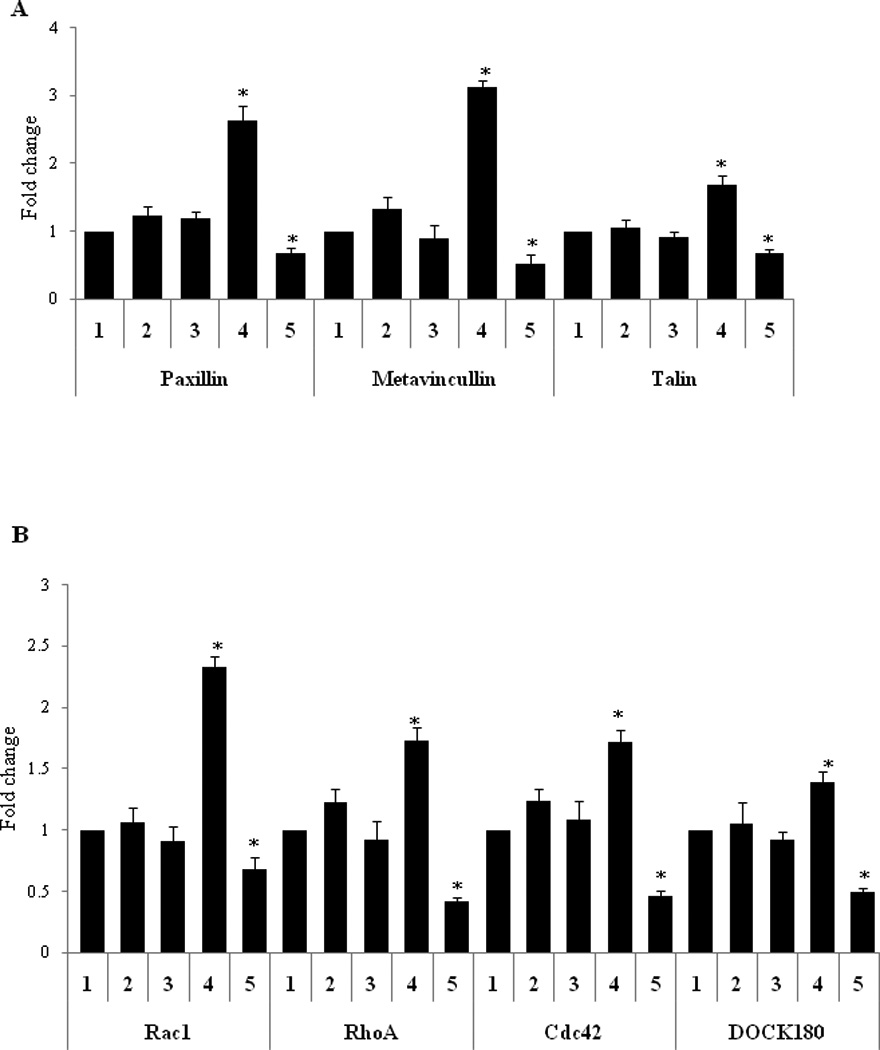
mRNA expression in A549 cells using qPCR. (A) paxillin, metavincullin, and talin. 1: untreated cells, 2: cells infected with Ad-GFP, 3: cells infected with Ad-GFP + control siRNA, 4: cells infected with Ad-PTTG, and 5: cells infected with Ad-PTTG cDNA + αV siRNA. (B) Rac1, RhoA, Cdc42, and DOCK180. Values were normalized with GAPDH. Columns indicated the mean (n = 3); error bars represent SEM. *p < 0.05.
Knockdown of integrin αV by αV siRNA results in decreased mRNA expression of its adhesion associated complex molecules paxillin, metavincullin, talin, Rac1, DOCK180, RhoA, and Cdc42
To confirm the role of PTTG in the induction of EMT through the activation of integrin αV, we knocked-out integrin αV mRNA by using siRNA (αV siRNA). Down regulation of integrin αV was confirmed by using immunohistochemical analysis (Fig. 5A). Infection of A549 cells with Ad-PTTG cDNA along with co-transfection using αV siRNA showed a significant decrease in mRNA expression of paxillin and metavincullin (Fig. 4A). Additionally, the downstream targets of paxillin, Rac1, RhoA, Cdc42, and DOCK180 were also down regulated (Fig. 4B), verifying the role of integrin αV in the assembly of the adhesion complex. Furthermore, down regulation of DOCK180 upon treatment with αV siRNA was confirmed by immunofluorescence (Fig. 5B).
Figure 5.
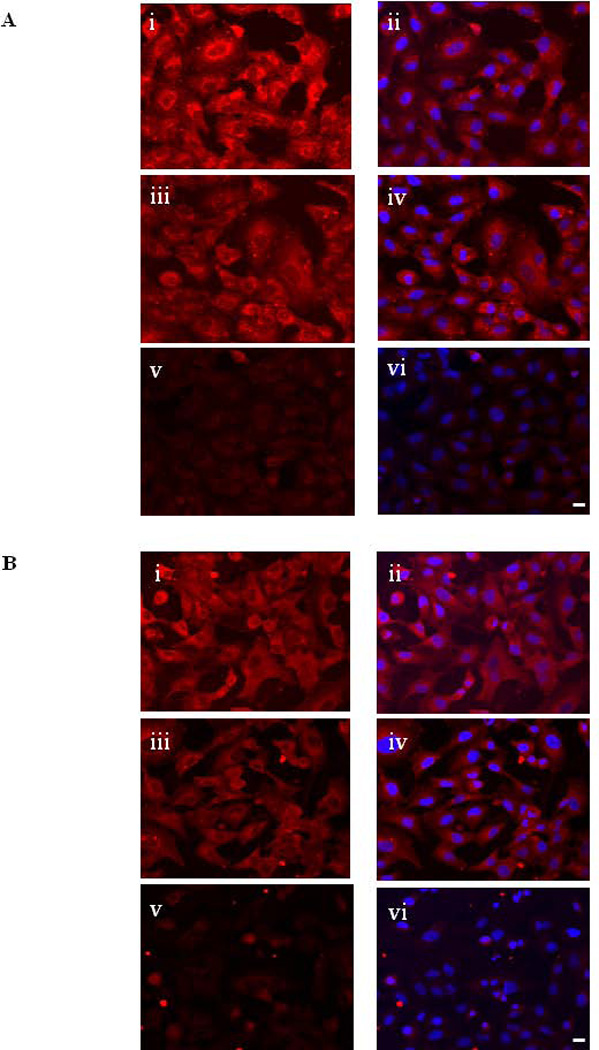
(A) Fluorescence staining for αV detected with Alexa Fluor 548 anti-mouse secondary antibody in A549 cells. i: un-transfected cells, ii: un-transfected cells counterstained with DAPI iii: cells transfected control siRNA, iv: control siRNA counterstained with DAPI, v: cells transfected with αV siRNA, vi: αV siRNA counterstained with DAPI. (B) Fluorescence staining for DOCK180 detected with Alexa Fluor 548 anti-mouse secondary antibody in A549 cells. i: un-transfected cells, ii: un-transfected cells counter stained with DAPI, iii: cells transfected with control siRNA, iv: control siRNA infected cells counter stained with DAPI, v: cells transfected with αV siRNA, vi: αV siRNA transfected cells countered stained with DAPI. White bar shown in the right panels is 20 µm.
Effect of knockdown of integrins αVβ3 by RGD peptides on expression of p-FAK
Activation and phosphorylation of FAK by ECM-integrins is an important phenomena for the cells to achieve migratory phenotype (McLean et al., 2005). In the present study, to investigate the potential role of PTTG in the induction of EMT through the activation of integrins and FAK pathway, we infected A549 and H1299 cells with Ad-PTTG cDNA or Ad-GFP and treated with echistatin, a specific RGD peptide, to block the activation of integrins. After 48 hr of total treatment, cells were harvested and protein extracts were analyzed for p-FAK and total FAK by western blot analysis. Both A549 and H1299 cell lines when infected with Ad-PTTG cDNA and RGD peptide showed a similar level of p-FAK to untreated cells (Fig.3C, D), indicating that RDG was able to block the effect of PTTG-induced FAK expression.
Effect of knockdown of integrin αV by siRNA on cell migration
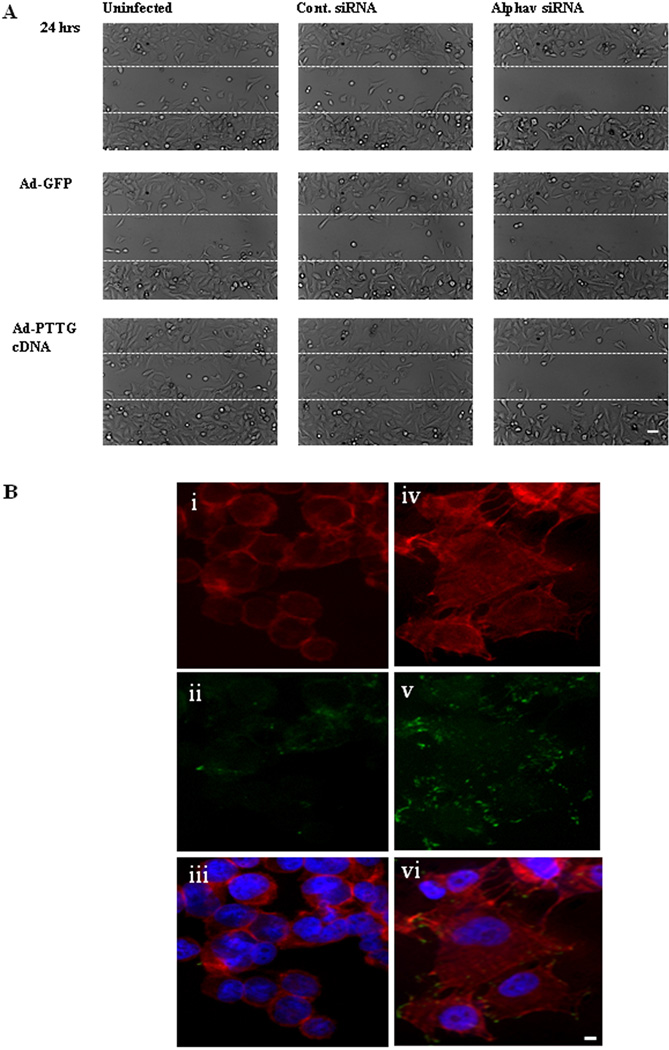
A549 cells were infected with Ad-PTTG cDNA or Ad-GFP control vector and co-transfected with αV siRNA or control siRNA. A wound was formed by scrapping away the cells and the cells were examined after 24 hr of wound formation. Overexpression of PTTG with and without control siRNA showed nearly complete healing of the wound after 24 hr compared to uninfected and Ad-GFP infected cells, which had fewer cells migrated into the gap (Fig. 6A). However, addition of αV siRNA significantly impaired the cells ability to migrate (Fig. 6A), suggesting that integrin αV is crucial to mediate the function of PTTG in tumor cell migration.
Figure 6.
(A) Wound migration assay in A549 cells. Cells were infected with Ad-GFP or Ad-PTTG cDNA and co-transfected with control siRNA or αV siRNA. Cells were examined after 24 hr post wound formation and photographed. White bar shown in the right panels is 20 µm. (B) Fluorescence microscopy of focal adhesion and actin cytoskeleton in A549 cells. F-actin was detected using TRITC-conjugated phalloidin (red). Focal contacts were revealed using anti-vinculin (green). i: uninfected cells with phalloidin, ii: uninfected cells with anti-vinculin, iii: overlay of i and ii with DAPI counter stain, iv: Ad-PTTG cDNA infected cells with phalloidin, v: AdPTTG cDNA infected cells with anti-vinculin, vi: overlay of iv and v with DAPI counter stain.
Actin cytoskeletal organization
In A549 cells, it has been shown that EMT leads to increased migratory and invasive abilities, which are characterized by re-organization of actin cytoskeleton through destruction and cellular protrusion formation (Keshamouni et al., 2006). To determine the role of PTTG in actin cytoskeletal reorganization, we infected A549 cells with Ad-PTTG cDNA and after 48 hr, cells were stained for F-actin and vinculin. Cells infected with Ad-PTTG cDNA showed morphological changes including formation of protrusions and destruction of actin filaments compared to uninfected cells (Fig. 6B), indicating a potential role of PTTG in the induction of EMT.
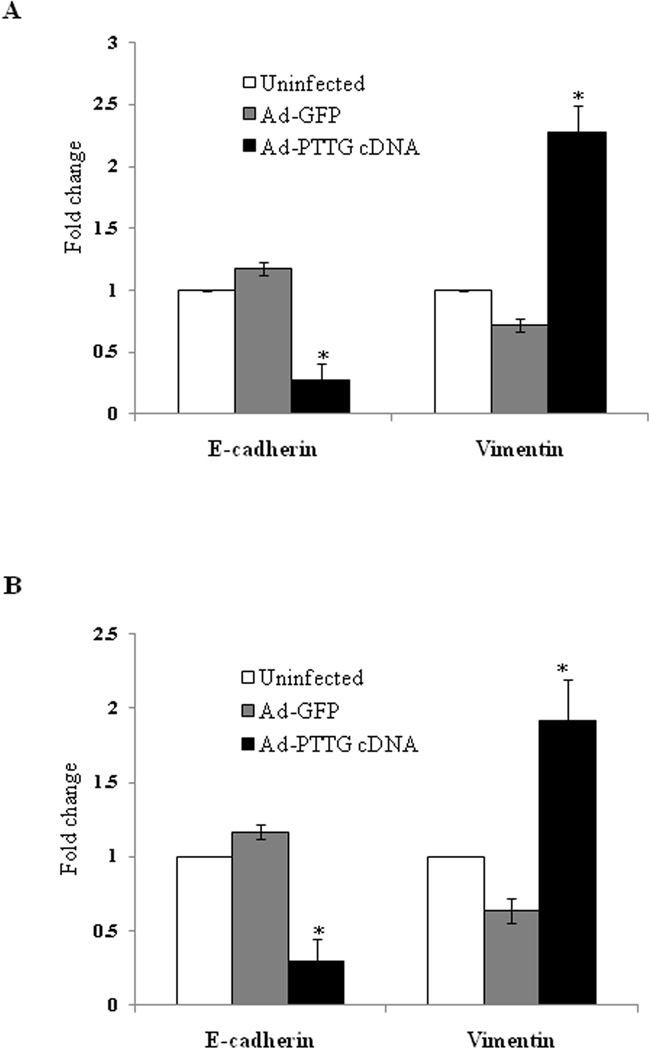
Expression of EMT markers E-cadherin and vimentin
To verify the induction of EMT, we tested the expression of epithelial marker E-cadherin and mesenchymal marker vimentin in A549 and H1299 cells on infection of cells with Ad-PTTG cDNA for 48 hr. In both cell lines, overexpression of PTTG resulted in a significant decrease in E-cadherin and increase in vimentin compared to Ad-GFP or untreated cells (Fig. 7), demonstrating the role of PTTG in the induction of EMT.
Figure 7.
E-cadherin and vimentin mRNA expression in (A) A549 and (B) H1299 using qPCR.
Discussion
Epithelial to mesenchymal transition (EMT) is a physiologic process that allows morphological and genetic changes of carcinoma cells from an epithelial to a mesenchymal phenotype. During EMT process, epithelial cell surface proteins that mediate cell-to cell contact, such as E-cadherin, are replaced by mesenchymal markers N-cadherin and vimentin, which provides more transient adhesive properties (Micalizzi et al., 2010). Numerous inducers of EMT in cancer cells have been identified including transforming growth factor-β (TGF-β), Wnt/β-catenin, Snail/Slug, Twist, and talin (Becam et al., 2005; Huber et al., 2005; Kim et al., 2002; Savagner et al., 1997; Yang et al., 2004; Zavadil and Bottinger, 2005). These factors have been reported to be critical as repressor of E-cadherin resulting in induction of EMT during developmental (Mercado-Pimentel and Runyan, 2007; Taneyhill et al., 2007). PTTG itself has been shown to increase cell proliferation and tumorigenesis in HEK293 cells, while mutation of PTTG decreased tumorigenesis (Hamid et al., 2005). In addition, down regulation of PTTG in lung cancer cells resulted in reduced colony formation and tumor formation in nude mice (Kakar and Malik, 2006). While PTTG can alter expression of MMP-2 (Malik and Kakar, 2006), co-expression of integrin αVβ3 with MMP-2 resulted in MMP-2 activation and correlated with progession of melanoma (Hofmann et al., 2000). Our results show that PTTG can act as an inducer of EMT by altering expression of E-cadherin and vimentin as well as integrin-mediated focal adhesion complex formation to alter the actin cytoskeleton. Adherin junctions incorporate the actin cytoskeleton allows the cells to “hold hands” and link cell to cell across the entire tissue, enabling a sheet of epithelial cells to move and function as a single cell (Vaezi et al., 2002). Therefore, changes in the actin cytoskeleton are a prerequisite for cell migration and invasion (Yilmaz and Christofori, 2009), and thus transition between an early stage neoplasm that is localized to the primary site to a late stage where metastasis has occurred. In addition to E-cadherin expression, alterations in the expression of integrins has been implicated in malignant transformation, tumor progression, and metastasis (Mizejewski, 1999). Integrins αVβ3 has been shown to be frequently overexpressed in tumor cells, including lung cancer, melanoma, glioblastoma, and breast cancer (Chen et al., 2005; Gladson and Cheresh, 1991; Rolli et al., 2003; Seftor et al., 1992). High level of expression of many integrins has been correlated with tumor progression and metastasis including α5β1, α6, and αVβ3 (Gong et al., 1997; Hofmann et al., 2000). In addition, mice treated with intetumumab, a monoclonal antibody to integrin αV, significantly reduced lung metastasis in A549 non-small cell lung cancer xenograft model (Ning et al., 2010). In the present study we demonstrated the potential role of PTTG in induction of EMT through the promotion of integrins αVβ3. Overexpression of PTTG resulted in a significant increase in level of integrins αV and β3 mRNA and protein (Fig. 2A–B, 3). In contrast down regulation of PTTG resulted in a reversal of the effects at both the mRNA and protein level (Fig. 2A–B), clearly demonstrating the involvement of PTTG in the induction of EMT through increased expression of integrin αVβ3. Thus this regulation occurs at the transcriptional level. No significant change in expression of other integrins such as α5, α6 or β1 was observed (Supplement Figure 1), indicating that αV and β3 probably are the main integrins in mediating the function of PTTG.
Integrin signaling is depending on the formation of adhesion complexes that includes both adaptor/scaffolding proteins as well as kinases that provide enzymatic activity (Berrier and Yamada, 2007). Proteins involved in adhesion complex formation include talin, paxillin, vinculin, α-actinin, FAK, and Src that interact either directly or indirectly to affect adhesion dependent responses (Playford and Schaller, 2004). Src interacts via its SH3 domain with integrin β3 to activate FAK signaling to promote tumor growth leading to cytoskeleton driven morphological changes (Huveneers et al., 2008). In addition to integrins, soluble growth factors also promote FAK activation, and thereby, FAK integrates growth factor receptor- and matrix-derived signals to elicit biological responses, including the induction of a migratory phenotype (McLean et al., 2005). FAK–Src complex has been shown to promote the phosphorylation of many FAK-associated Src substrates including CAS, paxillin, and p190RhoGAP, which have a central role in the reorganization of the actin cytoskeleton and migration (Playford and Schaller, 2004). So far several studies have reported the role of FAK signaling in the induction of EMT (Deng et al., 2010; Ponnusamy et al., 2010; Wendt et al., 2010). In our present study, we observed that overexpression of PTTG resulted in a significant increase in the total level of FAK and p-FAK (Fig. 3). Interestingly, knockout of integrins αVβ3 by using RGD peptide resulted in a significant decrease in the total level of FAK protein (Fig. 3) As a result of increased integrin and FAK expression by PTTG, we found a significant increase in the expression of adhesion complex proteins paxillin, metavincullin, and talin (Fig. 4A). Subsequent downstream molecules Rac1, RhoA, Cdc42, and DOCK180 were also overexpressed an effect which was reversed with the down regulation of integrin αV (Fig. 4B). The small GTPases Rac1, RhoA, and Cdc42 have been implicated in regulating the assembly and disassembly of the actin cytoskeleton (Altun-Gultekin et al., 1998; Berrier and Yamada, 2007; Klemke et al., 1998; Nakashima et al., 1999) while Cdc42-induced filopodia act as precursors for Rac-induced lamellipodia, establishing an integrin-dependent Rho GTPase activation in the regulation of cell spreading and migration (Guillou et al., 2008). DOCK180 has been shown to function as a guanine nucleotide exchange (GEF) factor for Rac to increase cell motility and enhance invasion (Jarzynka et al., 2007; Wang et al., 2010), a crucial step in the EMT process. Studies have shown that integrins αVβ3 and α5β1 differentially regulate RhoA activity in response to fibronectin binding (Danen et al., 2002). Consistent with our results, overexpression of integrins αVβ3 and subsequent overexpression of Rac1, RhoA, and Cdc42 as a result of PTTG overexpression leading to enhanced cell migration (Fig. 6A) and actin cytoskeleton destruction (Fig. 6B) as a step in induction of EMT.
Conclusion
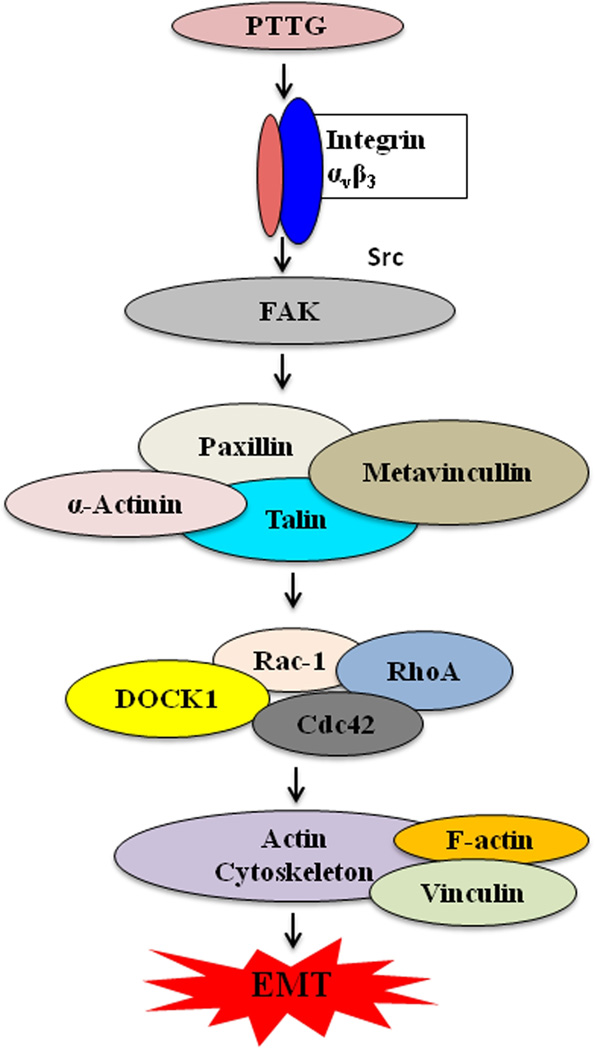
In the present study we have demonstrated for the first time the potential role of PTTG in the induction of EMT through the activation of integrin αVβ3 and its associated focal adhesion kinase pathway (Fig. 8).
Figure 8.
Schematic presentation of regulation of EMT by PTTG.
Materials and methods
Generation of plasmid and adenovirus constructs
The full length PTTG cDNA, PTTG siRNA, and control siRNA were sub-cloned into adenovirus shuttle vector (pShuttle). Positive clones were sequenced to confirm the orientation and authenticity of sequence as described previously (Panguluri and Kakar, 2009). The adenovirus expression systems were generated and purified in association with the Virus Vector Core Facility, Gene Therapy Center, University of North Carolina at Chapel Hill.
Cell culture and infection of cells with adenovirus expressing PTTG cDNA, GFP or PTTG-siRNA
Human non-small cell lung carcinoma cell line H1299 and adenocarcinomic human alveolar basal epithelial cancer cell line A549 were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in DMEM supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and 1% antibiotic/antimycotic (Sigma, St. Louis, MO, USA). The cell lines were routinely sub cultured every 3–4 days.
For infection of cells with adenovirus, cells were seeded in 6-well plates. After 24 hr, cells were infected with adenovirus expressing PTTG cDNA (Ad-PTTG cDNA), adenovirus expressing GFP (Ad-GFP), control siRNA (Ad-control siRNA) or PTTG-specific siRNA (AdPTTG siRNA) as described previously (Panguluri and Kakar, 2009).
Western blot analysis
Cells were plated in 6-well plates and infected with Ad-GFP or Ad-PTTG cDNA and treated with 100 nm echistatin (RGD peptide) after 24 hr of infection to block integrin αVβ3. After 48 hr of infection, cells were washed with PBS and lysed in chilled lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 1 mM Na3VO4, and 1 mM NaF] supplemented with Complete Mini Protease Inhibitor tablets (Roche Molecular Biochemical, Indianapolis, IN, USA). Protein was quantitated using BSA as a standard. Equal amount of protein (40 µg) from each sample was resolved on a 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA). Blots were probed with anti-FAK or anti-phospho-FAK (Tyr397) from Cell Signaling Technology (Danvers, MA, USA) at a dilution of 1:1,000 in Tris-buffered saline (pH 7.6) containing 0.1% Tween-20 (TBST). Immunoreactive proteins were visualized using the Enhanced Chemiluminescent Detection system (ECL) kit from GE Health System (Piscataway, NJ, USA) according to instructions provided by the supplier. The membrane was stripped by using western blot stripping reagent (BioRad, Hercules, CA, USA) and reprobed with GAPDH antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) to normalize the variation in loading of samples.
Immunofluorescence
Cells were cultured in chamber slides and infected with adenovirus as described above. After 48 hr of infection, cells were fixed with 4.0% paraformaldehyde in PBS for 30 min and then permeabilized with 0.2% Triton X-100 for 15 min at room temperature. Cells were rinsed twice with PBS then incubated for 1 hr with anti-PTTG polyclonal antibody (El-Naggar et al., 2007) at a dilution of 1:1,000 or anti-integrin αV (at a dilution of 1:1,000). After successive washes, cells were then incubated with Alexa Fluor 488 anti-rabbit or Alexa Fluor 548 anti-mouse secondary antibody. After incubation with secondary antibody for 45 min, cells were rinsed with PBS and incubated with DAPI for 20 min to label nuclei. Cells were then mounted with aquapolymount antifade solution (Sigma) and examined under a fluorescence microscope or with a MRC 600 confocal laser scanning microscope (Bio-Rad).
FACS analysis
FACS analysis was performed by using FACS Caliber Cytometer (BD Biosciences, San Jose, CA, USA). Briefly, after 48 hr of infection of A549 and H1299 cells with Ad-GFP or Ad-PTTG cDNA, cells were trypsinized and washed with wash buffer (PBS containing 0.1% BSA). After blocking for 1 hr, cells were incubated with anti-integrin αVβ3-Alexa Fluor 488 (Santa Cruz Biotechnology). The cells were washed with wash buffer followed by fixation for 1 hr. Cells were resuspended for analysis on a FACS Caliber cytometer and analyzed with FlowJo software.
Preparation of total RNA, synthesis of first strand cDNA, and quantitative real-time PCR
After 48 hr of infection with appropriate adenovirus, cells were harvested and total RNA was purified using trizol reagent (Sigma) according to the manufacturer’s protocol and as described previously (El-Naggar et al., 2007). First strand cDNA was synthesized using the iScript™ cDNA synthesis kit (BioRad) using 1 µg total RNA according to manufacturer’s protocol.
The real-time PCR reaction mixture was prepared in a Light Cycler® 480 (Roche Diagnostics) Multiwell 96 wells plate containing 1 µM of each primer, 10 µl of 2X master mix and 1µl of cDNA template, in a final reaction volume of 20 µl. The real-time PCR amplification was performed using the specific primers for PTTG, integrin αV, integrin β3, FAK, paxillin, metavincullin, talin, RhoA, Rac1, Cdc42, and DOCK180 (Table 1) using the following cycle parameters: enzyme activation at 95°C for 10 min; 45 cycles of 95°C for 10 s, 63°C for 10 s, and 72°C for 10 s. Following the amplification phase, a cooling step was performed at 40°C for 10 s (ramp rate of 1.5°C/s). Acquisition of the fluorescence signal was performed using the Mono Hydrolysis Probe setting (483–523 nm) following the 72°C extension phase of each cycle. GAPDH primers were included to normalize variation from sample to sample. All experiments were repeated three times using three independent preparations of cDNA.
Table I.
Primers Sequences for various genes
| Gene | Sequences |
|---|---|
| GAPDH | Sense 5’-TGA TGA CAT CAA GAA GGT GGT-3’ |
| Antisense 5’-TCC TTG GAG GCC ATG TGG GCC-3’ | |
| Integrin αV | Sense 5’-AATCTTCCAATTGAGGATATCAC-3’ |
| Antisense 5’-AAAACAGCCAGTAGCAACAAT-3’ | |
| Integrin β3 | Sense 5’-CCGTGACGAGATTGAGTCA-3’ |
| Antisense 5’-AGGATGGACTTTCCACTAGAA-3’ | |
| FAK | Sense 5’-GAA GTC TTC AGG GTC CGA TTG-3’ |
| Antisense 5’-CAT TCT CGT ACA CCT TAT CAT TCG-3’ | |
| Paxillin | Sense 5’-TGG CTT CGC TGT CGG ATT TC-3’ |
| Antisense 5’-GTC AAG GGC TGT CAC CAC TTT ATC-3’ | |
| Metavincullin | Sense 5’-CTT TCC CCT CTG ACA TGG AA-3’ |
| Antisense 5’-GAA TAA GTG CCC GCT TGG TA-3’ | |
| Talin | Sense 5’-ACC AGG TGA AGG TGA AGG TG-3’ |
| Antisense 5’-TTT TAG CGG CCT GAG TGA GT-3’ | |
| RhoA | Sense 5’-CGG AAT GAT GAG CAC ACA AGG-3’ |
| Antisense 5’-ATG TAC CCA AAA GCG CCA ATC-3’ | |
| Rac1 | Sense 5’-GCC GAT TGC CGA TGT GTT-3’ |
| Antisense 5’-CTC GGA TCG CTT CGT CAA A-3’ | |
| DOCK180 | Sense 5’-GGA AAT GAA AGG CGC TTC T-3’ |
| Antisense 5’-TCC ATT CCC ATC ATG CCG TTC-3’ | |
| Cdc42 | Sense 5’-AAG ACC CCA ATT TAC CTG AAA GC-3’ |
| Antisense 5’-TGG CGA AAG TCT CCA AGC G-3’ | |
| PTTG | Sense 5’-GCC TTA GAT GGG AGA TCT CA-3' |
| Antisense 5’-GCT TTA ACA GTC TTC TCA GT-3’ | |
| E-Cadherin | Sense 5’-TGA CAC CCG GGA CAA CGT TTA TTA-3’ |
| Antisense 5’-CTA GTC TAG ACC CCT AGT GGT CCT CG-3’ | |
| Vimentin | Sense 5’-GAC AAT GCG TCT CTG GCA CGT CTT-3’ |
| Antisense 5’-TCC TCC GCC TCC TGC AGG TTC TT-3’ |
Wound migration assay
Cells were treated with Ad-GFP or Ad-PTTG cDNA for 48 hr prior to wound formation. Wounds were formed by scraping away the cells and replacing the media. Cells were examined after 24 hr of wound formation.
Cytoskeleton organization analysis
Actin cytoskeleton staining was carried out by using Actin Cytoskeleton and Focal Adhesion staining kit purchased from Millipore (Temecula, CA, USA). Briefly, cells were cultured in chamber slides and infected with Ad-GFP or Ad-PTTG cDNA. After 48 hr of infection, cells were fixed with 4.0% paraformaldehyde in PBS for 30 min and then permeabilized with 0.2% Triton X-100 for 15 min at room temperature. After washing twice (5–10 min each) with washing buffer (PBS containing 0.05% Tween-20), followed by incubation with blocking solution (PBS containing 1% BSA) for 30 min. Cells were then incubated for 1 hr at room temperature with anti-vinculin at a dilution of 1:300. After two successive washes, cells were then exposed to corresponding FITC-conjugated secondary antibody at a dilution of 1:500 for 60 min at room temperature. For double labeling, cells were simultaneously incubated with TRITC-conjugated Phalloidin at the dilution of 1:1 000 to map the local orientation of actin filaments. After washing of cells with washing buffer, nuclei were counterstained with DAPI (diluted 1:500) for 20 min at room temperature followed by three washes (5–10 min each) with washing buffer. The cells were then mounted with aquapolymount antifade solution (Sigma) and observed under an Olympus IX50 fluorescence microscope or using a MRC 600 laser scanning microscope (Bio-Rad).
Statistical analysis
Data comparing differences between two groups were statistically analyzed using unpaired Student’s t-test. Differences were considered significant when p < 0.05.
Supplementary Material
Supplemental Figure 1. Effect of PTTG on expression of various integrins assessed by qPCR. mRNA from A549 uninfected cells, cells infected with Ad-GFP, or infected Ad-PTTG cDNA was subjected to real-time PCR.
Acknowledgements
The authors would like to thank Dr. Stanley D’Souza for his critical input. The work was supported by a grant from NCI CA 124630 (SSK).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Parag P. Shah, Email: ppshah04@louisville.edu.
Miranda Y. Fong, Email: myfong01@louisville.edu.
Sham S. Kakar, Email: sskaka01@louisville.edu.
References
- Agiostratidou G, Hulit J, Phillips GR, Hazan RB. Differential cadherin expression: potential markers for epithelial to mesenchymal transformation during tumor progression. J Mammary Gland Biol Neoplasia. 2007;12:127–133. doi: 10.1007/s10911-007-9044-6. [DOI] [PubMed] [Google Scholar]
- Altun-Gultekin ZF, Chandriani S, Bougeret C, Ishizaki T, Narumiya S, de Graaf P, et al. Activation of Rho-dependent cell spreading and focal adhesion biogenesis by the v-Crk adaptor protein. Mol Cell Biol. 1998;18:3044–3058. doi: 10.1128/mcb.18.5.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arold ST, Hoellerer MK, Noble ME. The structural basis of localization and signaling by the focal adhesion targeting domain. Structure. 2002;10:319–327. doi: 10.1016/s0969-2126(02)00717-7. [DOI] [PubMed] [Google Scholar]
- Becam IE, Tanentzapf G, Lepesant JA, Brown NH, Huynh JR. Integrin-independent repression of cadherin transcription by talin during axis formation in Drosophila. Nat Cell Biol. 2005;7:510–516. doi: 10.1038/ncb1253. [DOI] [PubMed] [Google Scholar]
- Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- Blanco D, Vicent S, Elizegi E, Pino I, Fraga MF, Esteller M, et al. Altered expression of adhesion molecules and epithelial-mesenchymal transition in silica-induced rat lung carcinogenesis. Lab Invest. 2004;84:999–1012. doi: 10.1038/labinvest.3700129. [DOI] [PubMed] [Google Scholar]
- Chamaon K, Kirches E, Kanakis D, Braeuninger S, Dietzmann K, Mawrin C. Regulation of the pituitary tumor transforming gene by insulin-like-growth factor-I and insulin differs between malignant and non-neoplastic astrocytes. Biochem Biophys Res Commun. 2005;331:86–92. doi: 10.1016/j.bbrc.2005.03.124. [DOI] [PubMed] [Google Scholar]
- Chen X, Sievers E, Hou Y, Park R, Tohme M, Bart R, et al. Integrin alpha v beta 3-targeted imaging of lung cancer. Neoplasia. 2005;7:271–279. doi: 10.1593/neo.04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho-Rok J, Yoo J, Jang YJ, Kim S, Chu IS, Yeom YI, et al. Adenovirus-mediated transfer of siRNA against PTTG1 inhibits liver cancer cell growth in vitro and in vivo. Hepatology. 2006;43:1042–1052. doi: 10.1002/hep.21137. [DOI] [PubMed] [Google Scholar]
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- Danen EH, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol. 2002;159:1071–1086. doi: 10.1083/jcb.200205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B, Yang X, Liu J, He F, Zhu Z, Zhang C. Focal adhesion kinase mediates TGF-beta1-induced renal tubular epithelial-to-mesenchymal transition in vitro. Mol Cell Biochem. 2010;340:21–29. doi: 10.1007/s11010-010-0396-7. [DOI] [PubMed] [Google Scholar]
- El-Naggar SM, Malik MT, Kakar SS. Small interfering RNA against PTTG: a novel therapy for ovarian cancer. Int J Oncol. 2007;31:137–143. [PubMed] [Google Scholar]
- Ezzell RM, Goldmann WH, Wang N, Parashurama N, Ingber DE. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp Cell Res. 1997;231:14–26. doi: 10.1006/excr.1996.3451. [DOI] [PubMed] [Google Scholar]
- Firrincieli D, Boissan M, Chignard N. Epithelial-mesenchymal transition in the liver. Gastroenterol Clin Biol. 2010;34:523–528. doi: 10.1016/j.gcb.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Wang D, Sun L, Zborowska E, Willson JK, Brattain MG. Role of alpha 5 beta 1 integrin in determining malignant properties of colon carcinoma cells. Cell Growth Differ. 1997;8:83–90. [PubMed] [Google Scholar]
- Guillou H, Depraz-Depland A, Planus E, Vianay B, Chaussy J, Grichine A, et al. Lamellipodia nucleation by filopodia depends on integrin occupancy and downstream Rac1 signaling. Exp Cell Res. 2008;314:478–488. doi: 10.1016/j.yexcr.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Hamid T, Malik MT, Kakar SS. Ectopic expression of PTTG1/securin promotes tumorigenesis in human embryonic kidney cells. Mol Cancer. 2005;4:3. doi: 10.1186/1476-4598-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I, Vuori K, Liddington RC. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat Struct Biol. 2002;9:101–106. doi: 10.1038/nsb755. [DOI] [PubMed] [Google Scholar]
- Heaney AP, Melmed S. Pituitary tumour transforming gene: a novel factor in pituitary tumour formation. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:367–380. doi: 10.1053/beem.1999.0028. [DOI] [PubMed] [Google Scholar]
- Hofmann UB, Westphal JR, Waas ET, Becker JC, Ruiter DJ, van Muijen GN. Coexpression of integrin alpha(v)beta3 and matrix metalloproteinase-2 (MMP-2) coincides with MMP-2 activation: correlation with melanoma progression. J Invest Dermatol. 2000;115:625–632. doi: 10.1046/j.1523-1747.2000.00114.x. [DOI] [PubMed] [Google Scholar]
- Honda S, Hayashi M, Kobayashi Y, Ishikawa Y, Nakagawa K, Tsuchiya E. A role for the pituitary tumor-transforming gene in the genesis and progression of non-small cell lung carcinomas. Anticancer Res. 2003;23:3775–3782. [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Huveneers S, Arslan S, van de Water B, Sonnenberg A, Danen EH. Integrins uncouple Src-induced morphological and oncogenic transformation. J Biol Chem. 2008;283:13243–13251. doi: 10.1074/jbc.M800927200. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jarzynka MJ, Hu B, Hui KM, Bar-Joseph I, Gu W, Hirose T, et al. ELMO1 and Dock180, a bipartite Rac1 guanine nucleotide exchange factor, promote human glioma cell invasion. Cancer Res. 2007;67:7203–7211. doi: 10.1158/0008-5472.CAN-07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar SS, Jennes L. Molecular cloning and characterization of the tumor transforming gene (TUTR1): a novel gene in human tumorigenesis. Cytogenet Cell Genet. 1999;84:211–216. doi: 10.1159/000015261. [DOI] [PubMed] [Google Scholar]
- Kakar SS, Malik MT. Suppression of lung cancer with siRNA targeting PTTG. Int J Oncol. 2006;29:387–395. [PubMed] [Google Scholar]
- Keshamouni VG, Michailidis G, Grasso CS, Anthwal S, Strahler JR, Walker A, et al. Differential protein expression profiling by iTRAQ-2DLC-MS/MS of lung cancer cells undergoing epithelial-mesenchymal transition reveals a migratory/invasive phenotype. J Proteome Res. 2006;5:1143–1154. doi: 10.1021/pr050455t. [DOI] [PubMed] [Google Scholar]
- Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a "molecular switch" for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada K, Nakamura T, Nagao K, Funabiki H, Nakagawa T, Yanagida M. Cut1 is loaded onto the spindle by binding to Cut2 and promotes anaphase spindle movement upon Cut2 proteolysis. Curr Biol. 1998;8:633–641. doi: 10.1016/s0960-9822(98)70250-7. [DOI] [PubMed] [Google Scholar]
- Lee IA, Seong C, Choe IS. Cloning and expression of human cDNA encoding human homologue of pituitary tumor transforming gene. Biochem Mol Biol Int. 1999;47:891–897. doi: 10.1080/15216549900201993. [DOI] [PubMed] [Google Scholar]
- Liang H, Zhong Y, Luo Z, Huang Y, Lin H, Luo M, et al. Assessment of biomarkers for clinical diagnosis of papillary thyroid carcinoma with distant metastasis. Int J Biol Markers. 25:38–45. doi: 10.1177/172460081002500106. [DOI] [PubMed] [Google Scholar]
- Liu G, Guibao CD, Zheng J. Structural insight into the mechanisms of targeting and signaling of focal adhesion kinase. Mol Cell Biol. 2002;22:2751–2760. doi: 10.1128/MCB.22.8.2751-2760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik MT, Kakar SS. Regulation of angiogenesis and invasion by human Pituitary tumor transforming gene (PTTG) through increased expression and secretion of matrix metalloproteinase-2 (MMP-2) Mol Cancer. 2006;5:61. doi: 10.1186/1476-4598-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KH, Boerner SA, Parsons JT. Regulation of focal adhesion targeting and inhibitory functions of the FAK related protein FRNK using a novel estrogen receptor "switch". Cell Motil Cytoskeleton. 2002;51:76–88. doi: 10.1002/cm.10018. [DOI] [PubMed] [Google Scholar]
- McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Runyan RB. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs. 2007;185:146–156. doi: 10.1159/000101315. [DOI] [PubMed] [Google Scholar]
- Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124–138. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- Nakashima N, Rose DW, Xiao S, Egawa K, Martin SS, Haruta T, et al. The functional role of CrkII in actin cytoskeleton organization and mitogenesis. J Biol Chem. 1999;274:3001–3008. doi: 10.1074/jbc.274.5.3001. [DOI] [PubMed] [Google Scholar]
- Ning S, Tian J, Marshall DJ, Knox SJ. Anti-alphav integrin monoclonal antibody intetumumab enhances the efficacy of radiation therapy and reduces metastasis of human cancer xenografts in nude rats. Cancer Res. 2010;70:7591–7599. doi: 10.1158/0008-5472.CAN-10-1639. [DOI] [PubMed] [Google Scholar]
- Nishiya N, Tachibana K, Shibanuma M, Mashimo JI, Nose K. Hic-5-reduced cell spreading on fibronectin: competitive effects between paxillin and Hic-5 through interaction with focal adhesion kinase. Mol Cell Biol. 2001;21:5332–5345. doi: 10.1128/MCB.21.16.5332-5345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando RA, Cheresh DA. Arginine-glycine-aspartic acid binding leading to molecular stabilization between integrin alpha v beta 3 and its ligand. J Biol Chem. 1991;266:19543–19550. [PubMed] [Google Scholar]
- Panguluri SK, Kakar SS. Effect of PTTG on endogenous gene expression in HEK 293 cells. BMC Genomics. 2009;10:577. doi: 10.1186/1471-2164-10-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pei L, Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG) Mol Endocrinol. 1997;11:433–441. doi: 10.1210/mend.11.4.9911. [DOI] [PubMed] [Google Scholar]
- Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Valles AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol. 2000;148:957–970. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- Ponnusamy MP, Lakshmanan I, Jain M, Das S, Chakraborty S, Dey P, et al. MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene. 2010;29:5741–5754. doi: 10.1038/onc.2010.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:9482–9487. doi: 10.1073/pnas.1633689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res. 2004;63:381–390. doi: 10.1016/j.cardiores.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Cell adhesion and tumor metastasis. Princess Takamatsu Symp. 1994;24:99–105. [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- Seftor RE, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, et al. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci U S A. 1992;89:1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Haruki N, Kuwabara Y, Nishiwaki T, Kato J, Shinoda N, et al. Expression of PTTG (pituitary tumor transforming gene) in esophageal cancer. Jpn J Clin Oncol. 2002;32:233–237. doi: 10.1093/jjco/hyf058. [DOI] [PubMed] [Google Scholar]
- Solbach C, Roller M, Fellbaum C, Nicoletti M, Kaufmann M. PTTG mRNA expression in primary breast cancer: a prognostic marker for lymph node invasion and tumor recurrence. Breast. 2004;13:80–81. doi: 10.1016/j.breast.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Taneyhill LA, Coles EG, Bronner-Fraser M. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development. 2007;134:1481–1490. doi: 10.1242/dev.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MH, Tan J, Cui FL. Expression of pituitary tumor transforming gene in human renal clear cell carcinoma and its significance. Nan Fang Yi Ke Da Xue Xue Bao. 30:1712–1714. [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Lin SJ, Cheng YM, Chen HM, Wing LY. Expression and functional analysis of pituitary tumor transforming gene-1 [corrected] in uterine leiomyomas. J Clin Endocrinol Metab. 2005;90:3715–3723. doi: 10.1210/jc.2004-2303. [DOI] [PubMed] [Google Scholar]
- Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- Valles AM, Beuvin M, Boyer B. Activation of Rac1 by paxillin-Crk-DOCK180 signaling complex is antagonized by Rap1 in migrating NBT-II cells. J Biol Chem. 2004;279:44490–44496. doi: 10.1074/jbc.M405144200. [DOI] [PubMed] [Google Scholar]
- Wang H, Linghu H, Wang J, Che YL, Xiang TX, Tang WX, et al. The role of Crk/Dock180/Rac1 pathway in the malignant behavior of human ovarian cancer cell SKOV3. Tumour Biol. 2010;31:59–67. doi: 10.1007/s13277-009-0009-9. [DOI] [PubMed] [Google Scholar]
- Wendt MK, Smith JA, Schiemann WP. Transforming growth factor-beta-induced epithelial-mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene. 2010;29:6485–6498. doi: 10.1038/onc.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff MA, Serrels B, Fincham VJ, Frame MC, Carragher NO. SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol Cell Biol. 2004;24:8113–8133. doi: 10.1128/MCB.24.18.8113-8133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Zhou C, Lou X, Xiao Z, Zhu H, Wang Q, et al. PTTG overexpression promotes lymph node metastasis in human esophageal squamous cell carcinoma. Cancer Res. 2009;69:3283–3290. doi: 10.1158/0008-5472.CAN-08-0367. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer and Metastasis Reviews. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- Zhang X, Horwitz GA, Prezant TR, Valentini A, Nakashima M, Bronstein MD, et al. Structure, expression, and function of human pituitary tumor-transforming gene (PTTG) Mol Endocrinol. 1999;13:156–166. doi: 10.1210/mend.13.1.0225. [DOI] [PubMed] [Google Scholar]
- Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]
- Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- Zuk A, Hay ED. Expression of beta 1 integrins changes during transformation of avian lens epithelium to mesenchyme in collagen gels. Dev Dyn. 1994;201:378–393. doi: 10.1002/aja.1002010409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Effect of PTTG on expression of various integrins assessed by qPCR. mRNA from A549 uninfected cells, cells infected with Ad-GFP, or infected Ad-PTTG cDNA was subjected to real-time PCR.