Abstract
Purpose of Review
This review will examine the unique susceptibility of premature infants to oxidative stress, the role of reactive oxygen species (ROS) in the pathogenesis of common disorders of the preterm infant, and potential for therapeutic interventions using enzymatic and/or non-enzymatic antioxidants.
Recent Findings
Oxidative stress is caused by an imbalance between the production of ROS and the ability to detoxify them with the help of antioxidants. The premature infant is especially susceptible to ROS-induced damage because of inadequate antioxidant stores at birth, as well as impaired upregulation in response to oxidant stress. Thus, the premature infant is at increased risk for the development of ROS-induced diseases of the newborn, such as bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis, and periventricular leukomalacia.
Summary
Potential therapies for ROS-induced disease include both enzymatic and non-enzymatic antioxidant preparations. More research is required to determine the beneficial effects of supplemental antioxidant therapy.
Keywords: Reactive oxygen species, antioxidants, oxidative stress, prematurity
INTRODUCTION
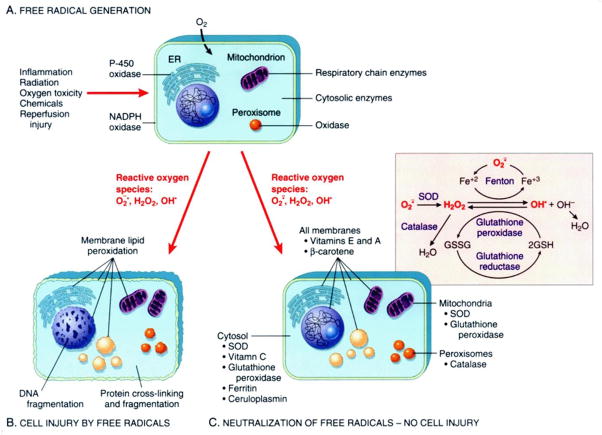
Under normal conditions, a delicate balance exists between the production of ROS and the antioxidant defenses that protect cells in vivo. The balance may be disturbed by increased ROS production or an inability to quench production because of inadequate antioxidant defenses. There is increasing evidence that links early exposure to oxidative stress with potentially lifelong consequences (1). Increased generation of ROS can occur as a result of many conditions affecting newborn infants, including hyperoxia, reperfusion, and/or inflammation (Figure). The premature infant is especially susceptible to ROS-induced damage for two major reasons. First, adequate concentrations of antioxidants may be absent at birth. Increases in antioxidant capacity occur in the latter part of gestation in preparation for the transition to extrauterine life. Second, the ability to increase synthesis of antioxidants in response to hyperoxia or other oxidant challenges is relatively impaired. This can lead to an increased risk for the development of ROS-induced diseases of the newborn, such as bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), and periventricular leukomalacia (PVL) (2). This article will review recent research involving ROS-mediated injury contributing to common neonatal disorders, as well as possible future therapeutic interventions using antioxidants.
Figure. Disruptions in Oxidant/Antioxidant Balance Can Cause Significant Cell Injury.
Production of reactive oxygen species can lead to significant cellular damage in the absence of antioxidants.
A–Free radical production occurs after cellular insult resulting from inflammation, radiation, oxygen toxicity, chemicals, or reperfusion injury.
B–Reactive oxygen species cause membrane lipid peroxidation that leads to cell injury through DNA and protein fragmentation.
C–Free radicals in the presence of antioxidants are neutralized and protect the cell from injury.
Normal Fetal and Neonatal Antioxidant Enzyme Maturation
Frank and Groseclose documented the development of antioxidant enzymes in the lungs of rabbits during late gestation. Enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) are important in scavenging ROS and have been shown to increase 150% during the last 15% of gestation (3). There are three forms of SOD that have been identified: copper-zinc superoxide dismutase (Cu/ZnSOD), present primarily in the cytoplasm, manganese superoxide dismutase (MnSOD) in the mitochondria, and extracellular superoxide dismutase (EC-SOD) located in the extracellular spaces in adults, but primarily intracellular in newborns.(4) The only known function of SOD is to convert extremely reactive superoxide radicals to hydrogen peroxide and water. Catalase, GPx, and glutathione reductase then convert hydrogen peroxide to water (Table).
TABLE.
Reactive Oxygen Species
| Radical | Symbola | Antioxidant |
|---|---|---|
| Superoxide anion | O2− | Superoxide dismutase, uric acid, vitamin E |
| Singlet oxygen | 1O2 | β-carotene, uric acid, vitamin E |
| Hydrogen peroxide | H2O2 | Catalase, glutathione peroxidase, glutathione |
| Hydroxyl radical | OH• | Vitamins C and E |
| Peroxide radical | LOO• | Vitamins C and E |
| Hydroperoxyl radical | LOOH | Glutathione transferase, glutathione peroxidase |
L, lipid
Antioxidant enzyme expression generally increases in most fetal compartments throughout the progression of pregnancy. Qanungo and colleagues found that SOD, catalase, GPx, and glutathione reductase activities increased with gestational age, as evidence of lipid peroxidation decreased in human placental and fetal tissues (5). Development of the antioxidant system during fetal life must also include redox signaling in the maintenance of pregnancy through uterine-placental-fetal interactions (6). There is evidence of regulation of antioxidant enzymes in the context of local nitric oxide (NO) generation via nitric oxide synthases and downstream NO-dependent signaling in the placenta, critically important to normal vascular development.
Preterm Birth and Oxidative Stress
Delivery constitutes a significant oxidative stress and the gestation of the newborn and circumstances of delivery will affect the overall burden (7, 8). Premature delivery often occurs before the normal upregulation of antioxidant systems and other ROS scavengers, such as glutathione and ceruloplasmin. This is in addition to relatively deficient uteroplacental transfer of nutrients important to antioxidant defenses and places the newborn at particular risk of ROS-induced injury (9). MnSOD mRNA appears to be induced in the fetal membranes following spontaneous labor and in the presence of chorioamnionitis (10). The effects of inflammatory stimuli may or may not induce placental antioxidant expression, depending on the stage of pregnancy. Antenatal corticosteroids used to accelerate lung maturation in threatened preterm birth might also lead to increased activity of SOD, catalase, and glutathione-S-transferase (11). This can help to counteract relative deficiencies in antioxidants stemming from preterm birth.
Oxidative Stress and Adverse Pregnancy Outcomes
Oxidative stress has been suggested as a causative agent in pregnancy-related disorders, such as recurrent pregnancy loss, pre-eclampsia, preterm premature rupture of membranes (pPROM), intrauterine growth restriction (IUGR), and fetal death (12). In utero stressors contribute to ROS production and resulting tissue damage. Markers of oxidative stress, such as non-protein bound iron, have been measured in cord blood as an indicator of intrauterine ROS production. These markers have been associated with the development of several postnatal disease processes, suggesting that in utero oxidative stress is a significant risk factor, especially in premature neonates (13). Identifying high risk neonates at birth may allow for early treatment with antioxidants and possible prevention of further oxidant damage. It is also reasonable to speculate that maternal deficiencies in antioxidant related micronutrients could contribute to reproductive disorders. Antioxidant deficiencies could induce an imbalance between ROS and diet-dependent antioxidants and supplementation may decrease this effect (14). However, randomized controlled trials using prenatal supplementation with vitamins C and E have failed to show a reduction in the risk of gestational hypertension or pre-eclampsia, and may in fact increase the risk of premature rupture of membranes (15–17). These findings might be due to the timing of supplementation after placentation has already occurred, inadequate dosing, or the fact that other antioxidants may have better function. Further research is needed to identify methods of decreasing oxidative stress in utero and reducing ROS induced diseases in the mother and her newborn infant.
Therapeutic Interventions with Antioxidants
Supplementation with enzymatic and/or non-enzymatic antioxidants might have beneficial effects in decreasing injury from excess production of ROS, particularly in disorders such as bronchopulmonary dysplasia, retinopathy of prematurity, periventricular leukomalacia, and necrotizing enterocolitis.
Bronchopulmonary Dysplasia
Although the pathogenesis of BPD is complex, studies do support a role for ROS-mediated damage. Vitamins A, C, and E are important factors in normal physiology as well as antioxidant defense (18). These vitamins are known to inhibit ROS-induced lipid peroxidation and scavenge ROS. In infants with BPD, plasma β-carotene and vitamin A concentrations are lower, likely reducing antioxidant protection. This may account for higher plasma 3-nitrotyrosine and protein carbonyls in those preterm infants at highest risk for developing BPD (19). Given that preterm infants are relatively deficient in antioxidant defenses, exogenous antioxidants such as vitamins A, E and recombinant human SOD (rhSOD) have been administered in attempts to prevent BPD (20). Although a Cochrane meta-analysis suggests that supplementation with vitamin A reduces BPD, neurodevelopmental and pulmonary outcomes at 18–22 months corrected gestational age (CGA) were not significantly different (21). Randomized controlled trials of vitamin E supplementation have also failed to show a reduction in the incidence of BPD (22). Trace elements, such as copper, zinc, iron, and selenium are also essential for normal antioxidant enzyme function and supplementation with these nutrients could optimize total antioxidant capacity (23). However, studies examining trace elements as active cofactors in extremely low birth weight infants showed that lower trace element concentrations did not substantially influence antioxidant enzyme concentration or the development of BPD. In addition, typical diseases of prematurity, including BPD were not associated with decreased antioxidant enzyme activities. (24)
Retinopathy of Prematurity
The developing retina in premature infants is particularly susceptible to damage mediated by ROS, as evidenced in many animal studies. Oxygen fluctuations can induce cells to express NADPH oxidase, which leads to increased ROS and apoptosis of endothelial cells, contributing to the avascular retina. N-acetylcysteine (NAC) has been shown to decrease lipid hydroperoxide (LHP) in a rat model, but was not found to significantly reduce avascularity or clock hours of neovascularization (25). Repeated oxygen fluctuations also increased retinal vascular endothelial growth factor (VEGF) and ROS. Neutralizing VEGF bioactivity reduced neovascularization and tortuosity, and inhibiting ROS with the NADPH oxidase inhibitor apocynin reduced the avascular retina by interfering with apoptosis (26). Resveratrol is a phytoalexin produced by a variety of plants in response to stress. Kim and associates investigated resveratrol as a nitric oxide-mechanism modulator as well as caffeic acid for retinal neovascularization anti-angiogenic activity and found some protective effects against the development of ROP (27). Further research demonstrates that early blocking of peroxynitrite-mediated tyrosine nitration and peroxynitrite formation by the use of epicatechin (a green tea extract) as well as NAC could also be considered a new therapeutic target for ischemic proliferative diseases of the retina (28).
Periventricular Leukomalacia (PVL)
Preterm infants are vulnerable to reperfusion-type injury and accompanying oxidative stress due to decreased regulation of cerebral perfusion. PVL is thought to develop after microglial activation leads to an accumulation of markers of oxidation in oligodendrocytes, such as nitrotyrosine and protein carbonyls (29). ROS have also been implicated in causing neuronal cell death. In vitro exposure to hyperoxia induces apoptosis in oligodendroglial cells in a developmentally dependent pattern. This is prevented by inhibition of lipoxygenase, with decreased expression of myelin basic protein in vivo in hyperoxia-exposed rat pups (30). Maternal lipopolysaccharide (LPS) exposure has been shown to stimulate the secretion of pro-inflammatory markers in maternal serum and amniotic fluid of pregnant mice, mimicking maternal infection. LPS-induced peroxisomal dysfunction depletes oligodendrocytes and exacerbates cerebral white matter injury in premature infants. NAC pretreatment attenuates this LPS-induced cerebral white matter injury by replenishment of reduced-GSH, ROS scavenging, and maintenance of peroxisomal proliferation/function via a peroxisome proliferators activated receptor-α (PPAR-α) dependent mechanism (31). LPS activates microglia cells which induces cell death and greatly impairs oligodendrocyte development, which may underlie selective white matter damage and hypomyelination in PVL (32). Melatonin has been studied as a neuroprotective agent in PVL in mouse models. In a recent report, agomelatine and melatonin did not prevent the initial appearance of white matter lesions, but they did promote secondary lesion repair. The effects of melatonin were only observed when given within the first two hours following the insult. However, agomelatine was still neuroprotective when administered eight hours after the insult. Although further research is needed, this may represent a promising new therapy for prevention of PVL (33).
Necrotizing Enterocolitis
While the etiology of NEC is multifactorial, inflammation and ROS production appear to play a key role. An increased incidence of NEC has recently been noted in infants who are born to mothers with chorioamnionitis (34). These findings suggest that prenatal infection/inflammation may predispose the intestine of the preterm infant to the development of NEC. In a neonatal rat model of NEC, LPS administration led to increased susceptibility to intestinal injury. This increase in intestinal injury appears to be mediated in part by inducible nitric oxide synthase (iNOS) and can be attenuated with the selective iNOS inhibitor aminoguanidine. During the early stages of NEC, NOS uncoupling becomes progressively worse, favoring production of ROS, vasoconstriction, intestinal ischemia, and NEC (35). It is possible that targeting iNOS or iNOS-derived NO may be of therapeutic benefit in preventing NEC. Enteral glutamine alone or in conjunction with arginine has been shown reduce oxidative stress in juvenile rat models. This occurs not only in hypoxia – reoxygenation, but also in healthy newborn rats. Therefore, enteral glutamine and arginine may be useful for preventing NEC in premature neonates, although further experimental and clinical studies are needed (36).
Potential Antioxidant Therapies in Premature Neonates
Antioxidants are critical in protecting against ROS-induced injury and several preclinical studies support antioxidant supplementation. Non-enzymatic proteins (transferrin, ferritin, ceruloplasmin), enzymes (superoxide dismutases, catalase, glutathione peroxidase), oxidizable molecules (glutathione, vitamins E, A, C, carotenoids, flavonoids), and trace elements (copper, zinc, selenium) all play a role in maintaining a delicate balance between ROS production and oxidant damage to tissues and organs (18).
Enzymatic
Enzymatic antioxidants are gestationally regulated, with premature newborns having decreased expression relative to full term neonates. Multiple models using transformed human alveolar epithelial cells have suggested that overexpression of antioxidants prevents ROS-induced injury. Increased expression of either MnSOD or CuZnSOD reverses the growth inhibitory effects of hyerpoxia in lung epithelial cells. (37). Overexpression of SOD not only reduced ROS production, but also mitigated the activation of the JNK/AP1 pathway which has been implicated in ROS-induced mitochondrial injury and apoptotic cell death (38). Melatonin is a pineal hormone that exhibits an indirect antioxidant effect by supporting SOD and glutathione peroxidase activity as well as direct effects through lipid peroxidation and scavenging oxygen-induced ROS (39). In a neonatal rat model, melatonin reduced ROS production and increased antioxidant levels in hyperoxia-induced lung damage, indicating a potential protective effect in BPD (40). Increasing SOD and catalase (CAT) activities have consistently been associated with protection against oxygen toxicity. Naturally derived commercial surfactants contain both SOD and CAT activity in significant concentrations. By adding additional SOD and CAT to surfactant preparations, the antioxidant effects are also potentiated (41). Pharmacologic antioxidant supplementation was indirectly tested in preterm infants who received supplemental cysteine in an attempt to stimulate glutathione synthesis. Despite significant increases in cysteine, glutathione concentrations did not increase and ROS-induced injury was not prevented (42). Davis and colleagues administered intratracheal rhSOD to premature infants. Although there were no differences in the incidence of death or BPD, there was a significant decrease in the number of patients who required asthma medications, had wheezing episodes, Emergency Room visits, or re-hospitalizations at 1 year CGA compared to controls (43). The failure to detect a significant difference in BPD was likely influenced by the absence of accepted guidelines for the clinical use of oxygen, rather than a failure of rhSOD to work, as evidenced by the improved outcomes at 1 year corrected age.
Non-enzymatic antioxidants
Resistance to oxidative stress also relies on non-enzymatic pathways. Non-enzymatic antioxidants are depleted in response to ROS-mediated stress. The effects of vitamin A are likely mediated through its action on retinol-binding protein and the retinoic acid receptor. NAC is a precursor of the antioxidant glutathione and a large multicenter trial showed no reduction in survival or incidence of BPD at 36 weeks CGA or improved pulmonary function at term (44). Ceruloplasm, transferrin, and ferroxidase all aid in the metabolism of iron, which can act as a potent oxidizing agent. Diminished function or bioavailability of these proteins may predispose the preterm infant to increased production of ROS (45).
New Antioxidants Under Investigation
There are multiple potential therapeutic antioxidants currently under investigation that could benefit premature infants. One protein under investigation, Pon3, was shown in laboratory studies to have antioxidant properties and to be up-regulated in rat, sheep, and human cord blood late in gestation. More research is needed, but Pon3 could serve as a potential therapeutic target in premature infants.(46) Clinical trials involving antioxidants currently registered with the NIH at www.clinicaltrials.gov include supplementation of preterm infants with lactoferrin and cysteine, examination of concentrations of beta-carotene, lutein, and lycopene in preterm infants fed formulas with mixed carotenoids and the effects on the developing eye, early administration of human erythropoietin in very preterm infants, NAC administration to women with intra-amniotic infection and/or inflammation, early enteral administration of vitamin E to extremely premature infants, and multiple trials involving inhaled nitric oxide. The results from these trials may change the way we treat many common neonatal conditions.
CONCLUSIONS
A delicate oxidant/antioxidant balance exists in the fetus and newborn. This balance can tip towards oxidant injury in the setting of preterm birth. Antioxidant enzymes are primarily upregulated in the latter part of gestation and preterm birth is associated with an increased generation of ROS. The use of supplemental antioxidants represents a logical strategy to prevent or ameliorate injury from excess production of ROS, but studies in animal models and in preterm infants have yielded mixed results. Caution must be taken since ROS are critical second messengers in various cell signaling pathways that control normal cellular functions, but strategies that maintain normal antioxidant balance may be beneficial to the preterm newborn.
KEY POINTS.
Premature infants are at increased risk of damage due to ROS due to inadequate antioxidant stores at birth as well as decreased production of antioxidants in response to oxidant stress
ROS-induced injury is important in the development of many common disorders of prematurity, such as BPD, ROP, NEC, and PVL
Supplementation with enzymatic and/or non-enzymatic antioxidants might have
beneficial effects in decreasing injury from excess production of ROS
Acknowledgments
We have no conflicts of interest to report and have no further acknowledgements.
Contributor Information
Jennifer W. Lee, Neonatal-Perinatal Fellow, The Floating Hospital for Children at Tufts Medical Center.
Jonathan M. Davis, Chief of Newborn Medicine, Program Director, Clinical and Translational Research Center, The Floating Hospital for Children at Tufts Medical Center, Professor of Pediatrics, Tufts University School of Medicine.
References
- 1.Sola A, Rogido MR, Deulofeut R. Oxygen as a neonatal health hazard: call for detente in clinical practice. Acta Paediatr. 2007 Jun;96(6):801–12. doi: 10.1111/j.1651-2227.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- **2.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med. 2010 Aug;15(4):191–5. doi: 10.1016/j.siny.2010.04.001. This article reviews the development of the antioxidant system in premature infants. It also discusses antioxidant enzymes as well as non-enzymatic antioxidants and indirect antioxidant supplementation. [DOI] [PubMed] [Google Scholar]
- 3.Frank L, Groseclose EE. Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. Pediatr Res. 1984 Mar;18(3):240–4. doi: 10.1203/00006450-198403000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed MN, Suliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, et al. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med. 2003 Feb 1;167(3):400–5. doi: 10.1164/rccm.200202-108OC. [DOI] [PubMed] [Google Scholar]
- 5.Qanungo S, Mukherjea M. Ontogenic profile of some antioxidants and lipid peroxidation in human placental and fetal tissues. Mol Cell Biochem. 2000 Dec;215(1–2):11–9. doi: 10.1023/a:1026511420505. [DOI] [PubMed] [Google Scholar]
- 6.Land SC. Oxygen-sensing pathways and the development of mammalian gas exchange. Redox Rep. 2003;8(6):325–40. doi: 10.1179/135100003225003348. [DOI] [PubMed] [Google Scholar]
- 7.Lurie S, Matas Z, Boaz M, Fux A, Golan A, Sadan O. Different degrees of fetal oxidative stress in elective and emergent cesarean section. Neonatology. 2007;92(2):111–5. doi: 10.1159/000100965. [DOI] [PubMed] [Google Scholar]
- 8.Georgeson GD, Szony BJ, Streitman K, Varga IS, Kovacs A, Kovacs L, et al. Antioxidant enzyme activities are decreased in preterm infants and in neonates born via caesarean section. Eur J Obstet Gynecol Reprod Biol. 2002 Jul 10;103(2):136–9. doi: 10.1016/s0301-2115(02)00050-7. [DOI] [PubMed] [Google Scholar]
- 9.Shah MD, Shah SR. Nutrient deficiencies in the premature infant. Pediatr Clin North Am. 2009 Oct;56(5):1069–83. doi: 10.1016/j.pcl.2009.08.001. [DOI] [PubMed] [Google Scholar]
- *10.Than NG, Romero R, Tarca AL, Draghici S, Erez O, Chaiworapongsa T, et al. Mitochondrial manganese superoxide dismutase mRNA expression in human chorioamniotic membranes and its association with labor, inflammation, and infection. J Matern Fetal Neonatal Med. 2009 Nov;22(11):1000–13. doi: 10.3109/14767050903019676. This cross-sectional study examined the differential expression of SOD2 in fetal membranes at term but not in labor, term in labor, preterm labor (PTL), PTL with chorioamnionitis, PPROM, and PPROM with chorioamnionitis. MnSOD mRNA expression in fetal membranes was higher in those in labor, PTL, and chorioamnionitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vento M, Aguar M, Escobar J, Arduini A, Escrig R, Brugada M, et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid Redox Signal. 2009 Dec;11(12):2945–55. doi: 10.1089/ars.2009.2671. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Agarwal A, Banerjee J, Alvarez JG. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. Obstet Gynecol Surv. 2007 May;62(5):335–47. doi: 10.1097/01.ogx.0000261644.89300.df. quiz 53–4. [DOI] [PubMed] [Google Scholar]
- 13.Perrone S, Tataranno ML, Negro S, Longini M, Marzocchi B, Proietti F, et al. Early identification of the risk for free radical-related diseases in preterm newborns. Early Hum Dev. 2010 Apr;86(4):241–4. doi: 10.1016/j.earlhumdev.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010 Jun 19; doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, et al. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG. 2009 May;116(6):780–8. doi: 10.1111/j.1471-0528.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- *16.Xu H, Perez-Cuevas R, Xiong X, Reyes H, Roy C, Julien P, et al. An international trial of antioxidants in the prevention of preeclampsia (INTAPP) Am J Obstet Gynecol. 2010 Mar;202(3):239, e1–e10. doi: 10.1016/j.ajog.2010.01.050. This is a multicenter RCT of vitamin supplementation to pregnant women to prevent pre-eclampsia. Supplementation with vitamins C and E did not reduce the risk of gestational hypertension and pre-eclampsia. Women in the group receiving vitamin supplementation had higher rates of PROM with an increased risk of the composite outcome “fetal loss or perinatal death” compared to the placebo group. [DOI] [PubMed] [Google Scholar]
- *17.McCance DR, Holmes VA, Maresh MJ, Patterson CC, Walker JD, Pearson DW, et al. Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): a randomised placebo-controlled trial. Lancet. 2010 Jul 24;376(9737):259–66. doi: 10.1016/S0140-6736(10)60630-7. This is a multicenter RCT of vitamin supplementation in women with type 1 diabetes preceding pregnancy. Diabetes mellitus is associated with increased oxidative stress and antioxidant depletion. However, supplementation with vitamins C and E did not reduce the risk of pre-eclampsia in women with type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debier C, Larondelle Y. Vitamins A and E: metabolism, roles and transfer to offspring. Br J Nutr. 2005 Feb;93(2):153–74. doi: 10.1079/bjn20041308. [DOI] [PubMed] [Google Scholar]
- 19.Ballard PL, Truog WE, Merrill JD, Gow A, Posencheg M, Golombek SG, et al. Plasma biomarkers of oxidative stress: relationship to lung disease and inhaled nitric oxide therapy in premature infants. Pediatrics. 2008 Mar;121(3):555–61. doi: 10.1542/peds.2007-2479. [DOI] [PubMed] [Google Scholar]
- 20.Tin W, Wiswell TE. Drug therapies in bronchopulmonary dysplasia: debunking the myths. Semin Fetal Neonatal Med. 2009 Dec;14(6):383–90. doi: 10.1016/j.siny.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999 Jun 24;340(25):1962–8. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 22.Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2003;(3):CD003665. doi: 10.1002/14651858.CD003665. [DOI] [PubMed] [Google Scholar]
- 23.Biniwale MA, Ehrenkranz RA. The role of nutrition in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006 Aug;30(4):200–8. doi: 10.1053/j.semperi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Loui A, Raab A, Maier RF, Bratter P, Obladen M. Trace elements and antioxidant enzymes in extremely low birthweight infants. J Trace Elem Med Biol. 2010 Apr;24(2):111–8. doi: 10.1016/j.jtemb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Mol Vis. 2007;13:840–53. [PMC free article] [PubMed] [Google Scholar]
- *26.Hartnett ME. The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model. Doc Ophthalmol. 2010 Feb;120(1):25–39. doi: 10.1007/s10633-009-9181-x. This study used a rat model of repeated fluctuations in oxygen which caused increased retinal hypoxia, and increased ROS and VEGF production. ROS trigger signaling of different pathways to cause an avascular retina and intravitreous neovascularization. Increased signaling of VEGF is important to the development of both avascular retina and intravitreous neovascularization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim WT, Suh ES. Retinal protective effects of resveratrol via modulation of nitric oxide synthase on oxygen-induced retinopathy. Korean J Ophthalmol. 2010 Apr;24(2):108–18. doi: 10.3341/kjo.2010.24.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al-Shabrawey M, El-Remessy AB. Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J Pharmacol Exp Ther. 2010 Jan;332(1):125–34. doi: 10.1124/jpet.109.157941. [DOI] [PubMed] [Google Scholar]
- 29.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008 Mar;93(2):F153–61. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerstner B, DeSilva TM, Genz K, Armstrong A, Brehmer F, Neve RL, et al. Hyperoxia causes maturation-dependent cell death in the developing white matter. J Neurosci. 2008 Jan 30;28(5):1236–45. doi: 10.1523/JNEUROSCI.3213-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: attenuation by N-acetyl cysteine. Exp Neurol. 2008 Apr;210(2):560–76. doi: 10.1016/j.expneurol.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Pang Y, Campbell L, Zheng B, Fan L, Cai Z, Rhodes P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. 2010 Mar 17;166(2):464–75. doi: 10.1016/j.neuroscience.2009.12.040. This study shows that LPS-activated microglia induce cell death and impair oligodendrocyte development in 2 phases. The early phase occurs within 24 h after LPS treatment and is mediated by NO-dependent oxidative damage and prevented by an inhibitor of nitric oxide synthase. The delayed cell death is evident 48 h after LPS treatment and is mediated by cytokines. [DOI] [PubMed] [Google Scholar]
- 33.Gressens P, Schwendimann L, Husson I, Sarkozy G, Mocaer E, Vamecq J, et al. Agomelatine, a melatonin receptor agonist with 5-HT(2C) receptor antagonist properties, protects the developing murine white matter against excitotoxicity. Eur J Pharmacol. 2008 Jun 24;588(1):58–63. doi: 10.1016/j.ejphar.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Goldenberg RL, Andrews WW, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth Project: placental histology in recurrent spontaneous and indicated preterm birth. Am J Obstet Gynecol. 2006 Sep;195(3):792–6. doi: 10.1016/j.ajog.2006.05.050. [DOI] [PubMed] [Google Scholar]
- *35.Whitehouse JS, Xu H, Shi Y, Noll L, Kaul S, Jones DW, et al. Mesenteric nitric oxide and superoxide production in experimental necrotizing enterocolitis. J Surg Res. 2010 Jun 1;161(1):1–8. doi: 10.1016/j.jss.2009.07.028. This study in rat pups found that reciprocal changes in NO and superoxide production by the mesenteric vasculature are NOS-dependent and suggest that NOS uncoupling becomes worse during the development of NEC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kul M, Vurucu S, Demirkaya E, Tunc T, Aydinoz S, Meral C, et al. Enteral glutamine and/or arginine supplementation have favorable effects on oxidative stress parameters in neonatal rat intestine. J Pediatr Gastroenterol Nutr. 2009 Jul;49(1):85–9. doi: 10.1097/MPG.0b013e318198cd36. [DOI] [PubMed] [Google Scholar]
- 37.Koo HC, Davis JM, Li Y, Hatzis D, Opsimos H, Pollack S, et al. Effects of transgene expression of superoxide dismutase and glutathione peroxidase on pulmonary epithelial cell growth in hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2005 Apr;288(4):L718–26. doi: 10.1152/ajplung.00456.2003. [DOI] [PubMed] [Google Scholar]
- 38.Joseph A, Li Y, Koo HC, Davis JM, Pollack S, Kazzaz JA. Superoxide dismutase attenuates hyperoxia-induced interleukin-8 induction via AP-1. Free Radic Biol Med. 2008 Oct 15;45(8):1143–9. doi: 10.1016/j.freeradbiomed.2008.07.006. [DOI] [PubMed] [Google Scholar]
- *39.Bonnefont-Rousselot D, Collin F. Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicology. 2010 Apr 24; doi: 10.1016/j.tox.2010.04.008. This review describes the antioxidant effects of melatonin and discusses positive results of previous studies of melatonin in newborns with asphyxia, sepsis, and those undergoing surgery. [DOI] [PubMed] [Google Scholar]
- 40.Pan L, Fu JH, Xue XD, Xu W, Zhou P, Wei B. Melatonin protects against oxidative damage in a neonatal rat model of bronchopulmonary dysplasia. World J Pediatr. 2009 Aug;5(3):216–21. doi: 10.1007/s12519-009-0041-2. [DOI] [PubMed] [Google Scholar]
- *41.Dani C, Buonocore G, Longini M, Felici C, Rodriguez A, Corsini I, et al. Superoxide dismutase and catalase activity in naturally derived commercial surfactants. Pediatr Pulmonol. 2009 Nov;44(11):1125–31. doi: 10.1002/ppul.21116. This study measured SOD and CAT activities, the scavenger activity against hydrogen peroxide, and its changes after the addition of SOD and CAT in four natural surfactants. [DOI] [PubMed] [Google Scholar]
- 42.te Braake FW, Schierbeek H, Vermes A, Huijmans JG, van Goudoever JB. High-dose cysteine administration does not increase synthesis of the antioxidant glutathione preterm infants. Pediatrics. 2009 Nov;124(5):e978–84. doi: 10.1542/peds.2008-2477. [DOI] [PubMed] [Google Scholar]
- 43.Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics. 2003 Mar;111(3):469–76. doi: 10.1542/peds.111.3.469. [DOI] [PubMed] [Google Scholar]
- 44.Sandberg K, Fellman V, Stigson L, Thiringer K, Hjalmarson O. N-acetylcysteine administration during the first week of life does not improve lung function in extremely low birth weight infants. Biol Neonate. 2004;86(4):275–9. doi: 10.1159/000080089. [DOI] [PubMed] [Google Scholar]
- 45.Collard KJ. Iron homeostasis in the neonate. Pediatrics. 2009 Apr;123(4):1208–16. doi: 10.1542/peds.2008-1047. [DOI] [PubMed] [Google Scholar]
- 46.Belteki G, Kempster SL, Forhead AJ, Giussani DA, Fowden AL, Curley A, et al. Paraoxonase-3, a putative circulating antioxidant, is systemically up-regulated in late gestation in the fetal rat, sheep, and human. J Clin Endocrinol Metab. Aug;95(8):3798–805. doi: 10.1210/jc.2010-0037. [DOI] [PubMed] [Google Scholar]