Abstract
Purpose
To evaluate relationships between retinal vessel caliber, AIDS-related factors, and mortality.
Design
Longitudinal, observational, cohort study.
Methods
We evaluated data for participants without ocular opportunistic infections at initial examination (baseline) in the Longitudinal Studies of the Ocular Complications of AIDS (1998–2008). Semi-automated evaluation of fundus photographs (1 eye/participant) determined central retinal artery equivalent (CRAE), central retinal vein equivalent (CRVE), and arteriole:venule ratio (AVR) at baseline. Multiple linear regression models, using forward selection, identified independent relationships between indices and various host- and disease-related variables.
Results
Included were 1250 participants. Mean follow-up for determination of mortality was 6.1 years. Smaller CRAE was related to increased age (p<0.001) and hypertension (p<0.001); larger CRAE was related to lower hematocrit (p=0.002). Larger CRAE and CRVE were associated with black race (p<0.001). Larger CRVE was related to smoking (p=0.004); smaller CRVE was related to age (p<0.001) and higher mean corpuscular volume (p=0.001). We observed the following relationships with AIDS-associated factors: smaller CRAE and larger CRVE with history of highly active antiretroviral therapy (HAART; p<0.001); and larger CRAE with lower CD4+ T-lymphocyte count (p=0.04). We did not identify independent relationships with HIV RNA blood levels. There was a 12% (95% CI, 2–21%) increase in mortality risk per quartile of decreasing AVR (p=0.02).
Conclusions
Variations in retinal vascular caliber are associated with AIDS-specific factors, and are markers for increased mortality risk. Relationships are consistent with the hypothesis that the vasculature is altered by known atherogenic effects of chronic HAART or the prolonged inflammatory state associated with AIDS.
Advances in digital image analysis software have enabled large, population-based studies to measure retinal vessel caliber reliably and reproducibly as a potent biomarker of vascular disease.1–3 Morphologic variations of retinal arterioles and venules are likely to reflect variations in cerebral and coronary vessels,4 with which they are anatomically and physiologically similar.5 Abnormalities in vessel caliber have been described in many disease states, including hypertension, diabetes mellitus, coronary artery disease, stroke, and renal dysfunction.6–12 Variations in the retinal vascular caliber have not been studied among people with AIDS, although this population is known to be at increased risk for cardiovascular morbidity.13, 14
In this study, we investigated relationships between vessel caliber indices and demographic, medical, and laboratory characteristics of participants in the Longitudinal Study of the Ocular Complications of AIDS (LSOCA). Based on relationships seen in the setting of other diseases,15 we hypothesize that narrower arterioles and more dilated venules will be related to systemic morbidity and mortality.
METHODS
Patient Population
LSOCA is an NIH-sponsored, prospective, epidemiological study of people with AIDS, which began in September 1998. A description of its design and methods, and a summary of data for study participants at study enrollment (baseline) have been published previously.16 Data were collected from study participants every 6 months per protocol. The current study includes data collected through December 31, 2008 for participants without ocular opportunistic infections at baseline.
Data Collection
We collected the following baseline demographic, medical, and laboratory information: age, gender, race/ethnicity (self-reported), hemoglobin, hematocrit, mean corpuscular volume (MCV), platelet count, leukocyte count, and absolute neutrophil count. We also collected the following AIDS-related information at baseline: time since AIDS diagnosis, lymphopenia as AIDS-defining illness, CD4+ T-lymphocyte count (baseline and nadir), CD8+ T-lymphocyte count, HIV RNA blood level (baseline and peak), use of highly active antiretroviral therapy (HAART; on HAART at baseline; ever on HAART), and Karnofsky score (a global measure of a one’s ability to conduct normal activities17). We identified the following potential co-morbidities at baseline: history of smoking, hypertension, diabetes mellitus, renal disease, coronary heart disease, peripheral vascular disease, and stroke.
Vessel caliber indices were determined in a semi-automated manner by certified graders at the University of Wisconsin Fundus Photograph Reading Center (IVAN software, Department of Ophthalmology and Visual Science, University of Wisconsin, Madison, WI) using a standardized protocol, described previously.1 Briefly, the six largest arterioles and venules in a ring-shaped area located between 0.5DD and 1.0 DD from the optic disc margin are identified. Computer software measures the caliber of these individual vessels, then combines them into two summary variables for the eye: the projected caliber size of the central retinal artery (central retinal artery equivalent [CRAE]), and the projected caliber size of the central retinal vein (central retinal vein equivalent [CRVE]), using formulas derived by Parr and Spears18, 19 and by Hubbard,1 with revision by Knudtson.20 These indices are used to calculate the arteriole:venule ratio (AVR), as CRAE/CRVE.
We categorized study participants who died during follow-up on the basis of immediate and contributing causes of death, using available death certificates; specifically, we identified whether death was associated with diseases characterized by vasculopathy (renal disease, cardiovascular disease, stroke), liver disease, AIDS-related opportunistic infections or malignancies, other AIDS-related disorders, or trauma, as described in a previous LSOCA publication about this cohort.21
Definitions
For purposes of this study, HAART was defined as the concurrent use of three or more antiretroviral drugs. Study definitions for the following conditions can be found in a previous publication about this cohort:21 diabetes mellitus; hypertension; cardiovascular disease; stroke; peripheral vascular disease; and renal disease.
Data Analysis and Statistical Techniques
Unless otherwise noted, the unit of analysis was the eye, and one eye per study participant was evaluated. Wong and associates have demonstrated a strong correlation between the eyes of an individual, for both CRAE and CRVE, and concluded that measurements from one eye accurately reflect a person’s systemic microvascular status.22 If vessel caliber indices could be determined for both eyes, the eye with better photographic quality was selected as the study eye. Values for each vessel caliber index were grouped by quartiles and modeled ordinally. With regard to CRAE and CRVE, the first quartile contained narrower arterioles and venules, respectively, while the fourth quartile contained wider arterioles and venules. With regard to AVR, the first quartile included relatively narrower arterioles vs. venules, while the fourth quartile included relatively wider arterioles vs. venules.
Ordered logistic regression was used to assess the cross-sectional relationship between baseline factors (demographic, medical, laboratory, visual function) and vessel caliber index quartiles as the response variable. A forward selection model with p-value entry criterion of 0.05 was used to create adjusted models, using the following covariates: age, black race, hematocrit, MCV, history of HAART (previous use, use at baseline, or both), time since diagnosis of AIDS, and history of smoking. Factors for which there were statistically significant associations on adjusted models were chosen as covariates in subsequent adjusted models.
Vessel caliber indices were used as predictors in (1) cross-sectional analyses using logistic regression of selected co-morbidities at baseline and linear regression of Karnofsky score at baseline; and (2) longitudinal analyses using Cox regression of incident death during follow-up. The Fisher exact test was used to examine the relationship between baseline vessel caliber indices and causes of, or factors contributing to, death.
Because diabetes mellitus can cause vascular disease similar to that seen in people with AIDS,23 we performed subgroup analyses, looking for significant (p<0.01) interactions between diabetes mellitus and relationships that involve vessel caliber. Similar subgroup analyses were performed to look for interactions with hypertension.
P-values were two-sided and were not adjusted for multiple comparisons. Statistical analyses were performed using SAS (SAS/STAT User’s Guide, Version 9.2, 2010; SAS Institute, Cary, North Carolina, USA) and Stata (Stata Statistical Software: Release 11, 2009; StataCorp LP, College Station, Texas, USA) statistical packages.
RESULTS
As of December 31, 2008, 2,221 individuals had enrolled in LSOCA, 1,712 of whom had no ocular opportunistic infections. The demographic, medical, laboratory, and ophthalmic examination data for this subpopulation are described in a previous publication.21 Median age of the cohort was 43 years (range 38–48 years), and 34% of participants self-reported race/ethnicity as being black. Participants were significantly more likely to be excluded if they were older (mean age 44±10 years for those excluded vs. 43±8 years for those included, p=0.03) or black (34% of black participants were excluded vs. 25% of non-black participants, p=0.0003). These differences were attributed to the quality of the fundus photographs; participants who were older or black were more likely to have problems with dilation, resulting in photographs of lower quality that could not be used to determine vascular caliber indices.
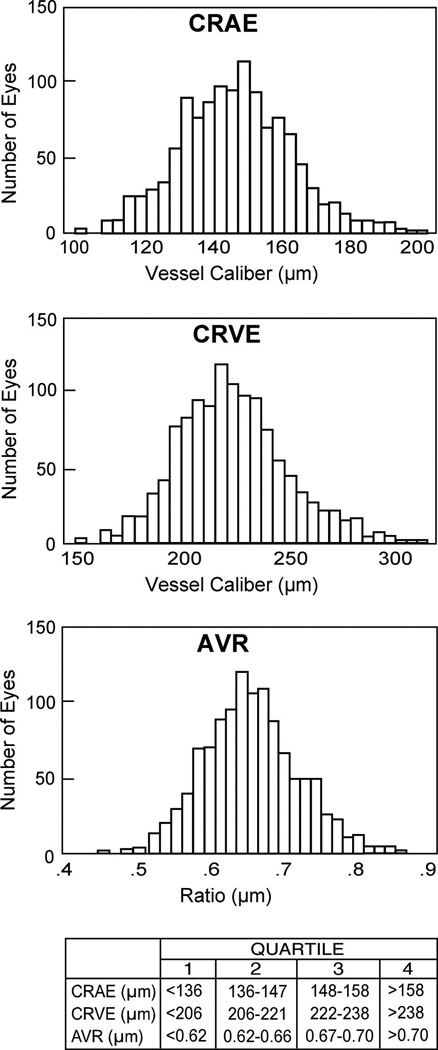
Median time since AIDS diagnosis was 4.2 years (range 1.6–7.1 years), and median CD4+ T-lymphocyte count at baseline was 192 cells/uL (range 81–350 cells/uL). HAART had been used before or at baseline in 90% of participants; 85% of participants were using HAART at baseline. Among the 1,712 eligible participants, 1250 eyes had vessel caliber measurements at baseline. The Figure illustrates the normal distribution of the vessel caliber indices.
Figure.
Distribution of central retinal artery equivalents (CRAE), central retinal vein equivalents (CRVE), and arteriole:venule ratio (AVR) at study enrollment (baseline). The ranges of values for the quartiles of each index are indicated below the bar graphs.
Table 1 shows relationships between vessel caliber indices and demographic, medical, and selected laboratory factors at baseline. Table 2 shows the relationships between vessel caliber indices and AIDS-specific factors. Crude (univariate) analyses demonstrated that at least one vessel caliber index was statistically associated with the following factors: age, gender, black race, weight, history of smoking, hemoglobin, hematocrit, MCV, time since AIDS diagnosis, lymphocytopenia, HIV RNA blood level, and history of HAART. On multivariate analyses, at least one vessel caliber index remained independently associated with the following factors: age, black race, hematocrit, MCV, history of smoking, time since AIDS diagnosis, and history of HAART. There was also an independent association between increased CRAE and decreased CD4+ T-lymphocyte count. There were no significant relationships between any vessel caliber index and cotton-wool spots (all p values ≥0.54, data not shown).
TABLE 1.
Relationships between Retinal Vessel Caliber Indices and Demographic and Laboratory Factors at Baseline for 1250 Study Participants without Ocular Opportunistic Infections in the Longitudinal Study of the Ocular Complications of AIDS
| Quartile | P Value | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Crude | Adjusteda | |
| Age (median, years) | ||||||
| CRAE | 45 | 44 | 42 | 41 | <0.001 | <0.001 |
| CRVE | 45 | 43 | 42 | 42 | <0.001 | <0.001 |
| AVR | 44 | 43 | 43 | 42 | <0.001 | <0.001 |
| Gender (percent female) | ||||||
| CRAE | 13 | 17 | 19 | 26 | <0.001 | -- |
| CRVE | 12 | 19 | 23 | 23 | <0.001 | -- |
| AVR | 20 | 15 | 18 | 22 | 0.28 | -- |
| Race/ethnicity (percent black) | ||||||
| CRAE | 24 | 30 | 38 | 44 | <0.001 | <0.001 |
| CRVE | 21 | 28 | 38 | 49 | <0.001 | <0.001 |
| AVR | 41 | 35 | 29 | 32 | 0.008 | 0.009 |
| Weight (median, kg) | ||||||
| CRAE | 77 | 74 | 75 | 74 | 0.02 | -- |
| CRVE | 75 | 75 | 74 | 74 | 0.04 | -- |
| AVR | 75 | 75 | 75 | 74 | 0.67 | -- |
| Smoking history (percent ever smoked cigarettes) | ||||||
| CRAE | 58 | 57 | 56 | 69 | 0.02 | -- |
| CRVE | 49 | 59 | 66 | 65 | <0.001 | 0.004 |
| AVR | 62 | 62 | 58 | 57 | 0.14 | -- |
| Hematology (medians) | ||||||
| Hemoglobin (g/dL) | ||||||
| CRAE | 14.1 | 14.0 | 13.6 | 13.2 | <0.001 | -- |
| CRVE | 13.9 | 13.8 | 13.7 | 13.5 | 0.002 | -- |
| AVR | 14.0 | 13.8 | 13.8 | 13.5 | 0.03 | -- |
| Hematocrit (percentage) | ||||||
| CRAE | 41.4 | 41.2 | 40.0 | 38.8 | <0.001 | 0.002 |
| CRVE | 41.0 | 40.8 | 40.1 | 39.5 | 0.005 | -- |
| AVR | 41.2 | 40.9 | 40.0 | 39.6 | 0.008 | 0.001 |
| Mean corpuscular volume (fL) | ||||||
| CRAE | 99.8 | 98.6 | 96.8 | 97.1 | 0.005 | -- |
| CRVE | 101.5 | 98.6 | 96.5 | 96.0 | <0.001 | 0.001 |
| AVR | 97.0 | 98.4 | 97.8 | 100.1 | 0.009 | 0.04 |
| Platelet count (×1000 cells/µL) | ||||||
| CRAE | 214 | 215 | 210 | 214 | 0.73 | -- |
| CRVE | 212 | 213 | 222 | 211 | 0.34 | -- |
| AVR | 220 | 214 | 209 | 215 | 0.42 | -- |
| Leukocyte count (×1000 cells/µL) | ||||||
| CRAE | 5.0 | 4.7 | 4.6 | 4.4 | 0.11 | -- |
| CRVE | 4.6 | 4.9 | 4.8 | 4.5 | 0.85 | -- |
| AVR | 4.8 | 4.7 | 4.8 | 4.4 | 0.24 | -- |
| Absolute neutrophil count (×1000 cells/µL) | ||||||
| CRAE | 2.6 | 2.4 | 2.3 | 2.2 | 0.32 | -- |
| CRVE | 2.3 | 2.5 | 2.5 | 2.2 | 0.87 | -- |
| AVR | 2.5 | 2.4 | 2.4 | 2.2 | 0.83 | -- |
AVR=arteriole:venule ratio
CRAE=central retinal arteriolar equivalent
CRVE=central retinal venular equivalent
HAART=highly active antiretroviral therapy
Variables selected from three separate ordered logistic regression models, regressing quartiles of CRAE, CRVE, and AVR on a candidate list of all baseline characteristics (n=1181 complete cases), using forward-selection entry criterion p<0.05. Significant variables for regression of CRAE are age, black race, hematocrit, HAART (ever used), and CD4+ T-lymphocyte count; for regression of CRVE, they are age, black race, history of smoking, mean corpuscular volume, HAART (ever used and current use); for regression of AVR, they are age, black race, hematocrit, mean corpuscular volume and current use of HAART.
TABLE 2.
Relationships between Retinal Vessel Caliber Indices and AIDS-Specific Medical and Laboratory Factors at Baseline for 1250 Study Participants without Ocular Opportunistic Infections in the Longitudinal Study of the Ocular Complications of AIDS
| Quartile | P Value | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Crude | Adjusteda | |
| AIDS history | ||||||
| Time since AIDS diagnosis (median, years) | ||||||
| CRAE | 4.5 | 4.4 | 4.4 | 3.4 | 0.15 | -- |
| CRVE | 4.6 | 4.5 | 3.9 | 3.6 | 0.05 | -- |
| AVR | 4.1 | 4.5 | 4.0 | 4.4 | 0.49 | -- |
| Lymphocytopenia as AIDS-defining illness (percentage) | ||||||
| CRAE | 65 | 67 | 68 | 60 | 0.16 | -- |
| CRVE | 68 | 66 | 66 | 60 | 0.03 | -- |
| AVR | 66 | 60 | 65 | 68 | 0.28 | -- |
| Immunology and virology | ||||||
| CD4+ T-lymphocyte count (median, cells/µL) | ||||||
| CRAE | 201 | 194 | 200 | 172 | 0.73 | 0.04 |
| CRVE | 207 | 197 | 184 | 182 | 0.13 | -- |
| AVR | 191 | 197 | 190 | 186 | 0.59 | -- |
| Nadir CD4+ T-lymphocyte count (median, cells/µL) | ||||||
| CRAE | 42 | 38 | 44 | 42 | 0.50 | -- |
| CRVE | 45 | 41 | 41 | 42 | 0.21 | -- |
| AVR | 39 | 41 | 46 | 42 | 0.33 | -- |
| CD8+ T-lymphocyte count (median, cells/µL) | ||||||
| CRAE | 784 | 773 | 798 | 747 | 0.09 | -- |
| CRVE | 756 | 753 | 808 | 766 | 0.78 | -- |
| AVR | 786 | 767 | 787 | 751 | 0.14 | |
| Peak HIV RNA blood level (median, log copies/mL) | ||||||
| CRAE | 5.3 | 5.3 | 5.3 | 5.3 | 0.50 | -- |
| CRVE | 5.3 | 5.3 | 5.3 | 5.4 | 0.37 | -- |
| AVR | 5.3 | 5.4 | 5.2 | 5.3 | 0.22 | -- |
| HIV RNA blood level (median, log copies/mL) | ||||||
| CRAE | 2.6 | 2.7 | 2.6 | 3.2 | <0.001 | -- |
| CRVE | 2.6 | 2.6 | 2.9 | 3.5 | <0.001 | -- |
| AVR | 2.9 | 2.6 | 2.8 | 2.7 | 0.37 | -- |
| Antiretroviral treatment history (percent individuals) | ||||||
| Ever on HAART | ||||||
| CRAE | 93 | 94 | 87 | 85 | <0.001 | <0.001 |
| CRVE | 94 | 92 | 90 | 83 | <0.001 | <0.001 |
| AVR | 89 | 91 | 88 | 90 | 0.82 | -- |
| Current HAART | ||||||
| CRAE | 89 | 88 | 82 | 80 | <0.001 | -- |
| CRVE | 91 | 87 | 83 | 77 | <0.001 | 0.01 |
| AVR | 79 | 85 | 88 | 86 | 0.01 | 0.004 |
AVR=arteriole:venule ratio
CRAE=central retinal arteriolar equivalent
CRVE=central retinal venular equivalent
HAART=highly active antiretroviral therapy
Variables selected from three separate ordered logistic regression models, regressing quartiles of CRAE, CRVE, and AVR on a candidate list of all baseline characteristics (n=1181 complete cases), using forward selection entry criterion p<0.05. Significant variables for regression of CRAE are age, black race, hematocrit, HAART (ever used), and CD4+ T-lymphocyte count; for regression of CRVE, they are age, black race, history of smoking, mean corpuscular volume, HAART (ever used and current use); for regression of AVR, they are age, black race, hematocrit, mean corpuscular volume and current use of HAART.
Table 3 shows the relationships between vessel caliber indices and the presence at baseline of selected systemic diseases that are characterized by vasculopathy. Only hypertension remained significantly associated with vessel caliber on multivariate analyses; for participants grouped by CRAE and AVR, those in the first quartiles (narrowest arterioles; smallest AVR) had the highest prevalence of hypertension. With regard to Karnofsky scores, we found that lower scores (worse health) were strongly related to larger CRVE (p=0.001) and smaller AVR (p<0.001; data not shown).
TABLE 3.
Relationships between Retinal Vessel Caliber Indices and Selected Co-Morbidities at Baseline for 1250 Study Participants without Ocular Opportunistic Infections in the Longitudinal Study of Ocular Complications of AIDS
| Quartile | Comparisons | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Crude | Adjusteda | |||
| ORb | P | ORb | P | |||||
| Co-morbidities | ||||||||
| Hypertension (percent individuals) | ||||||||
| CRAE | 27 | 21 | 17 | 14 | 0.76 | <0.001 | 0.75 | <0.001 |
| CRVE | 21 | 22 | 19 | 17 | 0.89 | 0.09 | 0.88 | 0.08 |
| AVR | 27 | 21 | 16 | 15 | 0.79 | <0.001 | 0.83 | 0.007 |
| Diabetes mellitus (percent individuals) | ||||||||
| CRAE | 9 | 8 | 10 | 6 | 0.89 | 0.23 | 0.92 | 0.41 |
| CRVE | 7 | 11 | 8 | 7 | 0.91 | 0.34 | 0.96 | 0.73 |
| AVR | 11 | 8 | 7 | 8 | 0.93 | 0.47 | 0.93 | 0.47 |
| Renal disease (percent individuals) | ||||||||
| CRAE | 5 | 4 | 6 | 5 | 1.03 | 0.83 | 1.00 | 0.97 |
| CRVE | 6 | 4 | 5 | 6 | 1.00 | 0.94 | 1.00 | 0.97 |
| AVR | 6 | 3 | 5 | 7 | 1.06 | 0.64 | 1.03 | 0.77 |
| Coronary heart disease (percent individuals) | ||||||||
| CRAE | 8 | 7 | 6 | 8 | 0.98 | 0.88 | 1.05 | 0.69 |
| CRVE | 8 | 7 | 7 | 8 | 1.01 | 0.90 | 1.04 | 0.77 |
| AVR | 7 | 7 | 8 | 6 | 0.98 | 0.88 | 1.00 | 0.97 |
| Peripheral vascular disease (percent individuals) | ||||||||
| CRAE | 5 | 3 | 5 | 3 | 0.89 | 0.45 | 0.90 | 0.52 |
| CRVE | 4 | 2 | 4 | 7 | 1.31 | 0.07 | 1.26 | 0.15 |
| AVR | 5 | 5 | 5 | 1 | 0.73 | 0.04 | 0.79 | 0.12 |
| Stroke (percent individuals) | ||||||||
| CRAE | 6 | 6 | 3 | 4 | 0.82 | 0.15 | 0.85 | 0.26 |
| CRVE | 7 | 4 | 6 | 4 | 0.86 | 0.27 | 0.88 | 0.38 |
| AVR | 3 | 9 | 5 | 3 | 0.95 | 0.73 | 0.96 | 0.77 |
AVR=arteriole:venule ratio
CRAE=central retinal arteriolar equivalent
CRVE=central retinal venular equivalent
Analyses involving CRAE were adjusted for age, race, hematocrit, time since diagnosis of AIDS, HAART (ever used), and CD4+ T-lymphocyte count; analyses involving CRVE were adjusted for age, race, mean corpuscular volume, history of smoking, and HAART (ever used and current use); analyses involving AVR were adjusted for age, race, mean corpuscular volume, hematocrit, and current use of HAART.
Odds ratio defined as change in odds of event per quartile of vascular measurement.
Table 4 shows the relationship of vessel caliber indices at baseline with death of 304 participants during follow-up. Larger CRVE (p=0.006) and smaller AVR (p<0.001) were strongly related to death on crude analyses; only the relationship with AVR remained significant after adjustment for comorbidities (p=0.02). HIV RNA blood level and HAART use at enrollment were the primary confounders that attenuated the effect of CRVE on mortality. The unadjusted relative risk (RR) for mortality per quartile of CRVE was 1.15 (p=0.006, as noted above); with adjustment, RR was 1.07 (p=0.21). During follow-up, 15 of 1250 participants developed cytomegalovirus (CMV) retinitis. Because AIDS-related CMV retinitis is associated with an increased risk of mortality,24 we repeated our analyses after excluding these 15 individuals, and the relationship between AVR and death remained significant (data not shown).
TABLE 4.
Retinal Vessel Caliber indices at Baseline as Predictors of Mortality among 1250 Study Participants without Ocular Oportunistic Infections in the Longitudinal Study of Ocular Complications of AIDSa
| Index | Crude | Adjustedb | ||||
|---|---|---|---|---|---|---|
| RRc death / quartile |
95% CI | P | RRc death / quartile |
95% CI | P | |
| CRAE | 0.98 | 0.89–1.09 | 0.77 | 0.95 | 0.86–1.06 | 0.40 |
| CRVE | 1.15 | 1.04–1.27 | 0.006 | 1.07 | 0.96–1.20 | 0.21 |
| AVR | 0.84 | 0.76–0.93 | <0.001 | 0.88 | 0.79–0.98 | 0.02 |
AVR=arteriole:venule ratio
CRAE=central retinal arteriolar equivalent
CRVE=central retinal venular equivalent
RR=relative risk
304 deaths among 1250 study participants; median follow-up, 6.8 years; rate, 3.6/100person-years.
Adjusted for the following variables at baseline: current use of HAART, CD4+ T-lymphocyte count, HIV RNA blood level; age, black race, mean corpuscular volume, hematocrit, time since diagnosis of AIDS, and hypertension (n=1152 because of missing values).
Relative risk estimated from Cox regression per increasing quartile.
Death certificates were available for 92 participants (30.3%). Table 5 shows the relationships between vessel caliber indices at baseline and causes of mortality during follow-up. There was a weak relationship between smaller CRAE and diseases characterized by microvasculopathy (cardiovascular disease, renal disease, and stroke) as causes of, or contributors to, death (p=0.08).
TABLE 5.
Relationships between Retinal Vessel Caliber Indices and Causes of Death during Follow-Up among 304 Study Participants in the Longitudinal Study of Ocular Complications of AIDS
| Quartile | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Number of deathsa | ||||
| CRAE | 78 | 81 | 66 | 79 |
| CRVE | 64 | 72 | 77 | 91 |
| AVR | 96 | 73 | 76 | 59 |
| Number of participants for whom death certificates were available | ||||
| CRAE | 30 | 22 | 23 | 17 |
| CRVE | 20 | 20 | 24 | 28 |
| AVR | 36 | 28 | 18 | 10 |
| Attributed causes of, or contributors to, death | ||||
| Diseases characterized by vasculopathyb | ||||
| Cardiovascular disease | ||||
| CRAE | 4 | 2 | 0 | 0 |
| CRVE | 2 | 2 | 2 | 0 |
| AVR | 3 | 3 | 0 | 0 |
| Renal disease | ||||
| CRAE | 1 | 0 | 1 | 1 |
| CRVE | 1 | 0 | 0 | 2 |
| AVR | 1 | 0 | 2 | 0 |
| Stroke | ||||
| CRAE | 1 | 1 | 0 | 0 |
| CRVE | 0 | 0 | 2 | 0 |
| AVR | 1 | 1 | 0 | 0 |
| Liver disease | ||||
| CRAE | 4 | 0 | 0 | 3 |
| CRVE | 3 | 0 | 2 | 2 |
| AVR | 4 | 1 | 2 | 0 |
| Other specified diseasesc | ||||
| CRAE | 4 | 3 | 2 | 4 |
| CRVE | 3 | 4 | 3 | 3 |
| AVR | 5 | 4 | 1 | 3 |
| AIDS-related opportunistic infection or malignancy | ||||
| CRAE | 9 | 6 | 7 | 4 |
| CRVE | 7 | 5 | 5 | 9 |
| AVR | 9 | 8 | 5 | 4 |
| AIDS-related, not otherwise specified | ||||
| CRAE | 7 | 9 | 11 | 5 |
| CRVE | 4 | 9 | 7 | 12 |
| AVR | 13 | 9 | 7 | 3 |
| Trauma | ||||
| CRAE | 0 | 1 | 2 | 0 |
| CRVE | 0 | 0 | 3 | 0 |
| AVR | 0 | 2 | 1 | 0 |
AVR=arteriole:venule ratio
CRAE=central retinal arteriolar equivalent
CRVE=central retinal venular equivalent
P-values comparing quartiles are 0.59, 0.77, and 0.81 for CRAE, CRVE, and AVR respectively.
P-values for the comparison of diseases characterized by vasculopathy vs. other causes are 0.08, 0.54, and 0.31 for CRAE, CRVE and AVR, respectively.
Includes organ diseases other than those listed in the table, plus sepsis and shock.
Of the 1250 participants, 249 (19.9%) had hypertension, and only 103 (8.2%) had diabetes mellitus. Neither hypertension nor diabetes mellitus had a significant effect on relationships between vessel caliber indices and any other factors demonstrated for the total population.
DISCUSSION
We studied variations in vessel caliber indices and their relationships to host and disease factors, but did not identify the prevalence of abnormal vessel caliber indices in our cohort, as normative data are not available.15 A number of large, population-based studies have identified various systemic, genetic, and environmental factors that influence vessel caliber indices;3, 6, 9–12, 25, 26 our results are consistent with these other studies with respect to the effects of age, race/ethnicity, and gender. Specifically, we found that older participants had significantly narrower arterioles, as shown in the Blue Mountain Eye Study (BMES),26 the Beaver Dam Eye Study (BDES),12 and the Multi-Ethnic Study of Atherosclerosis (MESA).11 We found a strong association between age and vessel caliber indices, despite the fact that the age range for individuals in our cohort was considerably narrower than ranges in previous studies. Both BMES and MESA found that women had larger CRAE; we observed this relationship in our population, as well, but it did not remain significant in our adjusted model. Black participants were more likely to have larger arterioles and venules, consistent with findings in MESA. Relationships between vessel caliber indices and age, race/ethnicity, and gender are poorly understood, but they may reflect unrecognized environmental or genetic influences.15 The fact that these relationships are present across different populations implies that not all of the variations in retinal vascular caliber observed in our cohort can be attributed directly to AIDS; for that reason, we adjusted for these factors in our analyses. Although there were significant differences in age and race/ethnicity of study participants who were and were not included in our analyses, the differences were small and not likely to influence our results or conclusions.
Infection with human immunodeficiency virus (HIV), its treatment, or both is thought to accelerate the aging process;27 thus, retinal vascular variations might occur earlier in life among people with AIDS than is seen in the general population. We did note that the distributions of vessel caliber indices for our cohort were similar to those for non-diabetics in the Wisconsin Epidemiological Study of Diabetic Retinopathy,28 despite the fact that the median age of that group (at least 55 years) was older than the median age of participants in our study (43 years).
Study participants with a self-reported history of smoking had significantly wider venules and smaller AVR, consistent with data from ARIC,29 MESA,11 Cardiovascular Health Study,10 and Rotterdam studies.9 It is known that smoking increases systemic markers of inflammation,30 which may be a contributor to retinal vessel damage.
Among the potential co-morbidities studied, only hypertension was statistically related to vessel caliber indices. Hypertensives were more likely to have narrower arterioles and smaller AVR, as reported in many other studies, including the BDES, which demonstrated a strong relationship between retinal arteriolar narrowing and nitricoxide-dependent endothelial dysfunction, thought to be the underlying mechanism of hypertension-related arteriolar changes.31 Prospective studies have found that retinal vascular variations predict development of clinical hypertension.32 It is likely that vessel caliber indices will predict similar changes in people with AIDS.
We also found that low hematocrit was associated with wider arterioles and larger AVR; we could not find previous reports of this association in other populations, based on a PubMed search. Anemia is an important clinical problem in people with AIDS; it is a prognostic marker for disease progression, and has been associated with increased mortality.33, 34 People with AIDS have multiple risk factors for anemia, including use of myelosuppressive drugs, poor nutritional status, and blood loss from gastrointestinal lesions.35 They commonly have other hematologic abnormalities, as well, including macrocytosis, which is associated with vitamin B12 or folate deficiency from intestinal malabsorption and with use of certain medications, such as zidovudine, stavudine, and ganciclovir.35, 36 We found that high MCV is independently related to the narrowing of retinal venules. It is possible that the relationships between these hematologic abnormalities and vessel caliber indices are not specific to AIDS, but were identified in our cohort because the abnormalities are more prevalent among people with AIDS than in other studied populations. It is possible that retinal vascular variations are caused by hypoxia, leading to vascular dilation,36 or to a complex interaction of hemorheologic factors (abnormal vessel wall shear,37 abnormal erythrocyte aggregation or deformability,38 leukocyte activation39), leading to altered blood flow and ischemia.
We also identified AIDS-specific risk factors for variations in vessel caliber indices. Participants who had ever been on HAART were more likely to have narrower arterioles and venules. The Rotterdam study has linked decreased CRAE to increased intima-media thickness, a marker for subclinical atherosclerosis, measured in carotid arteries.9 Intima-media thickening increases the risk of myocardial infarction and cardiovascular morbidity in HIV-infected individuals.40 Our findings are consistent with the notion that HAART has a pro-atherogenic effect on the vasculature.14 Vessel caliber indices were also weakly associated with duration of AIDS and low CD4+ T-lymphocyte count. Chronic HIV infection and HAART are associated with a heightened state of inflammation,41 which could be another mechanism for changes in the retinal vasculature.
A previous study of this cohort showed that abnormal contrast sensitivity is associated with increased mortality; evidence suggested that the association was based on an increased prevalence of life-threatening systemic diseases characterized by vasculopathies among those with abnormal contrast sensitivity.21 We also investigated whether variations in vessel caliber indices were markers for increased risk of death. Mortality increased by 12% per quartile of decreasing AVR (p=0.02). This relationship was significant, despite adjustment from known confounders, including hypertension, suggesting that AVR is an independent risk factor. We found that mortality risk also increased by 15% per quartile of retinal venular widening on crude analysis, but this relationship was not independent of other covariates. HIV RNA blood level and HAART use at enrollment were the primary confounders that attenuated the effect of CRVE on mortality; thus, variations in CRVE may be a specific marker for AIDS-related influences on mortality. These observations are similar to findings from the BDES and BMES; both studies found that smaller AVR and larger retinal venules were associated with an increased risk of death from cardiovascular disease.42, 43 We also identified a weak relationship between vessel caliber indices and death caused by diseases associated with vasculopathy.
There are several limitations to this study. Causation cannot be shown with cross-sectional analyses. Our ability to study the relationship between vessel caliber indices and causes of death was limited by the relatively small number of participants for whom death certificates were available. Lipids might influence the retinal vasculature, but we did not have serum lipid values at baseline for all participants. With regard to vessel caliber indices, values used in this study are not true measures of vessel caliber; they are based on the observable blood column, but do not take into account the immeasurable plasma cuff at the periphery of the vessel lumen. The normal systolic and diastolic cardiac cycle will alter retinal vessel caliber, but fluctuations are small and random, and not felt to be capable of causing major miscalculations.15 AVR has been used as a measure in previous cross-sectional studies of the retinal vasculature, and was therefore reported herein, to allow comparison to previous studies in other populations; however, there is a potential problem with use of AVR; CRAE and CRVE variations in the same direction could result in an unchanged ratio, masking retinal vascular abnormalities. Liew and associates have suggested that a statistical alternative is to report an interaction term between CRAE and CRAE, in addition to reporting each as a separate variable.44 Because AVR is based only on CRAE and CRVE, if AVR is significantly associated with a factor, either CRAE or CRVE will almost certainly be associated with the factor, after each index is adjusted for the other; thus, we are confident that the significant associations identified with AVR in the current study are relevant. Nevertheless, use of an interaction term in future studies may reveal additional associations, and may help to elucidate the specific type of vascular changes most closely associated with HIV-related disease.
In conclusion, our study has shown that people with AIDS have variations in the retinal vasculature that are related to duration of AIDS, treatment with antiretroviral drugs, hematologic abnormalities, and some measures of the severity of immunodeficiency. These vascular variations predict an increased risk of death. The fact that we saw many of the same relationships between vessel caliber indices and demographic factors seen in other populations supports the validity of our analysis techniques. The ability to visualize retinal vessels directly provides investigators with a potential tool to study early structural changes associated with life-threatening systemic vascular diseases. Such knowledge might lead to early risk factor intervention. Additional longitudinal studies are warranted to determine which of the vessel caliber indices will be most useful clinically for the study of people with AIDS, and whether changes in vascular caliber indices over time are even stronger predictors of adverse events.
ACKNOWLEDGEMENTS
Funding: This study was supported by Longitudinal Study of the Ocular Complications of AIDS (LSOCA) grant support from the National Eye Institute, Bethesda, Maryland to the Mount Sinai School of Medicine, New York, New York (U10 EY 08052); the Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland (U10 EY 08057); and the University of Wisconsin, Madison, Madison, Wisconsin (U10 EY 08067). Additional support provided by National Center for Research Resources through General Clinical Research Center Grants 5M01 RR 00350 (Baylor College of Medicine, Houston, Texas), 5M01 RR 05096 (Louisiana State University, Baton Rouge, Louisiana/Tulane /Charity Hospital, New Orleans, Louisiana), 5M01 RR00096 (New York University Medical Center, New York, New York), 5M01 RR 00865 (University of California, Los Angeles, California), 5M01 RR00046 (University of North Carolina, Chapel Hill, North Carolina), 5M01 RR00043 (University of Southern California, Los Angeles, California), ULI RR024996 (Weill Medical College of Cornell University, Ithaca, New York). Support also was provided through cooperative agreements U01 AI 27674 (Louisiana State University/Tulane), U01 AI 27660 (University of California, Los Angeles), U01 AI 27670 (University of California, San Diego, California), U01 AI 27663 (University of California, San Francisco, California), U01 AI25868 (University of North Carolina), U01 AI32783 (University of Pennsylvania, Philadelphia, Pennsylvania). Additional support was provided by the Skirball Foundation, New York, NY (Dr. Holland); The Elizabeth Taylor AIDS Foundation through a gift to the UCLA Herb Ritts, Jr. Memorial Vision Fund (Dr. Holland); the Jack H. Skirball Endowed Professorship (Dr. Holland); the Vernon O. Underwood Family Endowed Fellowship (Dr. Kalyani); and the Sybil Harrington Special Scholars Award from Research to Prevent Blindness, Inc., New York, NY (Dr. Thorne).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None of the authors have conflicts of interest with any aspect of this study. Funding entities had no role in the conduction or presentation of this study.
Contributions of Authors:
Study design: (PSK, AAF, MLVN, GNH).
Data collection: (SG, LDH, RPD, and the SOCA Research Group).
Data management and analysis: (SG, PSK, AAF, MLVN, LDH, RPD, GNH).
Data interpretation: (SG, PSK, AAF, MLVN, LDH, RPD, JET, GNH).
Preparation of initial draft of manuscript: (PSK, AAF, MLVN, GNH).
Review and approval of manuscript: All authors, as well as Study Officers of LSOCA, representing the SOCA Research Group, reviewed and approved the manuscript. The study was conducted with approval from the appropriate institutional review boards at each participating institution. Informed consent was obtained from all subjects, and the study was conducted in accordance with Health Insurance Portability and Accountability Act regulations.
The SOCA Research Group:
LSOCA is registered at http://www.clinicaltrials.gov (NCT00000168). A list of key personnel at participating clinical centers can be found in a prior printed publication.16
REFERENCES
- 1.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 2.Sherry LM, Wang JJ, Rochtchina E, et al. Reliability of computer-assisted retinal vessel measurementin a population. Clin Experiment Ophthalmol. 2002;30(3):179–182. doi: 10.1046/j.1442-9071.2002.00520.x. [DOI] [PubMed] [Google Scholar]
- 3.Couper DJ, Klein R, Hubbard LD, et al. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. Am J Ophthalmol. 2002;133(1):78–88. doi: 10.1016/s0002-9394(01)01315-0. [DOI] [PubMed] [Google Scholar]
- 4.Patton N, Aslam T, Macgillivray T, et al. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206(4):319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goto I, Katsuki S, Ikui H, Kimoto K, Mimatsu T. Pathological studies on the intracerebral and retinal arteries in cerebrovascular and noncerebrovascular diseases. Stroke. 1975;6(3):263–269. doi: 10.1161/01.str.6.3.263. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Moss SE, et al. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy: XIX: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2004;122(1):76–83. doi: 10.1001/archopht.122.1.76. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell P, Wang JJ, Wong TY, et al. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology. 2005;65(7):1005–1009. doi: 10.1212/01.wnl.0000179177.15900.ca. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Coresh J, Klein R, et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol. 2004;15(9):2469–2476. doi: 10.1097/01.ASN.0000136133.28194.E4. [DOI] [PubMed] [Google Scholar]
- 9.Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45(7):2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Br J Ophthalmol. 2002;86(9):1007–1013. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong TY, Islam FM, Klein R, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47(6):2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44(11):4644–4650. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- 13.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currier JS, Lundgren JD, Carr A, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54(1):74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114(4):787–793. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 17.Karnofsky SA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 18.Parr JC, Spears GF. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol. 1974;77(4):472–477. doi: 10.1016/0002-9394(74)90457-7. [DOI] [PubMed] [Google Scholar]
- 19.Parr JC, Spears GF. Mathematic relationships between the width of a retinal artery and the widths of its branches. Am J Ophthalmol. 1974;77(4):478–483. doi: 10.1016/0002-9394(74)90458-9. [DOI] [PubMed] [Google Scholar]
- 20.Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 21.Holland GN, Kappel PJ, Van Natta ML, et al. Association between abnormal contrast sensitivity and mortality among people with acquired immunodeficiency syndrome. Am J Ophthalmol. 2010;149(5):807–816. doi: 10.1016/j.ajo.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong TY, Knudtson MD, Klein R, et al. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111(6):1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 23.Pepose JS, Holland GN, Nestor MS, Cochran AJ, Foos RY. Acquired immune deficiency syndrome. Pathogenic mechanisms of ocular disease. Ophthalmology. 1985;92(4):472–484. doi: 10.1016/s0161-6420(85)34008-3. [DOI] [PubMed] [Google Scholar]
- 24.Puhan MA, Van Natta ML, Palella FJ, Addessi A, Meinert C. Excess mortality in patients with AIDS in the era of highly active antiretroviral therapy: temporal changes and risk factors. Clin Infect Dis. 51(8):947–956. doi: 10.1086/656415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124(1):87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- 26.Leung H, Wang JJ, Rochtchina E, et al. Relationships between age, blood pressure, and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci. 2003;44(7):2900–2904. doi: 10.1167/iovs.02-1114. [DOI] [PubMed] [Google Scholar]
- 27.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47(4):542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein R, Klein BE, Moss SE, Wong TY, Sharrett AR. Retinal vascular caliber in persons with type 2 diabetes: the Wisconsin Epidemiological Study of Diabetic Retinopathy: XX. Ophthalmology. 2006;113(9):1488–1498. doi: 10.1016/j.ophtha.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Klein R, Sharrett AR, Klein BE, et al. Are retinal arteriolar abnormalities related to atherosclerosis?: The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20(6):1644–1650. doi: 10.1161/01.atv.20.6.1644. [DOI] [PubMed] [Google Scholar]
- 30.Frohlich M, Sund M, Lowel H, et al. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95) Eur Heart J. 2003;24(14):1365–1372. doi: 10.1016/s0195-668x(03)00260-4. [DOI] [PubMed] [Google Scholar]
- 31.Xing C, Klein BE, Klein R, et al. Genome-wide linkage study of retinal vessel diameters in the Beaver Dam Eye Study. Hypertension. 2006;47(4):797–802. doi: 10.1161/01.HYP.0000208330.68355.72. [DOI] [PubMed] [Google Scholar]
- 32.Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ. 2004;329(7457):79. doi: 10.1136/bmj.38124.682523.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mocroft A, Kirk O, Barton SE, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13(8):943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 34.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:27S–43S. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Volberding PA, Levine AM, Dieterich D, et al. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38(10):1454–1463. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 36.Geene D, Sudre P, Anwar D, Goehring C, Saaidia A, Hirschel B. Causes of macrocytosis in HIV-infected patients not treated with zidovudine. Swiss HIV Cohort Study. J Infect. 2000;40(2):160–163. doi: 10.1053/jinf.1999.0628. [DOI] [PubMed] [Google Scholar]
- 37.Nagaoka T, Yoshida A. Noninvasive evaluation of wall shear stress on retinal microcirculation in humans. Invest Ophthalmol Vis Sci. 2006;47(3):1113–1119. doi: 10.1167/iovs.05-0218. [DOI] [PubMed] [Google Scholar]
- 38.Kim A, Dadgostar H, Holland GN, et al. Hemorheologic abnormalities associated with HIV infection: altered erythrocyte aggregation and deformability. Invest Ophthalmol Vis Sci. 2006;47(9):3927–3932. doi: 10.1167/iovs.06-0137. [DOI] [PubMed] [Google Scholar]
- 39.Dadgostar H, Holland GN, Huang X, et al. Hemorheologic abnormalities associated with HIV infection: in vivo assessment of retinal microvascular blood flow. Invest Ophthalmol Vis Sci. 2006;47(9):3933–3938. doi: 10.1167/iovs.06-0138. [DOI] [PubMed] [Google Scholar]
- 40.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23(14):1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross AC, Armentrout R, O'Riordan MA, et al. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. J Acquir Immune Defic Syndr. 2008;49(5):499–506. doi: 10.1097/QAI.0b013e318189a794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JJ, Liew G, Klein R, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28(16):1984–1992. doi: 10.1093/eurheartj/ehm221. [DOI] [PubMed] [Google Scholar]
- 43.Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110(5):933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 44.Liew G, Sharrett AR, Kronmal R, et al. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48(1):52–57. doi: 10.1167/iovs.06-0672. [DOI] [PubMed] [Google Scholar]