Abstract
Background
We sought to measure trends in Streptococcus pneumoniae (SP) carriage and antibiotic resistance in young children in Massachusetts communities after widespread adoption of heptavalent pneumococcal conjugate vaccine (PCV7) and before the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13).
Methods
We conducted a cross-sectional study including collection of questionnaire data and nasopharyngeal specimens among children <7 years in primary care practices from 8 Massachusetts communities during the winter season of 2008–9 and compared with to similar studies performed in 2001, 2003–4, and 2006–7. Antimicrobial susceptibility testing and serotyping were performed on pneumococcal isolates, and risk factors for colonization in recent seasons (2006–07 and 2008–09) were evaluated.
Results
We collected nasopharyngeal specimens from 1,011 children, 290 (29%) of whom were colonized with pneumococcus. Non-PCV7 serotypes accounted for 98% of pneumococcal isolates, most commonly 19A (14%), 6C (11%), and 15B/C (11%). In 2008–09, newly-targeted PCV13 serotypes accounted for 20% of carriage isolates and 41% of penicillin non-susceptible S. pneumoniae (PNSP). In multivariate models, younger age, child care, young siblings, and upper respiratory illness remained predictors of pneumococcal carriage, despite near-complete serotype replacement. Only young age and child care were significantly associated with PNSP carriage.
Conclusions
Serotype replacement post-PCV7 is essentially complete and has been sustained in young children, with the relatively virulent 19A being the most common serotype. Predictors of carriage remained similar despite serotype replacement. PCV13 may reduce 19A and decrease antibiotic-resistant strains, but monitoring for new serotype replacement is warranted.
Keywords: Streptococcus pneumoniae, pneumococcal conjugate vaccine, antibiotic resistance, serotype, colonization
Introduction
Following the introduction of heptavalent pneumococcal conjugate vaccine (PCV7) in 2000, the rates of invasive pneumococcal disease (IPD) in U.S. children have decreased substantially.[1–5] Rates of other childhood pneumococcal illnesses such as otitis media and non-invasive pneumonia have also decreased.[6–8] The adoption of PCV7 was also followed by reduced IPD rates in older children, adults and immunocompromised hosts through herd immunity.[3, 5, 9–10]
Nine years after introduction of PCV7, IPD rates in children remain lower than pre-vaccine levels,[11] but reductions in PCV7-type IPD have been partially offset by increases in serotypes not targeted by the vaccine.[3, 11–22] Non-vaccine serotypes, notably 19A, have rapidly filled the ecologic niche previously occupied by PCV7 serotypes, primarily by clonal expansion and, to a lesser extent, capsular switching; they now account for a substantial fraction of pneumococcal colonization and IPD in children and adults.[3, 12–19, 21, 23–25] Furthermore, antibiotic resistance has increasingly emerged among non-vaccine serotypes through both clonal expansion of previously resistant serotypes and acquisition of resistance by serotypes that were mainly susceptible in the past.[21, 23, 26]
Non-vaccine serotype replacement and the continued burden of pneumococcal disease have stimulated the development of a vaccine that targets a broader range of serotypes. In February 2010, the Food and Drug Administration (FDA) approved licensure of the 13-valent pneumococcal conjugate vaccine (PCV13),[27] which targets the same 7 serotypes as PCV7 and 6 additional serotypes (1, 3, 5, 6A, 7F and 19A), accounting for an estimated 63% of IPD in US children <5 years old in 2007.[11] Ongoing evaluation of the pneumococcal serotypes carried in a defined geographic area allows us to assess whether S. pneumoniae carriage serotypes have stabilized several years after PCV7 introduction and to anticipate the impact widespread use of PCV13 could have on carriage and disease.
Our prior work in Massachusetts communities in 2001[28], 2003–4[13] and 2006–7[21] showed that replacement of vaccine serotypes by non-vaccine serotypes in the nasopharynx was rapid and virtually complete by 2007.[21] Certain clones previously associated with vaccine serotypes persisted in the post-vaccine era by acquiring a non-vaccine serotype, including the multiply resistant ST 320 clone which newly expressed a 19A capsule.[29–31] Certain non-vaccine serotype clones associated with lowered susceptibility to penicillin (ST 199, ST 558) rapidly increased in carriage prevalence.[29] We now report the results of ongoing surveillance of S. pneumoniae carriage in the same Massachusetts communities. We evaluate ongoing changes in serotype distribution and antibiotic resistance in the context of universal use of PCV7 before the introduction of PCV13.
Materials and Methods
Study design and population
Children 3 months to <7 years of age seen in pediatric practices in 8 Massachusetts communities were enrolled during the “cold and flu” season from October 2008 to March 2009. Children in these 8 communities were similarly recruited for pneumococcal colonization assessments in young healthy children during comparable periods in 2001, 2003–04, and 2006–07.[13, 21, 28] Children from an additional 8 Massachusetts communities were also included in 2001 and 2003–4.
Parental consent was obtained by study staff members and nasopharyngeal specimens were obtained by trained nurses during well-child or sick visits. Parents of participating children completed a brief questionnaire regarding exposure to some potential predictors of pneumococcal carriage, including group child care, young siblings, recent antibiotic use, and concurrent illness.[28] Participants’ medical records were also reviewed for information on vaccines received, recent antibiotic use and symptoms and diagnoses at the time of the visit. All study procedures were approved by the Harvard Pilgrim Health Care institutional review board.
Identification of Serotypes and Antimicrobial Resistance Profiles
Nasopharyngeal swabs were plated within 24 hours for identification of S. pneumoniae. Serotype was primarily determined using the Quellung reaction with commercially available antisera (Serum Statens Institute, Copenhagen, Denmark), with confirmation by multi-locus sequence typing (MLST). All putative 6A specimens were then tested using monoclonal antibodies to identify which were actually the closely related 6C serotype.[32] Antibiotic susceptibility testing was performed using E-tests. Clinical and Laboratory Standards Institute (CLSI) susceptibility breakpoints were used to classify organisms as susceptible, intermediate, or resistant based on 2008 criteria to the following antibiotics: penicillin, amoxicillin, ceftriaxone, erythromycin, clindamycin, trimethoprim-sulfamethoxazole, levofloxacin, and vancomycin.[33–34] CLSI susceptibility breakpoints for penicillin were the following: susceptible (minimal inhibitory concentration [MIC] of ≤2 mg/L), intermediate (4 mg/L), and resistant (≥8.0 mg/L).[32] For comparison purposes across sampling periods, we also used pre-2008 penicillin susceptibility breakpoint criteria: susceptible (MIC of ≤0.06 mg/L), intermediate (>0.06 to 1.0 mg/L), and resistant (≥2.0 mg/L).[33] Penicillin non-susceptible S. pneumoniae (PNSP) was defined as any intermediate or resistant isolate when using either PNSP>0.06 or PNSP>2.0breakpoints.
Data Analysis
The proportions of children colonized with a) any S. pneumoniae, b) PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, 23F), c) additional PCV13 serotypes (1, 3, 5, 6A, 7F, 19A), and d) non-PCV13 serotypes were calculated for the 2008-09 season and compared to data from 2001, 2003–04, and 2006–07. As described previously, data from all available communities in each sampling period (16 communities in 2001 and 2003–04; subset of 8 communities in 2006–07 and 2008–09) were used to evaluate trends over time.[21] Because serotype information was missing for 35 S. pneumoniae isolates (which could not be re-grown) from 2001 and 7 isolates from 2003–04, the proportion of carriage among isolates with known serotypes are reported over time. Trends in antibiotic susceptibility were also described for S. pneumoniae across the 4 sampling periods; statistical tests for differences and for linear trends over time were performed using generalized linear mixed models controlling for community.
Potential predictors of overall pneumococcal carriage and PNSP carriage were gathered from questionnaires and chart review data. These included age group (3-<6mo, 6-<24mo, 24-<36mo, 36-<84mo), gender, group child care attendance, presence of young siblings <6 years, recent antibiotic use in last 2 months and acute respiratory tract infection (RTI) at time of sampling.[21, 28] Acute RTI was defined by reported symptoms on questionnaires or a diagnosis in the medical record of any upper or lower RTI, including otitis media, sinusitis, bronchitis, pharyngitis, cough illness or pneumonia. Because carriage patterns were similar in 2006–07 and 2008–09 in the context of widespread PCV7 use, predictors of carriage were evaluated for both seasons combined. Bivariate and multivariate logistic regression analyses used generalized linear mixed models to account for clustering by community (SAS 9.2, SAS Institute, Cary, NC).
Results
Study population
Nasopharyngeal specimens were collected from 1,011 children and compared with prior sampling periods. Characteristics of the communities across the 4 sampling periods are described in Supplemental Digital Content 1 (table), along with the proportion of study participants who carried S. pneumoniae. Study participants were similar in age distribution, gender, group child care attendance, and number of young siblings across sampling periods (see table, Supplemental Digital Content 2). The proportion of children with acute RTI (overall F statistic, p<0.001) and recent antibiotic use (overall F statistic, p<0.001) differed across the 4 sampling periods. PCV7 vaccine coverage (receipt of ≥1 dose) among participants increased from 50% in 2001 to 82% in 2003–04, before becoming nearly universal by 2006–07 (98%) and 2008–09 (99%).
Nasopharyngeal Carriage of S. pneumoniae
S. pneumoniae carriage in Massachusetts children (see table, Supplemental Digital Content 1) varied significantly over time (p = 0.006), decreasing in 2003–04 before returning to baseline values and remaining stable between 2006–07 and 2008–09. The trend in carriage across sampling periods was similar for children 3 to <24 months (2001: 26%; 2003–04: 25%; 2006–07: 32%; 2008–09: 31%) and children ≥24 months (2001: 28%; 2003–04: 22%; 2006–07: 28%; 2008– 09: 27%).
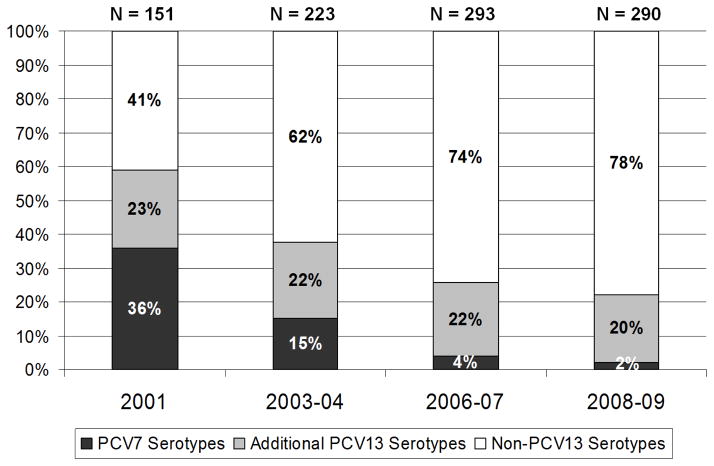
Figure 1 shows the proportion of pneumococcal carriage (among isolates with known serotypes) attributable to vaccine and non-vaccine serotypes. The proportion of PCV7 serotypes decreased significantly over the first three sampling periods (p < 0.001) and remained extremely low in 2008–09. Serotype replacement with strains not included in the PCV7 vaccine was virtually complete by 2006–07. Additional PCV13 serotypes (1, 3, 5, 6A, 7F, 19A) remained a stable proportion of all pneumococcal isolates across sampling periods, ranging from 20–23%. Carriage with non-PCV13 serotypes increased significantly from 2001 to 2006–07 (p<0.001) and remained stable between 2006–07 and 2008–09 (p=0.32).
Figure 1.
Percent of Streptococcus pneumoniae isolates within each sampling period by vaccine-included serotypes. Heptavalent pneumococcal conjugate vaccine (PCV7) serotypes are 4, 6B, 9V, 14, 18C, 19F, 23F. Additional 13-valent (PCV13) vaccine serotypes are 1, 3, 5, 6A, 7F, 19A. Non-PCV13 serotypes are all other serotypes.
Figure 2 shows trends in the proportions of the most commonly identified serotypes in 2008–09. Altogether, these 16 serotypes accounted for 89% of isolates during the 2008–09 sampling period. Serotype 19A (14%) was the most frequently identified pneumococcal type in 2008–09, followed by 6C (11%), 15B/C (11%), 23A (7%), 23B (7%), and 35B (7%). Of these, only 19A is directly targeted by PCV13. Significant increases in prevalence over time were noted for serotypes 19A (p=0.005), 7F (p=0.009), 6C (p<0.001), 23A (p=0.02), and 23B (p=0.002). In contrast, 6A (p<0.001) and 19F (p<0.001) declined across sampling periods.
Figure 2.
Distribution of the 16 most common pneumococcal serotypes (grouped by vaccine inclusion) accounting for 89% of all isolates in 2008–09, as proportions of total serotypes, by sampling period. P-values ≤0.1, based on generalized linear mixed model chi-square tests, evaluating differences in serotype-specific proportional carriage are indicated.
PCV7 serotypes continued to circulate in 2006–07, including 19F (2.4%), 23F (0.3%), 6B (0.7%), and 9V (0.3%), all of which were in children who were up-to-date on their PCV7 immunization series for their age. In 2008–09, the following PCV7 isolates were identified: 19F (1.4%), 6B (0.3%) and 14 (0.3%). All 4 children colonized with 19F in 2008–09 were up-to-date on PCV7 for their age, whereas the 2 children colonized with 14 and 6B were not.
Antimicrobial Susceptibility
The overall fraction of PNSPdid not vary significantly over time using either PNSP>0.06 (33% in 2001, 38% in 2003–04, 37% in 2006–07, and 34% in 2008–09, p=0.73) or PNSP>2.0 breakpoints (11% in 2001, 3% in 2003–04, 4% in 2006–07, and 6% in 2008–09, p=0.56). By 2006–07 or 2008–09, however, PCV13 serotypes were significantly less likely to be susceptible to penicillin (PNSP>0.06), erythromycin, clindamycin, trimethoprim-sulfamethoxazole, and ceftriaxone when compared to non-PCV13 serotypes (see table, Supplemental Digital Content 3). Although there was no discrimination in penicillin non-susceptibility using current PNSP>2.0 breakpoints, evidence of incremental rises in MIC were evident using PNSP>0.06 breakpoints. Using PNSP>0.06 breakpoints, increases in penicillin non-susceptibility were noted for 6C (p=0.09) and 15 B/C (p<0.001) across sampling periods (Table 1). However, the majority of penicillin non-susceptibility was due to increased prevalence of 19A and 35B. Among 19A isolates, non-susceptibility significantly increased over time for the following antibiotics: PNSP>2.00 (11%, 4%, 17%, 38%; p=0.01), erythromycin (0%, 36%, 30%, 69%; p = 0.001), clindamycin (0%, 4%, 15%, 38%; p < 0.001), ceftriaxone (13%, 8%, 20%, 52%; p = 0.003).
Predictors of Carriage
Potential risk factors associated with S. pneumoniae are described in Table 2. Younger age group, group child care attendance, young siblings, and respiratory tract infection were associated with pneumococcal carriage in multivariate models. Antibiotic exposure in the past two months was associated with decreased rates of pneumococcal carriage. We also evaluated predictors of carriage with penicillin non-susceptible pneumococci, using PNSP>2.0 breakpoints, among children swabbed in 2006–07 and 2008–09 (Table 2). Younger age and child care attendance were significantly associated with PNSP>2.0 carriage in multivariate models.
Discussion
In serially sampling Massachusetts communities during the past decade, we have noted a steady decline and near elimination of carriage of PCV7 serotypes in healthy children. However, following an initial decline in overall carriage due to loss of PCV7 serotypes, carriage returned to pre-PCV7 levels (30%) as a result of replacement by non-vaccine serotypes.. 19A carriage steadily increased to become a common colonizing pneumococcal serotype and the dominant source for invasive pneumococcal disease.[21] As PCV7 vaccination rates reached a plateau, the pneumococcal population structure in Massachusetts reached a new equilibrium comprised of non-PCV7 strains. Similar replacement with 19A has been reported following vaccination with PCV7 in the Netherlands, although it has not been uniform, and the factors that determine the precise serotypes involved in replacement in different populations remain poorly understood. [35–36]
In early 2010, PCV13 was approved for use in the U.S. to expand vaccine coverage to serotypes 1, 3, 5, 6A, 7F, and 19A. This new selective pressure will again alter the current equilibrium among pneumococcal carriage isolates. As PCV13 use increases during the next several years, we anticipate that overall rates of colonization may transiently drop, but eventual non-PCV13 serotype replacement may occur. The greatest impact of PCV13 in the United States is expected to be reductions in carriage and disease attributable to 19A since other PCV13 serotypes are infrequent sources of carriage and disease.[11, 24–25, 37]
The common replacement serotypes found here (19A, 6C, 15B/C, 23A, 23B, 35B) have arisen by expansion of existing clones, emergence of new clones, and serotype switching, whereby the capsular genes that determine serotype are replaced by those of another serotype.[28] As serotype 6A declined significantly, 6C became increasingly prevalent and is now the 2nd most common serotype in our sample. The decline in 6A has been attributed to cross-protection from PCV7, which includes 6B as a target.[32, 38] In similar fashion, PCV13 may provide cross-protection against 6C due to the inclusion of the closely-related serotype 6A.[38] If this occurs, additional reductions in pneumococcal colonization and IPD may occur. Nonetheless, we anticipate continued replacement of nasopharyngeal carriage by non-PCV13 serotypes as PCV13 use expands. Increases in IPD may or may not occur as a consequence, and will depend in part on the relative invasiveness of the most prevalent replacement serotypes.[39–41]
Widespread use of PCV7 led to an initial decline in antibiotic resistance, since PCV7 serotypes previously constituted the majority of antibiotic resistant strains.[5, 42–43] However, the emergence of non-PCV7 antibiotic resistant strains, particularly serotypes19A, 35B, and 15B/C, have mitigated the reduction of antibiotic resistance byPCV7. Serotype 19A now represents the most commonly carried non-susceptible serotype for penicillin, erythromycin and ceftriaxone resistance. Since 19A is now responsible for the largest fraction of PNSP>0.06 and PNSP>2.0 carriage, we anticipate that PCV13 use may result in a decline in overall antibiotic resistance rates. However, the long-term impact of PCV13 use on antibiotic resistance remains uncertain.[23, 44]
In the post-PCV7 era and before PCV13 use, the major predictors of pneumococcal carriage have remained similar, including young age, group child care attendance, having young siblings, concomitant respiratory tract infection, and lack of recent antibiotic exposure.[21] It appears from this and prior sampling periods that vaccination has not altered common risk factors for pneumococcal carriage.[13, 21]
Almost all carriage strains in our sample are classified as penicillin-susceptible by the revised 2008 breakpoints, which require a MIC of at least 2.0 μg/ml for non-susceptibility. However, young age and group child care were significantly associated with carriage of strains with higher penicillin MICs within the revised susceptible range. Under antibiotic pressure, such strains may increase following PCV13, similar to what occurred following PCV7.[21, 22, 44]
The results of this study should be interpreted in the context of several limitations. First, we did not examine the possibility that children carried multiple strains simultaneously, which might have altered the reported distribution of serotype and antibiotic resistance. Second, since we sampled during “cold and flu” seasons, we do not know whether S. pneumoniae carriage patterns are similar throughout the year or whether we adjusted completely for differences in respiratory illness rates between sampling periods. Third, we assume that carriage among children seeking care in physician offices reflects carriage in their community, and our method of sampling may bias our data toward serotypes that are cultured more easily during respiratory illnesses. Nevertheless, the frequency of participants having well-child visits and our adjustment for the presence of respiratory illness should have mitigated these effects. Furthermore, our ability to serially sample the same Massachusetts communities allows us to provide unique information regarding the pneumococcal population structure in colonized children.
In conclusion, PCV7 has resulted in near complete replacement with non-vaccine serotypes since 2007. 19A remains the predominant serotype since 2007 and is responsible for the majority of antibiotic resistance in our isolates, although other common replacement serotypes, 6C, 15B/C, and 35B, have begun to show evidence of increasing MICs to penicillin. We anticipate PCV13 use will reduce carriage with 19A and may decrease the prevalence of antibiotic resistant pneumococci in the community, at least for some time. Serotype replacement and other adaptive changes will need to be monitored in the presence of this newest selective pressure on S. pneumoniae.
Supplementary Material
Acknowledgments
We thank Katie Haffenreffer, Kristine Robin, and Verna Moran, RN for their dedication to this project, and extend our appreciation to the following participating practices: Alan Bulotsky MD & Associates (Brockton, MA), Berkshire Pediatric Associates (Pittsfield, MA), Cape Ann Pediatricians (Gloucester, MA), Children’s Health Care (Newburyport, MA), Harvard Vanguard Medical Associates (Chelmsford, MA), Medical Associates Pediatrics (Leominster, MA), Middleboro Pediatrics (Lakeville, MA), and Needham Pediatrics (Needham, MA). We also extend our gratitude to the many parents and children who made this study possible.
Footnotes
Conflicts of Interest and Source of Funding: This work was supported by the National Institutes of Health [R01 AI066304, Finkelstein]. Dr. Hanage (WPH) was funded as a Royal Society Research Fellow during this study. Dr. Pelton receives research support from Novartis, Intercell, Glaxo Smith Kline, and Pfizer, Inc. He has also received honoraria for participation in advisory board meetings on conjugate vaccines from Novartis, GSK and Pfizer, Inc. Dr. Lipsitch has received consulting support from AIR Worldwide, Pfizer, Novartis, and the Avian/Pandemic Flu Registry (Outcome Sciences), supported by Roche. All other authors report no disclosures (PW, GL, JF, WH, AS, SR, KK, MD, VH, ML, SH).
References
- 1.Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease--United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2005 Sep 16;54(36):893–7. [PubMed] [Google Scholar]
- 2.Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004 Jun;23(6):485–9. doi: 10.1097/01.inf.0000129685.04847.94. [DOI] [PubMed] [Google Scholar]
- 3.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007 Nov 1;196(9):1346–54. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 4.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006 Apr 12;295(14):1668–74. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 5.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003 May 1;348(18):1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 6.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001 Feb 8;344(6):403–9. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 7.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007 Apr 7;369(9568):1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 8.Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006 Sep;118(3):865–73. doi: 10.1542/peds.2006-0492. [DOI] [PubMed] [Google Scholar]
- 9.Flannery B, Heffernan RT, Harrison LH, et al. Changes in invasive Pneumococcal disease among HIV-infected adults living in the era of childhood pneumococcal immunization. Ann Intern Med. 2006 Jan 3;144(1):1–9. doi: 10.7326/0003-4819-144-1-200601030-00004. [DOI] [PubMed] [Google Scholar]
- 10.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005 Oct 26;294(16):2043–51. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 11.Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010 Jan 1;201(1):32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 12.Ghaffar F, Barton T, Lozano J, et al. Effect of the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae in the first 2 years of life. Clin Infect Dis. 2004 Oct 1;39(7):930–8. doi: 10.1086/423379. [DOI] [PubMed] [Google Scholar]
- 13.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005 Sep;116(3):e408–13. doi: 10.1542/peds.2004-2338. [DOI] [PubMed] [Google Scholar]
- 14.Pelton SI, Loughlin AM, Marchant CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2004 Nov;23(11):1015–22. doi: 10.1097/01.inf.0000143645.58215.f0. [DOI] [PubMed] [Google Scholar]
- 15.Emergence of antimicrobial-resistant serotype 19A Streptococcus pneumoniae--Massachusetts, 2001–2006. MMWR Morb Mortal Wkly Rep. 2007 Oct 19;56(41):1077–80. [PubMed] [Google Scholar]
- 16.Byington CL, Samore MH, Stoddard GJ, et al. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin Infect Dis. 2005 Jul 1;41(1):21–9. doi: 10.1086/430604. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs MR, Good CE, Beall B, Bajaksouzian S, Windau AR, Whitney CG. Changes in serotypes and antimicrobial susceptibility of invasive Streptococcus pneumoniae strains in Cleveland: a quarter century of experience. J Clin Microbiol. 2008 Mar;46(3):982–90. doi: 10.1128/JCM.02321-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SY, Moore MR, Bruden DL, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr Infect Dis J. 2008 Apr;27(4):335–40. doi: 10.1097/INF.0b013e318161434d. [DOI] [PubMed] [Google Scholar]
- 19.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007 Apr 25;297(16):1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 20.Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998-2005. MMWR Morb Mortal Wkly Rep. 2008 Feb 15;57(6):144–8. [PubMed] [Google Scholar]
- 21.Huang SS, Hinrichsen VL, Stevenson AE, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009 Jul;124(1):e1–11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011 Apr 12; doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanage WP, Huang SS, Lipsitch M, et al. Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae carriage isolates in the post-heptavalent conjugate vaccine era. J Infect Dis. 2007 Feb 1;195(3):347–52. doi: 10.1086/510249. [DOI] [PubMed] [Google Scholar]
- 24.Moore MR, Gertz RE, Jr, Woodbury RL, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008 Apr 1;197(7):1016–27. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 25.Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007 Jun;26(6):468–72. doi: 10.1097/INF.0b013e31803df9ca. [DOI] [PubMed] [Google Scholar]
- 26.Pai R, Moore MR, Pilishvili T, Gertz RE, Whitney CG, Beall B. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J Infect Dis. 2005 Dec 1;192(11):1988–95. doi: 10.1086/498043. [DOI] [PubMed] [Google Scholar]
- 27.Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children - Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010 Mar 12;59(9):258–61. [PubMed] [Google Scholar]
- 28.Finkelstein JA, Huang SS, Daniel J, et al. Antibiotic-resistant Streptococcus pneumoniae in the heptavalent pneumococcal conjugate vaccine era: predictors of carriage in a multicommunity sample. Pediatrics. 2003 Oct;112(4):862–9. doi: 10.1542/peds.112.4.862. [DOI] [PubMed] [Google Scholar]
- 29.Hanage WP, Bishop CJ, Huang SS, et al. Carried Pneumococci in Massachusetts Children: The Contribution of Clonal Expansion and Serotype Switching. Pediatr Infect Dis J. 2010 Nov 16; doi: 10.1097/INF.0b013e318201a154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janapatla RP, Hsu MH, Du JF, Hsieh YC, Lin TY, Chiu CH. Sequence types and antimicrobial susceptibility of invasive streptococcus pneumoniae isolates from a region with high antibiotic selective pressure and suboptimal vaccine coverage. Pediatr Infect Dis J. 2010 May;29(5):467–9. doi: 10.1097/INF.0b013e3181cb45f3. [DOI] [PubMed] [Google Scholar]
- 31.Pillai DR, Shahinas D, Buzina A, et al. Genome-wide dissection of globally emergent multi-drug resistant serotype 19A Streptococcus pneumoniae. BMC Genomics. 2009;10:642. doi: 10.1186/1471-2164-10-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nahm MH, Lin J, Finkelstein JA, Pelton SI. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis. 2009 Feb 1;199(3):320–5. doi: 10.1086/596064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Institute CaLS. Performance Standards for Antimicrobial Susceptibility Testing; Sixteenth Informational Supplement. 2004. [Google Scholar]
- 34.Institute CaLS. Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. CLSI; 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087–1898: 2007. [Google Scholar]
- 35.van Gils EJ, Veenhoven RH, Hak E, et al. Pneumococcal conjugate vaccination and nasopharyngeal acquisition of pneumococcal serotype 19A strains. JAMA. 2010 Sep 8;304(10):1099–106. doi: 10.1001/jama.2010.1290. [DOI] [PubMed] [Google Scholar]
- 36.Imohl M, Reinert RR, van der Linden M. Temporal Variations among Invasive Pneumococcal Disease Serotypes in Children and Adults in Germany (1992–2008) Int J Microbiol. 2010;2010:874189. doi: 10.1155/2010/874189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steenhoff AP, Shah SS, Ratner AJ, Patil SM, McGowan KL. Emergence of vaccine-related pneumococcal serotypes as a cause of bacteremia. Clin Infect Dis. 2006 Apr 1;42(7):907–14. doi: 10.1086/500941. [DOI] [PubMed] [Google Scholar]
- 38.Park IH, Moore MR, Treanor JJ, et al. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J Infect Dis. 2008 Dec 15;198(12):1818–22. doi: 10.1086/593339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis. 2003 May 1;187(9):1424–32. doi: 10.1086/374624. [DOI] [PubMed] [Google Scholar]
- 40.Hanage WP, Auranen K, Syrjanen R, et al. Ability of pneumococcal serotypes and clones to cause acute otitis media: implications for the prevention of otitis media by conjugate vaccines. Infect Immun. 2004 Jan;72(1):76–81. doi: 10.1128/IAI.72.1.76-81.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanage WP, Kaijalainen TH, Syrjanen RK, et al. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect Immun. 2005 Jan;73(1):431–5. doi: 10.1128/IAI.73.1.431-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan SL, Mason EO, Jr, Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics. 2004 Mar;113(3 Pt 1):443–9. doi: 10.1542/peds.113.3.443. [DOI] [PubMed] [Google Scholar]
- 43.Mufson MA, Stanek RJ. Epidemiology of invasive Streptococcus pneumoniae infections and vaccine implications among children in a West Virginia community, 1978-2003. Pediatr Infect Dis J. 2004 Aug;23(8):779–81. doi: 10.1097/01.inf.0000134536.15588.20. [DOI] [PubMed] [Google Scholar]
- 44.Hanage WP, Fraser C, Tang J, Connor TR, Corander J. Hyper-recombination, diversity, and antibiotic resistance in pneumococcus. Science. 2009 Jun 12;324(5933):1454–7. doi: 10.1126/science.1171908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.