Abstract
The amygdala is critically involved in detecting emotionally salient stimuli and in enhancing memory for emotional information. Growing evidence also suggests that the amygdala plays a crucial role in addiction, perhaps by strengthening associations between emotionally-charged drug cues and drug-seeking behavior. In the current study, by integrating functional MRI (fMRI), genetics, and outcome data from a large group of smokers who completed a smoking-cessation intervention and attempted to quit, we show that the amygdala also plays a role in quitting. Specifically, we demonstrate that the amygdala response to smoking-cessation messages in smokers trying to quit is a predictor of their post-intervention quitting outcome. We further show that the amygdala response is modulated by genetic variation in the serotonin transporter and mediates the impact of this genetic variation on quitting. These results point to a gene-brain-behavior pathway relevant to smoking cessation, and add to our understanding of the role of the amygdala in nicotine addiction.
Keywords: Amygdala, smoking cessation, serotonin transporter gene, fMRI, imaging genetics
Introduction
Cigarette smoking continues to be a leading preventable cause of morbidity and mortality in the U.S. (CDC, 2003, 2008). Many smokers attempt to quit but a majority relapse within 6 months (Quaak et al., 2009), highlighting a vital need for more effective interventions. Identification of brain predictors of relapse susceptibility could inform the design and selection of smoking-cessation interventions in the clinic, while also adding to our understanding of brain processes underlying nicotine addiction.
The goal of the current study was to examine the role of amygdala response to smoking-cessation messages in quitting as well as the genetic sources of variability in amygdala response. The amygdala plays a critical role in emotion processing, including response to emotionally salient stimuli and acquisition of emotional learning (LeDoux, 2000; Phelps, 2006). Emotionally salient information tends to be remembered better than emotionally neutral information (Cahill and McGaugh, 1995), and both animal and human studies suggest that the amygdala is required for this enhanced learning (McGaugh, 2004). For drug users, drug-related cues are emotionally arousing and are known to activate the amygdala (Childress et al., 1999; Franklin et al., 2007; Kilts et al., 2001). Therefore, drug-related information is more likely to be remembered by them and influence their behavior. Consistent with this view, growing evidence suggests that the amygdala is involved in drug addiction, by signaling the salience of drug-related cues and creating strong associations with drug-seeking behaviors (Robbins et al., 2008; See et al., 2003).
Previous neuroimaging studies in smokers have shown the link between amygdala response to smoking-related cues on the one side, and craving intensity (Franklin et al., 2011; Goudriaan et al., 2010) and relapse susceptibility (Janes et al., 2010) on the other side. In contrast, amygdala response to smoking-cessation messages has not yet been examined. We hypothesized that the amygdala may also play an important role in how smokers respond to smoking-cessation messages and how these messages influence their smoking behavior. Specifically, we expected that a greater amygdala response to smoking-cessation messages should be associated with enhanced memory of these messages, and therefore increased odds of quitting success. To test our hypothesis, we integrated functional MRI (fMRI) and outcome data from a large group of smokers who completed a smoking-cessation intervention, in order to examine whether the amygdala response to smoking-cessation messages predicted subsequent quitting outcome in these smokers.
We also investigated the genetic factors that may account for individual differences in amygdala response to smoking-cessation messages, using an imaging genetics approach (Bigos and Weinberger, 2010; Hariri, 2009; Hariri et al., 2006). Growing evidence from imaging genetics suggests that amygdala reactivity to emotionally salient stimuli (e.g., angry faces) is modulated by genetic variation in the serotonin transporter (Hariri et al., 2005; Hariri et al., 2002). Serotonin (or 5-hydroxytryptamine, 5-HT) is a major modulatory neurotransmitter in the mammalian brain, crucially involved in a range of brain processes, including stress response, arousal, motor activity, appetite, and mood (Jacobs and Azmitia, 1992)—but also cognitive control, cognitive flexibility, and decision making (Cools et al., 2008; Dayan and Huys, 2009; Kranz et al., 2010; Rogers, 2011). The serotonin transporter protein (5-HTT), responsible for reuptake of 5-HT from the synapse back into the presynaptic neuron, serves as a key regulator of 5-HT signaling.
Thus, in addition to testing if amygdala response to smoking-cessation messages predicted quitting, we also employed an imaging genetics approach to test whether two known functional polymorphisms in the 5-HTT gene (SLC6A4)—the 5-HTT-linked polymorphic region (5-HTTLPR) in the promoter (Heils et al., 1996; Lesch et al., 1996) and the serotonin transporter intron 2 (STin2) polymorphism (Fiskerstrand et al., 1999; Lesch et al., 1994)—modulated the amygdala response. The 5-HTTLPR is a 44-base pair insertion/deletion polymorphism in the promoter region, with the short allele (S) less efficiently transcribed than the long allele (L) (Heils et al., 1996; Lesch et al., 1996). In addition, the 5-HTTLPR includes an A→G single nucleotide substitution (rs25531), with the LG allele being functionally equivalent to the S allele, leaving the LA allele as the true high-transcription allele (Hu et al., 2006). The second functional polymorphism, STin2, is a 17-base pair insertion/deletion polymorphism in intron 2 of the 5-HTT gene SLC6A4, with the 12-repeat allele more efficiently transcribed than the 10-repeat allele (Fiskerstrand et al., 1999; Lesch et al., 1994). Based on previous evidence (Dannlowski et al., 2010; Hariri et al., 2005; Hariri et al., 2002), we hypothesized that low-transcription alleles of the 5-HTTLPR/rs25531 and STin2 would be associated with a relatively increased amygdala response compared to high-transcription alleles.
Materials and methods
Subjects
Ninety-one smokers interested in quitting (44 females and 47 males, mean age = 37.5 years, mean number of cigarettes per day = 16.7) were recruited for the study (Chua et al., 2011), which included a web-based tailored smoking-cessation intervention developed at the University of Michigan’s Center for Health Communications Research (Strecher et al., 2008). Smokers were eligible to participate if they smoked a minimum of 10 cigarettes per day and at least 100 cigarettes in their lifetime. Subjects were not enrolled in other smoking-cessation programs or taking pharmacological treatments for smoking cessation during study enrollment. All subjects were native English speakers, had normal vision and hearing, and had no history of head injury or mental illness. The study protocol was approved by the University of Michigan Medical School IRB and all subjects provided written informed consent.
Study design
The study involved 3 sessions plus a follow-up phone interview. In Session 1, subjects completed a baseline assessment of their smoking history and other health, demographic, and psychosocial characteristics relevant to smoking cessation. The responses were used to create tailored smoking-cessation messages for the subsequent intervention. In Session 2, subjects completed a Messages Task during functional MRI (fMRI). In Session 3, scheduled within one week of their fMRI session, subjects completed a web-based computer-tailored smoking-cessation intervention (Strecher et al., 2008) and started their quit attempt. All subjects also provided saliva for DNA extraction and genotyping, and received a 10-week supply of nicotine patches to help them quit. Four months after the intervention session, subjects were interviewed on the phone to determine their smoking-cessation outcome. The primary outcome measure was 7-day point-prevalence abstinence (cigarette free for the past 7 days), a metric that is highly correlated with both 24-hour point-prevalence abstinence and 30-day prolonged abstinence, as well as with physiological measures of smoking cessation (Velicer and Prochaska, 2004).
Messages Task
All subjects completed 5 runs of the Messages Task (Chua et al., 2011) during fMRI, with 2 blocks of each of the three types of messages (tailored smoking-cessation messages, untailored smoking-cessation messages, and neutral control messages) per run, 5 messages per block, for the total number of 150 messages. Each block lasted 24 seconds. The order of blocks in the Messages Task was pseudo-randomized within and across participants. Blocks were separated by fixations lasting 4–10 seconds (an average of 7 seconds). The messages were presented visually on the screen and simultaneously presented as an audio track. Subjects were instructed to pay attention to the messages but no response was required. Tailored smoking-cessation messages were created on the basis of the individual’s responses during a baseline assessment and made references to this person’s characteristics, experiences, and specific obstacles to quitting. Therefore, although of the same type, tailored smoking-cessation messages varied across subjects. Examples of tailored smoking-cessation messages include: You want to quit because you are tired of spending your money on cigarettes; You feel like your friend will help you stay on track once you quit; You have a very strong urge to smoke when you first wake. In contrast, untailored smoking-cessation messages were relevant to smokers in general and were identical for all subjects. Examples of untailored smoking-cessation messages include: Many people quit with another person so they can support each other; Many people relapse due to stress, alcohol, and cravings; Most people need to try more than once to quit smoking for good. Neutral control messages (i.e., world-knowledge messages not related to smoking) were also the same for all subjects. Examples of neutral messages include: The longest duration of a solar eclipse was 7 minutes, 31 seconds; Bali attracts more tourists than any other Indonesian island; Global warming caused the recent collapse of an Antarctic ice shelf.
Image acquisition
Scanning was performed on a 3T GE Signa Excite 2 scanner (Milwaukee, Wisconsin), beginning with a structural T1-overlay image (repetition time [TR] = 250 ms, echo time [TE] = 7 ms, flip angle [FA] = 75 degree, field of view [FOV] = 220 mm, 43 oblique axial slices, 256 × 256, slice thickness 3.0 mm). Functional scans were collected using a T2*-weighted spiral-in acquisition sequence (gradient echo, TR = 2000 ms, TE = 30 ms, FA = 90°, FOV = 220 mm, 64 × 64, slice thickness 3.0 mm) (Noll et al., 1998). High-resolution T1 scans were also obtained for precise anatomical localization (3D spoiled-gradient echo [3D-SPGR] with inversion recovery prep, time of inversion = 400 ms, TR = 9.0 ms, TE = 1.8 ms, FA = 15°, FOV = 260 mm, 128 slices, 256 × 256, 1.2 mm slice).
Image preprocessing
All functional scans were slice-time-corrected, motion-corrected, and realigned to the first scan using the MCFLIRT program (FSL Analysis Group, FMRIB, Oxford, UK). Subsequent processing was done using SPM (Wellcome Institute of Cognitive Neurology, London, UK). The T1-overlay was co-registered with a functional scan. The high-resolution 3D-SPGR image was co-registered to the T1-overlay and anatomically normalized to the Montreal Neurological Institute (MNI) 152 template. The resulting transformation parameters were then applied to the co-registered functional volumes. All functional volumes were smoothed with an overall Gaussian kernel with a full width at half maximum (FWHM) of 7 mm.
Genotyping procedures
Genomic DNA was obtained from saliva samples using Oragene collection system and extracted using the protocol provided (Genotek, Ontario, Canada). STin2 and 5-HTTLPR/rs25531 polymorphisms were genotyped using polymerase chain reaction and oligonucleotide primers. 5-HTTLPR was genotyped using primers from Yonan et al. (Yonan et al., 2006). PCR products were analyzed on 1.5% TBE agarose gels (expected band sizes: S – 415bp, L – 459bp). To additionally genotype the A-G SNP (rs25531), product was digested with MspI. Digest products were resolved on 3% TBE agarose gels (expected band sizes: LA – 331bp, 66 bp, and 62 bp; LG – 157 bp, 174 bp, 66 bp, and 62 bp; S – 287 bp, 66 bp, and 62 bp). STin2 was genotyped using primers from Kaiser et al. (Kaiser et al., 2001). PCR products were resolved for size on 1.5% agarose gels. Gels were visualized using ethidium bromide under UV light and reviewed by two independent people, with 100% concordance. Six of the samples were verified by Sanger sequencing, with 100% concordance.
Statistical analyses
After pre-processing, the functional data were analyzed using a modified General Linear Model (GLM) and a blocked design. Regressors of interests (tailored smoking-cessation messages, untailored smoking-cessation messages, and neutral messages) were convolved with a canonical hemodynamic response function (HRF) with a time derivative to account for between-subject and between-voxel variability in the response peak. Movement parameters were included as covariates. Statistical analyses were conducted in a series of steps using a random-effects model in SPM5. First, anatomically defined ROI masks of right and left amygdalae were constructed using WFU PickAtlas (Maldjian et al., 2003) (right amygdala: 87 voxels; left amygdala: 75 voxels). For each individual subject, we extracted the average parameter estimates (betas) for the tailored smoking-cessation messages – neutral messages contrast and the untailored smoking-cessation messages – neutral messages contrast for right and left amygdala ROIs. These individual parameter estimates, together with genotyping and outcome data, were then entered into second-level group analyses in SPSS 17.0. Race (Caucasian or not), gender, pre-intervention smoking severity (cigarettes smoked per day), and post-intervention use of nicotine patches (in days) were used as nuisance covariates in all analyses. The mediation analyses were conducted using the Sobel test of mediation and a boostrapping approach as implemented by an SPSS macro (Preacher and Hayes, 2008). We used a statistical significance threshold of p < 0.05 throughout.
Results
Smoking-cessation outcome
We present the results from the final sample of 82 subjects (38 females and 44 males; 77% Caucasian) for whom genotyping, fMRI, and full outcome data were available (Supplementary Results). Out of this final sample, 45 subjects were abstinent (and were classified as Quitters) and 37 were smoking (and were classified as Non-Quitters) at the 4-month follow-up. The two outcome groups did not differ in any demographic or smoking-related measures, including the length of use of nicotine patches after the intervention, except for a higher pre-intervention number of cigarettes smoked per day in Non-Quitters compared to Quitters (p = 0.053) (Supplementary Table 1).
Amygdala response to smoking-cessation messages predicts quitting
To test for emotional enhancement memory effect for smoking-cessation messages, and to verify that the subjects paid attention during the Messages Task, we gave them a surprise memory test after they left the scanner (see Supplementary Results). Both tailored and untailored smoking-cessation messages were remembered more accurately than neutral messages (p < 0.01), supporting an emotional enhancement effect for smoking-cessation messages.
We assessed the amygdala response to tailored and untailored smoking-cessation messages compared to neutral messages using blood-oxygenation-level dependent (BOLD) signal and the smoking-cessation messages > neutral messages contrast values extracted from anatomically defined right and left amygdala masks (Maldjian et al., 2003). To control for other potential sources of variability in susceptibility to relapse following a quit attempt, we included race, gender, pre-intervention cigarettes smoked per day, and post-intervention use of nicotine patches as nuisance covariates in all analyses.
Consistent with our main hypothesis, the mean amygdala response to smoking-cessation messages (averaged across right and left amygdala) was a significant predictor of subsequent post-intervention quitting outcome (B = 1.17, SE = 0.40, Wald χ2 = 8.32, p = 0.004, odds ratio = 3.21) (see Supplementary Table 2). A greater magnitude of the mean amygdala response to smoking-cessation messages predicted betters odds of quitting success. An omnibus test of model coefficients indicated that the model was significant (p = 0.001). The model explained 23% of the variance in quitting outcome (R square = 0.23) and correctly classified 33 out of 45 Quitters (73.3% accuracy). When tested with separate models, both the right and left amygdala responses to smoking-cessation messages also predicted subsequent quitting (right amygdala: B = 1.05, SE = 0.37, Wald χ2 = 8.31, p = 0.004, odds ratio = 2.87, classification accuracy = 73.3% of Quitters, Supplementary Table 3; left amygdala: B = 0.73, SE = 0.30, Wald χ2 = 5.84, p = 0.016, odds ratio = 2.08, classification accuracy = 75.6% of Quitters, Supplementary Table 4).
In addition, when we examined tailored and untailored smoking-cessation messages separately, the mean amygdala response to both types of messages was predictive of subsequent quitting (tailored messages: B = 0.96, SE = 0.33, Wald χ2 = 8.34, p = 0.004, odds ratio = 2.62, classification accuracy = 73.3% of Quitters, Supplementary Table 5; untailored messages: B = 0.63, SE = 0.28, Wald χ2 = 5.11, p = 0.024, odds ratio = 1.88, classification accuracy = 68.9% of Quitters, Supplementary Table 6). Both models were significant (p < 0.008) and explained 21% (tailored messages: R square = 0.21) and 18% (untailored messages: R square = 0.18) of the variance in quitting outcome, respectively.
Genotyping results
Next, we tested for modulation of amygdala response to smoking-cessation messages by genetic variation in the 5-HTT. Subjects provided saliva for DNA extraction and were genotyped for two common, functional polymorphisms in the regulatory regions of the 5-HTT gene (SLC6A4), 5-HTTLPR/rs22531 in the promoter and STin2 in intron 2.
We observed the following distribution of STin2 genotypes: 12 participants were 10/10 homozygotes, 38 were 10/12 heterozygotes, and 32 were 12/12 homozygotes. The observed 5-HTTLPR/rs25531 genotypes were functionally grouped as follows: 24 participants were LA/LA homozygotes, 41 were LA/LGS heterozygotes (i.e., LA/S or LA/LG), and 17 were LGS/LGS homozygotes (i.e., S/S, LG/S or LG/LG). No deviations from the Hardy-Weinberg Equilibrium were observed. The genotyping results are given in Supplementary Table 7.
Because the STin2 and 5-HTTLPR/rs25531 polymorphisms both affect the rate of transcription and may have combined effects on transcription efficiency (Hranilovic et al., 2004), both genotypes were included in the initial linear regression models to test if either genotype predicted amygdala response to smoking-cessation messages. Consistent with previous reports (Gelernter et al., 1999; Kazantseva et al., 2008), the STin2 and 5-HTTLPR/rs25531 loci were in linkage disequilibrium, i.e., the genotypes at the two loci were significantly associated with each other (χ2 = 27.80, p < 0.0001) (Table 1). Neither the STin2 genotype groups nor the 5-HTTLPR/rs22531 genotype groups differed in pre-intervention measures, except for a higher confidence in quitting in the LA/LA genotype group compared to the LGS carriers (t = 2.47, p = 0.015) (Supplementary Tables 8 and 9).
Table 1.
Linkage disequilibrium between the STin2 and 5-HTTLPR/rs25531 loci.
| STin2 Genotype (Number of 10 Alleles) | Total | ||||
|---|---|---|---|---|---|
| 12/12 0 |
12/10 1 |
10/10 2 |
|||
| 5-HTTLPR/rs22531 Genotype (Number of S or LG Alleles) |
LA/LA 0 |
7 | 7 | 10 | 24 |
|
LA/LGS 1 |
13 | 26 | 2 | 41 | |
|
LGS/LGS 2 |
12 | 5 | 0 | 17 | |
| Total | 32 | 38 | 12 | 82 | |
STin2 genotype predicts amygdala response to smoking-cessation messages
The STin2 genotype was a significant predictor of the mean amygdala response to smoking-cessation messages, when controlling for race, gender, pre-quit smoking severity, post-intervention use of nicotine patches, and for the 5-HTTLPR/rs25531 genotype (B = 0.35, SE = 0.17, t = 2.06, p = 0.042) (see Supplementary Table 10). The model including both STin2 and 5-HTTLPR/rs25531 was significant (p = 0.002) and accounted for 23% of the variance in the mean amygdala response to smoking-cessation messages (R square = 0.23). As hypothesized, a higher number of low-transcription alleles (STin2 10 alleles) predicted a greater magnitude of amygdala response. In contrast, the 5-HTTLPR/rs25531 genotype was not a significant predictor of the mean amygdala response when controlling for the STin2 genotype and the other covariates (p = 0.188). Because the genotypes at the two loci were highly associated with each other (χ2 = 27.80, p < 0.0001) (Table 1), we focused on the STin2 genotype and dropped the 5-HTTLPR/rs25531 from subsequent analyses.
Separate regression models for the right and left amygdala showed that the STin2 genotype alone significantly predicted the response to smoking-cessation messages both in the right amygdala (B = 0.50, SE = 0.15, t = 3.30, p = 0.001) and in the left amygdala (B = 0.41, SE = 0.18, t = 2.25, p = 0.027) (see Supplementary Tables 11–12). Both models were significant (p < 0.01) and explained 24% of the variance in the right amygdala response (R square = 0.24) and 18% of the variance in the left amygdala response (R square = 0.18), respectively.
The STin2 genotype alone was also a significant predictor of the mean amygdala response to tailored smoking-cessation messages (B = 0.38, SE = 0.15, t = 2.59, p = 0.012). The model was significant (p = 0.03) and explained 15% of the variance in the mean amygdala response (R square = 0.15). The effects were observed both in the right amygdala (B = 0.40, SE = 0.16, t = 2.43, p = 0.017) and in the left amygdala (B = 0.37, SE = 0.17, t = 2.18, p = 0.032) when tested with separate models (Supplementary Tables 13–15). The STin2 genotype also significantly predicted the mean amygdala response to untailored messages (B = 0.53, SE = 0.20, t = 2.60, p = 0.011). The model was significant (p = 0.002) and explained 22% of the variance in the mean amygdala response (R square = 0.22). For untailored messages, the STin2 effect was significant in the right amygdala (B = 0.60, SE = 0.19, t = 3.07, p = 0.003) and trend significant in the left amygdala (B = 0.46, SE = 0.24, t = 1.89, p = 0.063) (Supplementary Tables 16–18).
Furthermore, the results for STin2 modulation of the mean amygdala response remained essentially unchanged when we restricted the analyses to Caucasians (n = 63; 33 females and 30 males; STin2 genotypes: 10/10 = 12, 10/12 = 28, 12/12 = 23), controlling for gender, pre-intervention smoking severity, and post-intervention use of nicotine patches (all smoking-cessation messages: B = 0.47, SE = 0.18, t = 2.60, p = 0.012; tailored messages: B = 0.44, SE = 0.17, t = 2.59, p = 0.012; untailored messages: B = 0.49, SE = 0.23, t = 2.13, p = 0.037) (Supplementary Tables 19–21).
Amygdala response to smoking-cessation messages mediates STin2 effects on quitting
Because the mean amygdala response to smoking-cessation messages was both predictive of subsequent quitting outcome and modulated by the STin2 genotype in our data, we next tested for mediation effects. A mediation relationship is illustrated by a three-variable model with three causal pathways: Path a represents the effect of the predictor on the mediator; Path b represents the effect of the mediator on the outcome; and Path c represents the total effect of the predictor on the outcome (Baron and Kenny, 1986). Path c′ denotes the direct effect of the predictor on the outcome, controlling for the effect of the mediator. Evidence for mediation is obtained if we can reject the null hypothesis of no difference between the total effect (c) and the direct effect (c′), that is, c – c′ ≠ 0, demonstrating that the predictor affects the outcome at least in part through the mediator. The benefit of conducting a mediation analysis is that it allows us to test a hypothesis about a relationship between three different variables: thus, a significant mediation effect informs us about the process through which the predictor affects the outcome.
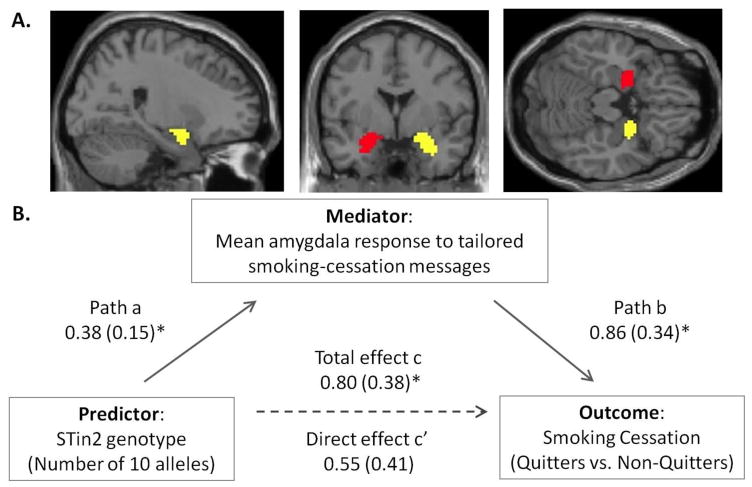
Using the Sobel test of mediation and a bootstrapping approach (Preacher and Hayes, 2008), we found that the mean amygdala response to tailored smoking-cessation messages was a significant mediator of STin2 genotype effects on quitting outcome (mean mediation effect value = 0.38, SE = 0.25, 95% CI: 0.02 – 0.92; p < 0.05) (Path diagram in Figure 1; see also Supplementary Results for a sample bootstrap output). Because the confidence interval for the mediation effect (c – c′) did not include zero when using the bootstrapping test of mediation effect, we could reject the null hypothesis of no difference between the total effect (c) and the direct effect (c′) in favor of the alternative hypothesis of mediation. Controlling for mediation effect in the amygdala reduced the association between the STin2 genotype and smoking cessation (total effect c: B = 0.80, SE = 0.38, p = 0.037; direct effect c′: B = 0.55, SE = 0.41, p = 0.173). In other words, a greater number of the low-transcription STin2 10 alleles was associated with a greater amygdala response to tailored smoking-cessation messages, and this greater amygdala response was associated with better odds of quitting success and mediated the impact of the STin2 genotype on quitting.
Figure 1.
Mean amygdala response to tailored smoking-cessation messages mediates the impact of STin2 genotype on smoking cessation. A. Anatomically defined region-of-interest masks for right and left amygdala shown against MNI 152 template. B. Mediation path diagram. Lines are labeled with unstandardized B coefficients, with standard errors in parentheses. Path b denotes the association of the mean amygdala response with smoking cessation, controlling for the effects of STin2 genotype. Direct effect c′ denotes the effect of STin2 genotype on smoking cessation, controlling for the mediation effect. *p < 0.05.
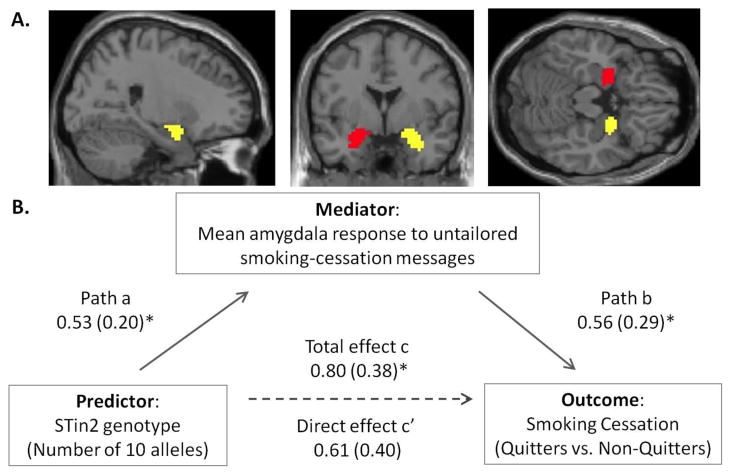
Similarly, the mean amygdala response to untailored smoking-cessation messages was a significant mediator of STin2 genotype effects on quitting outcome (mean mediation effect value = 0.35, SE = 0.25, 95% CI: 0.02 – 0.95; p < 0.05) (Path diagram in Figure 2, see also Supplementary Results for a sample bootstrap output). Controlling for mediation effect in the amygdala reduced the association between the STin2 genotype and smoking cessation (total effect c: B = 0.80, SE = 0.38, p = 0.037; direct effect c′: B = 0.61, SE = 0.40, p = 0.122). As before, a greater number of the STin2 10 alleles was associated with a greater amygdala response to untailored smoking-cessation messages, and this enhanced amygdala response was associated with better odds of successfully quitting and mediated the impact of the STin2 genotype on quitting.
Figure 2.
Mean amygdala response to untailored smoking-cessation messages mediates the impact of STin2 genotype on smoking cessation. A. Anatomically defined region-of-interest masks for right and left amygdala shown against MNI 152 template. B. Mediation path diagram. Lines are labeled with unstandardized B coefficients, with standard errors in parentheses. Path b denotes the association of the mean amygdala response with smoking cessation, controlling for the effects of STin2 genotype. Direct effect c′ denotes the effect of STin2 genotype on smoking cessation, controlling for the mediation effect. *p ≤ 0.05.
Voxel-wise results: Brain regions modulated by STin2 genotype
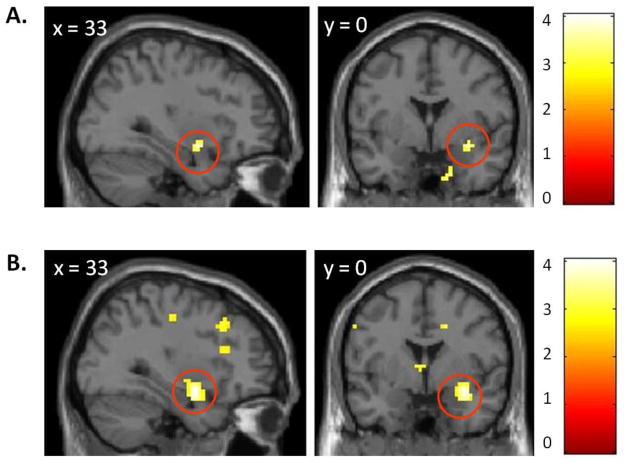
We also performed an exploratory, voxel-wise search for brain regions whose response to tailored and untailored smoking-cessation messages was modulated by the STin2 genotype, again controlling for the effects of race, gender, pre-intervention smoking severity, and post-intervention use of nicotine patches (p < 0.005, 10 contiguous voxels, uncorrected). A right amygdala cluster extending to parahippocampal gyrus was a global maximum in both searches, showing an increase in response magnitude with an increased number of the low-transcription STin2 10 alleles for both tailored messages (xyz coordinates of peak voxel: 33, 0, -12; cluster extent k = 16 voxels; T = 4.04, Z = 3.83; Figure 3A) and untailored messages (xyz coordinates of peak voxel: 33, 0, -12; cluster extent k = 94 voxels; T = 4.27, Z = 4.03; Figure 3B), confirming the results obtained with an anatomically defined mask described above.
Figure 3.
The right amygdala is a global maximum in a voxel-wise search for brain regions whose response to tailored smoking-cessation messages (A) and untailored smoking-cessation messages (B) is modulated by the STin2 genotype (thresholded at p = 0.005, 10 contiguous voxels). The results are shown against the MNI template brain. The scale represents t values.
Apart from the right amygdala/parahippocampal cluster, the number of STin2 10 alleles was also positively associated with response magnitude to tailored messages in the bilateral superior frontal gyrus, right fusiform gyrus, and right uncus (Supplementary Table 22), and with response magnitude to untailored messages in a number of cortical and subcortical regions, including bilateral medial frontal gyrus, precuneus, and mid cingulate gyrus, right middle frontal gyrus and postcentral gyrus, and left temporal cortex, caudate, putamen, precentral gyrus, and superior parietal lobule (Supplementary Table 23). In addition, an increase in the number of STin2 10 alleles was associated with decreased response to tailored messages in the left middle temporal gyrus, left cerebellum, and right middle frontal gyrus (Supplementary Table 22). No regions showed a decreased response to untailored messages with the increasing number of STin2 10 alleles.
Discussion
The goal of the current study was twofold: first, to examine the role of amygdala response to smoking-cessation messages in quitting; and second, to examine the genetic sources of variability in amygdala response. With respect to the first goal, using a “brain-as-predictor” approach, we demonstrate that neural response to smoking-cessation messages in bilateral, anatomically defined amygdala is a significant predictor of subsequent quitting outcome following a tailored smoking-cessation intervention. Smokers who showed a greater amygdala response to smoking-cessation messages had better odds of quitting success. Importantly, these results were obtained controlling for other factors that are known to affect quitting outcome, including gender, pre-quit smoking severity, and post-intervention use of nicotine patches.
The amygdala response to both tailored and untailored smoking-cessation messages significantly predicted subsequent quitting success. Tailored messages were tailored to individual participants based on their baseline assessment and thus varied between participants, while untailored messages were relevant to all smokers and were identical for all participants. In our view, amygdala engagement reflects that both types of messages were interpreted as emotionally salient by smokers. But because the content of both tailored and untailored messages was related to smoking and was also self-relevant to some degree, we were unable to dissociate the impact of these two attributes on the observed effects. Future studies treating smoking-relatedness and self-relevance as orthogonal factors may be needed to accomplish that, although this may prove difficult in the context of smoking cessation (e.g., it is possible that smoking-related stimuli are interpreted as self-relevant by smokers).
Our results suggest that smokers who show a greater amygdala response to smoking-cessation messages relative to neutral messages have less difficulty quitting. However, prior neuroimaging evidence also suggests that smokers who show a greater amygdala response to smoking-related cues relative to neutral cues are at a greater risk of slipping during their quit attempt (Janes et al., 2010). We propose that this apparent discrepancy can be explained as follows. The impact of amygdala response to emotionally salient stimuli on learning and behavior depends on the relation of these salient stimuli to current goals. If the emotionally salient stimuli are associated with behavioral responses consistent with the current goal, then amygdala activation to these stimuli should both enhance this goal representation and motivate (i.e., facilitate) the behaviors directed at attaining the goal. Thus, if a smoker has made a resolution to quit smoking, a greater amygdala response to smoking-cessation messages should increase his or her odds of quitting success. But if the emotionally salient stimuli are associated with behaviors that are contrary to the current goal, then we would expect that a greater amygdala activation to these salient stimuli may interfere with the attainment of the goal. For example, smoking-related cues are known to produce a subjective craving and trigger an urge to smoke in smokers (Ferguson and Shiffman, 2009; Rose, 2006), and amygdala activation to smoking-related cues as well as other drug-related cues is positively associated with such craving (Childress et al., 1999; Franklin et al., 2007; Kilts et al., 2001). Thus, a greater amygdala response to smoking-related cues should interfere with the goal to remain abstinent in smokers trying to quit.
Indeed, whereas exposure to drug-related cues is a key environmental trigger and a reliable predictor of relapse, including in smokers (Ferguson and Shiffman, 2009), the impact of this exposure is significantly moderated by individual differences in cue reactivity (Niaura et al., 1998). Our data and previous reports (Chua et al., 2011; Falk et al., 2010) suggest that individual differences in reactivity to smoking-cessation messages may similarly moderate the impact of such messages on quitting or reduction in smoking. Future studies could help elucidate the role of the amygdala by directly comparing the effectiveness of amygdala response to smoking-related cues vs. to smoking-cessation messages as a predictor of subsequent quitting outcome in smokers attempting to quit.
Of note, extensive evidence from both human and animal studies documents the role of the amygdala in detecting and responding to negative emotional stimuli and stressors (LeDoux, 2000; Phelps, 2006). And it is possible that it is specifically amygdala reactivity to negative emotional stimuli and stressors that determines the impact of smoking-cessation messages on subsequent quitting outcome in smokers. We certainly cannot rule out this possibility. However, because growing evidence also supports the role of the amygdala in detecting and responding to positive emotional stimuli (Baxter and Murray, 2002), and because we do not know if participants in our study perceived the smoking-cessation messages as negative or positive, we offer a more general interpretation focusing on amygdala reactivity to all emotionally salient stimuli, irrespective of perceived emotional valence.
Previous neuroimaging evidence from our group and others focused on the prefrontal cortical regions, and showed that the magnitude of response to smoking-cessation messages in the medial prefrontal cortex (MPFC) was a positive predictor of subsequent smoking cessation (Chua et al., 2011) or reduction in smoking (Falk et al., 2010). In the current report, we extend this evidence to the amygdala, a key subcortical region. Because the amygdala and the MPFC share bidirectional anatomical connections (Ghashghaei et al., 2007), and are thought to form a neural circuit of critical importance in addiction (Verdejo-Garcia and Bechara, 2009), future studies could examine whether variability in functional and structural connectivity in the amygdala–MPFC circuit also predicts subsequent quitting, above and beyond the responses of the two regions.
The second goal of the current study was to investigate the genetic sources of variation in amygdala response to smoking-cessation messages in the context of quitting. Here, we employed an imaging genetics approach to examine whether genetic variation in the serotonin transporter (5-HTT) could account for some of the observed individual differences in amygdala reactivity to smoking-cessation messages in smokers. Previous imaging genetics studies (Dannlowski et al., 2010; Hariri et al., 2005; Hariri et al., 2002) showed that the low-transcription S and LG alleles of the promoter polymorphism in that gene (5-HTTLPR/rs22531) were associated with an increased amygdala reactivity to emotionally salient stimuli, such as signals of threat. However, the impact of 5-HTT gene variation on amygdala response to smoking-cessation messages in smokers has not been examined.
We tested both the 5-HTTLPR/rs25531 and the STin2 polymorphisms, and found that the STin2 genotype was a significant independent predictor of the anatomically defined amygdala response to smoking-cessation messages (controlling for 5-HTTLPR/rs25531). The direction of the modulation was broadly consistent with previous studies: namely, the low-transcription allele was associated with an increase in amygdala response relative to the high-transcription allele. However, we found significant effects only for the STin2 genotype, whereas the 5-HTTLPR/rs25531 genotype (controlling for STin2) did not have a significant effect on amygdala response to smoking-cessation messages in our data.
In consideration of our sample size, we limited the genetic analyses to two common, functional polymorphisms in the 5-HTT gene. However, other polymorphisms in the 5-HTT gene have also been described and may contribute to the observed modulation of amygdala response. It is also possible that one or more yet unmeasured polymorphisms in the 5-HTT gene are responsible for the observed effects. A simultaneous examination of the effect of all of these polymorphisms, including their possible interactions, would require sample sizes beyond the scope of a neuroimaging study. Other, alternative approaches may have to be used to systematically examine the impact of 5-HTT gene variation on brain and behavior, including on amygdala response to smoking-cessation messages and smoking-related cues in the context of smoking cessation. For example, future studies could assess the impact of all functional variation in the 5-HTT gene using expression assays as a combined measure of all functional polymorphisms acting together to affect gene transcription. Alternatively, the 5-HTT gene could be sequenced and all observed variants could be coded into one aggregate measure of predicted transcription efficiency. Such aggregate measures could then be integrated with neuroimaging and outcome data.
To conclude, in this report we demonstrate that individual differences in amygdala response to smoking-cessation messages have an impact on subsequent real-life smoking cessation in smokers, and that genetic variation in the central serotonin system accounts for some of these individual differences in amygdala function. Taken together, these results point to a gene-brain-behavior pathway relevant to smoking cessation, and add to our understanding of the role of the amygdala in nicotine addiction. In addition, knowledge of such pathway could impact two important domains of translational research and clinical practice on smoking cessation: prediction and intervention. First, identification of brain predictors of smoking cessation and of genetic variants that modulate these brain processes could enable better predictive models and aid in treatment selection. And second, this knowledge could lead to the development of more effective smoking-cessation interventions tailored to the genetic and neural-response profiles of individual smokers for optimal intervention efficacy.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Drug Abuse Grant R21-DA024429-01 to H.F.C. and V.J.S. In addition, A.J.J. was supported by a William Orr Dingwall Foundation Fellowship and by a Sarah Winans Newman Scholarship from the Center for the Education of Women, University of Michigan. We also want to thank Drs. Margit Burmeister, Cindy Lustig, and Richard Gonzalez for helpful discussions of these results.
Footnotes
Financial disclosures
Drs. Jasinska, Chua, Ho, Rozek and Polk have no conflicts of interest to disclose. Dr. Strecher is the Chief Visionary Officer and Founder of HealthMedia, a company that develops and licenses computer-tailored health promotion, disease prevention, and disease management tools.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Weinberger DR. Imaging genetics--days of future past. Neuroimage. 2010;53:804–809. doi: 10.1016/j.neuroimage.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cogn. 1995;4:410–421. doi: 10.1006/ccog.1995.1048. [DOI] [PubMed] [Google Scholar]
- CDC. Cigarette smoking-attributable morbidity---United States, 2000. MMWR Morb Mortal Wkly Rep. 2003;52:842–844. [PubMed] [Google Scholar]
- CDC. Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, Strecher VJ. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nat Neurosci. 2011;14:426–427. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Konrad C, Kugel H, Zwitserlood P, Domschke K, Schoning S, Ohrmann P, Bauer J, Pyka M, Hohoff C, Zhang W, Baune BT, Heindel W, Arolt V, Suslow T. Emotion specific modulation of automatic amygdala responses by 5-HTTLPR genotype. Neuroimage. 2010;53:893–898. doi: 10.1016/j.neuroimage.2009.11.073. [DOI] [PubMed] [Google Scholar]
- Dayan P, Huys QJ. Serotonin in affective control. Annu Rev Neurosci. 2009;32:95–126. doi: 10.1146/annurev.neuro.051508.135607. [DOI] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Whalen D, Lieberman MD. Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychol. 2010 doi: 10.1037/a0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Fiskerstrand CE, Lovejoy EA, Quinn JP. An intronic polymorphic domain often associated with susceptibility to affective disorders has allele dependent differential enhancer activity in embryonic stem cells. FEBS Lett. 1999;458:171–174. doi: 10.1016/s0014-5793(99)01150-3. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Li Y, Suh JJ, Goldman M, Lohoff FW, Cruz J, Hazan R, Jens W, Detre JA, Berrettini W, O’Brien CP, Childress AR. Dopamine transporter genotype modulation of neural responses to smoking cues: confirmation in a new cohort. Addict Biol. 2011;16:308–322. doi: 10.1111/j.1369-1600.2010.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Cubells JF, Kidd JR, Pakstis AJ, Kidd KK. Population studies of polymorphisms of the serotonin transporter protein gene. Am J Med Genet. 1999;88:61–66. [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, de Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addict Biol. 2010;15:491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Stefulj J, Schwab S, Borrmann-Hassenbach M, Albus M, Jernej B, Wildenauer D. Serotonin transporter promoter and intron 2 polymorphisms: relationship between allelic variants and gene expression. Biol Psychiatry. 2004;55:1090–1094. doi: 10.1016/j.biopsych.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R, Tremblay PB, Schmider J, Henneken M, Dettling M, Muller-Oerlinghausen B, Uebelhack R, Roots I, Brockmoller J. Serotonin transporter polymorphisms: no association with response to antipsychotic treatment, but associations with the schizoparanoid and residual subtypes of schizophrenia. Mol Psychiatry. 2001;6:179–185. doi: 10.1038/sj.mp.4000821. [DOI] [PubMed] [Google Scholar]
- Kazantseva AV, Gaysina DA, Faskhutdinova GG, Noskova T, Malykh SB, Khusnutdinova EK. Polymorphisms of the serotonin transporter gene (5-HTTLPR, A/G SNP in 5-HTTLPR, and STin2 VNTR) and their relation to personality traits in healthy individuals from Russia. Psychiatr Genet. 2008;18:167–176. doi: 10.1097/YPG.0b013e328304deb8. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, Riederer P. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect. 1994;95:157–162. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addict Behav. 1998;23:209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Noll DC, Genovese CR, Vazquez AL, O’Brien JL, Eddy WF. Evaluation of respiratory artifact correction techniques in multishot spiral functional MRI using receiver operator characteristic analyses. Magn Reson Med. 1998;40:633–639. doi: 10.1002/mrm.1910400417. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Quaak M, van Schayck CP, Knaapen AM, van Schooten FJ. Genetic variation as a predictor of smoking cessation success. A promising preventive and intervention tool for chronic respiratory diseases? Eur Respir J. 2009;33:468–480. doi: 10.1183/09031936.00056908. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Rogers RD. The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology. 2011;36:114–132. doi: 10.1038/npp.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Strecher VJ, McClure JB, Alexander GL, Chakraborty B, Nair VN, Konkel JM, Greene SM, Collins LM, Carlier CC, Wiese CJ, Little RJ, Pomerleau CS, Pomerleau OF. Web-based smoking-cessation programs: results of a randomized trial. Am J Prev Med. 2008;34:373–381. doi: 10.1016/j.amepre.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer WF, Prochaska JO. A comparison of four self-report smoking cessation outcome measures. Addict Behav. 2004;29:51–60. doi: 10.1016/s0306-4603(03)00084-4. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56(Suppl 1):48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonan AL, Palmer AA, Gilliam TC. Hardy-Weinberg disequilibrium identified genotyping error of the serotonin transporter (SLC6A4) promoter polymorphism. Psychiatr Genet. 2006;16:31–34. doi: 10.1097/01.ypg.0000174393.79883.05. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.