Abstract
Background
While females disproportionately undergo bariatric surgery, rodent models investigating mechanisms of bariatric surgery have been limited to males. Female rodent models can also potentially allow us to understand the effects of surgical intervention on future generations of offspring. Sleeve gastrectomy is an attractive weight loss procedure for reproductive-age female patients as it avoids the malabsorption associated with intestinal bypass.
Objectives
We sought to evaluate the impact of sleeve gastrectomy on young female rats with diet-induced obesity.
Settings
David Geffen School of Medicine at UCLA
Methods
Sprague Dawley female rats were fed a 60% high-fat diet. At 12 weeks of age, animals underwent either sleeve gastrectomy or sham surgery. Animals were sacrificed four weeks after surgery. A chemistry panel was performed, and serum adipokines and gut hormones were assayed. Homeostasis model assessment score (HOMA) was calculated. Liver histology was graded for steatosis. Two-sample t-test was used to compare groups.
Results
Sleeve gastrectomy was associated with significant weight loss (5±6% vs. −4±6%; p<0.001), lower leptin levels (1.3±1.2 vs. 3.5±2.3 ng/ml; p<0.01), and higher adiponectin levels (0.43 ± 0.19 vs. 0.17 ± 0.14 ng/ml; p<0.004) when compared to sham animals. There were no significant differences in fasting ghrelin. Furthermore, we did not observe evidence of insulin resistance or steatohepatitis after 11 weeks of high-fat diet. Despite these limitations, further gender-specific studies are warranted given that the majority of bariatric surgeries are performed in females.
Conclusion
Sleeve gastrectomy appears to result in weight loss and improvements in adiponectin and leptin via mechanisms independent of ghrelin in a female model of diet-induced obesity.
INTRODUCTION
Obesity is a worldwide public health concern 1. It is associated with comorbidities including diabetes, hypertension, hyperlipidemia, sleep apnea, and degenerative joint disease. Bariatric surgery has proven to be safe and effective in the treatment of morbidly obesity as it leads to significant long-term weight loss, rapid resolution of comorbidities, and is associated with increased life expectancy 2–5.
The prevalence of extreme obesity is higher in women than in men (7% vs. 3%) 6. Furthermore, women are disproportionately more likely to undergo bariatric surgery 6. While the majority of patients undergoing bariatric surgery are women, no female gender-specific animal studies have been developed.
Female subjects are of particular interest because intervention prior to pregnancy has been demonstrated to have improved obstetrical and offspring outcomes 7,8. It has been proposed that maternal obesity may contribute to the development of offspring obesity via in-utero mechanisms 9,10. Thus, establishment of female models of diet-induced obesity and bariatric surgery could help to elucidate these mechanisms.
Although gastric bypass and biliopancreatic diversion are effective options for treating morbid obesity, many surgeons are reluctant to perform these procedures in reproductive-age women given concerns for malabsorption and theoretical concerns about the impact of nutritional deficiencies on offspring. Sleeve gastrectomy is an attractive surgical option for reproductive-age women as it has been proven to be efficacious, avoids intestinal bypass, and it can be used as a bridge to a more invasive procedure if needed 11–13. In this study, we sought to evaluate the impact of sleeve gastrectomy in a female rodent model of diet-induced obesity.
METHODS
Animal and Diet Model
Female Sprague Dawley rats (n=35) from Charles River Laboratory (Wilmington, MA) were utilized. All animals were maintained in an animal barrier as a non-breeding colony in a temperature and light controlled room, and were allowed free access to food and water. At three weeks of age, they were weaned and started on a 60% high-fat diet (Research Diets, D12492; New Brunswick, NJ). The animals were then randomized to undergo either sleeve gastrectomy or sham surgery. In order to minimize the risk of gastric obstruction, all animals were placed on a wire-floor (to help prevent them from eating bedding and feces) and fed an Ensure Plus diet for seven days pre- and post- operatively. On post-operative day seven, the high fat diet was reinstituted. Four weeks following the intervention, the animals were fasted overnight and sacrificed; isoflurane was administered, and approximately 5cc of blood was aspirated directly from the heart. Tissues (e.g. liver) were also harvested. The animals were sacrificed for tissue and blood collection four weeks following the surgery intervention. Animal weight was assessed weekly before and after surgical procedures. The use of the animals was approved by the UCLA Animal Research Committee (ARC 2008-062).
Sleeve Surgery Technique
Antibiotics, analgesics (buprenex), and fluid boluses were administered perioperatively. Anesthesia was induced with isoflurane and maintained for the same period in both the sleeve and sham surgery groups. A midline incision was made and the stomach exposed. A gastrotomy was performed in order to wash out the stomach (rodent hair and diet). A surgical stapler (2.0mm titanium staples; Autosuture Endo GIA; Norwalk, CT) was then used to remove an estimated 60–70% of the stomach. The staple line was oversewn with 5-0 PDS suture (Ethicon; Somerville, NJ). The omentum was wrapped around the suture line. The abdominal wall was closed in two layers with 4-0 Vicryl (Ethicon; Somerville, NJ).
Sham Surgery Technique
The stomach was exposed in a similar fashion to the sleeve surgery. A gastrotomy was performed and then closed with 5-0 PDS (Ethicon). The omentum was also wrapped around the suture line and the abdominal wall closed in two layers. Antibiotics and analgesics were administered perioperatively.
Laboratory Analysis and Assays
A fasting serum chemistry panel was performed. Serum adipokines IL-1β, IL-6, insulin, leptin, MCP-1, PAI-1 (total), and tumor necrosis factor-α (rat serum adipokine panel; #RADPK-81K, Milliplex Corporation; Billerica, MA). Amylin (active), gastric inhibitory protein (total), glucagon like peptide-1 (active), ghrelin (active), pancreatic polypeptide, and peptide YY (rat gut hormone panel; #RGT-88K, Milliplex Corporation; Billerica, MA). Homeostatic model assessment (HOMA) was calculated using the formula: fasting glucose in units of mmol/L * insulin in units of U/mL)/22.5] 14. Enyzme-linked immunosorbent assays were used to measure serum ghrelin and adiponectin (#EK-031-31 and #EK-ADI-02; Phoenix Pharmaceuticals, Inc.; Burlingame, CA).
Histology
Sections of liver were harvested from three different lobes of each rat. The specimens were fixed in formalin, and paraffin-embedded sections were stained with hematoxilyn and eosin. The histology was then graded for steatohepatitis. Grading of steatosis was performed as previously described15: Grade 0 = no steatosis, grade 1 <5%, grade 2 = 5–33%, grade 3 = 34–66%, and grade 4 = >66% [15]. A single pathologist reviewed all liver pathology. Three lobes from each animal were graded separately, and the mean grade was calculated.
Statistics
The mean and standard deviation were calculated for each group. Two-sample t-test was used to compare groups.
RESULTS
Surgery
Eighteen animals were randomized to sleeve gastrectomy, and seventeen animals were randomized to sham surgery. Thirteen of the sleeve animals survived to the endpoint of the study; one did not recover from anesthesia and four were euthanized prematurely because of distress secondary to peritonitis or abscess. Fifteen of the sham animals survived to the endpoint of the study; two did not recover from anesthesia. At the endpoint of the study, three of the sleeve animals were noted to have a >1cm abscess. These animals were excluded from analysis because of the potential impact of inflammation on study results.
Body Weight
Baseline body weights were similar between the sleeve and sham groups (Figure 1). There was a significant difference in the mean weight of animals in the sleeve (259 ± 30 grams) versus sham group (288 ± 27 grams) starting at post-operative week two (p < 0.02). At post-operative week four, sleeve animals had a mean weight of 280 ± 31 grams compared to 309 ± 29 grams (p < 0.02). This difference was also significant when measured as percent weight gain in sham (4 ± 6%) versus sleeve (−5 ± 6%) animals (p < 0.001).
Figure 1.
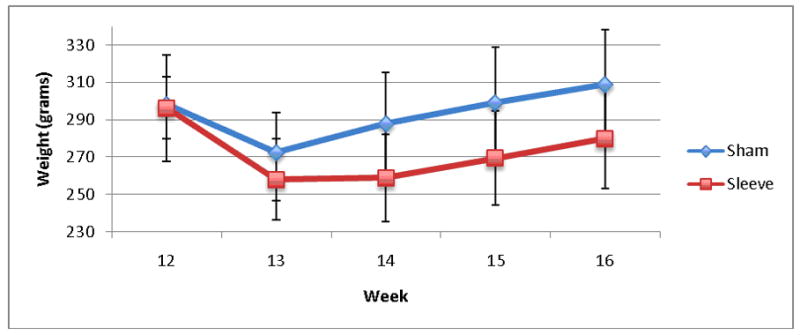
Weight.
Baseline weights at the time of surgery were similar between sleeve and sham groups (week 12). There was a significant difference in weight between the sleeve and sham groups after surgery (week 16), p<0.02.
Adipokines
Leptin levels were significantly lower in the sleeve group compared with the sham group (1.3 ± 1.2 vs. 3.5 ± 2.3 ng/ml; p < 0.01). Conversely, adiponectin levels in the sleeve group were higher than the sham group (0.43 ± 0.19 vs. 0.17 ± 0.14 ng/ml; p < 0.004). There were no significant differences in IL-1β, IL-6, MCP-1, PAI-1, and TNF-α.
Markers of Glucose Metabolism
There were no significant differences in fasting serum glucose, insulin, or HOMA scores between sleeve and sham groups (Table 1).
Table 1.
Glucose Metabolism.
There were no significant differences noted in glucose, insulin, or homeostatic model assessment (HOMA).
| Glucose | Insulin | HOMA | |
|---|---|---|---|
| Units | mg/dl | μg/ml | |
| Sham | 130 ± 12 | 472 ± 176 | 1.9 ±1.4 |
| Sleeve | 134 ± 22 | 426 ± 231 | 2.1 ±1.8 |
| p-value | p=0.63 | p=0.61 | p=0.78 |
Gut Hormones
There were no differences in ghrelin levels between fasting sleeve and sham groups using two different commercially available assays (Table 2). Similarly, there were no significant differences between sleeve and sham groups in fasting serum gastric inhibitory protein, glucagon like peptide-1, peptide YY, and pancreatic polypeptide.
Table 2.
Gut Hormones.
There were no significant differences between sleeve and sham groups in fasting ghrelin levels using two different commercially available kits (Ghrelin 1 – Phoenix; Ghrelin 2 – Milliplex). Similarly, there were no significant difference between sleeve and sham groups in fasting serum gastric inhibitory protein (GIP), glucagon like peptide-1 (GLP-1), peptide YY (PYY), and pancreatic polypeptide (PP).
| Ghrelin 1 | Ghrelin 2 | GIP | GLP-1 | PYY | PP | |
|---|---|---|---|---|---|---|
| Units | ng/ml | pg/ml | pg/ml | pg/ml | pg/ml | pg/ml |
| Sham | 9.5 ± 2.6 | 39 ± 63 | 41 ± 29 | 32 ± 14 | 46 ± 25 | 71 ± 63 |
| Sleeve | 12 ± 5.2 | 39 ± 49 | 28 ± 12 | 40 ± 25 | 43 ± 20 | 47 ± 23 |
| p-value | 0.19 | 0.99 | 0.17 | 0.42 | 0.77 | 0.22 |
Liver Weight, Laboratories, and Histology
There was a nonsignificant trend toward lower liver weights in the sleeve group (7.6 + 1.5 grams) when compared with the sham group (8.3 + 1.1 grams). However, these differences resolved after taking into account the liver to body weight ratio (0.026 ± 0.002 vs. 0.027 ± 0.003). There were no significant differences between sleeve and sham groups in cholesterol, aminotransferases, or bilirubin. Minimal steatosis was noted in both sleeve and sham groups with no significant difference in mean grade between the two groups (1.2 ± 1.0 vs. 0.82 ± 0.93; p=0.40).
DISCUSSION
Our female model of sleeve gastrectomy demonstrated significant weight loss and an improvement in adipokine profile (decreased leptin and increased adiponectin). These findings are consistent with other rodent and human studies of sleeve gastrectomy 16,17. Improvements in adipokine profile have been associated with decreased inflammation. However, we did not observe differences in inflammatory cytokines.
Interestingly, we observed no differences in ghrelin levels between sleeve and sham animals. Ghrelin is an orexigenic hormone mainly produced by A cells of the gastric mucosa in the fundus and gastric body. Therefore, ghrelin levels should theoretically decrease after sleeve gastrectomy. Indeed, a decrease in ghrelin levels after sleeve gastrectomy has been documented in humans 18. However, an opposite effect has been noted in rodent models of sleeve gastrectomy 19,20. Anatomic differences between the rat and human stomach may account for some of these differences as a large portion of the rodent’s stomach is composed of an aglandular forestomach. Therefore, sleeve gastrectomy in rodents may result in the removal of less ghrelin-producing glandular stomach than in humans. In addition, extragastric production of ghrelin in rodents has been proposed 21.
This study is unique in that it focuses on female rodents, which demographically represent the gender most likely to undergo bariatric surgery in humans 6. Nearly 80% of patients undergoing bariatric surgery are female and 35% are of reproductive age (less than 40 years of age). Given these demographics, it is important to evaluate both the potentially beneficial and deleterious effects of bariatric surgery on reproductive-age women. Reproductive-age women are also of particular interest because of the link between maternal obesity, gestational diabetes, adverse perinatal outcomes, and transgenerational transmission of metabolic phenotype to offspring. Despite previous concerns about the potentially deleterious impact of bariatric surgery on maternal nutrition, bariatric surgery has been demonstrated to improve maternal outcomes and likely improves neonatal outcomes 22. Studies of women undergoing bariatric surgery demonstrated a 50% reduction in the prevalence of obesity and metabolic syndrome in offspring 23. It is unclear whether improvements in offspring health following bariatric surgery are related to changes in the uterine environment, the post-natal environment, or the epigenome.
Our study may be limited by the observation that young female Sprague Dawley rats appear to be resistant to the development of insulin resistance, hypercholesterolemia, and steatohepatitis when fed a 60% high-fat diet for 11 weeks. In fact, estrogen may be protective against development of insulin resistance in female rodents 24–26. Thus, unlike other bariatric surgery studies in male rodents, our study may have been unable to demonstrate improvements in glucose metabolism, inflammatory markers, gut hormones, or steatohepatitis because the baseline metabolic phenotype was relatively normal in the population we evaluated 27. Future studies to evaluate the impact of bariatric surgery on glucose metabolism and steatohepatitis in female rodents may require: 1) a more diabetogenic diet; 2) a diabetes-prone strain of rodent; 3) more sensitive measures of insulin resistance, and 4) a more prolonged period of exposure to high-fat diet. In fact, both obese and non-obese rodents models of diabetes do exist but the majority are genetically determined and, therefore, may not represent an accurate model of human diet-induced obesity and diabetes 20. Furthermore, the diabetic phenotype in these strains is still more pronounced in males than in females. In addition to using alternative diets and strains of rodents, one could employ more sensitive measures of insulin resistance. These include glucose and insulin tolerance tests and hyperinsulinemic-euglycemic clamp studies. Similarly, additional studies evaluating gut-hormone response to meals may represent a more sensitive means of assessing the impact of surgery on incretins and other gut-hormones than measuring fasting levels alone. The relative protection against insulin resistance noted in female rodents may hinder our ability to use an animal model to investigate the impact of bariatric surgery on maternal and offspring health.
One of the limitations of this study is the short follow up of four weeks. However, this time period is the equivalent of three human years 28. While we can not exclude the potential for weight regain following sleeve gastrectomy, other investigators have demonstrated that weight loss is maintained at a 15-week timepoint following sleeve gastrectomy in rodents 16,17. Another limitation of the study is a relatively high complication rate in these relatively young female rats. These results were obtained after several pilot studies, refinements of technique, and development of a rigorous perioperative-care protocol as delineated in the methods. Despite these efforts, we observed that the aglandular forestomach in rodents remains susceptible to the development of a leak or abscess. Our data will need further confirmation once the animal model is better established with a lower complication rate.
In summary, this study demonstrates the limitations of a female rodent model of diet-induced obesity. However, it suggests that sleeve gastrectomy can induce weight loss and improve the adiponectin/leptin profile via mechanisms independent of ghrelin and intestinal bypass. Additional studies including pair-feeding will be required to determine whether the affects of sleeve gastrectomy are independent of caloric intake.
Figure 2.
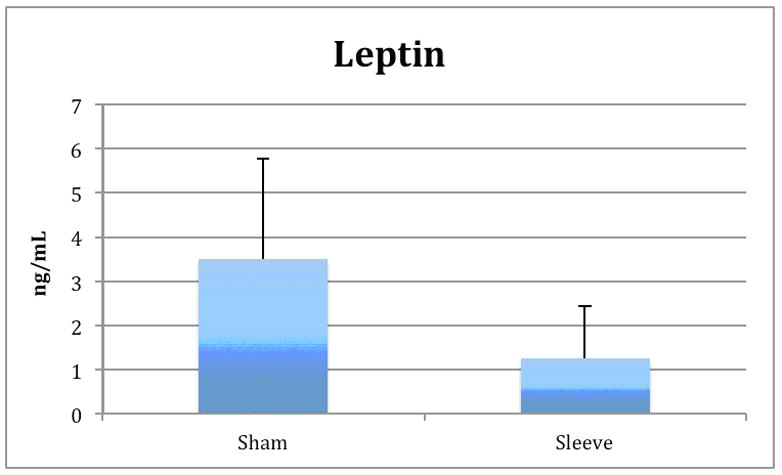
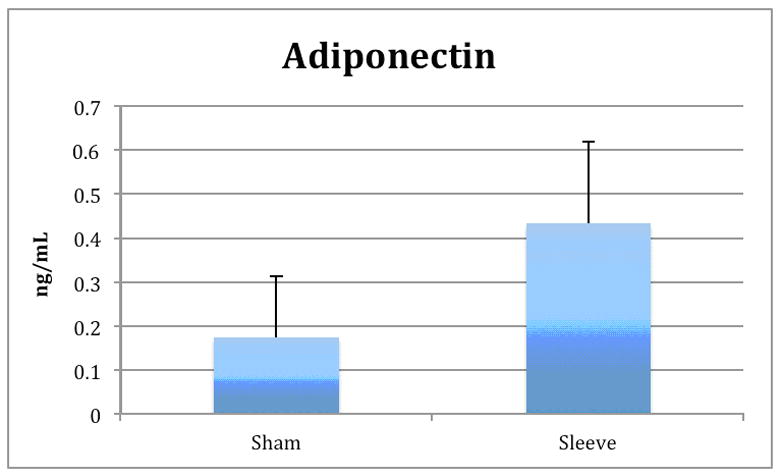
Adipokines.
Significant differences were observe in leptin (Figure 2a) and adiponectin levels (Figure 2b) between sleeve and sham groups (p<0.01). There was no significant difference in ghrelin levels between groups.
Acknowledgments
This research was funded by the American Society of Metabolic and Bariatric Surgery Research Grant and the UCSD/UCLA Diabetes Endocrine Research Center (DERC) Pilot and Feasibility Grant (NIH P30 DK063491).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang Y, Monteiro C, Popkin BM. Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China, and Russia. Am J Clin Nutr. 2002;75:971–7. doi: 10.1093/ajcn/75.6.971. [DOI] [PubMed] [Google Scholar]
- 2.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–59. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 3.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 5.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 6.Martin M, Beekley A, Kjorstad R, Sebesta J. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surg Obes Relat Dis. 2010;6:8–15. doi: 10.1016/j.soard.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286–96. doi: 10.1001/jama.2008.641. [DOI] [PubMed] [Google Scholar]
- 8.Smith J, Cianflone K, Biron S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009;94:4275–83. doi: 10.1210/jc.2009-0709. [DOI] [PubMed] [Google Scholar]
- 9.Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology. 2011;152:2228–36. doi: 10.1210/en.2010-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poston L, Harthoorn LF, Van Der Beek EM. Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement. Pediatr Res. 2011;69:175–80. doi: 10.1203/PDR.0b013e3182055ede. [DOI] [PubMed] [Google Scholar]
- 11.Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;5:469–75. doi: 10.1016/j.soard.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)-a prospective study. Obes Surg. 2010;20:447–53. doi: 10.1007/s11695-009-0068-4. [DOI] [PubMed] [Google Scholar]
- 13.Hamoui N, Anthone GJ, Kaufman HS, Crookes PF. Sleeve gastrectomy in the high-risk patient. Obes Surg. 2006;16:1445–9. doi: 10.1381/096089206778870157. [DOI] [PubMed] [Google Scholar]
- 14.Marroquin OC, Kip KE, Kelley DE, et al. Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women: a report from the Women’s Ischemia Syndrome Evaluation. Circulation. 2004;109:714–21. doi: 10.1161/01.CIR.0000115517.26897.A7. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Kotidis EV, Koliakos GG, Baltzopoulos VG, Ioannidis KN, Yovos JG, Papavramidis ST. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment--a prospective study. Obes Surg. 2006;16:1425–32. doi: 10.1381/096089206778870058. [DOI] [PubMed] [Google Scholar]
- 17.Patrikakos P, Toutouzas KG, Perrea D, et al. A surgical rat model of sleeve gastrectomy with staple technique: long-term weight loss results. Obes Surg. 2009;19:1586–90. doi: 10.1007/s11695-009-9965-9. [DOI] [PubMed] [Google Scholar]
- 18.Langer FB, Reza Hoda MA, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–9. doi: 10.1381/0960892054621125. [DOI] [PubMed] [Google Scholar]
- 19.Pereferrer FS, Gonzalez MH, Rovira AF, Blasco SB, Rivas AM, del Castillo Dejardin D. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg. 2008;18:97–108. doi: 10.1007/s11695-007-9351-4. [DOI] [PubMed] [Google Scholar]
- 20.Lopez PP, Nicholson SE, Burkhardt GE, Johnson RA, Johnson FK. Development of a sleeve gastrectomy weight loss model in obese Zucker rats. J Surg Res. 2009;157:243–50. doi: 10.1016/j.jss.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereferrer FS, Gonzalez MH, Rovira AF, Blasco SB, Rivas AM, del Castillo Dejardin D. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg. 2008;18:97–108. doi: 10.1007/s11695-007-9351-4. [DOI] [PubMed] [Google Scholar]
- 22.Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: A systematic review. JAMA. 2008;300:2286–96. doi: 10.1001/jama.2008.641. [DOI] [PubMed] [Google Scholar]
- 23.Smith J, Cianflone K, Biron S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009;94:4275–83. doi: 10.1210/jc.2009-0709. [DOI] [PubMed] [Google Scholar]
- 24.Hevener A, Reichart D, Janez A, Olefsky J. Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes. 2002;51:1907–12. doi: 10.2337/diabetes.51.6.1907. [DOI] [PubMed] [Google Scholar]
- 25.Ribas V, Nguyen MT, Henstridge DC, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab. 2010;298:E304–319. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Liu J. Sleeve gastrectomy relieves steatohepatitis in high-fat-diet-induced obese rats. Obes Surg. 2009;19:921–5. doi: 10.1007/s11695-008-9663-z. [DOI] [PubMed] [Google Scholar]
- 28.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]


