Abstract
Current evidence indicates that the ability of physical activity to sustain a normal phenotype of arterial endothelial cells (ECs) plays a central role in the beneficial effects of exercise (Ex) on atherosclerotic disease. Here we evaluate the strength of evidence that shear stress (SS) and/or circumferential wall stress (stretch) are the primary signals, produced by bouts of Ex, that signal altered gene expression in arterial ECs, thereby resulting in a less atherogenic EC phenotype. Current literature indicates that SS is a signal for expression of antiatherogenic genes in cultured ECs, in ECs of isolated arteries, and in ECs of arteries in intact animals. Furthermore, SS levels in the arteries of humans during Ex are in the range that produces beneficial changes. In contrast, complex flow profiles within recirculation zones and/or oscillatory flow patterns can cause proatherogenic gene expression in ECs. In vivo evidence indicates that Ex decreases oscillatory flow/SS in some portions of the arterial tree but may increase oscillatory flow in other areas of the arterial tree. Circumferential wall stress can increase expression of some beneficial EC genes as well, but circumferential wall stress also increases production of reactive oxygen species and increases the expression of adhesion factors and other proatherogenic genes. Interactions of arterial pressure and fluid SS play an important role in arterial vascular health and likely contribute to how Ex bouts signal changes in EC gene expression. It is also clear that other local and circulating factors interact with these hemodynamic signals during Ex to produce the healthy arterial EC phenotype. We conclude that available evidence suggests that exercise signals formation of beneficial endothelial cell phenotype at least in part through changes in SS and wall stretch in the arteries.
Keywords: nitric oxide, prostacyclin, endothelial nitric oxide synthase, shear stress, pressure
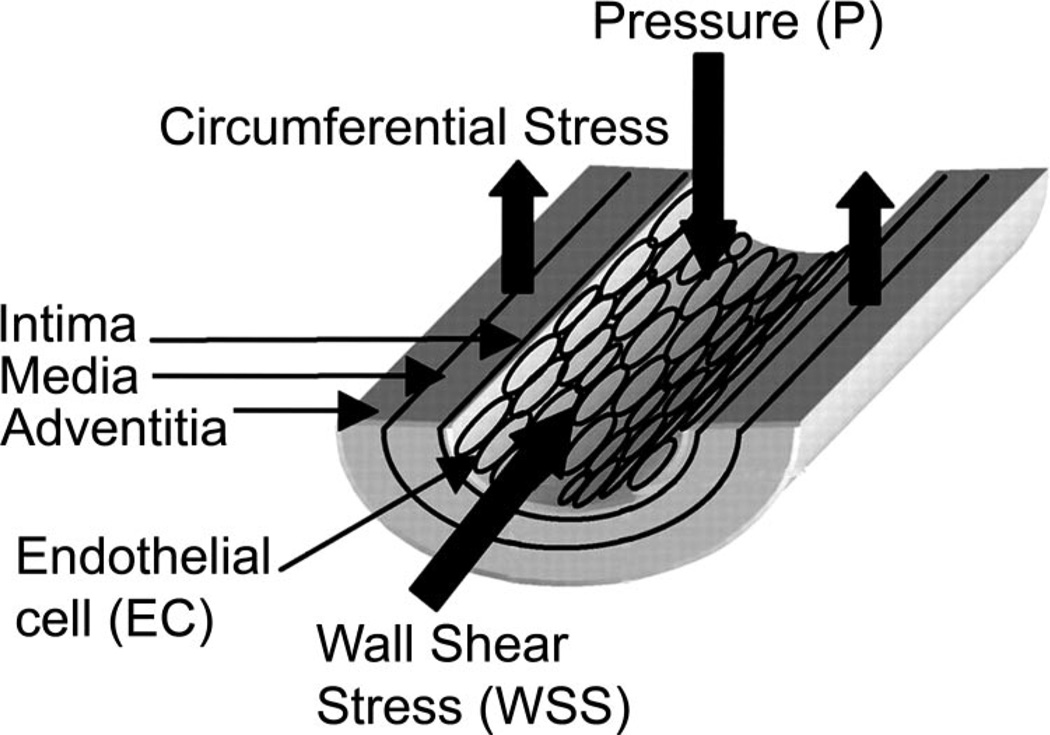
Atherosclerotic Cardiovascular disease is responsible for over 19 million deaths annually worldwide and coronary artery disease (CAD) remains the leading cause of death among cardiovascular diseases (93). Physical inactivity is recognized as a risk factor for the development of atherosclerotic CAD, and exercise training programs have been shown to be effective in prevention and treatment of CAD (7, 26, 29, 34, 88, 89, 94, 95, 102, 105, 129). Given these facts, there is growing interest in determining the mechanisms whereby physical activity and exercise produce beneficial effects in prevention and treatment of atherosclerosis. The primary mechanisms that underlie the effectiveness of physical activity in prevention and/or treatment of CAD are now being examined on several fronts but have not been fully established. Current evidence indicates that the ability of physical activity to sustain a normal phenotype of arterial endothelial cells plays a central role in the beneficial effects of physical activity (47). The purpose of this paper is to evaluate the strength of evidence that shear stress on the artery wall and/or circumferential wall stress (stretch on the artery wall) (Fig. 1) are the primary signals, produced by bouts of exercise, that signal altered gene expression in arterial endothelial cells, thereby resulting in a less atherogenic endothelial cell phenotype. There are several excellent, recent reviews outlining the general and specific effects of shear stress and stretch on endothelial cells (16, 51, 115, 136). Here we focus on the importance of these two hemodynamic signals in exercise training effects on endothelial cell phenotype. Available literature indicates that endothelial cell phenotype in vivo is determined via an intricate orchestration of many signals that interact with shear stress and cyclic strain in determining endothelial cell gene expression. The central hypothesis of our discussion is that shear stress and stretch, produced by exercise, modulate endothelial cell phenotype in a net antiatherogenic manner.
Fig. 1.
Mechanical effects of hemodynamic forces on the vascular wall. Figure is adapted from and used with permission of Dancu et al. (19).
Before discussing the effects of these hemodynamic forces on endothelial cell phenotype, we first summarize evidence demonstrating the importance of normal endothelial phenotype in artery health and of changes in endothelial cell phenotype associated with development and progression of atherosclerosis. Next, we review evidence that the beneficial effects of physical activity on atherosclerosis and CAD are the result of exercise training-induced antiatherogenic endothelial cell phenotype. With this background we then discuss evidence in the literature that supports the hypothesis that shear stress and/or circumferential wall stress (stretch) are important signals responsible for exercise-induced expression of antiatherogenic genes in endothelial cells. First we discuss evidence that shear stress can signal antiatherogenic gene expression in cultured endothelial cells. We then summarize evidence indicating that in vivo shear stresses produced in the human arterial tree during exercise are in the range known to cause these beneficial effects on gene expression in endothelial cells. We also discuss how recirculating flow patterns and oscillatory flow can have deleterious effects on endothelial cell phenotype. After the discussion of shear stress we consider evidence that circumferential stress on the walls of arteries can signal antiatherogenic gene expression in arterial endothelial cells. Finally, we evaluate evidence that the interaction of these hemodynamic signals, as occurs in vivo, is critical in producing the antiatherogenic phenotype in endothelium of arteries of physically active subjects.
Importance of Endothelial Cell Phenotype in Artery Health
It is generally accepted that an early event in the pathogenesis of atherosclerosis is endothelial cell dysfunction. Endothelial cell dysfunction can be characterized by one or more of the following features: 1) reduced endothelium-mediated vasodilation, 2) enhanced endothelial cell turnover, 3) increased expression of adhesion molecules and other inflammatory genes of endothelial cells, 4) increased oxidant stress, and 5) increased permeability characteristics of the endothelial barrier. These changes in endothelial function represent the change in endothelial cell phenotype that, in this paper, we will refer to as proatherogenic phenotype.
Current evidence indicates that endothelial dysfunction is present prior to and throughout all stages of atherosclerosis, suggesting that the change of endothelial cell phenotype from normal to proatherogenic plays a key role in initiating atherosclerosis and in the development of atherosclerosis (20, 27, 32, 77, 130–132). Indeed, it has been proposed that decreased endothelium-dependent dilation and/or nitric oxide (NO) bioavailability are key components of the proatherogenic endothelial cell phenotype as one develops this disease. The progressive atherosclerotic process involving endothelial cell-leukocyte adhesion and transmigration, vessel wall inflammation, lipid accumulation/foam cell formation, and conversion to the synthetic smooth muscle cell phenotype has been reviewed in detail previously (26, 35, 74, 93, 117, 118). Accumulating evidence demonstrates, however, that the progression of atherosclerosis and CAD can be slowed, stopped, or even reversed by various interventions, including regular physical activity, and that these effects are accompanied by improved endothelium-dependent vasodilation and/or NO bioavailability (48, 117, 118).
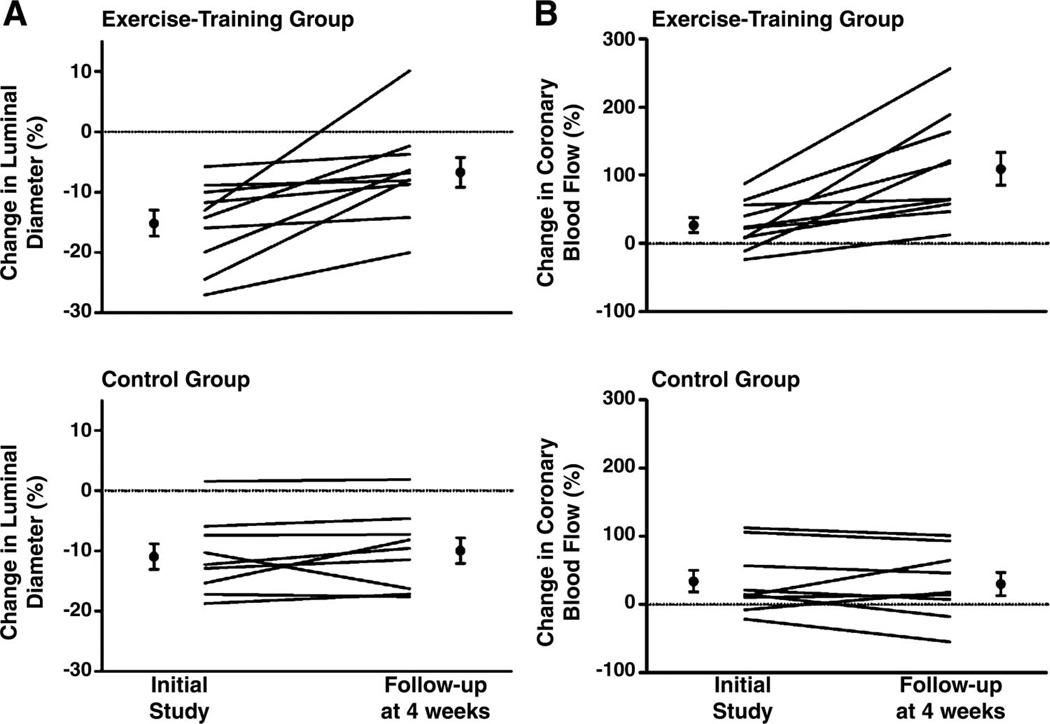
Several studies have examined the effects of exercise training in patients with advanced atherosclerotic CAD (37, 38, 47, 49, 101). For example, Hambrecht and colleagues (49) reported that 4 wk of exercise training resulted in improved endothelium-dependent dilation in conduit arteries (Fig. 2A) and resistance arteries/arterioles (Fig. 2B) in CAD patients. In conduit arteries intracoronary infusion of acetylcholine (ACh) produced less constriction after 4 wk of exercise training and exercise training increased ACh-induced dilation in the coronary microcirculation as shown by measures of total coronary blood flow obtained with a Doppler wire in the coronary artery (Fig. 2B) (49). Lifestyle changes, including increased physical activity, have also been reported to decrease the rate of progression of CAD and/or reverse CAD in patients (38, 48, 101). In summary, research over the past few years provides substantial and increasing evidence that these beneficial effects of exercise are the result of effects of exercise on endothelial cells (75, 80, 90).
Fig. 2.
Changes in coronary artery luminal diameter (A) and coronary blood flow (B) in response to intracoronary administration of acetylcholine in exercise-trained (top) and sedentary control (bottom) subjects. Lines show changes in individual subjects and black dots show mean ± SE for the groups. Data are taken with permission from Hambrecht et al. (49) ©[2000] Massachusetts Medical Society. All rights reserved.
Exercise Training Produces Antiatherogenic Endothelial Cell Phenotype in Atherosclerotic Arteries
The balance of proatherogenic and antiatherogenic factors in artery tissue determine whether or not areas of lipid-laden foam cells in the intima of the artery develop into atherosclerotic lesions (35, 75, 117, 118). As outlined above, the proatherogenic phenotype in arterial endothelial cells is characterized by reduced endothelium-dependent dilation (i.e., endothelial dysfunction), decreased expression of enzymes involved in endothelial cell signaling pathways (i.e., pathways for producing NO, endothelium-derived hyperpolarizing factor (EDHF), and prostacyclin), increased expression of adhesion factors, inflammatory mediators, increased oxidative stress, and increased expression of other atherogenic genes (51, 75, 131, 132). Hypercholesterolemia has been reported to cause signs of proatherogenic endothelial cell phenotype (decreased endothelium-dependent relaxation) in conduit arteries of humans (8, 9), monkeys (52, 76), and pigs (6, 17, 63, 113, 114, 124, 139, 140). In addition, the established risk factors (i.e., hypertension, diabetes, smoking, obesity, etc.) also cause signs of proatherogenic endothelial cell phenotype and decreased endothelium dependent relaxation (51, 75, 131, 132). A growing body of evidence indicates that disruption of the NO synthase (NOS) pathway (26, 86, 95) and/or reduced bioavailability of NO are important components of the proatherogenic endothelial cell phenotype (75, 131, 132).
Sustained physical activity has been shown to improve or maintain normal endothelial function in peripheral arteries (57, 68, 80, 84, 90, 119, 140) and coronary arteries (91, 104, 134) and to have similar effects in the presence of vascular disease (47, 124, 139). This improved endothelial function produced by exercise appears to be partially the result of changes in regulation of the endothelial NOS (eNOS) pathway (68, 71, 73, 91, 139) that involve increased expression of eNOS protein (47, 72) and activation by phosphorylation of eNOS serine 1177 by Akt (47). It is important to emphasize that in the key studies of Hambrecht and colleagues (47, 49) as well as studies in experimental animals (68, 71, 73, 91, 139) outlined above, the increased expression and activity of eNOS was associated with improved endothelial function. Other signs of an antiatherogenic endothelial cell phenotype induced by increased physical activity include increased expression and activity of superoxide dismutases (SOD) (31, 110, 111) and of catalase (40) and decreased oxidative stress (75). These changes would serve to increase NO bioavailability by reducing reactive oxygen species (ROS) that catabolize NO to peroxynitrite leading to the formation of nitrotyrosine (51, 75, 110). Work examining the effects of exercise training on the development of atherosclerosis in rabbit aortas demonstrated that exercise training reduces atherosclerosis and improves endothelial function and phenotype as shown by decreased expression of adhesion molecules (e.g., P-selectin, VCAM-1) as well as inflammatory markers such as MCP-1 (16) and inducible NOS (iNOS) (143, 144). The opposing effects of exercise and hypercholesterolemia on eNOS expression, NO bioavailability, and endothelial function in arteries are consistent with the hypothesis that exercise has its beneficial effects through modulation of endothelial gene expression.
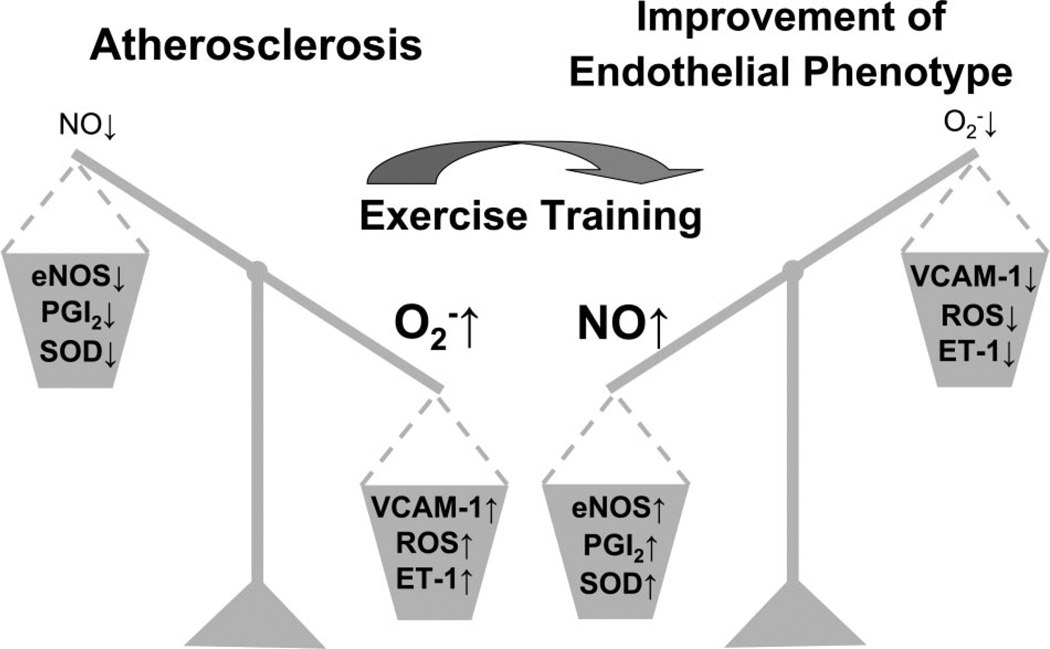
Thus there is substantial epidemiological and clinical evidence indicating that exercise training has beneficial effects in prevention and treatment of CAD (75) and, as discussed above, that these effects are associated with changes in endothelial cell phenotype. As shown in Fig. 3, we interpret currently available data as support for the hypothesis that the effects of exercise on expression of endothelial genes making the cells antiatherogenic (reflected as improvement of endothelial phenotype) represents a major mechanism whereby exercise limits or reverses atherosclerosis. The preponderance of evidence in this regard supports the widely held contention that the exercise related signal for antiatherogenic endothelial gene expression is increased shear stress produced by the increased cardiac output and regional blood flows required to provide oxygen to active cardiac and skeletal muscle during exercise (75). Locomotory exercise at intensities that produce maximal levels of oxygen consumption is associated with dramatic stress on the cardiovascular system. The demands for muscle blood flow cause dramatic increases in cardiac output (5–6 l/min to 25 l/min) and increases in shear stress, produced by movement of blood along the walls of the arteries. Also, high-intensity exercise increases stretch on the artery walls associated with increases in systolic arterial pressures and increases in heart rate that alter the magnitude and frequency of systolic stretch. Because of the magnitude of these hemodynamic effects of exercise on the conduit arteries, many have proposed that the beneficial effects of exercise on endothelial phenotype are the result of these hemodynamic, mechanical signals (36, 75).
Fig. 3.
Interactive effects of atherosclerosis and exercise training on markers of endothelial cell phenotype. eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; NO, nitric oxide; , superoxide; PGI2, prostacyclin; ROS, reactive oxygen species; SOD, superoxide dismutase; VCAM-1, vascular cell adhesion molecule-1. ↑, increase; ↓, decrease.
Shear Stress as a Signal for Altered Endothelial Cell Phenotype
Regular exposure to increased “shear stress” on endothelial cells that results from increases in blood flow during bouts of exercise is considered by many investigators in this field to be the primary signal for exercise training-induced adaptations of endothelial function and phenotype (50, 87, 92, 112). Acute changes in shear stress on the wall of arteries are a well-known signal for flow-induced vasodilation (62, 65, 66, 109). Wall shear stress is increased by an increase in blood flow (Q̇) as described by the following equation: (where SS is shear stress, η is viscosity, and Ri is internal radius of the artery).
Accordingly, at a given vessel radius, when flow increases, shear stress at the vessel wall also increases (Fig. 1). Acutely, flow-induced dilation of the artery increases radius and thereby returns shear stress toward normal levels. It appears that substances that signal remodeling and altered phenotype of endothelial and coronary smooth muscle cells are also released in response to increased shear stress (50, 75, 77).
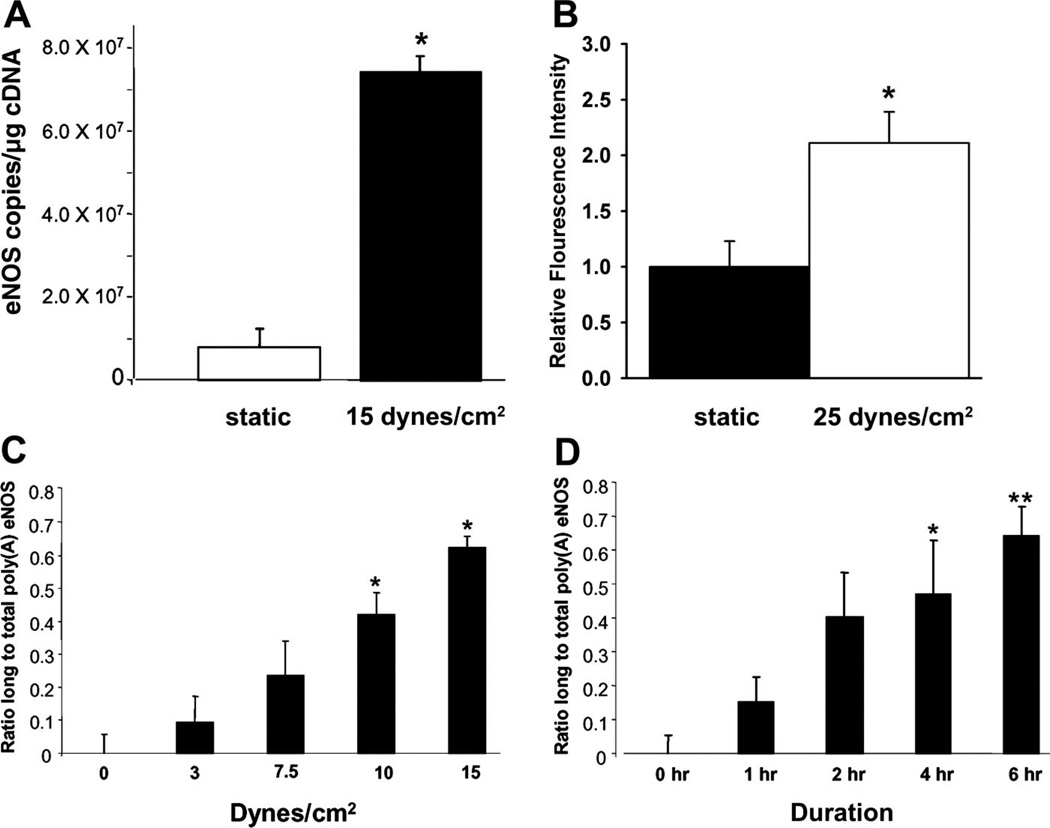
There is a growing body of evidence that increased shear stress is a signal for increased eNOS, decreased endothelin-1 (ET-1) and decreased VCAM-1 gene expression in endothelial cells. Early studies involving cultured endothelial cells suggested that this may underlie training-induced increases in endothelium-dependent vasodilation. In 1992, Harrison and colleagues (96) reported that increases in eNOS mRNA and eNOS protein were observed when cultured endothelial cells were exposed to 24 h of a sustained increase in shear stress. These investigators subsequently demonstrated that as little as 3 h of increased shear stress promoted increased eNOS gene transcription (125, 127, 128) and that eNOS mRNA expression exhibited a dose-response relationship with shear. These findings were later confirmed by other investigators (97, 107). Several intracellular signaling mechanisms have been identified that mediate increased transcription of the eNOS gene in endothelial cells when exposed to shear stress (51). These mechanisms include G proteins (82), Ca2+ (82), and c-Src (21, 22, 51). c-Src is a proto-oncogene product that acts as an intracellular signaling molecule via its tyrosine kinase activity. In addition to an increase in transcription of the eNOS gene, exercise training may lead to elevated eNOS mRNA levels via posttranscriptional mechanisms. One such mechanism is increased mRNA stability as shown in Fig. 4. Recent work by Harrison and colleagues (51) has shown that cultured endothelial cells exhibit increased poly(A) tail length in response to shear stress, inferring a prolonged half-life on eNOS mRNA.
Fig. 4.
Unidirectional shear stress increases expression of eNOS mRNA. A: amount of eNOS mRNA in endothelial cells exposed to no shear stress or to 6 h of shear stress at 15 dyn/cm2. *P < 0.05 vs. static. B: exposure of endothelial cells to shear stress for 6 h increased the amount of eNOS protein as reflected in eNOS immunofluorescence intensity in bovine aortic endothelial cells. Data from Ranjan et al. (107). *P < 0.05 vs. static. C: dose-response relationship of the effects of shear stress on eNOS mRNA polyadenylation. Increasing amounts of shear produce progressively longer transcripts. *P < 0.05 vs. dyn/cm2. D: increasing the duration of exposure of endothelial cells to shear stress also results in increased eNOS mRNA polyadenylation. Increasing duration of shear stress exposure at 15 dyn/cm2 produce progressively longer transcripts. *P < 0.05 vs. 0 h. **P < 0.05 vs. 0 h. Data for A, C, and D are taken with permission from Weber et al. (135) and data illustrated in B are taken from Ranjan et al. (107).
Recent evidence obtained with chip array analysis indicates that “… mean shear stress significantly affects the expression of about 3,000 endothelial cell genes” (53). Space does not allow us to discuss all the endothelial cell genes for which shear stress has been shown to influence expression. For the remainder of this discussion we focus on expression of eNOS and SODs as markers of an antiatherogenic endothelial phenotype and on expression of ET-1, adhesion molecules (VCAM-1, ICAM-1, selectins), and ROS as markers of a proatherogenic endothelial cell phenotype. Shear stress has been demonstrated to signal increased expression of antioxidant genes (96, 97, 107), increased production of prostacyclin (30, 39, 147), and increased expression of COX-1 and PGI2 synthase (98) in cultured endothelial cells, in addition to increased expression of eNOS. Also, increased shear stress in isolated perfused coronary arterioles produced increased eNOS and SOD-1 expression, demonstrating that it is not just cultured endothelial cells that exhibit these effects of increased shear stress (138). There is limited evidence from in vivo experiments evaluating the effects of shear stress on endothelial phenotype. There is evidence that chronic increases in blood flow produced by arteriovenous fistulas in dogs result in increased endothelium dependent relaxation, decreased production of endothelin and altered vasomotor responses to endothelin (87). Also, in rats arteriovenous fistulas have been reported to cause increased eNOS activity, mRNA levels, and protein content (92). Although the results of these experiments support the hypothesis that shear stress may signal antiatherogenic endothelial cell phenotype in vivo, they did not evaluate the effect of shear stress on other anti- or proatherogenic genes.
As summarized above, shear stress has been shown to produce increases in eNOS expression in cultured endothelial cells, in a dose-related manner, across the range of shear stress from 4 to 15 dyn/cm2 (128). Although it is clear that mean wall shear stress levels are not uniform in the arterial tree of mammals (11), recent results indicate that shear stress levels are generally in the range of 1 to 15 dyn/cm2 in human arteries. Areas prone to atherosclerosis in the arterial circulation generally exhibit low shear stress that can be caused by low velocity of laminar flow due to low blood flow levels and/or secondary recirculating flow patterns (separation, reattachment, and recirculation) around branch points and distal to stenoses (136). During exercise both causes of low shear in arteries appear to be decreased. For example, MR imaging allows measurement of shear stress in human arteries at rest and during exercise (12, 25, 122) and indicate that at rest, shear stress is in the range of 1 to 2 dyn/cm2 in the human abdominal aorta and increases to an average of 7 to 20 dyn/cm2 during moderate exercise (122). Also, Cheng et al. (11) report that review of the literature indicates that in nonatherosclerotic human arteries mean wall shear stress levels are in the range of 2 to 15 dyn/cm2 and that wall shear stress is inversely related to the diameter of the artery. Thus the magnitude of shear stress in humans during exercise spans the range of values that produce antiatherogenic endothelial gene expression in cultured cells, suggesting that shear stress could be an exercise-induced signal in humans and large mammals.
The effects of body size on cardiovascular hemodynamics should be considered as one evaluates available literature relative to the magnitude of shear stress produced in the arteries of the mammal. For example, in contrast to the values of shear stress in the aorta of humans outlined above, values of shear stress are much greater in the aortas of rats and mice. Greve et al. (45) report that shear stress values range between 60 and 120 dyn/cm2 in rodent aortas. Also, Cheng et al. (11) report that average mean wall shear stress levels in the common carotid artery of small rodents (rat and mouse) are in the range of 50 to 70 dyn/cm2 whereas larger mammals (human and dog) are in the range of 10 to 20 dyn/cm2. Given these observations, exercise-induced changes in shear stress may have a relatively different effect on endothelium in rodents than in arteries of large mammals.
Considering effects of exercise on shear stress, it is important to remember that increases in blood flow do not necessarily produce increased shear stress if radius of the artery also increases. This may explain some reports in the literature of no increase in eNOS expression in some arteries after exercise training (58, 83, 99, 100, 103).
Another important effect of exercise on shear stress may be that exercise can influence the frequency and magnitude of pulsations in flow (unidirectional) and/or oscillatory flow (antegrade and retrograde flow) and resulting shear stresses. It is well known that under some circumstances coronary blood flow can be retrograde and that retrograde flow also occurs in other areas of the cardiovascular system (12, 13, 43, 122). Oscillatory shear stress, as occurs with retrograde and ante-grade flow, has been reported to produce the opposite effects on expression of many endothelial genes as does unidirectional shear stress (46). For example, unidirectional shear stress decreases expression of ET-1 (64, 81) and VCAM-1 (53) whereas oscillatory shear stress produces increased expression of ET-1 (147), increases expression of adhesion molecules (i.e., VCAM-1) (10, 53), decreases eNOS expression (even below zero shear stress levels) (55), increases expression of enzymes that produce ROS (i.e., NADPH oxidase) (23, 55), and increases the release of superoxide (85). As summarized in Table 1, current literature clearly demonstrates that oscillatory shear stress produces an increased expression of proatherogenic genes in cultured endothelial cells and a decrease in antiatherogenic genes (51).
Table 1.
Oscillatory flow effects on markers of endothelial cell inflammation, adhesion, and phenotype
| Stimulus |
||||
|---|---|---|---|---|
| Study | Model | Flow Type and Magnitude, dyn/cm2 | Duration, h | Oscillatory Shear Effects (Vs. Unidirectional) |
| Chappell et al. (10) | HUVEC in parallel plate chamber | Unidirectional: 5 ± 5 | 24 | ● ↑ VCAM-1, ICAM-1, and E-selectin mRNA |
| Oscillatory: 0 ± 5 | ● ↑ monocyte adhesion | |||
| DeKeulenaer et al. (23) | HUVEC in parallel plate chamber | Unidirectional: 5 | 1, 5, and 24 | ● ↑ production |
| Oscillatory: 0 ± 5 | ● SOD mRNA/protein expression unchanged | |||
| Hsiai et al. (54) | BAEC in flow channel | Unidirectional: 50 ± 40 | 4 | ● ↑ ICAM-1, P-selectin, and MCP-1 mRNA |
| Oscillatory: 0 ± 2.6 | ● ↑ monocyte adhesion | |||
| Hwang et al. (55) | BAEC in flow channel | Unidirectional: 25 | 4 and 8 | ● ↑ production |
| Oscillatory: 0 ± 3 | ● ↓ eNOS mRNA | |||
| ● ↑ gp91phox, NOX4, and MCP-1 mRNA | ||||
| ● ↑ monocyte adhesion | ||||
| McNally et al. (85) | BAEC and MAEC in cone and plate viscometer | Unidirectional: 15 | 4 | ● ↑ production by xanthine oxidase |
| Oscillatory: 0 ± 15 | ● ↑ H2O2 production | |||
| Silacci et al. (116) | Perfused BAEC tubes | Unidirectional: 0.3 ± 0.1 and 6 ± 3 | 4 and 24 | ● ↓ eNOS mRNA |
| Oscillatory: 0.3 ± 3 | ● ↓ p22phox mRNA | |||
| ● No change in production or overall oxidative state | ||||
| Ziegler et al. (147) | Perfused BAEC tubes | Unidirectional: 6 ± 3 | 1, 4, and 24 | ● ↑ ET-1 mRNA |
| Oscillatory: 0.3 ± 3 | ● ↓ eNOS mRNA | |||
| Ziegler et al. (148) | Perfused BAEC tubes and EA hy.926 cells | Unidirectional: 6 ± 6 | 24 | ● ↓ eNOS mRNA |
| Oscillatory: 0.3 ± 6 | ● ↓ eNOS promoter activation | |||
| Gambillara et al. (33) | Perfused swine carotid | Unidirectional: 0.3 ± 0.1 and 6 ± 3 | 72 | ● ↓ eNOS mRNA/protein |
| Oscillatory: 0.3 ± 3 | ● ↓ Vasodilation to bradykinin | |||
BAEC, bovine aortic endothelial cells; eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; HUVEC, human umbilical vein endothelial cells; ICAM-1, intracellular adhesion molecule 1; MAEC, mouse aortic endothelial cells; MCP-1, monocyte chemoattractant protein-1; SOD, superoxide dismutase; VCAM-1, vascular cell adhesion molecule-1.
The effects of exercise on oscillatory flow and shear stress appear to be heterogeneous in different segments of the arterial tree. For example, in the human aorta, available information indicates that exercise decreases oscillatory flow and shear stress as total shear increases (122). Indeed, in human subjects, mild exercise was reported to abolish retrograde flow and shear in the supraceliac and infrarenal segments of the abdominal aorta (12). This is important because, whereas both oscillatory and steady shear stress stimulate increased NO production and eNOS expression in cultured cells, oscillatory shear stress produces substantial increases in the production of ROS whereas steady shear stress does not (51, 85). The effects of exercise on oscillatory flow in conduit arteries that perfuse active skeletal muscle are very complicated because exercise effects are dependent on interactions between the cardiac cycle and skeletal muscle contractions. For instance, Lutjemeier et al. (78) demonstrated that the amount of antegrade and retrograde blood flow during single-leg knee extension exercise was dependent on the point in the cardiac cycle where muscle contraction was initiated. Blood flow became oscillatory during contractions as the contraction would impede flow followed by augmented flow during skeletal muscle relaxation. As a result of these interactions of different combinations of timing of cardiac cycle and muscle contraction, blood flow patterns vary substantially during rhythmic exercise (78). Available evidence indicates that when going from rest to exercise antegrade flow increases to a greater extent than retrograde flow in arteries perfusing exercising skeletal muscle. The effects of exercise on oscillatory flow in conduit arteries that are not providing blood flow to exercising muscle tissue are also complex. For example, exercise has been reported to increase retrograde flow in the brachial arteries of humans performing leg exercise (42), in contrast to the decrease in oscillatory shear rate produced by exercise in the abdominal aorta.
Currently literature also suggests that oscillatory flow and shear stress may have different effects on endothelium in vivo than those observed in cell culture experiments (Table 1). For example, it has been reported that leg exercise produces increased blood flow and increased contribution of NOS generated NO release in brachial arteries of humans (42–44). Green et al. (44) demonstrated that leg cycling exercise produced oscillatory, antegrade/retrograde, blood flow patterns in the brachial artery and, importantly, that these conditions were associated with a larger contribution of NOS generated NO release from the brachial circulation. Green et al. concluded that these results may suggest an important role of oscillatory shear stress-mediated release of NO from the endothelium of the brachial circulation during leg exercise. On the basis of the literature summarized above, it is reasonable to propose that repetitive enhancement of NO production by brachial artery endothelial cells produced by leg exercise training would lead to an upregulation of eNOS expression, i.e., an antiatherogenic endothelial cell phenotype. However, at this time it is not possible to establish for sure that the increased NO production seen under these conditions in the arm vasculature is the result of the oscillatory shear, as proposed, or due to other stimuli in the arms during leg exercise, i.e., systemic effects of exercise on endothelial cell phenotype. Therefore, it is important that future work determine whether the increase in NO production in the arm, reported to occur during leg exercise, is evidence that oscillatory shear stress in the arm vasculature is a signaling mechanism underlying production of an antiatherogenic endothelial cell phenotype. More work is needed to understand these hemodynamic interactions and to pinpoint the impact of oscillatory flow and shear on endothelial cell phenotype in different arteries in vivo.
In summary, review of the current literature indicates that shear stress produces increased expression of antiatherogenic genes and decreased expression of proatherogenic genes in cultured endothelial cells and in endothelial cells of arteries in vivo. Also, the magnitudes of shear stress required for these alterations in gene expression are in the range of shear stress values reported in arteries during moderate levels of exercise in conscious humans. These observations indicate that shear stress during exercise is sufficient to induce antiatherogenic gene expression and are consistent with the hypothesis that shear stress is an important signal for exercise induced changes in endothelial cell gene expression. In cultured endothelial cells, oscillatory shear stress produces proatherogenic gene expression. Conversely, exercise appears to reduce retrograde flow in some arteries, thus decreasing oscillatory shear stress signals, whereas exercise increases retrograde flow in other arteries. More focused research in humans is needed to establish the importance of oscillatory flow in the effects of exercise on endothelial cell phenotype. Despite this current limitation in our knowledge, we conclude that available evidence strongly supports the hypothesis that shear stress is one signal for exercise-induced expression of antiatherogenic endothelial cell phenotype in arteries.
Pressure and Circumferential Stress (Stretch) as a Signal for Altered Endothelial Cell Phenotype
It is well known that arterial pressure fluctuates with systolic pressures of 120 mmHg and diastolic pressures of 80 mmHg at rest. During exercise peak systolic pressures can approach 200 mmHg and diastolic pressures remain normal or decrease (108). Arterial pressure waves pass through the arterial tree, and the magnitude of pulse pressure is determined by interactions between the compliance of the segments of the arterial tree and harmonics of the pressure waves. Thus arterial systolic and diastolic pressures in the femoral arteries can be significantly different from those measured in the aorta (108). Blood pressure can influence endothelial cells in at least two manners. First, cell culture experiments have demonstrated that exposure of endothelial cells to pressure affects growth rate of endothelial cells. Pressures in the range of 20 to 100 mmHg cause increased growth rates and higher pressures (> 130 mmHg) cause inhibition of endothelial cell growth (115, 133). In addition to these effects on growth rates, pressure can signal altered endothelial cell gene expression. For example, exposure of endothelial cells in culture to pressures of 135 mmHg results in increased eNOS and iNOS protein content (133).
A second manner whereby changes in pressure can alter gene expression of endothelial cells results from stretch of the arteries. Because arteries are compliant, changes in pressure can stretch endothelial cells producing circumferential stress (strain) and the pulsatile nature of arterial blood pressure results in cyclic strain on the arteries. It appears that cyclic strain applied to endothelial cells produces even more dramatic effects on endothelial cell phenotype than does just compression (direct effects of pressure on the cells), as discussed in detail below.
Endothelial cells are exposed to cyclic circumferential stress (strain) from distention of arteries caused by increased blood pressure (which increases transmural pressure across the arterial wall) (25) and/or by relaxation of smooth muscle in the wall, which allows the blood vessel to increase in diameter, producing stretch of the endothelial cells lining the vessel. In vivo, arterial endothelial cells are exposed to cyclic distention during the cardiac cycle. Cyclic strain and wall movement have been measured in human thoracic aorta with MRI, and results demonstrate that peak strain usually occurs after peak flow in the aorta (25). The frequency and magnitude of this distention increases during exercise because of increased systolic pressure and heart rate (108). As summarized in Table 2, there is a growing body of evidence that cyclic strain alters endothelial cell gene expression patterns. Awolesi et al. demonstrated that cyclic strain increased transcription of eNOS in cultured endothelial cells (3) and increased eNOS enzyme activity of cultured endothelial cells (2). Since these observations, it has been proposed that distention (increased intraluminal pressure) of arteries is a signal for increased expression of the eNOS gene (148). There is also evidence that stretch increases expression of EDHF synthase (CYP450) (106), suggesting that cyclic strain may increase endothelium-dependent dilator pathways in general. Considering factors that may be proatherogenic, however, as summarized in Table 2, cyclic strain has also been reported to increase release of ROS (15) and to increase expression of adhesion molecules including ICAM (14, 145), selectin (145), and MCP-1 (141, 142, 145). These observations are consistent with many reports in the literature that indicate that chronic increases in blood pressure are associated with impaired endothelial function and increased progression of atherosclerosis (28). Also, Dancu et al. (19) reported that cyclic strain applied to cultured endothelial cells grown in silicon tubes caused a decrease in eNOS expression, in contrast to reports of studies using other methods of inducing stretch on cultured cells (3).
Table 2.
Cyclic strain effects on markers of endothelial cell inflammation, adhesion, and phenotype
| Stimulus |
||||
|---|---|---|---|---|
| Study | Model | Cyclic Strain, % | Duration, h | Cyclic Strain Effects (vs. static) |
| Awolesi et al. (2) | BAEC on flexible membrane | 10 and 24% | 24 | ● ↑ eNOS activity |
| ● ↑ eNOS protein | ||||
| Awolesi et al. (3) | BAEC on flexible membrane | 6 and 10% | 0–24 | ● ↑ eNOS mRNA |
| ● ↑ eNOS protein | ||||
| Wung et al. (141) | HUVEC on flexible membrane | 12% | 0.5, 1, and 24 | ● ↑ production |
| ● ↑ MCP-1 | ||||
| Wung et al. (142) | HUVEC on flexible membrane | 0.5 and 2 | ● ↑ NO production | |
| ● ↓ signaling of MCP-1 | ||||
| Cheng et al. (14) | HUVEC on flexible membrane | 9, 11, and 12% | 24–48 | ● ↑ soluble ICAM-1 release |
| ● ↑ surface ICAM-1 expression | ||||
| ● ↑ ICAM-1 mRNA | ||||
| Cheng et al. (15) | HUVEC on flexible membrane | 12% | 0.25–20 | ● ↑ ICAM-1 mRNA |
| ● ↑ production | ||||
| ● ↑ antioxidant activity | ||||
| Yun et al. (145) | HUVEC on flexible membrane | 15 and 25% | 0.5–24 | ● ↑ E-selectin expression |
| ● ↑ ICAM-1 expression | ||||
| ● ↑ monocyte adhesion | ||||
| Ziegler et al. (148) | Perfused BAEC silicon tubes | 4% | 24 | ● ↓ eNOS promoter activation |
| ● No change in eNOS mRNA | ||||
| Ziegler et al. (147) | Perfused BAEC silicon tubes | 4% | 1, 4, and 24 | ● No change in ET-1 mRNA |
| ● No change in eNOS mRNA | ||||
It is clear that the effects of cyclic strain on endothelial cells are complex and can be modified by a number of important variables. One important consideration in interpreting cyclic strain literature is evaluation of direct and indirect effects. For example, when endothelial cells in an intact artery are “stretched” because of increased pressure, there is an increase in superoxide and other forms of ROS in the artery (5) and an increase in adhesion molecules, such as VCAM-1 (126). The ROS produced by cyclic strain may indirectly cause increased expression of eNOS (4, 15). The major effect of rhythmic circumferential strain on endothelial cells appears to be proatherogenic, even though the changes in eNOS expression alone would be antiatherogenic because the increased ROS production and expression of adhesion molecules override the effects of increased eNOS expression (51). The increased eNOS expression, although insufficient to correct function, may be a compensation for the loss of bioactive NO through the actions of superoxide and other ROS species. Consistent with the proposal that increased eNOS expression may be secondary to ROS effects are the reports that increased blood pressure increases eNOS expression in rat aorta (4) in association with increased oxidant stress in the aorta as well (5). Exercise bouts are usually of 1- 2-h duration. Conversely, many studies of the effects of cyclic strain on endothelial cell phenotype have been designed so that the cells are exposed to cyclic strain 24 h/day. These different exposure times should be considered when interpreting results. For example, brief increases in blood pressure and ROS associated with bouts of exercise may signal increased eNOS expression and other beneficial effects of exercise, whereas chronic increases in blood pressure may elevate ROS chronically. Hence chronic elevations in blood pressure may trigger different changes in endothelial phenotype than do episodic increases in pressure and wall stretch. This hypothetically may explain the paradoxical enhancement in endothelial function associated with exercise, relative to the impaired endothelial function associated with primary hypertension.
In summary, cyclic strain on endothelial cells causes increased expression of eNOS. However, cyclic strain also signals upregulation of proatherogenic genes including adhesion molecules, MCP-1 expression, and markers of oxidant stress as summarized in Table 2. Also, chronic increases in blood pressure have been shown to result in proatherogenic endothelial cell phenotypes, and increased blood pressure is recognized as a risk factor for development of atherosclerosis (28). Therefore, we conclude that at this time it is unclear whether increased wall strain participates in signaling an antiatherogenic endothelial cell phenotype in physically active subjects. Given the duration of exposure to cyclic strain produced by exercise for 1 or 2 h/day, future research is required to determine whether increases in systolic blood pressures and heart rate produce important signals for the beneficial changes in endothelial cell phenotype associated with exercise training.
Interactions of Shear Stress and Circumferential Stress (Stretch) in Altered Endothelial Cell Phenotype
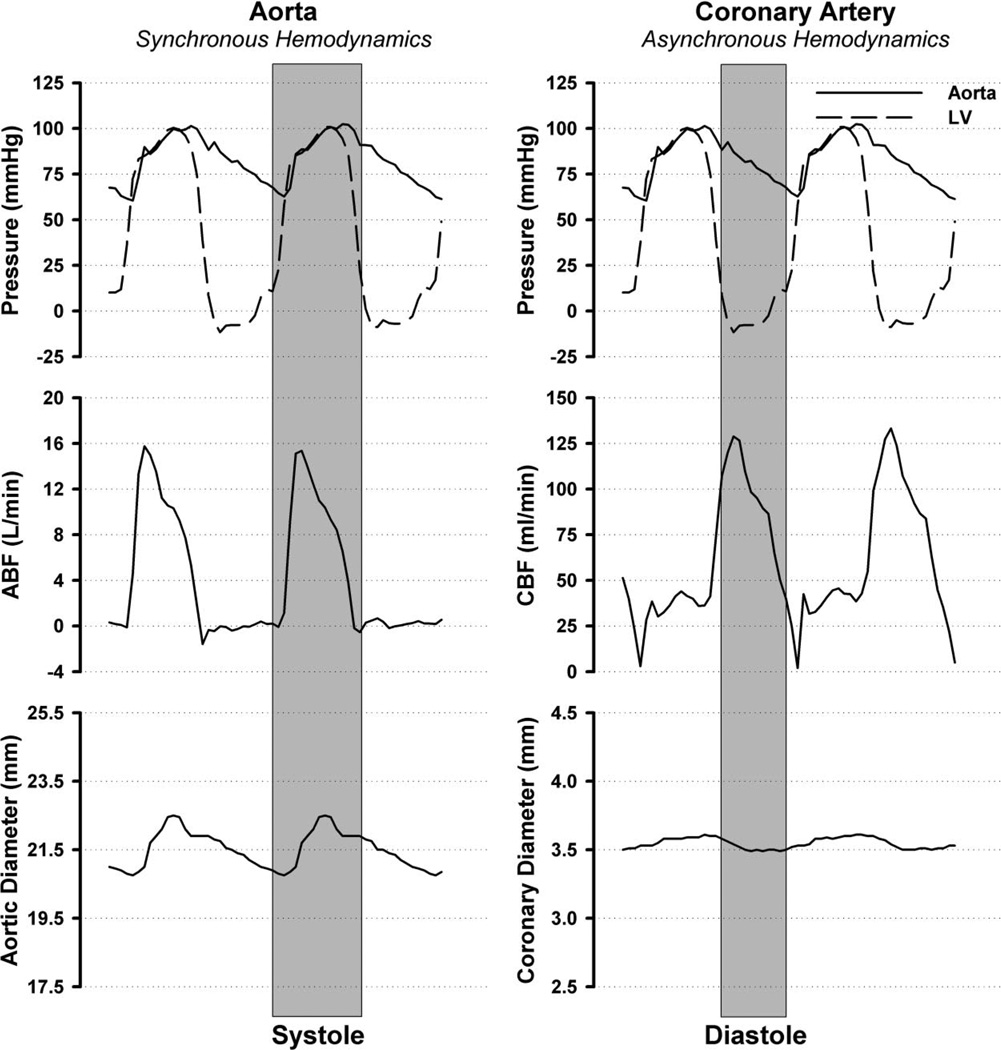
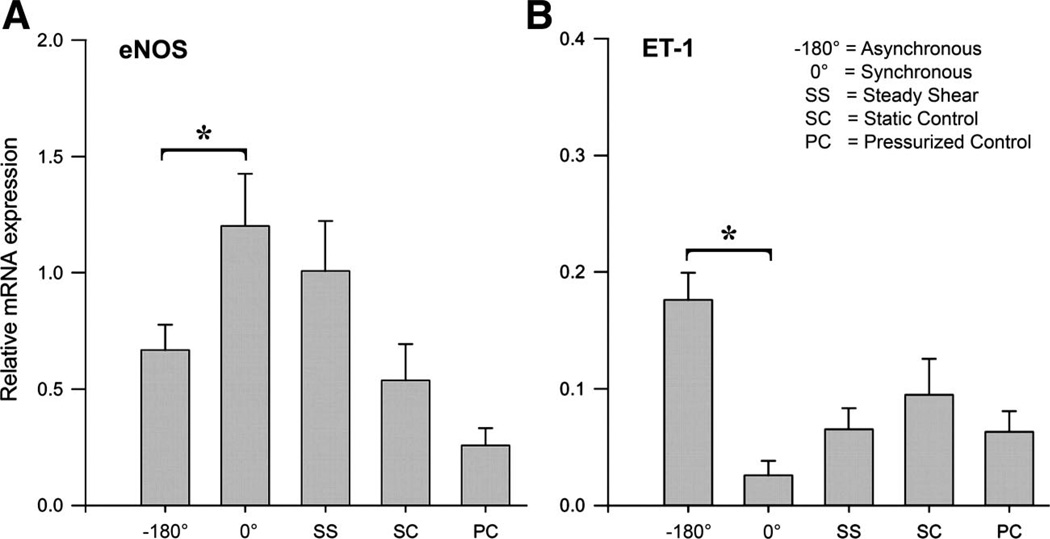
As outlined above, exercise produces complex hemodynamics that 1) increase the magnitude of blood flow and shear stress, 2) the frequency of pulsatile changes in pressures and flows, and 3) increased arterial systolic pressures. Available evidence indicates that the complexity of hemodynamics can contribute to expression of a proatherogenic endothelial cell phenotype especially when these hemodynamic parameters are asynchronous. Dancu et al. demonstrated that when pulsatile changes in diameter, flow and blood pressure occur synchronously these hemodynamic forces have different effects on endothelium lining silicon tubes than when the pulsatile changes are asynchronous (19). Figure 5 illustrates synchronous hemodynamics (on the left) and asynchronous hemodynamics on the right from data obtained in chronically instrumented, conscious pigs. Note on the left that aortic blood pressure, aortic blood flow, and aortic diameter have peak values during systole. This example is not truly synchronous because the time courses of the changes are similar though not identical. Truly synchronous hemodynamics would exist if these parameters in the aorta had the same time courses, i.e., minimal and maximal values at the same time points, so that peak pressure, flow, and diameter occurred at nearly the same time. In contrast to synchronous hemodynamics, an example of asynchronous hemodynamics is shown on the right of Fig. 5 in the coronary artery. Peak coronary flow occurs during early diastole, when pressure and coronary artery diameter are less than their peak values, i.e., asynchronous hemodynamics as defined by Dancu et al. (18). Dancu et al. examined the effects of synchronous and asynchronous changes in pressure, tube diameter, and gene expression on endothelial cells cultured in silicon tubes (18, 19). As shown in Fig. 6, Dancu et al.’s results demonstrate that asynchronous pulsations in shear stress and circumferential strain results in decreased eNOS expression but increased ET-1 expression in endothelial cells (18, 19). It is well known that asynchronous hemodynamics is the normal condition in the coronary circulation as illustrated in Fig. 5. That is, most coronary blood flow occurs during diastole, when blood pressure and coronary artery diameters are low (Fig. 5). Because of this, peak values of shear stress and circumferential strain are normally asynchronous in the coronary circulation. Perhaps of more interest, it is not uncommon to observe retrograde flow in the coronary arteries during early left ventricular systole at rest (59). These conditions may explain, in part, the propensity of the conduit coronary arteries to develop atherosclerosis. During exercise, coronary blood flow is normally no longer oscillatory but becomes pulsatile with positive flow in both systole and diastole. The pulsations in flow and pressure remain asynchronous during exercise, and it is not clear that the asynchrony of shear stress and circumferential strain would be decreased in the coronary arterial tree during exercise. It seems possible, however, that the relatively greater effect of exercise on shear stress (due to the four- to sixfold increases in coronary blood flow) and the fact that coronary blood flow is not oscillatory during exercise predominate during bouts of exercise resulting in a net antiatherogenic signal (69).
Fig. 5.
Synchronous and asynchronous hemodynamic environments as observed in a chronically instrumented pig during moderate treadmill exercise. On the left, synchronous hemodynamics as defined by Dancu et al. (18) are illustrated with measures of aortic pressure, left ventricular (LV) pressure, aortic blood flow (ABF), and aortic diameter. In contrast to this nearly synchronous hemodynamics, asynchronous hemodynamics are illustrated on the right with measures of aortic pressure, LV pressure, coronary blood flow (CBF), and coronary artery diameter. Systole and diastole were defined by the LV pressure trace obtained from a high-fidelity Konigsberg pressure transducer. Aortic and coronary flows were determined with Transonic flow probes, and aortic pressure was measured through a fluid-filled catheter.
Fig. 6.
Effect of 12-h exposure of endothelial cell-coated silicon elastic tubes to synchronous and asynchronous hemodynamic environments on expression of eNOS and ET-1 mRNA. Values are mean ± SE, *P < 0.05 vs. synchronous (0°) conditions. Data are used with permission from Dancu et al. (19).
Enhanced external counterpulsation is a noninvasive treatment for CAD patients that has been shown to increase diastolic coronary blood flow and total coronary blood flow. Enhanced external counterpulsation increases diastolic pressures above the diaphragm by compressing the tissues below the diaphragm and driving blood toward the heart. In patients who received daily 1-h treatments of enhanced external counterpulsation for 6 wk, it was reported that plasma nitrate and nitrite levels were increased (indicative of increased release of NO in the blood) and ET-1 levels were decreased, suggesting improved endothelial function and phenotype in these patients (1). Similar results have been reported for enhanced external counterpulsation treatment in an animal model of CAD (146). Zhang et al. (146) proposed that enhanced external counterpulsation in a porcine model of CAD applied to the hindlimbs and hips of the pigs would increase shear stress and improve endothelial cell function/phenotype in the coronary and brachial arteries. They found that enhanced external counterpulsation resulted in a doubling of peak diastolic shear stress in the brachial artery. Application of enhanced external counterpulsation for 2 h a day for 7 wk also resulted in a more antiatherogenic endothelial cell phenotype in the left anterior descending coronary artery (146). On the basis of the analysis of the effects of hemodynamic stress presented above, it would be expected that the arteries in the hindlimb of these pigs and perhaps in the abdominal aorta, would have retrograde flow during counterpulsation, which drives blood back into the thoracic aorta. If this hypothesis is correct, we would further expect proatherogenic changes in endothelial cell gene expression in these caudal arteries. Zhang et al. did not report whether proatherogenic changes in endothelial gene expression were seen in the abdominal aorta and arteries of the hindlimb. Therefore, this hypothesis will have to be tested in future work.
Systemic Distribution of Exercise-Induced Arterial Vascular Adaptations
There are reports of exercise training-induced adaptations in vascular beds providing blood flow to tissues/muscle not heavily involved in training bouts (44, 56, 57, 61, 67, 69, 70) and within skeletal muscle that does not exhibit increased oxidative capacity with exercise training (62, 67, 137). For example, most (24, 79, 120) but not all (123) studies have reported that leg exercise training of healthy and diseased human populations produces improved endothelium-dependent dilation in conduit arteries of their relatively inactive arms. Such observations may suggest a systemic effect of exercise training on arterial endothelial cell phenotype. However, modest increases in blood flow (42), oscillatory shear rate (42), pulse rate (41, 42), and blood pressure have been reported in the arms of humans during lower extremity exercise (42, 60, 121). These results make it difficult to appreciate the importance of primary hemodynamic signals (shear stress and cyclic strain) that may signal phenotypic changes to endothelial cells in the brachial artery during leg exercise. In addition, it is unclear at this time whether hemodynamic forces or circulating factors are the underlying mechanisms involved in vasculature adaptations in conduit arteries that do not perfuse cardiac or skeletal muscle active during exercise. As a result, it has yet to be determined whether one or a combination of the four hemodynamic forces mentioned above are the underlying mechanism involved in the apparent systemic effects of exercise on endothelial cell function/phenotype.
Conclusions
In conclusion, the available literature reviewed herein shows that shear stress is a signal for expression of antiatherogenic genes in endothelial cells in culture, in isolated arteries, and in arteries of intact animals. Furthermore, shear stress levels in the arteries of humans during exercise are in the range that produces beneficial changes. In contrast, oscillatory flow patterns can cause proatherogenic gene expression in cultured endothelial cells. In vivo evidence indicates that exercise decreases oscillatory flow/shear in some portions of the arterial tree but may increase oscillatory flow in other areas of the arterial tree. We conclude that current evidence indicates that increased shear stress during exercise may be one signal produced by exercise that contributes to an antiatherogenic phenotype of arterial endothelial cells.
Our review of current literature indicates that circumferential wall stress can also increase expression of some beneficial antiatherogenic endothelial cell genes. However, this literature equally provides evidence that circumferential wall stress increases production of ROS and increases the expression of adhesion factors and other proatherogenic genes. These data suggest that circumferential wall stress produced during exercise may contribute to exercise-induced modulation of endothelial cell phenotype. It appears that interactions of arterial pressure and fluid shear stress may play an important role in arterial vascular health and likely contribute to how exercise bouts signal changes in endothelial cell gene expression. Given that the timing of these signals during the cardiac cycle (i.e., whether or not the changes are asynchronous or synchronous) can have major effects on the impact of these signals on endothelial phenotype, more detailed analysis of these hemodynamic variables during bouts of exercise training is required. We conclude that available evidence suggests that exercise signals formation of beneficial endothelial cell phenotype at least in part through these mechanical signals: shear stress and wall stretch. However, it is also clear that other local and circulating factors interact with these hemodynamic signals during exercise to produce the healthy arterial endothelial cell phenotype. Future research promises to reveal these factors and how they interact with hemodynamic forces.
Acknowledgments
GRANTS
This work was supported by National Institutes of Health Grants RR-18276, HL-36088, HL-52490, and AR-048523.
REFERENCES
- 1.Akhtar M, Wu GF, Du ZM, Zheng ZS, Michaels AD. Effect of external counterpulsation on plasma nitric oxide and endothelin-1 levels. Am J Cardiol. 2006;98:28–30. doi: 10.1016/j.amjcard.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 2.Awolesci MA, Widmann MD, Sessa WC, Sumpio BE. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery. 1994;116:439–444. [PubMed] [Google Scholar]
- 3.Awolesi MA, Sessa WC, Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest. 1995;96:1449–1454. doi: 10.1172/JCI118181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton CH, Ni Z, Vaziri ND. Effect of severe aortic banding above the renal arteries on nitric oxide synthase isotype expression. Kidney Int. 2001;59:654–661. doi: 10.1046/j.1523-1755.2001.059002654.x. [DOI] [PubMed] [Google Scholar]
- 5.Barton CH, Ni Z, Vaziri ND. Enhanced nitric oxide inactivation in aortic coarctation-induced hypertension. Kidney Int. 2001;60:1083–1087. doi: 10.1046/j.1523-1755.2001.0600031083.x. [DOI] [PubMed] [Google Scholar]
- 6.Best PJ, Lerman LO, Romero JC, Richardson D, Holmes DR, Jr, Lerman A. Coronary endothelial function is preserved with chronic endothelin receptor antagonism in experimental hypercholesterolemia in vitro. Arterioscler Thromb Vasc Biol. 1999;19:2769–2775. doi: 10.1161/01.atv.19.11.2769. [DOI] [PubMed] [Google Scholar]
- 7.Blair SN. Physical activity, fitness, and coronary heart disease. In: Bouchard C, Shephard RJ, Stephens T, editors. Physical Activity, Fitness and Health. Champaign, IL: Human Kinetics; 1994. pp. 579–590. [Google Scholar]
- 8.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 9.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 10.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 11.Cheng C, Helderman F, Tempel D, Segers D, Hierck B, Poelmann R, van Tol A, Duncker DJ, Robbers-Visser D, Ursem NTC, van Haperen R, Wentzel JJ, Gijsen F, van der Steen AFW, de Crom R, Krams R. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis. 2007;195:225–235. doi: 10.1016/j.atherosclerosis.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Cheng CP, Herfkens RJ, Taylor CA. Abdominal aortic hemodynamic conditions in healthy subjects aged 50–70 at rest and during lower limb exercise: in vivo quantification using MRI. Atherosclerosis. 2003;168:323–331. doi: 10.1016/s0021-9150(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 13.Cheng CP, Herfkens RJ, Taylor CA. Comparison of abdominal aortic hemodynamics between men and women at rest and during lower limb exercise. J Vasc Surg. 2003;37:118–123. doi: 10.1067/mva.2002.107. [DOI] [PubMed] [Google Scholar]
- 14.Cheng JJ, Wung BS, Chao YJ, Wang DL. Cyclic strain enhances adhesion of monocytes to endothelial cells by increasing intercellular adhesion molecule-1 expression. Hypertension. 1996;28:386–391. doi: 10.1161/01.hyp.28.3.386. [DOI] [PubMed] [Google Scholar]
- 15.Cheng JJ, Wung BS, Chao YJ, Wang DL. Cyclic strain-induced reactive oxygen species involved in ICAM-1 gene induction in endothelial cells. Hypertension. 1998;31:125–130. doi: 10.1161/01.hyp.31.1.125. [DOI] [PubMed] [Google Scholar]
- 16.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 17.Cohen RA, Zitnay KM, Haudenschild C, Cunningham LD. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ Res. 1988;63:903–910. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- 18.Dancu M, Berardi D, Vanden Heuvel J, Tarbell J. Atherogenic endothelial cell eNOS and ET-1 responses to asynchronous hemodynamics are mitigated by conjugated linoleic acid. Ann Biomed Eng. 2007;35:1111–1119. doi: 10.1007/s10439-007-9290-1. [DOI] [PubMed] [Google Scholar]
- 19.Dancu MB, Berardi DE, Vanden Heuvel JP, Tarbell JM. Asynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:2088–2094. doi: 10.1161/01.ATV.0000143855.85343.0e. [DOI] [PubMed] [Google Scholar]
- 20.Daniel TO, Ives HE. Endothelial control of vascular function. News Physiol Sci. 1994;4:139–142. [Google Scholar]
- 21.Davis ME, Cai H, Drummond GR, Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res. 2001;89:1073–1080. doi: 10.1161/hh2301.100806. [DOI] [PubMed] [Google Scholar]
- 22.Davis ME, Cai H, McCann L, Fukai T, Harrison DG. Role of c-Src in regulation of endothelial nitric oxide synthase expression during exercise training. Am J Physiol Heart Circ Physiol. 2003;284:H1449–H1453. doi: 10.1152/ajpheart.00918.2002. [DOI] [PubMed] [Google Scholar]
- 23.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 24.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 25.Draney MT, Herfkens RJ, Hughes TJ, Pelc NJ, Wedding KL, Zarins CK, Taylor CA. Quantification of vessel wall cyclic strain using cine phase contrast magnetic resonance imaging. Ann Biomed Eng. 2002;30:1033–1045. doi: 10.1114/1.1513566. [DOI] [PubMed] [Google Scholar]
- 26.Dzau VJ. Pathobiology of atherosclerosis and plaque complications. Am Heart J. 1994;128:1300–1304. doi: 10.1016/0002-8703(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 27.Dzau VJ, Gibbons GH. The role of the endothelium in vascular remodeling. In: Rubanyi GM, editor. Cardiovascular Significance of Endothelium-Derived Vasoactive Factors. Mount Kisco, NY: Futura; 1991. pp. 281–291. chapt. 13. [Google Scholar]
- 28.Dzau VJ, Gibbons GH, Morishita R, Pratt RE. New perspectives in hypertension research. Hypertension. 1994;23:1132–1140. doi: 10.1161/01.hyp.23.6.1132. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher GF, Blair SN, Blumenthal J, Caspersen C, Chaitman B, Epstein S, Falls H, Sivarajan Froelicher ES, Froelicher VF, Pina IL. Statement on exercise. Benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1992;86:340–344. doi: 10.1161/01.cir.86.1.340. [DOI] [PubMed] [Google Scholar]
- 30.Frangos JA, Eskin SG, McIntyre LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 31.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 33.Gambillara V, Chambaz C, Montorzi G, Roy S, Stergiopulos N, Silacci P. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol. 2006;290:H2320–H2328. doi: 10.1152/ajpheart.00486.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 35.Geng YJ, Libby P. Progression of atheroma: a struggle between death and procreation. Arterioscler Thromb Vasc Biol. 2002;22:1370–1380. doi: 10.1161/01.atv.0000031341.84618.a4. [DOI] [PubMed] [Google Scholar]
- 36.Gielen S, Hambrecht R. Effects of exercise training on vascular function and myocardial perfusion. Cardiol Clin. 2001;19:357–368. doi: 10.1016/s0733-8651(05)70222-8. [DOI] [PubMed] [Google Scholar]
- 37.Gokce N, Vita JA, Bader DS, Sherman DL, Hunter LM, Holbrook M, O’Malley C, Keaney JF, Balady GJ. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. Am J Cardiol. 2002;90:124–127. doi: 10.1016/s0002-9149(02)02433-5. [DOI] [PubMed] [Google Scholar]
- 38.Gould KL, Ornish D, Scherwitz L, Brown S, Edens RP, Hess MJ, Mullani N, Bolomey L, Dobbs F, Armstrong WT, Merritt T, Ports T, Sparler S, Billings J. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA. 1995;274:894–901. doi: 10.1001/jama.1995.03530110056036. [DOI] [PubMed] [Google Scholar]
- 39.Grabowshi EF, Jaffer EA, Weksler BB. Prostacyclin production by cultured endothelial cell monolayers exposed to step increases in shear stress. J Lab Clin Med. 1985;105:36–43. [PubMed] [Google Scholar]
- 40.Graham DA, Rush JWE. Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J Appl Physiol. 2004;96:2088–2096. doi: 10.1152/japplphysiol.01252.2003. [DOI] [PubMed] [Google Scholar]
- 41.Green D, Cheetham C, Henderson C, Weerasooriya R, O’Driscoll G. Effect of cardiac pacing on forearm vascular responses and nitric oxide function. Am J Physiol Heart Circ Physiol. 2002;283:H1354–H1360. doi: 10.1152/ajpheart.00050.2002. [DOI] [PubMed] [Google Scholar]
- 42.Green D, Cheetham C, Mavaddat L, Watts K, Best M, Taylor R, O’Driscoll G. Effect of lower limb exercise on forearm vascular function: contribution of nitric oxide. Am J Physiol Heart Circ Physiol. 2002;283:H899–H907. doi: 10.1152/ajpheart.00049.2002. [DOI] [PubMed] [Google Scholar]
- 43.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O’Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol. 2005;562:617–628. doi: 10.1113/jphysiol.2004.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greve JM, Les AS, Tang BT, Draney Blomme MT, Wilson NM, Dalman RL, Pelc NJ, Taylor CA. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am J Physiol Heart Circ Physiol. 2006;291:H1700–H1708. doi: 10.1152/ajpheart.00274.2006. [DOI] [PubMed] [Google Scholar]
- 46.Guo D, Chien S, Shyy JYJ. Regulation of endothelial cell cycle by laminar versus oscillatory flow: distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circ Res. 2007;100:564–571. doi: 10.1161/01.RES.0000259561.23876.c5. [DOI] [PubMed] [Google Scholar]
- 47.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003:3118–3120. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 48.Hambrecht R, Niebauer J, Marburger C, Grunze M, Kalberer B, Hauer K, Schlierf G, Kubler W, Schuler G. Various intensities of leisure time physical activity in patients with coronary artery disease: effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol. 1993;22:468–477. doi: 10.1016/0735-1097(93)90051-2. [DOI] [PubMed] [Google Scholar]
- 49.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 50.Harrison DG, Sayegh H, Ohara Y, Inoue N, Venema RC. Regulation of expression of the endothelial cell nitric oxide synthase. Clin Exp Pharmacol Physiol. 1996;23:251–255. doi: 10.1111/j.1440-1681.1996.tb02606.x. [DOI] [PubMed] [Google Scholar]
- 51.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 52.Heistad DD, Armstrong ML, Marcus ML, Piegors DJ, Mark AL. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atherosclerotic monkeys. Circ Res. 1984;54:711–718. doi: 10.1161/01.res.54.6.711. [DOI] [PubMed] [Google Scholar]
- 53.Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H645–H653. doi: 10.1152/ajpheart.01087.2006. [DOI] [PubMed] [Google Scholar]
- 54.Hsiai TK, Cho SK, Wong PK, Ing M, Salazar A, Sevanian A, Navab M, Demer LL, Ho CM. Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB J. 2003;17:1648–1657. doi: 10.1096/fj.02-1064com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ingjer F. Capillary supply and mitochondrial content of different skeletal muscle fiber types in untrained and endurance trained men. A histochemical and ultrastructural study. Eur J Appl Physiol. 1979;40:197–209. doi: 10.1007/BF00426942. [DOI] [PubMed] [Google Scholar]
- 57.Jasperse JL, Laughlin MH. Endothelial function and exercise training: evidence from studies using animal models. Med Sci Sports Exerc. 2006;38:445–454. doi: 10.1249/01.mss.0000191187.24525.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson LR, Laughlin MH. Chronic exercise training does not alter pulmonary vasorelaxation in normal pigs. J Appl Physiol. 2000;88:2008–2014. doi: 10.1152/jappl.2000.88.6.2008. [DOI] [PubMed] [Google Scholar]
- 59.Kajiya F, Yada T, Matsumoto T, Goto M, Ogasawara Y. Intramyocardial influences on blood flow distributions in the myocardial wall. Ann Biomed Eng. 2000;28:897–902. doi: 10.1114/1.1308487. [DOI] [PubMed] [Google Scholar]
- 60.Kingwell BA, Sherrard B, Jennings GL, Dart AM. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol Heart Circ Physiol. 1997;272:H1070–H1077. doi: 10.1152/ajpheart.1997.272.3.H1070. [DOI] [PubMed] [Google Scholar]
- 61.Klausen K, Andersen LB, Pelle I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol Scand. 1981;113:9–16. doi: 10.1111/j.1748-1716.1981.tb06854.x. [DOI] [PubMed] [Google Scholar]
- 62.Koller A, Huang A, Sun D, Kaley G. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles. Role of endothelial nitric oxide and prostaglandins. Circ Res. 1995;76:544–550. doi: 10.1161/01.res.76.4.544. [DOI] [PubMed] [Google Scholar]
- 63.Komori K, Shimokawa H, Vanhoutte PM. Hypercholesterolemia impairs endothelium-dependent relaxations to aggregating platelets in porcine iliac arteries. J Vasc Surg. 1989;10:318–325. [PubMed] [Google Scholar]
- 64.Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am J Physiol Heart Circ Physiol. 1993;264:H150–H156. doi: 10.1152/ajpheart.1993.264.1.H150. [DOI] [PubMed] [Google Scholar]
- 65.Kuo L, Chilian WM, Davis MJ, Laughlin MH. Endotoxin impairs flow-induced vasodilation of porcine coronary arterioles. Am J Physiol Heart Circ Physiol. 1992;262:H1838–H1845. doi: 10.1152/ajpheart.1992.262.6.H1838. [DOI] [PubMed] [Google Scholar]
- 66.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol. 1990;259:H1063–H1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- 67.Lash JM, Bohlen HG. Time-and order-dependent changes in functional and NO-mediated dilation during exercise training. J Appl Physiol. 1997;82:460–468. doi: 10.1152/jappl.1997.82.2.460. [DOI] [PubMed] [Google Scholar]
- 68.Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc. 1995;27:1135–1144. [PubMed] [Google Scholar]
- 69.Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: 1996. Control of blood flow to cardiac and skeletal muscle during exercise; pp. 705–769. Am. Physiol. Soc., sect. 12, chapt. 16. [Google Scholar]
- 70.Laughlin MH, McAllister RM, Delp MD. Heterogeneity of blood flow in striated muscle. In: Crystal RG, West JB, editors. The Lung. Philadelphia, PA: Lippincott-Raven; 1997. pp. 1945–1955. [Google Scholar]
- 71.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 72.Laughlin MH, Turk JR, Schrage WG, Woodman CR, Price EM. Influence of coronary artery diameter on eNOS protein content. Am J Physiol Heart Circ Physiol. 2003;284:H1307–H1312. doi: 10.1152/ajpheart.00792.2002. [DOI] [PubMed] [Google Scholar]
- 73.Laughlin MH, Welshons WV, Sturek M, Rush JW, Turk JR, Taylor JA, Judy BM, Henderson KK, Ganjam VK. Gender, exercise training, and eNOS expression in porcine skeletal muscle arteries. J Appl Physiol. 2003;95:250–264. doi: 10.1152/japplphysiol.00061.2003. [DOI] [PubMed] [Google Scholar]
- 74.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 75.Linke A, Erbs S, Hambrecht R. Exercise and the coronary circulation—alterations and adaptations in coronary artery disease. Prog Cardiovasc Dis. 2006;48:270–284. doi: 10.1016/j.pcad.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Lopez JAG, Armstrong ML, Peigors DJ, Heistad DD. Effect of early and advanced atherosclerosis on vascular responses to serotonin, thromboxane A2, and ADP. Circulation. 1989;79:698–705. doi: 10.1161/01.cir.79.3.698. [DOI] [PubMed] [Google Scholar]
- 77.Luscher TF, Vanhoutte PM. The Endothelium: Modulator of Cardiovascular Function. Boca Raton, FL: CRC; 1990. [Google Scholar]
- 78.Lutjemeier BJ, Miura A, Scheuermann BW, Koga S, Townsend DK, Barstow TJ. Muscle contraction-blood flow interactions during upright knee extension exercise in humans. J Appl Physiol. 2005;98:1575–1583. doi: 10.1152/japplphysiol.00219.2004. [DOI] [PubMed] [Google Scholar]
- 79.Maiorana A, O’Driscoll G, Dembo L, Cheetham C, Goodman C, Taylor R, Green D. Effect of aerobic and resistance exercise training on vascular function in heart failure. Am J Physiol Heart Circ Physiol. 2000;279:H1999–H2005. doi: 10.1152/ajpheart.2000.279.4.H1999. [DOI] [PubMed] [Google Scholar]
- 80.Maiorana A, O’Driscoll G, Taylor R, Green D. Exercise and the nitric oxide vasodilator system. Sports Med. 2003;33:1013–1035. doi: 10.2165/00007256-200333140-00001. [DOI] [PubMed] [Google Scholar]
- 81.Malek A, Izumo S. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am J Physiol Cell Physiol. 1992;263:C389–C396. doi: 10.1152/ajpcell.1992.263.2.C389. [DOI] [PubMed] [Google Scholar]
- 82.Malek AM, Jiang L, Lee I, Sessa WC, Izumo S, Alper SL. Induction of nitric oxide synthase mRNA by shear stress requires intracellular calcium and G-protein signals and is modulated by PI 3 kinase. Biochem Biophys Res Commun. 1999;254:231–242. doi: 10.1006/bbrc.1998.9921. Erratum Biochem Biophys Res Commun 256: 255, 1999. [DOI] [PubMed] [Google Scholar]
- 83.McAllister RM, Kimani JK, Webster JL, Parker JL, Laughlin MH. Effects of exercise training on responses of peripheral and visceral arteries in swine. J Appl Physiol. 1996;80:216–225. doi: 10.1152/jappl.1996.80.1.216. [DOI] [PubMed] [Google Scholar]
- 84.McAllister RM, Laughlin MH. Short-term exercise training alters responses of porcine femoral and brachial arteries. J Appl Physiol. 1997;82:1438–1444. doi: 10.1152/jappl.1997.82.5.1438. [DOI] [PubMed] [Google Scholar]
- 85.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 86.Meredith IT, Yeung AC, Weidinger FF, Anderson TJ, Uehata A, Ryan TJJ, Selwyn AP, Ganz P. Role of impaired endothelium-dependent vasodilation in ischemic manifestations of coronary artery diseases. Circulation. 1993;87:56–66. [Google Scholar]
- 87.Miller VM, Burnett JC., Jr Modulation of NO and endothelin by chronic increases in blood flow in canine femoral arteries. Am J Physiol Heart Circ Physiol. 1992;263:H103–H108. doi: 10.1152/ajpheart.1992.263.1.H103. [DOI] [PubMed] [Google Scholar]
- 88.Morris JN, Clayton DG, Everitt MG, Semmence AM, Burgess EH. Exercise in leisure time: coronary attack and death rates. Br Heart J. 1990;63:325–334. doi: 10.1136/hrt.63.6.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morris JN, Heady JA, Raffle P, Roberts CG, Parks JW. Coronary heart disease and physical activity of work. Lancet. 1953;2:1053–1057. doi: 10.1016/s0140-6736(53)90665-5. [DOI] [PubMed] [Google Scholar]
- 90.Moyna NM, Thompson PD. The effect of physical activity on endothelial function in man. Acta Physiol Scand. 2004;180:113–123. doi: 10.1111/j.0001-6772.2003.01253.x. [DOI] [PubMed] [Google Scholar]
- 91.Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise- trained pigs. Circulation. 1994;89:2308–2314. doi: 10.1161/01.cir.89.5.2308. [DOI] [PubMed] [Google Scholar]
- 92.Nadaud S, Philippe M, Arnal JF, Michel JB, Soubrier F. Sustained increase in aortic endothelial nitric oxide synthase expression in vivo in a model of chronic high blood flow. Circ Res. 1996;79:857–863. doi: 10.1161/01.res.79.4.857. [DOI] [PubMed] [Google Scholar]
- 93.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 94.National Institutes of Health. Physical activity and cardiovascular health. NIH Consensus Statement. 1995;13:1–33. [PubMed] [Google Scholar]
- 95.Nava E, Noll G, Luscher TF. Nitric oxide in cardiovascular diseases. Ann Med. 1995;27:343–351. doi: 10.3109/07853899509002587. [DOI] [PubMed] [Google Scholar]
- 96.Nishida K, Harrison DG, Navas JP, Fisher AA, Dockery SP, Uematsu M, Nerem RM, Alexander RW, Murphy TJ. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992;90:2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noris M, Morigi M, Donadelli R, Aiello S, Foppolo M, Todeschini M, Orisio S, Remuzzi G, Remuzzi A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ Res. 1995;76:536–543. doi: 10.1161/01.res.76.4.536. [DOI] [PubMed] [Google Scholar]
- 98.Okahara K, Sun B, Kambayashi J. Upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:1922–1926. doi: 10.1161/01.atv.18.12.1922. [DOI] [PubMed] [Google Scholar]
- 99.Oltman CL, Parker JL, Adams HR, Laughlin MH. Effects of exercise training on vasomotor reactivity of porcine coronary arteries. Am J Physiol Heart Circ Physiol. 1992;263:H372–H382. doi: 10.1152/ajpheart.1992.263.2.H372. [DOI] [PubMed] [Google Scholar]
- 100.Oltman CL, Parker JL, Laughlin MH. Endothelium-dependent vasodilation of proximal coronary arteries from exercise-trained pigs. J Appl Physiol. 1995;79:33–40. doi: 10.1152/jappl.1995.79.1.33. [DOI] [PubMed] [Google Scholar]
- 101.Ornish D, Scherwitz LW, Billings JH, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, Hogeboom C, Brand RJ. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 102.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–401. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 103.Parker JL, Mattox ML, Laughlin MH. Contractile responsiveness of coronary arteries from exercise-trained rats. J Appl Physiol. 1997;83:434–443. doi: 10.1152/jappl.1997.83.2.434. [DOI] [PubMed] [Google Scholar]
- 104.Parker JL, Oltman CL, Muller JM, Myers PR, Adams HR, Laughlin MH. Effects of exercise training on regulation of tone in coronary arteries and arterioles. Med Sci Sports Exerc. 1994;26:1252–1261. [PubMed] [Google Scholar]
- 105.Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health. 1987;8:253–287. doi: 10.1146/annurev.pu.08.050187.001345. [DOI] [PubMed] [Google Scholar]
- 106.Quilley J, Fulton D, McGiff JC. Hyperpolarizing factors. Biochem Pharmacol. 1997;54:1059–1070. doi: 10.1016/s0006-2952(97)00039-7. [DOI] [PubMed] [Google Scholar]
- 107.Ranjan V, Xiao Z, Diamond SL. Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. Am J Physiol Heart Circ Physiol. 1995;269:H550–H555. doi: 10.1152/ajpheart.1995.269.2.H550. [DOI] [PubMed] [Google Scholar]
- 108.Rowell LB. Human Cardiovascular Control. Oxford, UK: Oxford University Press; 1993. [Google Scholar]
- 109.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- 110.Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol. 2003;284:H1378–H1387. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- 111.Rush JWE, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol. 2000;279:H1890–H1897. doi: 10.1152/ajpheart.2000.279.5.H2068. [DOI] [PubMed] [Google Scholar]
- 112.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 113.Shimokawa H, Vanhoutte PM. Hypercholesterolemia causes generalized impairment of endothelium-dependent relaxation to aggregating platelets in porcine arteries. J Am Coll Cardiol. 1989;13:1402–1408. doi: 10.1016/0735-1097(89)90318-5. [DOI] [PubMed] [Google Scholar]
- 114.Shimokawa H, Vanhoutte PM. Impaired endothelium-dependent relaxation to aggregating platelets and related vasoactive substances in porcine coronary arteries in hypercholesterolemia and atherosclerosis. Circ Res. 1989;64:900–914. doi: 10.1161/01.res.64.5.900. [DOI] [PubMed] [Google Scholar]
- 115.Shin HY, Gerritsen ME, Bizios R. Regulation of endothelial cell proliferation and apoptosis by cyclic pressure. Ann Biomed Eng. 2002;30:297–304. doi: 10.1114/1.1458595. [DOI] [PubMed] [Google Scholar]
- 116.Silacci P, Desgeorges A, Mazzolai L, Chambaz C, Hayoz D. Flow pulsatility is a critical determinant of oxidative stress in endothelial cells. Hypertension. 2001;38:1162–1166. doi: 10.1161/hy1101.095993. [DOI] [PubMed] [Google Scholar]
- 117.Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20:1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 118.Stary HC, Chandler AB, Glasgov S, Guyton JR, Insull W, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A report from the committee on vascular lesions of the council on arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 119.Sun D, Huang A, Koller A, Kaley G. Adaptation of flow-induced dilation of arterioles to daily exercise. Microvasc Res. 1998;56:54–61. doi: 10.1006/mvre.1998.2083. [DOI] [PubMed] [Google Scholar]
- 120.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 121.Tanaka H, Shimizu S, Ohmori F, Muraoka Y, Kumagai M, Yoshizawa M, Kagaya A. Increases in blood flow and shear stress to nonworking limbs during incremental exercise. Med Sci Sports Exerc. 2006;38:81–85. doi: 10.1249/01.mss.0000191166.81789.de. [DOI] [PubMed] [Google Scholar]
- 122.Tang BT, Cheng CP, Draney MT, Wilson NM, Tsao PS, Herfkens RJ, Taylor CA. Abdominal aortic hemodynamics in young healthy adults at rest and during lower limb exercise: quantification using image-based computer modeling. Am J Physiol Heart Circ Physiol. 2006;291:H668–H676. doi: 10.1152/ajpheart.01301.2005. [DOI] [PubMed] [Google Scholar]
- 123.Thijssen DHJ, de Groot PCE, Smits P, Hopman MTE. Vascular adaptations to 8-week cycling training in older men. Acta Physiol (Oxf) 2007;190:221–228. doi: 10.1111/j.1748-1716.2007.01685.x. [DOI] [PubMed] [Google Scholar]
- 124.Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JWE, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol. 2004;96:1114–1126. doi: 10.1152/japplphysiol.00768.2003. [DOI] [PubMed] [Google Scholar]
- 125.Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996;16:1256–1262. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- 126.Tropea BI, Huie P, Cooke JP, Tsao PS, Sibley RK, Zarins CK. Hypertension-enhanced monocyte adhesion in experimental atherosclerosis. J Vasc Surg. 1996;23:596–605. doi: 10.1016/s0741-5214(96)80038-3. [DOI] [PubMed] [Google Scholar]
- 127.Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281:H1380–H1389. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- 128.Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol Cell Physiol. 1995;269:C1371–C1378. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- 129.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 130.Vita JA, Keaney JF. Exercise—toning up the endothelium? N Engl J Med. 2000;342:503–505. doi: 10.1056/NEJM200002173420710. [DOI] [PubMed] [Google Scholar]
- 131.Vita JA, Keaney JF JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 132.Vita JA, Loscalzo J. Shouldering the risk factor burden: infection, atherosclerosis, and the vascular endothelium. Circulation. 2002;106:164–166. doi: 10.1161/01.cir.0000023452.26135.34. [DOI] [PubMed] [Google Scholar]
- 133.Vouyouka AG, Jiang Y, Rastogi R, Basson MD. Ambient pressure upregulates nitric oxide synthase in a phosphorylated-extracellular regulated kinase- and protein kinase C-dependent manner. J Vasc Surg. 2006;44:1076–1084. doi: 10.1016/j.jvs.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 134.Wang J, Wolin MS, Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res. 1993;73:829–838. doi: 10.1161/01.res.73.5.829. [DOI] [PubMed] [Google Scholar]
- 135.Weber M, Hagedorn CH, Harrison DG, Searles CD. Laminar shear stress and 3′ polyadenylation of eNOS mRNA. Circ Res. 2005;96:1161–1168. doi: 10.1161/01.RES.0000170651.72198.fa. [DOI] [PubMed] [Google Scholar]
- 136.White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Phil Trans R Soc B: Biol Sci. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wiegman DI, Harris PD, Joshua IG, Miller FN. Decreased vascular sensitivity to norepinephrine following exercise. J Appl Physiol. 1981;52:282–287. doi: 10.1152/jappl.1981.51.2.282. [DOI] [PubMed] [Google Scholar]
- 138.Woodman CR, Muller JM, Rush JW, Laughlin MH, Price EM. Flow regulation of ecNOS and Cu/Zn SOD mRNA expression in porcine coronary arterioles. Am J Physiol Heart Circ Physiol. 1999;276:H1058–H1063. doi: 10.1152/ajpheart.1999.276.3.H1058. [DOI] [PubMed] [Google Scholar]
- 139.Woodman CR, Turk JR, Rush JWE, Laughlin MH. Exercise attenuates the effects of hypercholesterolemia on endothelium-dependent relaxation in female porcine coronary arteries. J Appl Physiol. 2004;96:1105–1113. doi: 10.1152/japplphysiol.00767.2003. [DOI] [PubMed] [Google Scholar]
- 140.Woodman CR, Turk JR, Williams DP, Laughlin MH. Exercise training preserves endothelium-dependent relaxation in brachial arteries from hyperlipidemic pigs. J Appl Physiol. 2003;94:2017–2026. doi: 10.1152/japplphysiol.01025.2002. [DOI] [PubMed] [Google Scholar]
- 141.Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL. Cyclic straininduced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res. 1997;81:1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- 142.Wung BS, Cheng JJ, Shyue SK, Wang DL. NO modulates monocyte chemotactic protein-1 expression in endothelial cells under cyclic strain. Arterioscler Thromb Vasc Biol. 2001;21:1941–1947. doi: 10.1161/hq1201.099428. [DOI] [PubMed] [Google Scholar]
- 143.Yang AL, Chen HI. Chronic exercise reduces adhesion molecules/iNOS expression and partially reverses vascular responsiveness in hypercholesterolemic rabbit aortae. Atherosclerosis. 2003;169:11–17. doi: 10.1016/s0021-9150(03)00013-3. [DOI] [PubMed] [Google Scholar]
- 144.Yang AL, Jen CJ, Chen HI. Effects of high-cholesterol diet and parallel exercise training on the vascular function of rabbit aortas: a time course study. J Appl Physiol. 2003;95:1194–1200. doi: 10.1152/japplphysiol.00282.2003. [DOI] [PubMed] [Google Scholar]
- 145.Yun JK, Anderson JM, Ziats NP. Cyclic-strain-induced endothelial cell expression of adhesion molecules and their roles in monocyteendothelial interaction. J Biomed Mater Res. 1999;44:87–97. doi: 10.1002/(sici)1097-4636(199901)44:1<87::aid-jbm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Y, He X, Chen X, Ma H, Liu D, Luo J, Du Z, Jin Y, Xiong Y, He J, Fang D, Wang K, Lawson WE, Hui JCK, Zheng Z, Wu G. Enhanced external counterpulsation inhibits intimal hyperplasia by modifying shear stress responsive gene expression in hypercholesterolemic pigs. Circulation. 2007;116:526–534. doi: 10.1161/CIRCULATIONAHA.106.647248. [DOI] [PubMed] [Google Scholar]
- 147.Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:686–692. doi: 10.1161/01.atv.18.5.686. [DOI] [PubMed] [Google Scholar]
- 148.Ziegler T, Silacci P, Harrison VJ, Hayoz D. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension. 1998;32:351–355. doi: 10.1161/01.hyp.32.2.351. [DOI] [PubMed] [Google Scholar]