Abstract
Objective
Vascular Endothelial Growth Factor Receptor-3 (VEGFR-3) is a critical regulator of developmental and adult vasculogenesis and lymphangiogenesis through its interactions with select members of the VEGF family. The goal of this study was to investigate how VEGFR-3 expression is regulated during inflammatory lymphangiogenesis.
Methods and Results
In this study, we present for the first time evidence that VEGFR-3 can be negatively regulated by a mirtron, hsa-miR-1236 (miR-1236), which is expressed in primary human lymphatic endothelial cells (HLEC). In HLEC, miR-1236 is upregulated in response to IL-1β, a negative regulator of VEGFR-3. miR-1236 binds the 3′ untranslated region of Vegfr3, resulting in translational inhibition. Overexpression of miR-1236 significantly decreased expression of VEGFR-3, but not VEGFR-2, in HLEC. Compared to a control miR, overexpression of miR-1236 also led to decreased VEGFR-3 signaling. However, VEGFR-2-specific signaling was not affected. miR-1236 can attenuate HLEC migration and tube formation, as well as in vivo lymphangiogenesis.
Conclusion
Our data suggest that miR-1236 may function as a negative regulator of VEGFR-3 signaling during inflammatory lymphangiogenesis.
Keywords: VEGFR-3, miR-1236, lymphangiogenesis, vascular biology
INTRODUCTION
The Vascular Endothelial Growth Factor (VEGF) family of ligands and its tyrosine kinase receptors are critical regulators of angiogenesis and lymphangiogenesis. Of the VEGF receptors, VEGFR-3 has been shown to be critical for developmental angiogenesis and lymphangiogenesis, as VEGFR-3 null mice die due to a failure in remodeling of the primary vascular plexus 1. VEGFR-3 expression is maintained in blood endothelial cell lymphatic precursors but becomes restricted to lymphatic endothelial cells with the exception of few blood capillaries in some organs 2. As a receptor for Vascular Endothelial Growth Factor-C (VEGF-C), VEGFR-3 is essential for proper development of the lymphatic vasculature 3–5. The lymphatic vasculature is an integral part of normal physiology. Lymphatic vessels act as a conduit for immune cells for assistance in immune surveillance, return extravasated interstitial fluid to the blood, and absorb dietary lipids in the intestine 2, 6–8. In the adult mouse, VEGFR-3 is critical for signaling in response to VEGF-C/D-induced lymphatic vessel growth 9, which occurs mostly as a result of inflammation 2. Recent work has demonstrated that the transcription factors Prox1, NF-κB and Tbx1 are critical for VEGFR-3 gene expression during vascular development10, 11. However, little is known about the mechanisms by which VEGFR-3 expression is controlled during inflammatory lymphangiogenesis 12 and is the subject of this study.
Recently, post-transcriptional regulators of gene expression have gained attention. MicroRNAs (miRNAs) are endogenous, non-protein coding small RNAs that play important roles in the post-transcriptional regulation of target genes by directing target mRNAs for translational repression or destabilization. The collection of miRNA-class regulatory RNAs in invertebrates and mammals was expanded by the finding of short hairpin introns known as mirtrons 13, 14. Mirtrons bypass Drosha cleavage by using the spliceosome to generate their precursor ends (pri-miRNAs). Although generated differently from conventional microRNAs, mirtrons are also cleaved by Dicer to generate functional microRNAs that can also regulate genes post-transcriptionally. Recent work has indicated a role for microRNAs in angiogenesis 15 and determination of lymphatic endothelial cell lineage fate from blood endothelial cells by regulation of Prox-1 16, 17. However, it is not known if microRNAs can directly regulate VEGFR-3 –a critical receptor involved in lymphangiogenesis and angiogenesis.
In this study, we show that miR-1236 is expressed in endothelial cells and binds to the 3′ untranslated region (UTR) of the Vegfr3 gene, resulting in translational inhibition of Vegfr3. This work reveals that miR-1236 may represent a post-transcriptional mechanism by which VEGFR-3 expression is fine-tuned in lymphatic endothelial cells. Our work is the first example of a microRNA that targets Vegfr3 and may have important implications for the control of VEGFR-3 during inflammatory lymphangiogenesis.
METHODS
DNA constructs/Reporter gene assays
From HLEC, cDNAs encoding the entire 3′UTR of Vegfr3 (1.69kb) was amplified by RT-PCR from HLEC RNA and was directionally subcloned into the NotI-XhoI sites downstream of the Renilla luciferase in the psiCHECK2 vector that also contains a constitutively expressed firefly luciferase gene. Construct was confirmed by sequencing at WM Keck Facility at Yale University. Next, the region complementary to the miR-1236 predicted seed sequence in position 144–150 of the human Vegfr3 3′UTR, GGAAGA, was scrambled to TCTAGA (mut Vegfr3 3′UTR), using the QuikChange™ Site-Directed Mutagenesis Kit. This construct was also confirmed by sequencing. Luciferase output was quantified with a Berthold Lumat LB 9501 luminometer and renilla luciferase activity was normalized to the corresponding firefly luciferase activity using a Dual-Glo Luciferase Assay System (Promega) and reported as the percentage of control cells cotransfected with the same concentration of control mimic or miR-1236 mimic. Experiments were performed 4 times using triplicates.
VEGFR-3 lentiviral expression construct
Full-length human Vegfr3 was amplified using PCR from a VEGFR-3 mammalian expression plasmid, and BamHI and Not1 restriction sites were introduced at either end of the cDNA. The PCR product was subcloned into the target lentiviral vector pLex (OpenBiosystems), resulting in pLex-VEGFR-3. The VEGFR3 cDNA insert was confirmed by DNA sequencing and VEGFR3 protein expression from pLex-VEGFR3 was confirmed by Western blotting upon transient transfection into 293T cells.
MicroRNA analysis
MicroRNAs were isolated from cells using miRNeasy kit (Qiagen) according to the manufacturer’s instructions. Quantitative reverse transcription-PCR (qRT-PCR) assays were performed using a TaqMan miRNA assay kit (Applied Biosystems) for the mature miRNA using the target-specific stem-loop RT primer. The forward primer sequences for hsa-miR-1236 and RNU6B were purchased from Applied Biosystems and used for PCR validation. qRT-PCR was performed by using iQ SYBR Green Supermix on C1000 Thermal Cycler (Bio-Rad).
Statistical analyses
All data are expressed as mean ± SEM. Statistical analysis was performed with a two-tailed Student’s paired t-test. Statistical significance for p-values are as follows: *, <0.05; **, <0.01; ***, <0.001. For analysis, positive staining and area was quantified with Image Pro Plus Software (MediaCybernetics).
The detailed methods that we have published are provided in the online supplement. These methods include lymphatic EC culture, transfection, immunoblotting for VEGFR2/3 signaling, immunofluorescence, cell migration/tube formation assays, and in vivo lymphangiogenic assays as we described previously 18.
RESULTS
Inflammatory lymphangiogenesis in vivo is transient
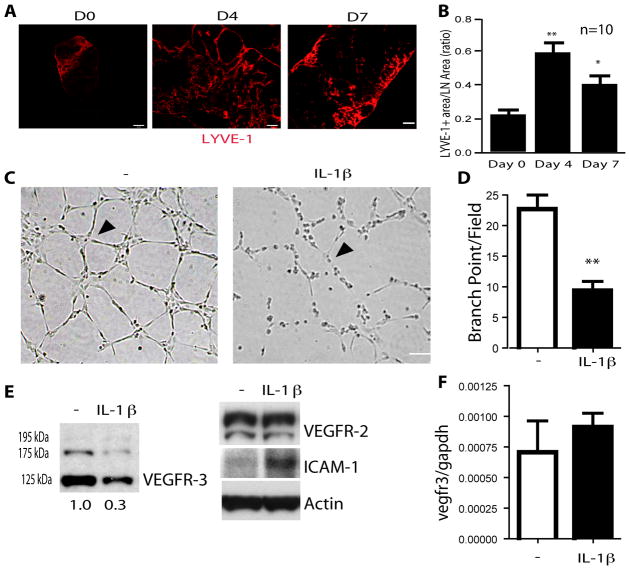
We have observed that lymphangiogenesis is transient in all models we have examined, including VEGF-induced cornea lymphangiogenesis 18, VEGF-induced lymph node (LN) lymphangiogenesis 18 and inflammation-mediated LN lymphangiogenesis by immunization by skin painting with oxazolone (Fig. 1A). In the oxazolone skin painting model, oxazolone-induced inflammation elicits a profound lymph node lymphangiogenic response 19. In this system, LN lymphangiogenesis peaked on days 3–4 followed by regression by day 7 (Fig. 1A with quantifications in Fig. 1B). We determined the levels of lymphangiogenic growth factors (VEGF-A and VEGF-C) by qRT-PCR and found that their expressions in lymph nodes were even higher at day 7 compared to day 3 post oxazolone challenge (Supplemental Fig. IA). Recently, it has been shown that inflammation can decrease VEGFR-3 protein and transcript during oxazolone challenge, concomitant with the expression of inflammatory cytokines 20, 21. Therefore, we hypothesized that inflammatory cytokines may inhibit oxazolone-induced lymphangiogenesis. Consistent with a recent report 20, we found that proinflammatory cytokines such as tumor necrosis factor-α (TNF), interleukin-1 β (IL-1β) and interferon γ (IFN-γ) were increased after oxazolone challenge (Supplemental Fig. IB) with IL-1β at a much higher level. While TNF and IFN-γ have been shown to drive and inhibit lymphangiogenesis, respectively 22, 23, the effect of IL-1β on lymphangiogenesis is not well-characterized. We determined a direct effect of IL-1β on lymphangiogenesis in a VEGF-C-induced human lymphatic EC (HLEC) tube formation assay. In the presence of IL-1β, VEGF-C-induced tube formation in a Matrigel was significantly suppressed (Fig. 1C with quantification in Fig. 1D). To determine the mechanism for decreased tube formation by IL-1β, we analyzed protein expression of the VEGF-C receptors- VEGFR-2 and VEGFR-3. As we reported recently 18, protein analysis of VEGFR-3 by Western blot revealed 3 bands (Fig. 1E). While the band at 175 kDa is considered to be the intracellular and unglycosylated precursor, the band with molecular weight 125 kDa represents the mature form of VEGFR-3 24, 25. Stimulation of HLEC with IL-1β for 24 h strongly reduced expression of VEGFR-3, but not VEGFR-2 (Fig. 1E). A similar effect was found by stimulation with other inflammatory cytokines, namely IL-1α and TNF (Supplemental Fig. IC). However, IL-1β did not significantly affect VEGFR-3 mRNA as measured by qRT-PCR (Fig. 1F). These results suggested that IL-1β may regulate VEGFR-3 at a post-transcriptional level.
Fig. 1. Inflammatory lymphangiogenesis in vivo is transient.
A–B. 4% oxazolone in acetone was administered on shaved skin on the abdomen in proximity to inguinal and brachial lymph nodes of WT mice. Lymph nodes (LNs) were collected at day 4 and 7. Lymphatic vessels were visualized by immunostaining with anti-LYVE-1 and representative images are shown in A. Scale bar, 200 μm. LYVE-1-positive area were quantified in B. Data are mean ± SEM, n=10 for time points, *, p<0.05 and **, p<0.01. C–D. Tube formation assays in a Matrigel. HLEC were stimulated with IL-1β (5 ng) for 24 h. Cells were then seeded to Matrigel and cultured with 3% serum + VEGF-C (100 ng/ml) for 6 h and representative images are shown in C. Branch points as indicated by arrowheads were quantified in D. Data are mean ± SEM from three independent experiments, **, p<0.01. Scale bar, 100 μm. E–F. Effects of IL-1β on VEGFR-3 protein and mRNA. HLEC were stimulated with IL-1β (5 ng) for 24 h. VEGFR-3 and VEGFR-2, and ICAM-1 proteins were detected by Western blot with respective antibodies. β-actin was used as a loading control and relative levels of VEGFR-3 are indicated below the blot with untreated group as 1.0 (E). VEGFR-3 mRNA was assessed by qRT-PCR with normalization to gapdh mRNA (F). Data are mean ± SEM from three independent experiments.
Induction of miR-1236 by IL-1β
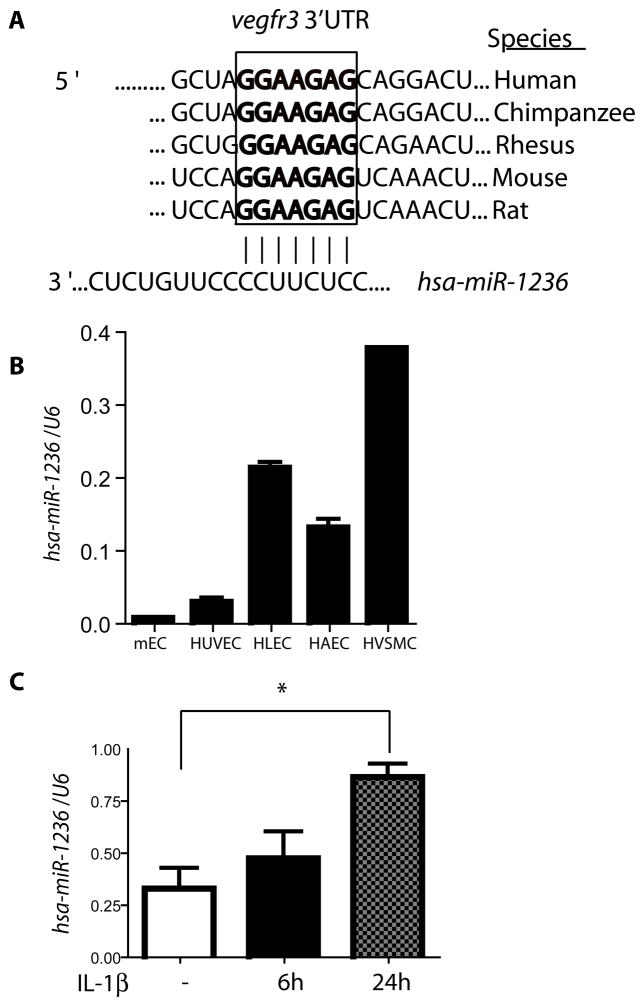
The results above prompted us to examine if VEGFR-3 expression can be regulated by miRNA. To this end, TargetScan (www.targetscan.org) and other microRNA prediction algorithms were used to search for miRNA binding sites in the Vegfr3 3′UTR. In silico analysis revealed a highly conserved site in the Vegfr3 3′UTR as a putative target of a 7mer-m8 site in the seed region of miR-1236 (Fig. 2A). We chose miR-1236 as a candidate because of its relatively high context percentile score and the possibility of being functionally testable in vivo, as the predicted binding site is also conserved in mice. In contrast, miR-132, miR 323-5p, miR 200b and miR200c, which are in the higher percentile score than miR-1236, do not have predicted 3′UTR target sites that are conserved in mice, making it difficult to test the effectiveness in vivo. Using qRT-PCR with TaqMan primers that recognize the mature miRNA species, we detected a pan-endothelial expression of miR-1236. HLEC had higher expression of miR-1236 than human vein endothelial cells (HUVEC) and human aortic EC (HAEC), but lower than human smooth muscle cells (Fig. 2B). Expression of miR-1236 was specific to human cells, using mouse ECs as a control (Fig. 2B). Since lymphatic cells express endogenous VEGFR-3, we used HLEC to determine the effect of miR-1236 on VEGFR-3 expression. Next, we determined if miR-1236 is regulated by inflammatory cytokines in HLEC. Stimulation of HLEC with inflammatory cytokines that downregulate VEGFR-3 caused an upregulation of miR-1236 (Supplemental Fig. IIA). Stimulation of HLEC with IL-1β led to the most significant (>3-fold) upregulation of miR-1236, peaking at 24 h post-stimulation (Fig. 2C). Expression of the host gene of miR-1236, Negative Elongation Factor-E (NELF-E), was also confirmed in HLEC. Furthermore, we observed a trend in the upregulation of host gene, NELF-E upon stimulation with IL-1β (Supplemental Fig. IIB). The kinetics of miR-1236 induction is consistent with IL-1β-induced downregulation of VEGFR-3 at 24h (Fig. 1E). These data suggest that IL-1β-induced miR-1236 may negatively regulate VEGFR-3 expression in HLEC.
Fig. 2. Endogenous expression and induction of miR-1236 by IL-1β.
A. Cross species sequence alignment of the putative miR-1236 binding site located in the 3′UTR (nucleotide 140–157 following the stop codon shown) of the Vegfr3 transcript. B. Expression of miR-1236 in mouse endothelium (mEC), venous EC (HUVEC), lymphatic EC (HLEC), arterial EC (HAEC), and vascular smooth muscle cells (HVSMC). TaqMan primers were used to probe miR-1236 expression, which was normalized to RNU6B expression using the comparative Ct method. C. Expression of miR-1236 in HLEC after stimulation with IL-1β (5 ng) for 6 h and 24 h compared to unstimulated cells. Data in B and C are mean ± SEM from duplicates in three independent experiments. *, p<0.05.
Endogenous miR-1236 in primary human lymphatic endothelial cells regulates VEGFR-3 expression by binding to the vegfr3 3′UTR
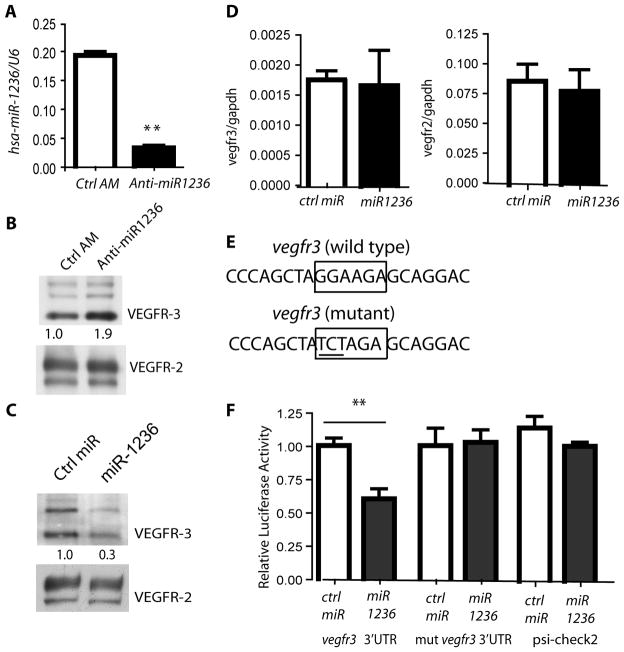
We were able to efficiently transfect siRNA into HLEC (Supplemental Fig. III). To verify if miR-1236 targets VEGFR-3, HLEC were transfected with a control antagomir or antagomir against miR-1236. Inhibition of endogenous miR-1236 was confirmed by qRT-PCR (Fig. 3A). Total VEGFR-3 was modestly increased in HLEC (Fig. 3B), suggesting that endogenous miR-1236 in primary human lymphatic endothelial cells may partially contribute to regulation of VEGFR-3 protein expression. To further determine how miR-1236 regulates VEGFR-3 expression in HLEC, we took an overexpression approach. Introduction of miR-1236 to HLEC in culture resulted in significant down-regulation of total VEGFR-3 protein, but not mRNA, indicating that miR-1236 targets the Vegfr3 gene for translational inhibition (Fig. 3C-D). Importantly, neither VEGFR-2 transcript nor protein was significantly decreased after overexpression of miR-1236 (Fig. 3C-D).
Fig. 3. Endogenous miR-1236 in primary human lymphatic endothelial cells regulates VEGFR-3 protein expression.
A-B. Effects of antagomir against miR-1236 on VEGFR-3 expression. HLEC were transfected with ctrl anti-miR or anti-miR-1236 (60 nM) for 48 h. Expression of miR-1236 was determined by qRT-PCR (A). Data are representative of 3 independent experiments. **, p< 0.01. VEGFR-3 and VEGFR-2 protein expression was determined by Western blot with respective antibodies (B). Similar results were obtained from additional three experiments. C–D. Effects of miR-1236 on VEGFR-3 expression. Primary HLEC were transfected with control miRNA mimics or miR-1236 mimics (40 nM) for 48 h. Western Blot was used to detect VEGFR-3 and VEGFR-2 protein (C). Relative levels of VEGFR-3 (normalized with VEGFR-2) are indicated below the blot with Ctrl miR as 1.0. D. qRT-PCR for Vegfr2 and Vegfr3 mRNA and data are mean ± SEM from three independent experiments. E. Construct design. cDNAs encoding the entire 3′UTR of vegfr-3 including the region complementary to the miR-1236 predicted seed sequence in position 144–150 of the human Vegfr3 3′UTR (wild type Vegfr3 3′UTR). The putative binding sequence, GGAAGA, was scrambled to TCTAGA (mut Vegfr3 3′UTR). F. Luciferase reporter assay COS-7 cells. Ctrl or miR-1236 mimics (60 nM), were co-transfected with the wild type or mutated 3′ UTR of Vegfr3, or with the psiCHECK2 vector backbone. Renilla luciferase signal is normalized to the internal firefly luciferase. Data represent the mean ± SEM from 4 independent experiments. **, p<0.01.
In order to confirm Vegfr3 as a bona fide direct target of miR-1236, we sought to determine whether miR-1236 was able to bind to and repress Vegfr3 mRNA. Therefore, the entire 1.69 kb Vegfr3 3′UTR, containing the predicted consensus miR-1236 binding site was cloned into the psiCHECK2 luciferase reporter construct. Next, we mutated the putative binding site of the original construct by substituting three base pairs (Fig. 3E). We then tested these predicted miRNA/mRNA interactions based on luciferase activity as subsequently measured in COS cells. In contrast to co-transfection of the VEGFR-3 reporter construct together with a control microRNA, co-transfection of the VEGFR-3 reporter construct together with miR-1236 significantly reduced luciferase activity (Fig. 3F). This decrease in luciferase activity was seen in a concentration dependent manner (not shown). Furthermore, co-transfection of miR-1236 with a mutant Vegfr3 3′UTR reporter construct into which only a 3-base pair mutation in the miR-1236 binding site was introduced, did not repress luciferase activity as seen by miR-1236 on the wild-type construct (Fig. 3F, mut vegfr3 3′UTR). In addition, no significant repression was found by co-transfection of the psiCHECK2 vector backbone together with miR-1236 (Fig. 3F). Taken together, these data suggest that miR-1236 directly binds to Vegfr3 to negatively regulate VEGFR-3 expression.
miR-1236 negatively regulates VEGFR-3-dependent signaling in primary human lymphatic endothelial cells
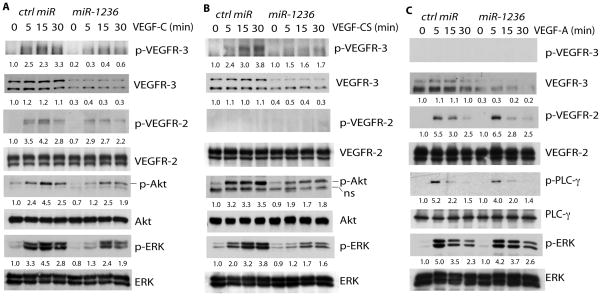
We then determined if miR-1236 regulates VEGFR-3 signaling in HLEC. PI3K-Akt, PLCγ and MAPK (ERK1/2) signaling pathways are reported to be the major downstream effectors in VEGFR-3 signaling. We have recently shown in HLEC that VEGF-A activates VEGFR-2 whereas VEGF-C activates VEGFR-3 strongly and VEGFR-2 weakly. However, a mutant form of VEGF-C (C156S, CS) specifically binds to and activates VEGFR-3 but not VEGFR-2 18 (Also see Fig. 4). We have also reported that VEGF-C and VEGF-CS strongly activate the VEGFR-3-Akt axis but not the PLC-γ axis. In contrast, VEGF-A strongly activates VEGFR-2-PLC-γ/ERK1/2 pathways 18. Therefore, we examined effects of miR-1236 on VEGF-C, VEGF-CS and VEGF-A-induced signaling in HLEC. Overexpression of miR-1236 in HLEC significantly reduced the total level of VEGFR-3 as well as VEGF-C or VEGF-CS-induced phosphorylation of VEGFR-3 (Fig. 4A-B). The reduced phosphorylation of VEGFR-3 was due to reduced total levels of VEGFR-3, as overexpression of VEGFR-3 lacking the 3′UTR rescued VEGFR-3 signaling (Supplemental Fig. IV). miR-1236 also weakly reduced VEGF-C-induced phosphorylation of VEGFR-2 without effects on the total levels of VEGFR-2 (Fig. 4A), perhaps partially due to heterodimerization between VEGFR2 and VEGFR3 26. miR-1236-mediated reduction of p-VEGFR-2 was also rescued by overexpression of VEGFR-3 (Supplemental Fig. IV). Moreover, VEGF-C and VEGF-CS-induced phosphorylation of downstream effectors Akt and ERK1/2 were also decreased by miR-1236 (Fig. 4A-B). In contrast, miR-1236 had no significant effect on VEGF-A-induced VEGFR-2-PLC-γ/ERK1/2 signaling (Fig. 4C). With the exception of VEGFR-3, total levels of VEGFR-2, Akt, PLC-γ and ERK1/2 were not altered by miR-1236 (Fig. 4A–C). These data suggest that miR-1236 specifically regulates VEGFR-3-dependent signaling in LEC.
Fig. 4. miR-1236 regulates VEGFR-3 dependent signaling in primary human lymphatic endothelial cells.
A-C. HLEC were transfected with ctrl miR or miR 1236. After 48 h, cells were subsequently serum-starved overnight followed by treatment with VEGF-C (100 ng/ml) (A), VEGF-C Cys156Ser (VEGF-CS, 250 ng/ml) (B) or VEGF-A (50 ng/ml) (C) for indicated times. Phospho- and total VEGFR-3, VEGFR-2, Akt, ERK and PLCγ were detected by Western blot with respective antibodies. Similar results were obtained from additional two experiments. Relative levels of phospho- and total proteins were quantified by taking untreated Ctrl miR as 1.0. Similar results were obtained from additional two experiments.
miR-1236 regulates VEGFR-3-dependent functions in primary human lymphatic endothelial cells
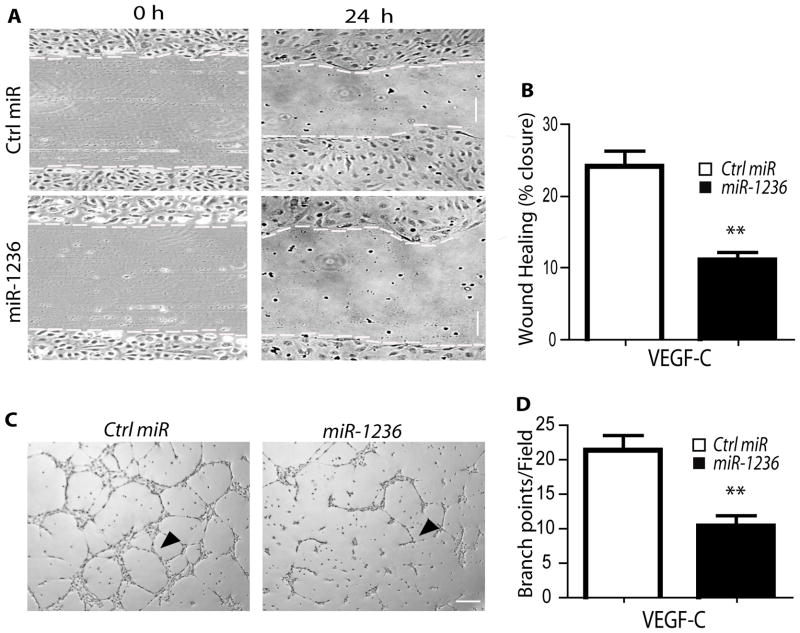
VEGFR-3 has been shown to be important for growth, survival and migratory signals in HLEC27. Therefore, we determined if miR-1236 repression of VEGFR-3 signaling would have negative functional outcomes in HLEC on two well-known functions for LEC - migration and tube formation. A control miR or miR-1236 was transfected into HLEC, and cell migration was assessed by a scratch wound assay. Overexpression of miR-1236 significantly reduced scratch closure by HLECs in response to VEGF-C compared to the control miR (Fig. 5A with quantification in 5B). Proliferation might contribute a minimal effect in this assay as cell cycle analysis showed miR-1236 weakly inhibit VEGF-C (but not VEGF-A)-induced cell proliferation (Supplemental Fig. V). Next, we determined the effect of miR-1236 on tube formation by HLEC in a Matrigel assay, in which endothelial cells align to form elongated tube-like structures. Compared to the control miR, overexpression of miR-1236 in HLEC led to a significant reduction in the ability of HLEC to form tube-like structures in a Matrigel in response to VEGF-C (Fig. 5C with quantification in 5D). Taken together, these results suggest that miR-1236 can attenuate VEGF-C/VEGFR-3 mediated functions in HLEC.
Fig. 5. miR-1236 regulates VEGFR-3 dependent functions in primary human lymphatic endothelial cells.
A–B. HLEC monolayer migration assay. 48 h after transfection with ctrl miR or miR-1236, confluent monolayers of HLEC were subjected to “wound” injury assay in the presence of 3% serum + VEGF-C (100 ng/ml) for 24 h. Representative images are shown in A. Scale bar, 100 μm. Cell migration distances (mm) were measured and % wound closure was quantified in B. Data are mean±SEM from duplicates (10 different areas in each well) of three independent experiments. **, P<0.01. C–D. Tube formation assays in a Matrigel. HLEC were transfected with ctrl miR or miR-1236 for 48 h. Cells were then seeded to Matrigel and cultured with 3% serum + VEGF-C (100 ng/ml) for 6 h and representative images are shown in C. Branch points as indicated by arrowheads were quantified in D. Data are mean ± SEM from three independent experiments, **, p<0.01. Scale bar, 100 μm.
miR-1236 reduces lymphangiogenesis and angiogenesis in vivo
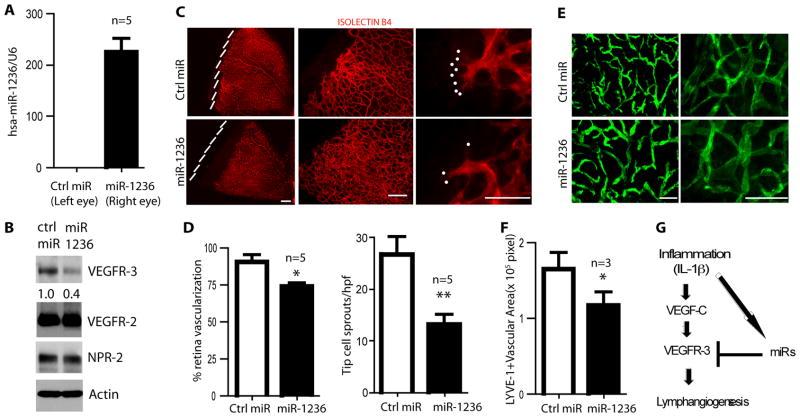
Development of the murine retina is well described 28. Interestingly, VEGFR-3 is expressed on the developing retina 29. Since there is no murine homologue to miR-1236, we injected miR-1236 into mouse tissues to determine an in vivo effect. Intravitreous injection of FITC-labeled miRNA was taken up by the retina (Supplemental Fig. VI). After a single intravitreous injection of miR-1236, we found that miR-1236 was detectable up to 5 days (Fig. 6A), indicating that miR-1236 was stable in the vitreous chamber and retina. Importantly, VEGFR-3, but not VEGFR-2 or neuropillin-2 (NPR-2, a VEGF-A co-receptor), was decreased from mouse retinas that were injected with miR-1236, relative to control mimics (Fig. 6B). Isolectin B4 staining revealed a tortuous and disorganized pattern of blood vessels in the right eye (miR-1236) compared to the left eye (control miR) (Fig. 6C). Furthermore, miR-1236-treated retina showed decreased vascularization with a reduced number of sprouts on the leading edge (tip cells) and (Fig. 6C with quantification in 6D), consistent with the phenotypes upon VEGFR-3 inhibition as described by Tammela et al 29. To assess the effects of miR-1236 on ear skin lymphangiogenesis, we treated 12d-old mice undergoing postnatal ear lymphangiogenesis with two injections of miR-1236 and monitored vascular growth 8d later. At the site of injection, LYVE-1 positive lymphatic vessels in the ear were decreased approximately 30% relative to the control miR (Fig. 6E with quantification in 6F). These results suggest that overexpression of miR-1236 can decrease lymphangiogenesis in vivo, through inhibition of VEGFR-3 signaling.
Fig. 6. miR-1236 regulates VEGFR-3 and (lymph)angiogenesis in vivo.
A–B. 4 day-old pups received an intraorbital injection of control miR, left eye (0.3 μg) or miR-1236, right eye (0.3 μg). Retinas were harvested on day 4 post-injection and miR-1236 was assessed by qRT-PCR with normalization to RNU6B (A). VEGFR2, VEGFR-2, NPR-2 and β-actin were detected by Western blot with respective antibodies. C–D. Retinas were harvested on day 4 post-injection, dissected, fixed, and stained with isolectin B4. Retina edges at low power images (10x) are indicated by dashed lines (left panel). A 20x images are shown in the middle. Tip cell sprouts at high power images (40x) are indicated by dots (right panel). Scale bar, 100 μm. % retina vascularization areas towards edge were quantified from the low power images (10x) and number of tip cell sprouts (indicated by dots) was quantified from the high power images (40x, right) are presented in D. Data in A-D presented are from 5 retinas for each group. **, p<0.01. E–F. Control miR or miR-1236 (0.3 μg) was injected into the left ear and right ear, respectively, of 12d-old mice. Ears of 20-day old mice were collected and stained with anti-LYVE-1 with quantification of LYVE-1+ areas in E (n=3 mice per group). *, p< 0.05 and. Scale bar, 50 μm. G. A model for miR as a negative regulator in inflammation/IL-1β mediated inhibition on VEGFR-3-dependent lymphangiogenesis (see text for details).
DISCUSSION
In the study presented here, we define a novel mechanism of Vegfr3 post-transcriptional regulation mediated by cytokine and microRNA. It was recently found that in the setting of acute skin inflammation in mice, VEGFR-3 mRNA and protein is strongly downregulated in inflamed lymphatics. This decrease correlates with the appearance of inflammatory cytokines, including IL-1β 20. IL-1β, a multifunctional mediator of the inflammatory response, is among the cytokines produced during oxazolone-induced inflammation 20. Despite IL-1β having a lymphangiogenic response in vivo, it was reported to be indirect, through production of VEGFs 30–32. To determine the direct effects of IL-1β on HLEC, we introduced IL-1β to HLEC in culture. Interestingly, IL-1β downregulated VEGFR-3 protein but not the transcript in cultured lymphatic ECs, leading to decreased HLEC function. To investigate a potential role for microRNA in VEGFR-3 regulation in HLEC, in silico analysis using TargetScan predicted a conserved binding site in the 3′ UTR of Vegfr3, complementary to hsa-miR-1236. hsa-miR-1236 is an experimentally cloned mirtron, first mentioned by Berezikov et al. using a computational screen for conserved mammalian mirtrons 33. We cloned the 3′ UTR of Vegfr3 and subsequent luciferase assays identified a miR-1236 binding site between nucleotides 144 and 150 of the human Vegfr3 3′ UTR. This region of the 3′UTR is conserved across species including analyses of the human, chimpanzee, rhesus, mouse and rat Vegfr3 UTRs. From our in vitro studies, we conclude that the Vegfr3 transcript is a target of miR-1236. miR-1236 was able to decrease VEGFR-3 protein, but not Vegfr3 transcript. Compared to the control miR, miR-1236 caused reduction of VEGFR-3 protein and led to a significant reduction in the signaling response to the lymphangiogenic growth factor, VEGF-C. No significant difference was found after stimulation with a VEGFR-2 specific ligand, VEGF-A, suggesting a VEGFR-3 specific effect. Importantly, miR-1236-mediated inhibition on VEGFR-3 signaling resulted in reduced lymphatic EC migration and tube formation, as well as lymphangiogenesis in vivo.
Another important finding is that miR-1236 is inducible by IL-1β signaling in LEC. These results support a model that miR-1236 functions as a negative regulator of VEGF-C/VEGFR-3 signaling and inflammation-induced lymphangiogenesis (Fig. 6G). Inflammatory cytokines such as IL-1β contribute to initial lymphangiogenesis, in part, by inducing expression of VEGFs and adhesion molecules such as ICAM-1, which recruits inflammatory cells. IL-1β also induces miR-1236, which in turn suppresses VEGFR-3-dependent signaling. Negative feedback mechanisms have been previously demonstrated for VEGF/VEGFR signaling. Specifically, VEGF-A via VEGFR-2 induces activation/expression of Notch, which is turn suppresses VEGFR-2 expression/activity, therefore negatively regulating angiogenesis 34–36. In addition, VEGFR-2/3 is also negatively regulated by many other mechanisms, including phosphatases, endocytosis, and ubiquitination 37–39. VEGFR-2 has been recently shown to be regulated by microRNA-424 40. Our study has demonstrated, for the first time, that VEGFR-3 signaling is feedback regulated by microRNA. Our data suggests that miR-1236 activity could play a role in the regulation of VEGFR-3 expression in lymphatic and blood vasculature during human hemangiogenesis. Modulation of VEGFR-3 levels using miR-1236 may represent a promising approach for the treatment of human disease in which a decrease in VEGFR-3 would be beneficial.
We cannot rule out other targets of miR-1236, as exogenous VEGFR-3 failed to completely rescue tube formation and migration despite rescuing VEGFR-3 signaling (Supplemental Fig. IV). However, analysis of miR-1236 targets did not yield signature genes necessary for lymphatic signaling in response to VEGF-C such as neuropilin-2. Furthermore, we confirmed that neuropillin-2 levels were not altered by miR-1236 in HLEC and in retina (Fig. 6). Since 3′ UTR analysis revealed that this site is evolutionarily conserved in vertebrates, we decided to test the effect of miR-1236 in vivo. Administration of miR-1236 in vivo caused a significant reduction in tip cells and angiogenesis of blood vessels. Moreover, lymphatic density was decreased during postnatal vascular development in tissues examined. These results are consistent with the importance of VEGFR-3 being important for lymphangiogenesis and angiogenesis 29.
miR-1236 is an evolutionary recent microRNA as it is only found in Pongo pygmaeus, Pan troglodytes, and Homo sapiens. Since mirtrons are thought to be newly evolved regulators in various animal species with a preexisting canonical miRNA pathway, it is thought that the contribution of mirtrons to the miRNA-mediated regulatory network is smaller than that of canonical miRNAs, owing to their generally modest expression levels41. Our data is consistent with this finding. By Northern blot, absolute expression of miR-1236 is low in HLEC. Furthermore, this may explain why knockdown of miR-1236 had a modest effect on VEGFR-3 protein and function in HLEC (Supplemental Figure VII). It is possible that there may be more mirtrons and conventional microRNAs that target Vegfr3 mRNA to add to the regulation provided by more highly expressed microRNAs in the traditional pathway. Indeed, knockdown of Dicer in HLECs led to an increase of VEGFR-3 in steady state conditions (not shown), supporting this hypothesis. Nevertheless, our study indicates that miR-1236 is a miR that targets VEGFR-3, and should provide a foundation for us to define more miRNAs regulating VEGFR-3 signaling and lymphangiogenesis in the context of inflammation. Moreover, the seemingly dual role of IL-1β and other inflammatory cytokines in the resolution of lymphangiogenesis and/or lymphatic dysfunction is intriguing. While IL-1β and other inflammatory cytokines mediate inflammation, the kinetics and interplay between these cytokines and lymphangiogenic factors needs further investigation.
Supplementary Material
Acknowledgments
We thank Dr. Mark Saltzman and Christopher Cheng for labeled RNAs.
Source of funding: This work was supported by NIH grant R01 HL065978 and a National Nature Science Foundation of China ((81170863). D. Jones is supported by a National Research Service Award (National Cancer Institute, Ruth Kirschstein Predoctoral Fellowship F31 CA 136316).
Footnotes
Disclosure: None.
References
- 1.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 2.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 3.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. Embo J. 1996;15:1751. [PMC free article] [PubMed] [Google Scholar]
- 5.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 6.Oliver G. Lymphatic vasculature development. Nat Rev Immunol. 2004;4:35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- 7.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 8.Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6:109–122. doi: 10.1089/lrb.2008.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA, Alitalo K. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. Embo J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, Pepper MS, Zawieja DC, Ran S. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood. 2010;115:418–429. doi: 10.1182/blood-2008-12-196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Fulcoli FG, Tang S, Baldini A. Tbx1 regulates proliferation and differentiation of multipotent heart progenitors. Circ Res. 2009;105:842–851. doi: 10.1161/CIRCRESAHA.109.200295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones D, Min W. An overview of lymphatic vessels and their emerging role in cardiovascular disease. J Cardiovasc Dis Res. 2011;2:141–152. doi: 10.4103/0975-3583.85260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamura K, Hagen JW, Duan H, Tyler DM, Lai EC. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007;130:89–100. doi: 10.1016/j.cell.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass Drosha processing. Nature. 2007;448:83–86. doi: 10.1038/nature05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suarez Y, Fernandez-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedrioli DM, Karpanen T, Dabouras V, Jurisic G, van de Hoek G, Shin JW, Marino D, Kalin RE, Leidel S, Cinelli P, Schulte-Merker S, Brandli AW, Detmar M. miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol Cell Biol. 2010;30:3620–3634. doi: 10.1128/MCB.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazenwadel J, Michael MZ, Harvey NL. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood. 2010 doi: 10.1182/blood-2009-12-256297. [DOI] [PubMed] [Google Scholar]
- 18.Jones D, Xu Z, Zhang H, He Y, Kluger MS, Chen H, Min W. Functional Analyses of the Nonreceptor Kinase Bone Marrow Kinase on the X Chromosome in Vascular Endothelial Growth Factor-Induced Lymphangiogenesis. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 20.Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, Werner S, Alitalo K, Detmar M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood. 2011;117:4667–4678. doi: 10.1182/blood-2010-10-316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vigl B, Aebischer D, Nitschke M, Iolyeva M, Rothlin T, Antsiferova O, Halin C. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood. 2011;118:205–215. doi: 10.1182/blood-2010-12-326447. [DOI] [PubMed] [Google Scholar]
- 22.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH, Koh GY. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34:96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Pajusola K, Aprelikova O, Pelicci G, Weich H, Claesson-Welsh L, Alitalo K. Signalling properties of FLT4, a proteolytically processed receptor tyrosine kinase related to two VEGF receptors. Oncogene. 1994;9:3545–3555. [PubMed] [Google Scholar]
- 25.Bando H, Brokelmann M, Toi M, Alitalo K, Sleeman JP, Sipos B, Grone HJ, Weich HA. Immunodetection and quantification of vascular endothelial growth factor receptor-3 in human malignant tumor tissues. Int J Cancer. 2004;111:184–191. doi: 10.1002/ijc.20211. [DOI] [PubMed] [Google Scholar]
- 26.Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, Alitalo K, Claesson-Welsh L. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem. 2003;278:40973–40979. doi: 10.1074/jbc.M304499200. [DOI] [PubMed] [Google Scholar]
- 27.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. Embo J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Lofqvist C, Hellstrom A, Smith LE. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Yla-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 30.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter RM, Ishibashi T, Kuwano M, Ono M. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watari K, Nakao S, Fotovati A, Basaki Y, Hosoi F, Bereczky B, Higuchi R, Miyamoto T, Kuwano M, Ono M. Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem Biophys Res Commun. 2008;377:826–831. doi: 10.1016/j.bbrc.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 32.Lin CH, Lu J, Lee H. Interleukin-1beta expression is required for lysophosphatidic Acid-induced lymphangiogenesis in human umbilical vein endothelial cells. Int J Inflam. 2010;2011:351010. doi: 10.4061/2011/351010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol Cell. 2007;28:328–336. doi: 10.1016/j.molcel.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 36.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, McKinney R, Fukai T, Ushio-Fukai M. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102:1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruns AF, Herbert SP, Odell AF, Jopling HM, Hooper NM, Zachary IC, Walker JH, Ponnambalam S. Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic. 2010;11:161–174. doi: 10.1111/j.1600-0854.2009.01001.x. [DOI] [PubMed] [Google Scholar]
- 39.Karpanen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, Tamagnone L, Alitalo K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. Faseb J. 2006;20:1462–1472. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- 40.Chamorro-Jorganes A, Araldi E, Penalva LO, Sandhu D, Fernandez-Hernando C, Suarez Y. MicroRNA-16 and MicroRNA-424 Regulate Cell-Autonomous Angiogenic Functions in Endothelial Cells via Targeting Vascular Endothelial Growth Factor Receptor-2 and Fibroblast Growth Factor Receptor-1. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin R, Smibert P, Yalcin A, Tyler DM, Schafer U, Tuschl T, Lai EC. A Drosophila pasha mutant distinguishes the canonical microRNA and mirtron pathways. Mol Cell Biol. 2009;29:861–870. doi: 10.1128/MCB.01524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.