Abstract
Objectives
Recent studies suggest adverse events associated with aprotinin in adults may not occur in children, and there is interest in further pediatric study of aprotinin. However, there are limited contemporary data comparing aprotinin to other available antifibrinolytics [aminocaproic acid (ACA) and tranexamic acid (TXA)] to guide current practice and aid in potential trial design. We performed a comparative analysis in a large multicenter cohort.
Methods
The Society of Thoracic Surgeons Congenital Heart Surgery Database (2004–2008) was linked to medication data from the Pediatric Health Information Systems Database. Efficacy and safety outcomes were evaluated in multivariable analysis adjusting for patient and center factors overall, and in neonates and those undergoing redo sternotomy.
Results
There were 22,258 patients (25 centers) included: median age 7.6m (interquartile range 2.6–43.4). Aprotinin (vs. no drug) was associated with a significant reduction in combined hospital mortality/bleeding requiring surgical intervention overall (OR 0.81 95%CI 0.68–0.91), and in the redo sternotomy subgroup (OR 0.57 95%CI 0.40–0.80). There was no benefit in neonates, and no difference in renal failure requiring dialysis in any group. In comparative analysis, there was no difference in outcome in aprotinin vs. ACA recipients. TXA (vs. aprotinin) was associated with significantly reduced mortality/bleeding requiring surgical intervention overall (OR 0.47 95%CI 0.30–0.74) and in neonates (OR 0.30 95%CI 0.15–0.58).
Conclusions
These observational data suggest aprotinin is associated with reduced bleeding and mortality in children undergoing heart surgery with no increase in dialysis. Comparative analyses suggest similar efficacy of ACA and improved outcomes associated with TXA.
Introduction
Aprotinin was the most commonly utilized antifibrinolytic medication in children undergoing heart surgery, until it was taken off the market in 2007 following adult studies reporting increased mortality and renal failure (1,2). As a result, aprotinin is also no longer available to children undergoing heart surgery (3). Recent studies have suggested that the adverse events associated with aprotinin in adults may not occur in children. We previously evaluated safety outcomes in >30,000 children undergoing heart surgery, and found that aprotinin was not associated with increased mortality or dialysis (4). This previous study was limited by the use of administrative data, focused on safety outcomes only, and did not compare aprotinin to other antifibrinolytic medications [aminocaproic acid (ACA) and tranexamic acid (TXA)]. The few previous comparative analyses evaluating these medications have been limited by small sample size (5,6).
Thus, while there is interest in further prospective study of aprotinin in children, there are limited contemporary data comparing aprotinin to other available antifibrinolytic medications to guide current practice and aid in planning future prospective study. The purpose of the present study was to evaluate the comparative outcomes associated with aprotinin, ACA, and TXA in a large multicenter cohort. We first evaluated outcomes associated with aprotinin vs. no drug prior to 2007 when aprotinin was most widely used. We then subsequently assessed comparative outcomes associated with aprotinin, ACA, and TXA during 2007–2008 when utilization transitioned from aprotinin to the other 2 medications. We evaluated the overall cohort of children undergoing heart surgery, and the subgroups undergoing redo sternotomy and neonates as these groups may be more prone to bleeding (3,7).
Methods
Data Source
De-identified data from the Pediatric Health Information Systems (PHIS) Database and Society of Thoracic Surgeons Congenital Heart Surgery (STS-CHS) Database were used for this analysis. Data from 30 centers participating in both databases from 2004–2008 were linked using the method of “indirect identifiers” as previously described and verified (8). Overall, data on 90% of 45,830 eligible patients at these centers were successfully linked using this methodology. Through linking these data, we are able to capitalize upon the strengths of both datasets and utilize the detailed diagnosis and procedure information in the STS-CHS Database and medication data from the PHIS Database, as described below.
The PHIS Database is a large administrative database containing inpatient data from 41 pediatric hospitals in the US affiliated with the Child Health Corporation of America. The database currently contains information from >4.6 million inpatient discharges. Data quality and reliability are assured through a joint effort between Child Health Corporation of America and participating hospitals. Data collected include demographics, diagnoses and procedures [using International Classification of Diseases, Ninth Revision (ICD-9) coding], in-patient outcomes data, and resource utilization data from the hospital bill including pharmaceuticals, imaging, laboratory studies, and hospital charges.
The STS-CHS Database is the largest clinical pediatric heart surgery data registry in the world. It currently contains data on >160,000 surgeries performed since 1998. The STS-CHS Database contains pre-operative, operative, and outcomes data on all patients undergoing pediatric heart surgery at participating centers. Diagnoses and procedures are coded using the International Pediatric and Congenital Cardiac Code, which was developed through an international collaborative effort of pediatric cardiologists and congenital heart surgeons (9). Data quality and reliability are assured through intrinsic verification of data as well as a formal process of site visits and data audits (10).
This study was approved by the institutional review boards at Duke University Medical Center and The Children’s Hospital of Philadelphia with waiver of informed consent. The study was also reviewed and approved by the STS-CHS Database Task Force, and Child Health Corporation of America in compliance with their PHIS Database External Use Guidelines.
Study Population
Thirty centers (n=41,371 patients with successfully linked data as described above) were eligible for inclusion. Centers with >15% missing data for any STS-CHS study variable were excluded (n=5 centers); while the STS-CHS Database contains nearly complete data for the required standard data fields regarding procedure and in-hospital mortality, not all centers submit complete data for the other variables in the STS-CHS Database, and it is therefore standard practice to exclude centers with >15% missing data for key study variables in order to maximize data integrity and minimize missing data). This left 25 centers (n=32,660 patients) eligible for inclusion. Patients with missing PHIS medication data (n=1805) were excluded. STS-CHS data were used to identify patients undergoing any surgery classified in the Society of Thoracic Surgeons-European Association for Cardiothoracic Surgery (STS-EACTS) risk stratification system (category 1 = lowest mortality risk, category 5 = highest mortality risk) (11). This system was recently developed based on empiric data from nearly 80,000 patients. It includes a greater number of operations compared with other risk stratification systems (11). Ninety-seven percent of the remaining 30,855 eligible patients were classified in the STS-EACTS system; only those patients undergoing surgery involving cardiopulmonary bypass (n=22,485) were included. Only the first cardiovascular operation of the admission was analyzed, and patients who received more than one of the drugs of interest on the day of surgery, and those with missing weight data were excluded. This left a cohort of 22,258 patients from 25 centers.
Data Collection
Data were collected from both the STS-CHS and PHIS Databases (as noted below for each variable), including: age (PHIS), prematurity (PHIS, collected for neonates only using ICD-9 codes for gestational age <37 weeks), sex (PHIS), race (PHIS), weight (STS-CHS), the presence of any non-cardiac/genetic abnormality (STS-CHS), any pre-operative risk factor (as defined in the STS-CHS Database), any previous cardiothoracic surgery (STS-CHS), STS-EACTS risk category for the primary procedure performed (STS-CHS), the use of peri-operative corticosteroids (PHIS), and center average annual surgical volume of STS-EACTS classified surgeries (STS-CHS) (12).
Primary exposure
The primary exposure variable was receipt of aprotinin, ACA, TXA, or none of these medications on the day of surgery (PHIS).
Outcomes
The primary efficacy endpoint was a composite of in-hospital mortality (PHIS) or bleeding requiring surgical intervention during the hospitalization (STS-CHS). These outcomes were also examined individually. Other outcomes evaluated included postoperative total and intensive care unit length of stay (combination of STS-CHS and PHIS data). Post-operative duration of mechanical ventilation in days was also collected (PHIS). The primary safety endpoint was acute renal failure requiring temporary or permanent dialysis (STS-CHS). Due to reports in adults of possible neurologic side effects of TXA, post-operative neurologic impairment (transient or permanent neurologic deficit or new onset seizure; STS-CHS) was also evaluated (13).
Analysis
Variables were described using standard summary statistics. Unadjusted outcomes were compared between groups using Chi-square and Wilcoxon Rank-Sum tests. Multivariable analysis was also performed to evaluate outcomes associated with aprotinin, ACA, and TXA (as described below). All models were adjusted for age, weight z-score, race, sex, any non-cardiac/genetic abnormality or other STS-CHS pre-operative risk factors, any previous cardiothoracic surgery, STS-EACTS surgical risk category, and use of peri-operative corticosteroids. Models for the neonatal subgroup were also adjusted for prematurity. Our analytic approach was chosen specifically to account for confounding by center, as it was noted from initial inspection of the data that choice of treatment appeared largely center dependent, and historically it has been shown that both hospital care and outcome can vary by center. For dichotomous outcome variables, conditional logistic regression stratified by center was used to account for potential confounding effect of center (this methodology also accounts for potential differences in center volume). Odds ratios and 95% confidence intervals are reported. For continuous outcome variables, linear regression with center modeled as a main effect was utilized. Length of stay and duration of ventilation were not normally distributed and were log transformed for analysis. Regression coefficients from the linear regression models were exponentiated and the ratio of length of stay or duration of ventilation between groups with 95% confidence intervals reported. In cases where there was a significant difference between groups, estimates of the difference in actual days at the median value of length of stay or duration of ventilation were also calculated. All analyses were performed in the overall cohort and in two selected subgroups: 1) neonates, 2) those with a history of previous cardiothoracic surgery undergoing redo sternotomy.
Outcomes were first evaluated in those who received aprotinin vs. no drug from 2004–2007 (the time period when aprotinin was commonly used). Univariable and multivariable analyses were performed as outlined above. Next, the second analysis focused on the years 2007–2008 when there was a shift in use of aprotinin to use of ACA or TXA after aprotinin was taken off the market. Outcomes associated with ACA and TXA were compared with aprotinin in univariable and multivariable analysis, again as outlined above.
An instrumental variable analysis was then performed as an additional method for comparative analysis of the three drugs, in an attempt to further minimize potential confounding center effects. For this analysis, data from 2008 were compared to 2007, restricting to centers who switched primarily (>75% use) from aprotinin to ACA, or from aprotinin to TXA, and patients at these centers who received any antifibrinolytic medication. All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC). Patients with missing data for a study endpoint were excluded from analysis involving that endpoint. A p-value <0.05 was considered statistically significant. The p-values presented are without adjustment for multiple comparisons.
Results
Study population characteristics
A total of 22,258 patients (25 centers) were included. Patient characteristics overall, and for those who received each antifibrinolytic medication are displayed in Table 1. Change in medication utilization patterns is displayed in Figure 1. During 2004–2007, 49% of patients overall received an antifibrinolytic medication (42% aprotinin, 7% ACA or TXA). In 2008 after aprotinin was taken off the market, a similar proportion of patients overall received an antifibrinolytic medication (43%), but only 5% received aprotinin, while 20% received ACA and 18% received TXA. Overall, during the study period, 23 centers used aprotinin, 20 centers used ACA, and 11 centers used TXA. Of note, mortality in 2004–2007 vs. 2008 was similar (3.6% vs. 3.4%).
Table 1.
Patient and center characteristics in the overall cohort
| Overall (n=22258) | Aprotinin (n=7329) | TXA (n=1486) | ACA (n=1667) | No drug (n=11766) | |
|---|---|---|---|---|---|
| Age at surgery, months | 7.6 (2.6–43.4) | 8.1 (2.3–46.0) | 6.5 (3.5–30.9) | 7.9 (3.2–49.1) | 7.6 (2.5–42.8) |
| Sex, male | 12431 (55.9%) | 4121 (56.2%) | 837 (56.3%) | 935 (56.1%) | 6538 (55.9%) |
| Race, white | 15729 (70.7%) | 5272 (71.9%) | 1064 (71.6%) | 1250 (75.0%) | 8143 (69.2%) |
| Weight, kg | 6.9 (4.2–14.5) | 7.1 (4.1–14.8) | 6.4 (4.6–12.4) | 7.0 (4.4–15.1) | 6.9 (4.2–14.4) |
| Weight-for-age z-score | −1.1 (−2.1, −0.2) | −1.1 (−2.1, −0.2) | −1.3 (−2.3, −0.3) | −1.2 (−2.1, −0.2) | −1.1 (−2.2, −0.2) |
| Non-cardiac/genetic abnormality | 6376 (28.7%) | 1982 (27.0%) | 496 (33.4%) | 538 (32.3%) | 3360 (28.5%) |
| Prematurity* | 574 (14.0%) | 215 (14.4%) | 26 (12.6%) | 37 (13.6%) | 296 (13.9%) |
| Any STS pre-op risk factor | 4955 (22.3%) | 1599 (21.8%) | 357 (24.0%) | 402 (24.1%) | 2597 (22.1%) |
| Previous CT surgery | 7232 (32.5%) | 2909 (39.7%) | 554 (37.3%) | 639 (38.3%) | 3130 (26.6%) |
| STS-EACTS risk category | |||||
| 1 | 5465 (24.6%) | 1496 (20.4%) | 333 (22.4%) | 367 (22.0%) | 3269 (27.8%) |
| 2 | 6354 (28.6%) | 2177 (29.7%) | 428 (28.8%) | 499 (29.9%) | 3250 (27.6%) |
| 3 | 4112 (18.5%) | 1364 (18.6%) | 319 (21.5%) | 352 (21.1%) | 2077 (17.6%) |
| 4 | 5063 (22.8%) | 1786 (24.4%) | 350 (23.6%) | 355 (21.3%) | 2572 (21.8%) |
| 5 | 1264 (5.7%) | 506 (6.9%) | 56 (3.8%) | 94 (5.6%) | 608 (5.2%) |
| Peri-operative corticosteroids | 13890 (62.4%) | 5321 (72.6%) | 1301 (87.6%) | 1243 (74.6%) | 6025 (51.2%) |
| Annual center volume, median operations/year | 376 (328–649) | 370 (324–501) | 649 (350–708) | 376 (342–398) | 393 (341–649) |
Data are displayed as frequency and percent for dichotomous variables, and median and interquartile range for continuous variables.
neonates only
CT=cardiothoracic
Figure 1.
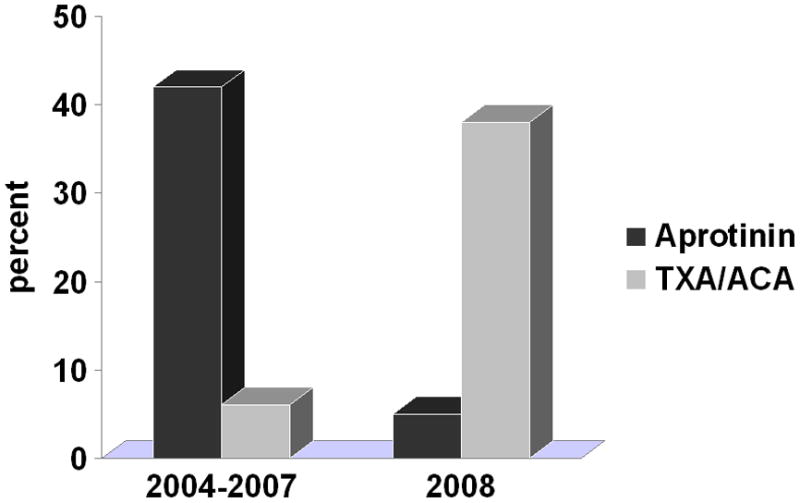
Antifibrinolytic use in children undergoing heart surgery
Subsequent analyses were performed both in the overall cohort of patients as well as two subgroups: neonates (n=4,426) and those undergoing redo sternotomy (n=7,232). The most common procedures performed in the neonate subgroup were: Norwood operation (23%), arterial switch operation (23%), coarctation/interrupted aortic arch repair (13%), repair of total anomalous pulmonary venous connection (10%), and truncus arteriosus repair (4%). The most common procedures performed in the re-operation subgroup were: Fontan operation (17%), bidirectional cavopulmonary anastomosis (14%), conduit operation (10%), pulmonary valve replacement (4%), and subvalvar aortic stenosis repair (4%).
Aprotinin vs. no drug
We first evaluated outcomes associated with aprotinin vs. no drug in 2004–2007, the time period when aprotinin was most commonly used. Unadjusted and adjusted outcomes are displayed in Table 2. In multivariable analysis, aprotinin was associated with a significant reduction in the composite endpoint of in-hospital mortality/bleeding requiring surgical intervention in the overall cohort. There was a statistically significant difference between groups in duration of ventilation (0.1 days), likely not clinically meaningful (Table 2). There were no other differences in outcome detected in the overall cohort including no difference in renal failure requiring dialysis.
Table 2.
Unadjusted and adjusted post-operative outcomes associated with aprotinin vs. no drug
| Unadjusted Results | Adjusted Results | ||||
|---|---|---|---|---|---|
| Aprotinin | No drug | p-value | Aprotinin vs. No Drug | p-value | |
| Overall | (n=7077) | (n=8644) | |||
| In-hospital mortality | 242 (3.4%) | 317 (3.7%) | 0.40 | 0.83 (0.68–1.04) | 0.15 |
| Bleeding requiring surgical invervention | 122 (1.7%) | 173 (2.0%) | 0.20 | 0.76 (0.57–1.02) | 0.07 |
| Composite* | 340 (4.8%) | 455 (5.3%) | 0.19 | 0.81 (0.68–0.97) | 0.02 |
| Dialysis | 106 (1.5%) | 102 (1.2%) | 0.08 | 1.03 (0.74–1.45) | 0.85 |
| Neurologic deficit | 113 (1.6%) | 95 (1.1%) | 0.007 | 1.18 (0.84–1.64) | 0.34 |
| Total LOS, days | 8.7 (4.0–13.0) | 7.9 (4.0–11.0) | <0.0001 | 1.02 (1.00–1.05) | 0.11 |
| ICU LOS, days | 5.1 (2.0–8.0) | 4.4 (2.0–7.0) | <0.0001 | 1.03 (0.99–1.06) | 0.12 |
| Duration of ventilation, days | 3.4 (1.0–5.0) | 3.0 (1.0–5.0) | <0.0001 | 1.04 (1.01–1.09)a | 0.02 |
| Redo Sternotomy Subgroup | (n=2819) | (n=2239) | |||
| In-hospital mortality | 64 (2.3%) | 67 (3.0%) | 0.11 | 0.57 (0.37–0.87) | 0.009 |
| Bleeding requiring surgical invervention | 45 (1.6%) | 61 (2.7%) | 0.006 | 0.50 (0.30–0.85) | 0.01 |
| Composite* | 102 (3.6%) | 118 (5.3%) | 0.004 | 0.56 (0.40–0.80) | 0.001 |
| Dialysis | 29 (1.0%) | 21 (0.9%) | 0.74 | 0.96 (0.45–2.02) | 0.91 |
| Neurologic deficit | 51 (1.8%) | 27 (1.2%) | 0.08 | 1.22 (0.68–2.20) | 0.51 |
| Total LOS, days | 7.5 (4.0–10.0) | 8.1 (5.0–11.0) | 0.001 | 0.94 (0.90–0.98)b | 0.006 |
| ICU LOS, days | 3.8 (2.0–6.0) | 4.1 (2.0–6.0) | 0.0002 | 0.90 (0.84–0.95)c | 0.0004 |
| Duration of ventilation, days | 2.3 (1.0–3.0) | 2.7 (1.0–4.0) | 0.005 | 0.93 (0.87–1.00) d | 0.04 |
| Neonate Subgroup | (n=1517) | (n=1700) | |||
| In-hospital mortality | 165 (10.9%) | 207 (12.2%) | 0.25 | 0.88 (0.66–1.17) | 0.38 |
| Bleeding requiring surgical invervention | 49 (3.2%) | 72 (4.2%) | 0.14 | 0.91 (0.56–1.49) | 0.71 |
| Composite* | 198 (13.1%) | 256 (15.1%) | 0.10 | 0.88 (0.67–1.14) | 0.32 |
| Dialysis | 70 (4.6%) | 70 (4.1%) | 0.48 | 0.95 (0.61–1.48) | 0.83 |
| Neurologic deficit | 49 (3.2%) | 38 (2.2%) | 0.08 | 1.23 (0.71–2.12) | 0.46 |
| Total LOS, days | 19.0 (9.0–29.0) | 19.2 (9.0–30.0) | 0.60 | 0.99 (0.92–1.07) | 0.85 |
| ICU LOS, days | 13.4 (6.0–20.0) | 12.8 (6.0–20.0) | 0.04 | 0.99 (0.91–1.07) | 0.80 |
| Duration of ventilation, days | 7.3 (4.0–11.0) | 7.1 (3.0–11.0) | 0.02 | 1.00 (0.92–1.09) | 0.93 |
--Unadjusted results are displayed as frequency (percent) for dichotomous variables, and 10% trimmed means (interquartile range) for continuous variables. Adjusted results (from conditional logistic regression for dichotomous variables and linear regression with center modeled as a main effect for continuous variables) are displayed as adjusted OR (95%CI) for dichotomous variables and adjusted ratio of LOS or duration of ventilation (days) between groups for continuous variables. In those groups where aprotinin was associated with a significant difference in LOS or duration of ventilation, adjusted estimates of the difference in actual days (aprotinin vs. no drug) at the median value of LOS or duration of ventilation were also calculated:
Overall group, duration of ventilation (+ 0.1 days); Reoperation subgroup,
total LOS (−0.5 days),
ICU LOS (−0.3 days),
duration of ventilation (−0.1 days).
Composite endpoint = in-hospital mortality or bleeding requiring re-operation, ICU= intensive care unit, LOS = length of stay
In the redo sternotomy subgroup, aprotinin was associated with a significant reduction in mortality and bleeding requiring surgical intervention, as well as reduced length of total and intensive care unit length of stay. There were no differences detected in dialysis or neurologic complications (Table 2). In the neonatal subgroup, aprotinin was not associated with a significant difference in any outcome evaluated (Table 2).
Aprotinin vs. TXA and ACA
Efficacy and safety outcomes were then compared in patients who received aprotinin, TXA, or ACA in 2007–2008 (the time period when use shifted from aprotinin to TXA or ACA) in unadjusted and adjusted analysis (Table 3). In multivariable analysis, there was generally no difference detected in efficacy or safety outcomes comparing aprotinin with ACA overall or in the two subgroups, with the exception of greater in-hospital mortality associated with ACA in the re-operation subgroup and greater bleeding requiring surgical invervention in the neonatal subgroup (Table 3). In contrast, TXA was generally associated with improved outcomes in comparison to aprotinin. This included reduced in-hospital mortality, bleeding requiring surgical invervention, intensive care unit length of stay, and dialysis associated with TXA vs. aprotinin in the overall cohort (Table 3). In the neonatal subgroup, similar differences were seen when comparing TXA with aprotinin. In the redo sternotomy subgroup, no significant differences between TXA and aprotinin were detected (Table 3).
Table 3.
Unadjusted and adjusted post-operative outcomes associated with TXA, ACA, and aprotinin
| Unadjusted Results | Adjusted Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Aprotinin | TXA | ACA | p-value | TXA vs. aprotinin | p-value | ACA vs. aprotinin | p-value | |
| Overall | (n=2247) | (n=1140) | (n=1478) | |||||
| In-hospital mortality | 89 (4.0%) | 23 (2.0%) | 57 (3.9%) | 0.009 | 0.39 (0.21–0.71) | 0.002 | 1.30 (0.82–2.08) | 0.27 |
| Bleeding requiring surgical invervention | 52 (2.3%) | 18 (1.6%) | 38 (2.6%) | 0.21 | 0.42 (0.22–0.77) | 0.005 | 1.62 (0.91–2.88) | 0.10 |
| Composite* | 132 (5.9%) | 41 (3.6%) | 79 (5.4%) | 0.02 | 0.47 (0.30–0.74) | 0.001 | 1.13 (0.77–1.67) | 0.53 |
| Dialysis | 38 (1.7%) | 9 (0.8%) | 23 (1.6%) | 0.10 | 0.23 (0.10–0.55) | 0.0008 | 1.01 (0.51–1.98) | 0.99 |
| Neurologic deficit | 56 (2.5%) | 29 (2.5%) | 25 (1.7%) | 0.21 | 0.92 (0.51–1.65) | 0.78 | 0.97 (0.54–1.74) | 0.91 |
| Total LOS, days | 8.9 (4.0–14.0) | 7.6 (4.0–11.0) | 9.3 (5.0–14.0) | <0.0001 | 0.95 (0.89–1.01) | 0.09 | 0.95 (0.90–1.01) | 0.10 |
| ICU LOS, days | 5.2 (2.0–8.0) | 4.3 (2.0–7.0) | 5.3 (2.0–8.0) | <0.0001 | 0.92 (0.85–1.00)a | 0.04 | 0.99 (0.92–1.06) | 0.68 |
| Duration of ventilation, days | 3.3 (1.0–5.0) | 2.7 (1.0–4.0) | 3.3 (1.0–5.0) | <0.0001 | 0.80 (0.73–0.88)b | <0.0001 | 1.00 (0.92–1.08) | 0.98 |
| Redo Sternotomy Subgroup | (n=953) | (n=469) | (n=573) | |||||
| In-hospital mortality | 18 (1.9%) | 7 (1.5%) | 19 (3.3%) | 0.09 | 0.50 (0.16–1.57) | 0.24 | 2.59 (1.04–6.45) | 0.04 |
| Bleeding requiring surgical invervention | 20 (2.1%) | 6 (1.3%) | 12 (2.1%) | 0.51 | 0.40 (0.14–1.10) | 0.08 | 1.42 (0.58–3.47) | 0.44 |
| Composite* | 35 (3.7%) | 13 (2.8%) | 26 (4.5%) | 0.32 | 0.63 (0.28–1.42) | 0.26 | 1.31 (0.67–2.54) | 0.43 |
| Dialysis | 8 (0.8%) | 4 (0.9%) | 4 (0.7%) | 0.95 | NA | NA | ||
| Neurologic deficit | 21 (2.2%) | 18 (3.8%) | 9 (1.6%) | 0.05 | 1.57 (0.66–3.73) | 0.31 | 1.12 (0.40–3.10) | 0.83 |
| Total LOS, days | 7.5 (4.0–11.0) | 7.1 (4.0–10.0) | 8.2 (5.0–11.0) | 0.0004 | 1.02 (0.94–1.12) | 0.62 | 0.99 (0.91–1.08) | 0.85 |
| ICU LOS, days | 3.9 (2.0–6.0) | 3.3 (2.0–5.0) | 4.3 (2.0–7.0) | <0.0001 | 1.02 (0.91–1.14) | 0.76 | 1.03 (0.93–1.15) | 0.55 |
| Duration of ventilation, days | 2.2 (1.0–3.0) | 2.2 (1.0–3.0) | 2.4 (1.0–4.0) | 0.58 | 0.91 (0.80–1.04) | 0.18 | 1.10 (0.97–1.25) | 0.13 |
| Neonate Subgroup | (n=481) | (n=206) | (n=271) | |||||
| In-hospital mortality | 67 (13.9%) | 13 (6.3%) | 32 (11.8%) | 0.02 | 0.31 (0.14–0.69) | 0.004 | 1.13 (0.59–2.18) | 0.71 |
| Bleeding requiring surgical invervention | 25 (5.2%) | 6 (2.9%) | 19 (7.0%) | 0.14 | 0.30 (0.11–0.84) | 0.02 | 2.81 (1.12–7.09) | 0.03 |
| Composite* | 86 (17.9%) | 19 (9.2%) | 42 (15.5%) | 0.02 | 0.30 (0.15–0.58) | 0.0004 | 1.31 (0.73–2.34) | 0.37 |
| Dialysis | 27 (5.6%) | 4 (1.9%) | 15 (5.5%) | 0.09 | 0.12 (0.04–0.41) | 0.0006 | 0.73 (0.30–1.77) | 0.49 |
| Neurologic deficit | 29 (6.0%) | 8 (3.9%) | 10 (3.7%) | 0.26 | 0.55 (0.20–1.51) | 0.25 | 0.83 (0.30–2.27) | 0.72 |
| Total LOS, days | 20.2 (9.0–32.0) | 16.3 (9.0–23.0) | 22.3 (11.0–36.0) | 0.002 | 0.87 (0.75–1.01) | 0.07 | 0.96 (0.83–1.11) | 0.61 |
| ICU LOS, days | 14.3 (6.0–22.0) | 12.2 (6.0–18.0) | 14.6 (6.0–24.0) | 0.18 | 0.82 (0.69–0.98)c | 0.03 | 0.99 (0.84–1.17) | 0.94 |
| Duration of ventilation, days | 7.0 (3.0–10.0) | 5.5 (3.0–8.0) | 8.3 (4.0–12.0) | 0.0002 | 0.62 (0.52–0.74)d | <0.0001 | 0.92 (0.78–1.10) | 0.37 |
--Unadjusted results are displayed as frequency (percent) for dichotomous variables, and 10% trimmed means (interquartile range) for continuous variables. Adjusted results (from conditional logistic regression for dichotomous variables and linear regression with center modeled as a main effect for continuous variables) are displayed as adjusted OR (95%CI) for dichotomous variables and adjusted ratio of LOS or duration of ventilation (days) between groups for continuous variables. In those groups where aprotinin was associated with a significant difference in LOS or duration of ventilation, adjusted estimates of the difference in actual days at the median value of LOS or duration of ventilation were also calculated: Overall group:
ICU LOS, TXA vs. aprotinin (−0.3 days);
duration of ventilation, TXA vs. aprotinin (−0.4 days). Neonate subgroup:
ICU LOS, TXA vs. aprotinin (−1.8 days);
duration of ventilation, TXA vs. aprotinin (−2.3 days).
Composite endpoint=in-hospital mortality or bleeding requiring re-operation. NA=not enough events to model, ICU=intensive care unit, LOS=length of stay
Instrumental variable analysis
An instrumental variable analysis was performed as a second method for comparative analysis of the three drugs, in an attempt to further minimize potential confounding center effects. Data from 2008 was compared to 2007, restricting to centers who switched primarily (>75% use) to ACA from aprotinin (n=7; 1553 patients) or switched primarily to TXA from aprotinin (n=3; 653 patients) and patients who received any antifibrinolytic medication at these centers. Due to the smaller number of patients, only the composite endpoint could be modeled in the overall cohort in multivariable analysis. Among centers who switched primarily to TXA from aprotinin, there was not a significant difference detected in in-hospital mortality or bleeding requiring surgical intervention in 2008 (primarilyTXA) vs. 2007 (primarily aprotinin): adjusted OR 0.53 (95% CI 0.27–1.05, p=0.07). However, the point estimate was in the same direction as the primary analysis noted above favoring TXA. Among centers who switched primarily to ACA from aprotinin, there was no difference detected in in-hospital mortality or bleeding requiring surgical intervention in 2008 (primarily ACA) vs. 2007 (primarily aprotinin): adjusted OR 0.85 (95% CI 0.52–1.41, p=0.54). There were too few dialysis events to model in multivariable analysis; however in unadjusted analysis in centers who switched primarily to TXA from aprotinin there was a reduction in renal failure requiring dialysis in 2008 (primarily TXA) vs. 2007 (primary aprotinin): 0.7% vs. 3.1%, p=0.03). In centers who switched primarily to ACA from aprotinin, there was not a significant difference detected in the proportion with renal failure requiring dialysis in 2008 (primarily ACA) vs. 2007 (primarily aprotinin): 1.2% vs. 2.7%, p=0.07. The overall number of patients requiring dialysis in both cases was small (9 and 32, respectively).
Discussion
This multi-center contemporary analysis of antifibrinolytic medications in children undergoing heart surgery suggests aprotinin is associated with reduced bleeding requiring surgical intervention and mortality with no increase in dialysis. Comparative analyses suggest similar efficacy of ACA and improved outcomes associated with TXA.
Several previous studies of aprotinin in the pediatric population have suggested aprotinin is effective in reducing bleeding following heart surgery, and a meta-analysis of 12 randomized controlled trials (n=626) found that aprotinin reduced the proportion of children who received transfusions during cardiac surgery by 33% (14). Our analysis of a contemporary cohort of >20,000 children suggests that aprotinin is associated with reduced bleeding requiring re-operation and mortality. These effects were most prominent in the subgroup of patients undergoing redo sternotomy. Previous analyses have suggested differential efficacy of aprotinin, particularly in those at highest risk for bleeding (7). Costello and colleagues found that only those undergoing re-operation had a significant decrease in transfusions associated with aprotinin in an observational study of 112 children undergoing heart surgery (7). Previous studies have also reported a reduction in inflammatory markers in patients who receive aprotinin, as well as a reduction in post-operative myocardial dysfunction and inotropic support (15,16,17). These properties, along with the impact of aprotinin on bleeding, may have contributed to the decreased length of stay and duration of mechanical ventilation we observed in the redo sternotomy subgroup.
However, many of the previous investigations have been limited by important methodologic concerns including small sample size, heterogeneous patient population, lack of standardized transfusion protocols, and varying doses of aprotinin used, and not all studies have demonstrated a beneficial effect of aprotinin (14). In addition, in a previous analysis of safety outcomes conducted by our group using an administrative datasource, we did not find a significant impact of aprotinin on mortality overall or in those undergoing re-operation (4). Analyses involving administrative data must rely upon ICD-9 codes from the hospital bill to identify children undergoing heart surgery. In contrast, in the present study we were able to utilize data from a clinical registry to identify the study population. Using our linked dataset, which contains both the clinical registry data in the STS-CHS Database and the ICD-9 codes from the administrative database (PHIS) for each patient, we found that 10% of our overall study cohort would not have been identified if relying upon ICD-9 codes alone, including 18% of patients in the redo sternotomy cohort and 13% of patients in higher STS-EACTS risk categories (categories 3–5) vs. 6% of patients in lower STS-EACTS risk categories (categories 1–2). It is possible that these differences may in part explain differences in study results.
Regarding the safety of aprotinin, we did not find any difference in renal failure requiring dialysis in comparison with no drug. Our data support results of previous single-center observational analyses. Guzzetta et al. evaluated 200 neonates undergoing congenital heart surgery and found that aprotinin was not significantly associated with post-operative creatinine levels or dialysis (18). Backer et al. evaluated 1251 children and adolescents undergoing congenital heart surgery, and found that aprotinin was not associated with post-operative renal failure or dialysis compared with historical controls (19). Evaluation of safety was limited in the small randomized trials of aprotinin in children, and in the recent meta-analysis combining results from 12 trials, mortality and renal failure could not be assessed (14).
In comparative analyses, our observational data suggested that in general ACA was associated with similar or worse outcome compared with aprotinin. There are few previous studies comparing outcomes in patients receiving different antifibrinolytic medications and it is difficult to draw conclusions due to differences in study design, dosing, and outcomes, and small sample sizes. Chauhan and colleagues compared aprotinin to ACA in 300 children undergoing heart surgery and found no difference in blood loss, transfusion, and need for re-exploration (5).
In contrast, we found that TXA appeared to be associated with improved outcomes compared with aprotinin. These results differ from a previous analysis of 100 children undergoing heart surgery which found no difference in blood loss or transfusion in those randomized to aprotinin vs. TXA (6). This study included fewer patients compared with our analysis and evaluated different outcomes. The reasons for potential greater efficacy of TXA are unclear. There are limited pediatric pharmacokinetic data available and in our study we were unable to evaluate medication doses. It has been previously reported that TXA may be a more potent inhibitor of fibrinolysis than ACA (20,21). In addition, TXA may have anti-inflammatory properties (22). In regard to safety, we did not detect any difference comparing ACA to aprotinin. TXA appeared to be associated with a lower rate of dialysis compared with aprotinin. However the overall number of events was small and it may be premature to draw any conclusions from these data. There were also few neurologic events in our study. Nonetheless, we did not find any difference comparing ACA, TXA, and aprotinin. Previous case reports have suggested that TXA may be associated with an increase in post-operative seizures in adult patients undergoing cardiac surgery (13).
Limitations
This study is subject to the limitations of all observational analyses. Our analytic strategy attempted to account for known patient confounders; however it is likely that there are other variables not captured in these databases which may impact both the receipt of anti-fibrinolytic medication and outcome. These may include differences in bypass strategies. We also attempted to account for center effects through our analytic approach utilizing conditional logistic regression and instrumental variable analysis. However, these strategies may not account for all differences between centers, and our results may not be generalizable to all centers performing congenital heart surgery.
Our study is also subject to the limitations of the databases. While our large sample size, multi-center data, and combined data from both a clinical registry and administrative database allowed evaluation of endpoints such as mortality and other safety outcomes not able to be assessed in previous single-center studies, we were not able to evaluate other outcomes such as volume of blood loss, transfusions, and more mild degrees of renal impairment, as these data are not collected in the databases utilized. In addition, the datasources used for this study also do not contain information on dosing or indication for administration. Thus, we were not able to compare specific doses of antifibrinolytic medications. Rather, these data represent outcomes associated with these medications in routine clinical practice across multiple centers.
Finally, although this analysis represents the largest evaluation of these medications in the pediatric population, the small sample size in certain subgroups, and small number of events, limited our analysis and power to detect differences between groups in some cases. For example, we were unable to perform adjusted analyses of dialysis in the instrumental variable analysis; thus these data are not adjusted for patient risk, and must be interpreted with caution. In addition, our analysis involved multiple comparisons. We chose not to adjust the p-values or to specify a p-value cut-off for “significance” as this methodology can be somewhat subjective; however we cannot rule out the possibility that some of our findings may be due to chance alone.
Conclusions
This large observational analysis of children undergoing heart surgery suggests that aprotinin is associated with reduced bleeding requiring surgical intervention and mortality with no increase in dialysis in comparison to no drug. Comparative analyses suggest similar efficacy of ACA and improved outcomes associated with TXA. These findings should be evaluated in an adequately powered clinical trial.
Acknowledgments
Funding Sources/Disclosures:
This study was supported by National Heart, Lung, and Blood Institute Grant 1RC1HL099941-01, under the 2009 American Recovery and Reinvestment Act. Dr. Pasquali: Grant support National Heart, Lung, and Blood Institute (1K08HL103631-01), and American Heart Association Mid-Atlantic Affiliate Clinical Research Program. Dr. Shah: Grant support National Institute of Allergy and Infectious Diseases (K01 AI73729), and Robert Wood Johnson Foundation Physician Faculty Scholar program. Dr. J Jacobs: Chair, Society of Thoracic Surgeons Congenital Heart Surgery Database Task Force, and medical advisor and shareholder, CardioAccess Dr. Peterson: Principal Investigator, Society of Thoracic Surgeons National Databases Analytic Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fergusson DA, Hebert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, et al. A comparison of aprotinin and lysine analgues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–2331. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 2.Mangano D, Tudor JC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 3.Petaja J, Lundstrom U, Leijala M, Peltola K, Siimes MA. Bleeding and use of blood products after heart operations in infants. J Thorac and Cardiovas Surg. 1995;109:524–529. doi: 10.1016/S0022-5223(95)70284-9. [DOI] [PubMed] [Google Scholar]
- 4.Pasquali SK, Hall M, Li JS, Peterson ED, Jaggers J, Lodge AJ, et al. Safety of aprotinin in congenital heart operations: Results from a large multicenter database. Ann Thorac Surg. 2010;90:14–21. doi: 10.1016/j.athoracsur.2010.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan S, Kumar BA, Rao BH, Rao MS, Dubey B, Saxena N, et al. Efficacy of aprotinin, epsilon aminocaproic acid, or combination in cyanotic heart disease. Ann Thorac Surg. 2000;70:1308–1312. doi: 10.1016/s0003-4975(00)01752-5. [DOI] [PubMed] [Google Scholar]
- 6.Bulutcu FS, Ozbek U, Polat B, Yalcin Y, Karaci AR, Bayindir O. Which may be effective to reduce blood loss after cardiac operations in cyanotic children: tranexamic acid, aprotinin or a combination? Paediatr Anaesth. 2005;15:41–46. doi: 10.1111/j.1460-9592.2004.01366.x. [DOI] [PubMed] [Google Scholar]
- 7.Costello JM, Backer CL, de Hoyos A, Binns HJ, Mavroudis C. Aprotinin reduces operative closure time and blood product use after pediatric bypass. Ann Thorac Surg. 2003;75:1261–1266. doi: 10.1016/s0003-4975(02)04667-2. [DOI] [PubMed] [Google Scholar]
- 8.Pasquali SK, Jacobs JP, Shook GJ, O’Brien SM, Hall M, Jacobs ML, et al. Linking clinical registry data with administrative data using indirect identifiers: Implementation and validation in the congenital heart surgery population. Am Heart J. 2010;160:1099–1104. doi: 10.1016/j.ahj.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franklin RC, Jacobs JP, Krogmann ON, Beland MJ, Aiello VD, Colan SD, et al. Nomenclature for congenital and paediatric cardiac disease: historical perspectives and The International Pediatric and Congenital Cardiac Code. Cardiol Young. 2008;18 (Suppl 2):70–80. doi: 10.1017/S1047951108002795. [DOI] [PubMed] [Google Scholar]
- 10.Clarke DR, Breen LS, Jacobs ML, Franklin RC, Tobota Z, Maruszewski B, et al. Verification of data in congenital cardiac surgery. Cardiol Young. 2008;18 (Suppl2):177–187. doi: 10.1017/S1047951108002862. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 12. [Accessed November 15, 2010.];STS Congenital Database Full Specifications. http://www.sts.org/documents/pdf/Congenital_DataSpecs_250.pdf.
- 13.Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analog. 2010;110:350–353. doi: 10.1213/ANE.0b013e3181c92b23. [DOI] [PubMed] [Google Scholar]
- 14.Arnold DM, Fergusson DA, Chan AK, Cook RJ, Faser GA, Lim W, et al. Avoiding transfusions in children undergoing cardiac surgery: A meta-analysis of randomized trials of aprotinin. Anesth Analg. 2006;102:731–737. doi: 10.1213/01.ane.0000194954.64293.61. [DOI] [PubMed] [Google Scholar]
- 15.Tassani P, Augustin N, Barankay A, Braun SL, Zaccaria F, Richter JA. High dose aprotinin modulates the balance between proinflammatory and anti-inflammatory response during coronary artery bypass graft surgery. J Cardiovasc Thorac Surg. 2000;14:682–686. doi: 10.1053/jcan.2000.18328. [DOI] [PubMed] [Google Scholar]
- 16.Wippermann CF, Schmid FX, Eberle B, Huth RG, Kampmann C, Schranz D, et al. Reduced inotropic support after aprotinin therapy during pediatric cardiac operations. Ann Thorac Surg. 1999;67:173–176. doi: 10.1016/s0003-4975(98)00974-6. [DOI] [PubMed] [Google Scholar]
- 17.Tweddell J, Berger S, Frommelt PC, Pelech AN, Lewis DA, Fedderly RT, et al. Aprotinin improves outcome of single ventricle palliation. Ann Thorac Surg. 1996;62:1329–1336. doi: 10.1016/0003-4975(96)00670-4. [DOI] [PubMed] [Google Scholar]
- 18.Guzzetta NA, Evans FM, Rosenberg ES, Fazlollah TM, Baker MJ, Wilson EC, et al. The impact of aprotinin on postoperative renal dysfunction in neonates undergoing cardiopulmonary bypass. Anesth Analg. 2009;108:448–455. doi: 10.1213/ane.0b013e318194007a. [DOI] [PubMed] [Google Scholar]
- 19.Backer CL, Kelle AM, Stewart RD, Suresh SC, Ali FN, Cohn RA, et al. Aprotinin is safe in pediatric patients undergoing cardiac surgery. J Thorac and Cardiovas Surg. 2007;134:1421–1426. doi: 10.1016/j.jtcvs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Fiechtner BK, Nuttall GA, Johnson ME, Dong Y, Sujirattanawimol N, Oliver WC, et al. Plasma tranexamic acid concentrations during cardiopulmonary bypass. Anesth Analg. 2001;92:1131–1136. doi: 10.1097/00000539-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 21.McNicol G, Fletcher A, Alkjaersig N, Sherry S. The absorption, distribution, and excretion of epsilon-aminocaproic acid following oral or intravenous administration to man. J Lab Clin Med. 1962;59:15–24. [Google Scholar]
- 22.Jimenez JJ, Iribarren JL, Lorente L, Rodriguez JM, Hernandez D, Nassar I, et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care. 2007;11:R117. doi: 10.1186/cc6173. [DOI] [PMC free article] [PubMed] [Google Scholar]


