Abstract
We tested the hypothesis that short-term increases in intraluminal pressure improve endothelium-dependent dilation and increase endothelial nitric oxide (NO) synthase (eNOS) expression in senescent soleus muscle feed arteries (SFA). SFA isolated from young (4 mo) and old (24 mo) Fischer 344 rats were cannulated and pressurized at 90 (p90) or 130 (p130) cmH2O for 4 h. At the end of the 4-h protocol, pressure in p130 SFA was lowered to 90 cmH2O for examination of endothelium-dependent (flow- or ACh-induced) vasodilation. Flow- and ACh-induced dilations were blunted in old p90 SFA relative to young p90 SFA. Pretreatment with increased pressure (p130) improved flow- and ACh-induced dilations in old SFA, such that vasodilator responses were similar to those in young SFA. In the presence of Nω-nitro-l-arginine (l-NNA) or l-NNA + indomethacin (Indo), flow-induced dilation was inhibited in old p130 SFA, such that the response was not greater than the response in old p90 SFA. In old p130 SFA, ACh-induced dilation was inhibited by l-NNA + Indo (not l-NNA alone). In a separate experiment, SFA were pressurized at 70, 90, 110, or 130 cmH2O for 4 h, and eNOS mRNA and protein content were assessed. Increased pressure induced eNOS mRNA expression in young (not old) SFA. eNOS protein content was not altered in young or old SFA. These results indicate that short-term increases in intraluminal pressure improve endothelium-dependent dilation in senescent SFA, in part by enhancing NO bioavailability; however, the beneficial effect was not associated with increased eNOS expression.
Keywords: endothelial dysfunction, endothelial nitric oxide synthase, exercise
Aging induces endothelial dysfunction in conduit arteries and arterioles (4–6, 9–11, 15, 20, 22, 23). The age-related decline in endothelial function is characterized by impaired endothelium-dependent vasodilator responses and is often associated with an impaired ability of vascular endothelial cells to produce and release nitric oxide (NO) (3, 5, 7, 15, 19, 23).
Previous studies indicate that endurance exercise training attenuates or reverses the detrimental effects of aging on endothelial function in some vascular beds (9, 16–19). The exercise-induced improvements in endothelial function in senescent arteries are associated with increased expression of endothelial NO synthase (eNOS) (18) and improved NO-mediated, endothelium-dependent dilation (18, 19).
The mechanism/signal(s) whereby exercise training induces eNOS expression and improves vasodilator responses in senescent arteries is not fully understood; however, it has been proposed that short-term increases in shear stress and/or perfusion pressure associated with individual bouts of exercise may act directly on vascular endothelial cells to induce eNOS expression and enhance the potential for NO-mediated dilation (9). This speculation is supported by evidence indicating that increased intraluminal shear stress in vitro induces eNOS expression and improves endothelium-dependent dilation in senescent skeletal muscle arteries by increasing NO bioavailability (24); however, the efficacy of short-term increases in intraluminal pressure to induce eNOS expression and enhance the potential for endothelium-dependent dilation in senescent arteries is not known. Therefore, the purpose of the present study was to test the hypothesis that short-term increases in intraluminal pressure, within a range of pressure believed to be present during muscle contractions, improve endothelium-dependent dilation and induce eNOS expression in senescent skeletal muscle arteries. Soleus muscle feed arteries (SFA) were studied, because they play an integral role in the control of soleus muscle blood flow at rest and during exercise (21) and because an age-related decline in NO-mediated, endothelium-dependent dilation is well documented in these arteries (22–24).
METHODS
Animals
Before initiation of the present study, approval was received from the Institutional Animal Care and Use Committee at the University of Missouri. Male Fischer 344 rats (4 and 24 mo of age, n = 26/group) were purchased from a commercial dealer (Harlan Sprague-Dawley, Indianapolis, IN) and housed in the College of Veterinary Medicine’s Animal Care Facility. The facility was maintained at 24°C with a 12:12-h light-dark cycle. Food and water were provided ad libitum, and the rats were examined daily by the investigators and by veterinarians affiliated with the College of Veterinary Medicine. Fischer 344 rats were selected for use in this study, in part, because previous studies indicate that this model of aging is not complicated by age-related changes in blood pressure (8).
Isolation of Feed Arteries
Procedures used to isolate SFA have been described previously in detail (22–24). Briefly, rats were anesthetized with an injection of pentobarbital sodium (50 mg/kg body wt ip). Soleus muscles from the left and right hindlimb were removed and placed in MOPS-buffered physiological saline solution (PSS) containing (in mM) 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, and 25.0 MOPS (pH 7.4). SFA were dissected free of paired veins and connective tissue under a dissection microscope, cut on both ends, and transferred to a Lucite chamber containing MOPS-PSS (4°C) for cannulation.
Experimental Series 1: Determination of Vasodilator Responses
Preparation of arteries
Twenty (10 young and 10 old) rats were used in experimental series 1. The old rats weighed significantly more than the young rats (420 ± 17 vs. 346 ± 13 g). SFA were cannulated with two resistance-matched glass micropipettes and secured with 11-0 surgical silk, as described previously (22, 23). The micropipettes were subsequently attached to separate hydrostatic pressure reservoirs filled with MOPS-PSS supplemented with albumin (1 g/100 ml). Initially, all SFA were pressurized to 60 cmH2O (1 mmHg = 1.36 cmH2O) and checked for leaks by verification that intraluminal pressure remained constant when the valves to the reservoirs were closed. When an SFA was determined to be leak free, intraluminal pressure was raised to 90 cmH2O (p90) or 130 cmH2O (p130) for 4 h. The rationale for selecting these pressures was based on previous studies by Williams and Segal (21) indicating that, under resting conditions, pressure in rat SFA is ~90 cmH2O, whereas systemic pressure is ~130 cmH2O. During electrically stimulated muscle contractions, these investigators reported that SFA dilated, while systemic pressure remained constant. As SFA dilate, intraluminal pressure would increase toward and, as a limit, equilibrate with systemic pressure (i.e., 130 cmH2O). Therefore, we pressurized young and old SFA at 130 cmH2O to approximate the likely upper limit of intraluminal pressure in SFA during soleus muscle activity. At the end of the 4-h protocol, intraluminal pressure of p130 SFA was lowered to 90 cmH2O, and SFA were allowed to develop tone. SFA that did not develop ≥25% spontaneous tone were constricted with phenylephrine.
Endothelium-dependent dilation
Procedures used to assess vasodilator responses in SFA have been published previously (12, 22, 23). Endothelium-dependent dilation was assessed in SFA by measurement of vasodilator responses to increases in intraluminal flow or increasing doses of ACh. Intraluminal flow was established in SFA by changing proximal and distal pressures in equal, but opposite, directions while maintaining constant pressure at the midpoint of the artery (13). Vasodilator responses were assessed at pressure gradients of 0, 2, 4, 6, 8, 10, 15, 20, 30, and 40 cmH2O, corresponding to flow rates of 0–62 µl/min. Each flow rate was maintained for 3–5 min to ensure that SFA reached a steady diameter. ACh-induced dilation was assessed in SFA by addition of increasing doses of ACh to the bath solution in cumulative doses over the range 10−9–10−4 M in whole-log increments. A total of four SFA were studied in parallel from each rat. SFA 1 was exposed to normal (90 cmH2O) intraluminal pressure for 4 h, and vasodilator responses were subsequently assessed in the absence of enzyme inhibitors. SFA 2 was exposed to high (130 cmH2O) intraluminal pressure for 4 h and then reset to normal (90 cmH2O) pressure, and vasodilator responses were assessed in the absence of enzyme inhibitors. SFA 3 was exposed to high (130 cmH2O) intraluminal pressure for 4 h and then reset to normal (90 cmH2O) pressure, and vasodilator responses were assessed in the presence of 300 µM Nω-nitro-l-arginine (l-NNA) to block NO synthase (NOS). SFA 4 was exposed to high (130 cmH2O) intraluminal pressure for 4 h and then reset to normal (90 cmH2O) pressure, and vasodilator responses were assessed in the presence of l-NNA + 5 µM indomethacin (Indo) to block NOS and cyclooxygenase. The experimental protocol was designed such that intraluminal flow was always administered first followed by ACh.
Endothelium-independent dilation
Endothelium-independent dilation was assessed by addition of increasing doses of sodium nitroprusside (SNP) to the bath solution in cumulative doses over the range 10−9–10−4 M in whole-log increments.
Passive diameter
At the end of each experiment, SFA were incubated for 30 min in Ca2+-free PSS for determination of passive diameter at an intraluminal pressure of 90 cmH2O (Table 1).
Table 1.
Characteristics of SFA from young and old rats
| Parameter | p90 | p130 | p130 + l-NNA | p130 + l-NNA + Indo |
|---|---|---|---|---|
| Maximum diameter, µm | ||||
| Young | 182.4±11.4 | 166.1±15.6 | 171.2±6.9 | 191.1±13.9 |
| Old | 188.9±11.1 | 192.5±11.6 | 177.2±15.2 | 164.0±13.3 |
| Initial tone, % | ||||
| Flow | ||||
| Young | 47.0±5.5 | 54.8±8.4 | 69.7±6.3 | 62.6±6.4 |
| Old | 54.6±9.5 | 51.7±5.8 | 64.0±4.8 | 57.6±9.9 |
| ACh | ||||
| Young | 34.4±4.2 | 54.4±11.9 | 82.3±4.6†‡ | 70.8±10.4† |
| Old | 45.9±9.8 | 46.1±7.3 | 68.6±4.4* | 68.5±8.8 |
Values are means ± SE; n = 8–9 rats/group [young (4 mo old) and old (24 mo old)]. SFA, soleus muscle feed arteries; l-NNA, Nω-nitro-l-arginine; Indo, indomethacin; p90 and p130, normal (90 cmH2O) and high (130 cmH2O) intraluminal pressure, respectively.
P ≤ 0.05 vs. young.
P ≤ 0.05 vs. p90.
P ≤ 0.05 vs. p130.
Experimental Series 2: eNOS and SOD-1 mRNA Expression
Sixteen (8 young and 8 old) rats were used in experimental series 2. The old rats weighed significantly more than the young rats (416 ± 12 vs. 384 ± 4 g). SFA were cannulated with two resistance-matched glass micropipettes and secured with 11-0 surgical silk as described in Experimental Series 1. The micropipettes were attached to separate hydrostatic pressure reservoirs filled with MOPS-PSS supplemented with albumin (1 g/100 ml). Initially, all SFA were pressurized to 60 cmH2O and checked for leaks. When an SFA was determined to be leak free, intraluminal pressure was raised to 70, 90, 110, or 130 cmH2O for 4 h. Normal resting pressure in SFA is 90 cmH2O (21); thus the range of pressures used in these experiments encompassed one pressure below and two pressures above resting in vivo pressure. In addition, the range of pressures was within the range of pressures believed to be present in SFA during soleus muscle contractions (21). The PSS bathing the artery was maintained at 37°C and changed at 30-min intervals. At the end of the 4-h experimental protocol, SFA were removed from the pipettes, and the ends of the artery that had been tied onto the pipettes were removed with surgical scissors. Each SFA was then placed in an RNase-free microcentrifuge tube and frozen at −80°C for RNA isolation.
Quantification of eNOS and SOD-1 mRNA expression
Relative differences in eNOS and SOD-1 mRNA expression in young and old SFA were assessed using a real-time RT-PCR, as described previously (24). All mRNA data were standardized by expression of eNOS or SOD-1 relative to GAPDH. The primer sequences were as follows: 5′-CCA CAA TCC TGG TGC GTC-3′ (sense) and 5′-GCC TTT TTC CAG TTG TTC CA-3′ (antisense) for eNOS, 5′-CCA GCG GAT GAA GAG AGG-3′ (sense) and 5′-CAA TCA CAC CAC AAG CCA AG-3′ (antisense) for SOD-1, and 5′-ACT CTA CCC ACG GCA AGT TC-3′ (sense) and 5′-TAC TCA GCA CCA GCA TCA CC-3′ (antisense) for GAPDH. PCR for eNOS, SOD-1, and GAPDH were initiated with a denaturing step at 95°C (10 min) followed by 40 cycles at 95°C (30 s), 60°C (60 s), and 75°C (60 s). A melt curve, ramping from 60 to 95°C, was performed after each RT-PCR to test for the presence of primer dimers. When primer dimer formation was detected, the PCR was rerun using a separate aliquot of cDNA.
Experimental Series 3: eNOS and SOD-1 Protein Content
Eighteen (9 young and 9 old) rats were used in experimental series 3. The old rats weighed significantly more than the young rats (414 ± 13 vs. 343 ± 15 g). SFA were cannulated, pressurized to 60 cmH2O, and checked for leaks as described in Experimental Series 1. When an SFA was determined to be leak-free, intraluminal pressure was raised to 70, 90, 110, or 130 cmH2O for 4 h. The PSS bathing the artery was maintained at 37°C and changed at 30-min intervals. At the end of the 4-h experimental protocol, SFA were removed from the pipettes, and the ends of the artery that had been tied onto the pipettes were removed with surgical scissors. Each SFA was then placed in a microcentrifuge tube and frozen at −80°C for use in immunoblot analysis.
Quantification of eNOS and SOD-1 protein content
Relative differences in eNOS and SOD-1 protein content were assessed in SFA using immunoblot analysis, as described previously in detail (12, 14). eNOS protein content was evaluated with a monoclonal antibody (1:1,600 dilution; catalog no. N30020, Transduction Laboratories). SOD-1 protein content was assessed with a polyclonal antibody (1:1,600 dilution; catalog no. SOD-100, Stressgen). Immunoblots were evaluated by enhanced chemiluminescence (ECL, Amersham) and densitometry with use of an LAS-3000 Luminescent Image Analyzer and Mutli-Gauge Image Analysis Software (FUJIFILM Medical Systems). All data are expressed as densitometric units. GAPDH was used as an internal standard to control for small differences in protein loading. GAPDH protein content was assessed with a monoclonal antibody (1:10,000 dilution; catalog no. MAB374, Chemicon).
Statistical Analysis
Values are means ± SE. Between-group differences in eNOS and SOD-1 mRNA expression, eNOS and SOD-1 protein content, and body weight were assessed using one-way ANOVA. Two-way ANOVA with repeated measures on one factor (dose) was used to determine whether dilation to flow, ACh, or SNP differed by group. Concentration-response data are expressed as a percentage of maximal possible dilation. Percent possible dilation was calculated as follows: [(Ddose − DB)/(DP − DB)] × 100, where Ddose is measured diameter for a given dose, DB is baseline diameter before a given dose, and DP is maximal passive diameter. A total of 80 SFA were used in experimental series 1 to determine whether pretreatment with short-term increases in intraluminal pressure improves vasodilator responses. Seven (2 young and 5 old) SFA required phenylephrine to achieve 25% tone. Deletion of these arteries from the statistical analyses did not alter interpretation of the results; therefore, all 80 SFA were included in the final analyses. When a significant F value was obtained, post hoc analyses were performed with Duncan’s multiple-range test. Statistical significance was set at P ≤ 0.05.
RESULTS
Flow-Induced Dilation
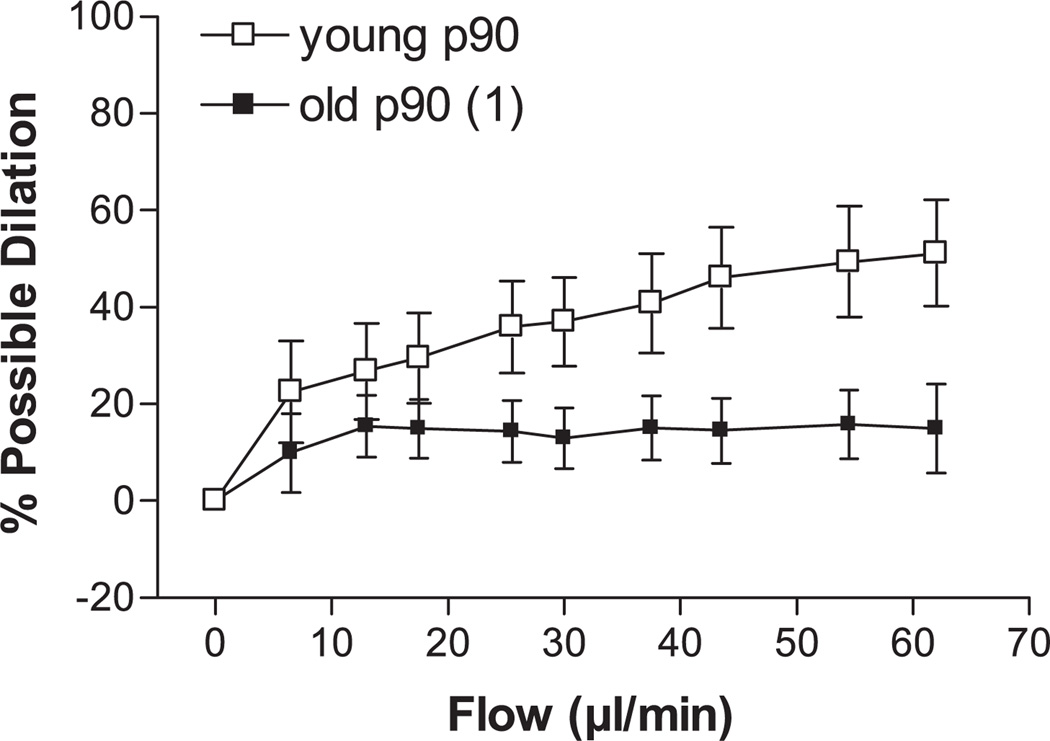
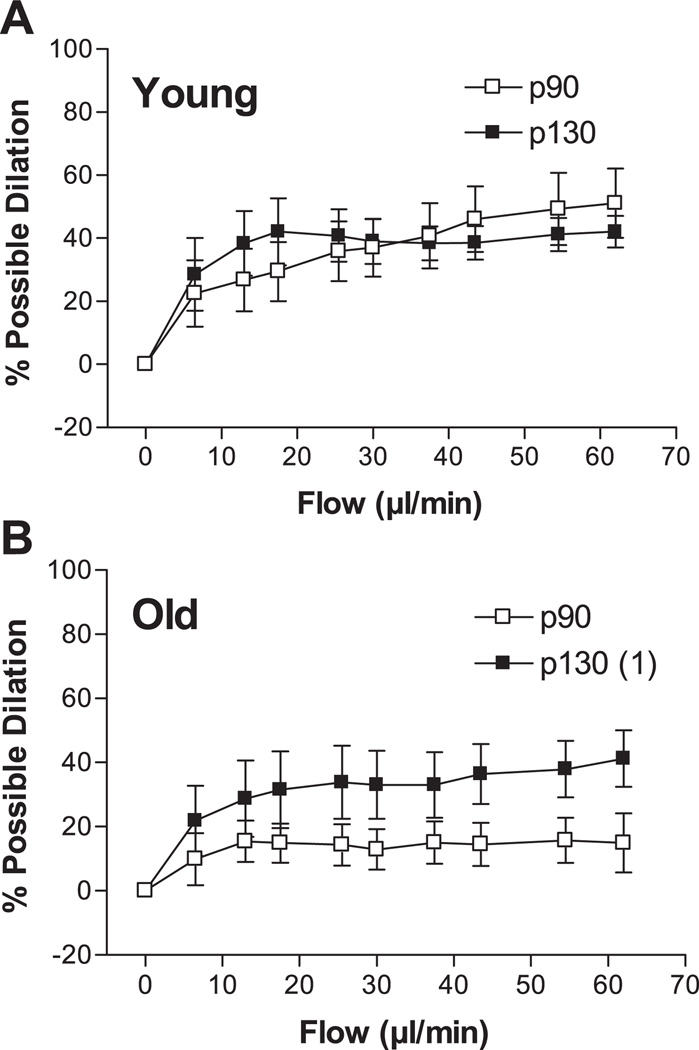
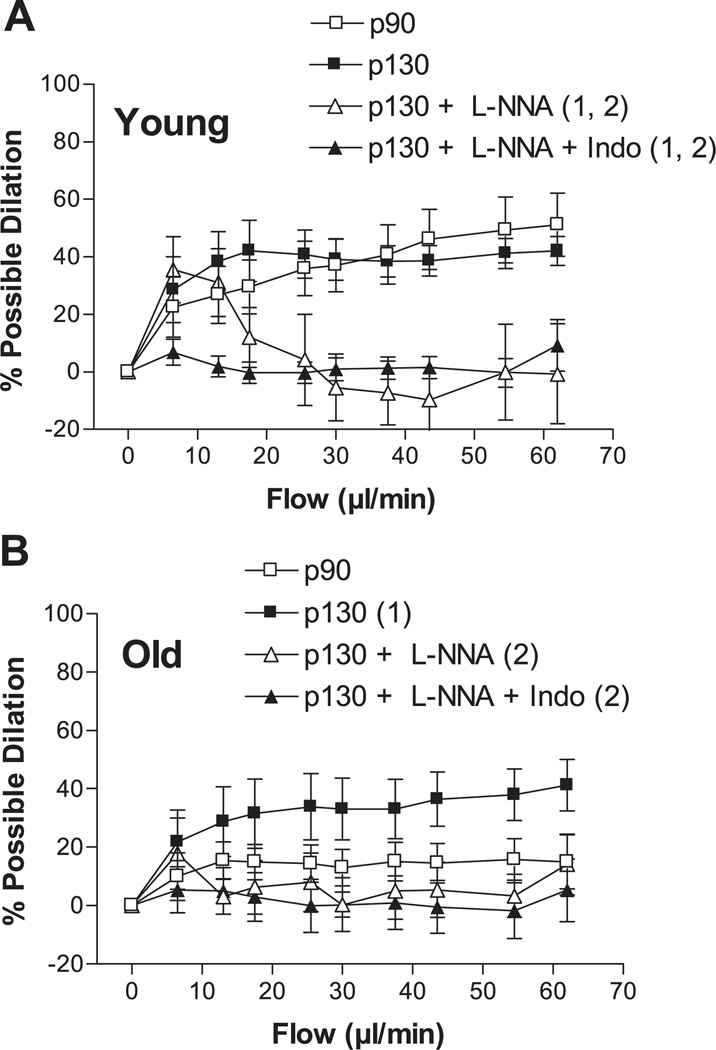
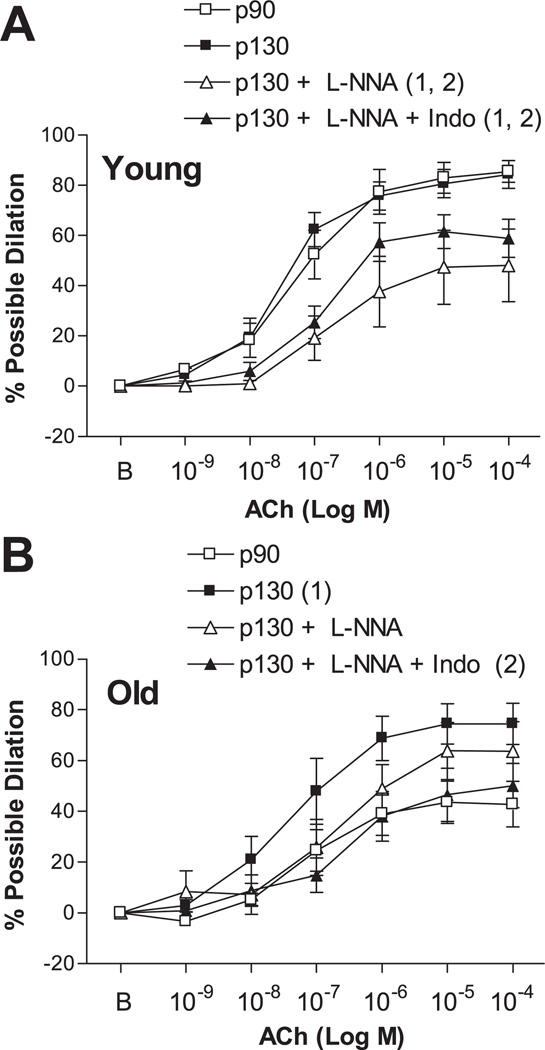
Step increases in intraluminal flow elicited dilation of young and old p90 SFA; however, flow-induced dilation was significantly blunted in the old p90 SFA (Fig. 1). After 4 h of pretreatment with increased intraluminal pressure, flow-induced dilation was improved in old (not young) SFA, such that flow-induced dilation was greater in old p130 SFA than in old p90 SFA (Fig. 2). In addition, flow-induced dilation of old p130 SFA was not different from that of young p90 or young p130 SFA (Fig. 2). In young p130 SFA, flow-induced dilation was inhibited by l-NNA and l-NNA + Indo (Fig. 3A). In old p130 SFA, flow-induced dilation was inhibited by l-NNA and l-NNA + Indo, such that the response was no longer greater than in old p90 SFA (Fig. 3B).
Fig. 1.
Effect of aging on flow-induced dilation in soleus muscle feed arteries (SFA). All SFA were pressurized to 90 cmH2O (p90) for 4 h before assessment of endothelial function. Values are means ± SE; n = 8–9 rats/group. 1Significantly different from young p90, P ≤ 0.05.
Fig. 2.
Effect of a short-term increase in intraluminal pressure on flow-induced dilation in young (A) and old (B) SFA. SFA were pressurized to 90 (p90) or 130 (p130) cmH2O for 4 h. At the end of the 4-h treatment period, intraluminal pressure in p130 SFA was reset to 90 cmH2O. Flow-induced dilation was then assessed in all SFA at an intraluminal pressure of 90 cmH2O. Values are means ± SE; n = 8–9 rats/group. 1Significantly different from p90, P ≤ 0.05.
Fig. 3.
Flow-induced dilation in young (A) and old (B) SFA pressurized to 90 or 130 cmH2O for 4 h. Vasodilator responses were assessed in the absence of enzyme inhibitors, in the presence of 300 µM Nω-nitro-l-arginine (l-NNA), or in the presence of l-NNA + 5 µM indomethacin (Indo). Values are means ± SE; n = 8–9 rats/group. Significantly different from 1p90 and 2p130, P ≤ 0.05.
ACh-Induced Dilation
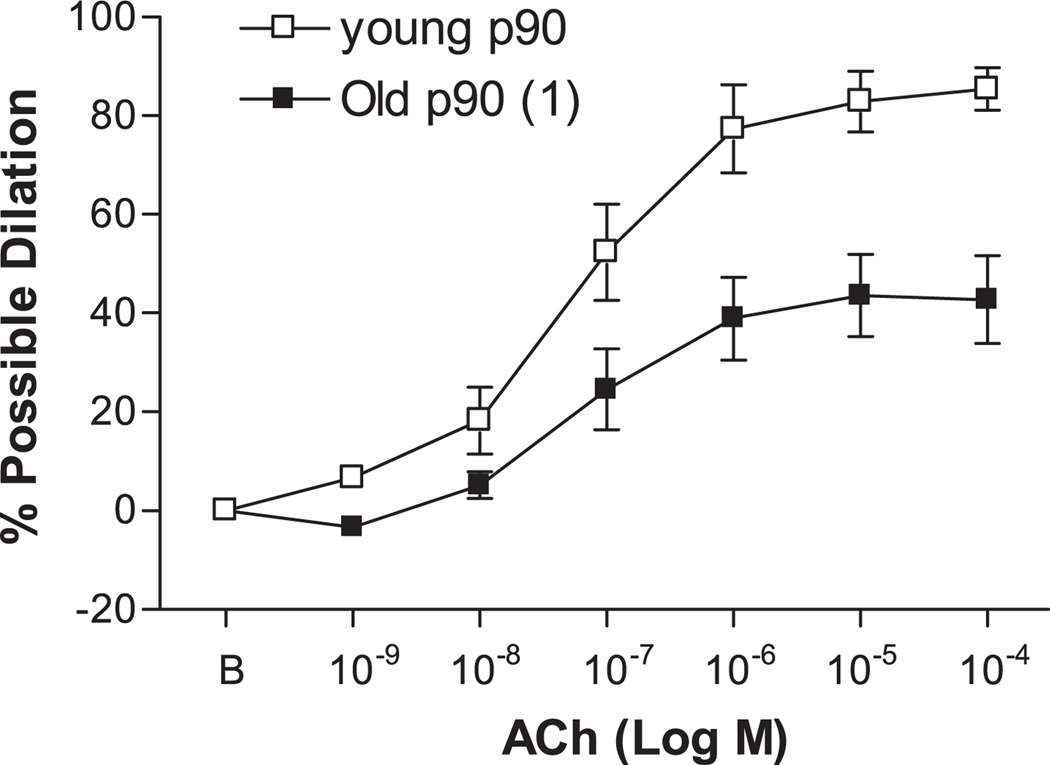
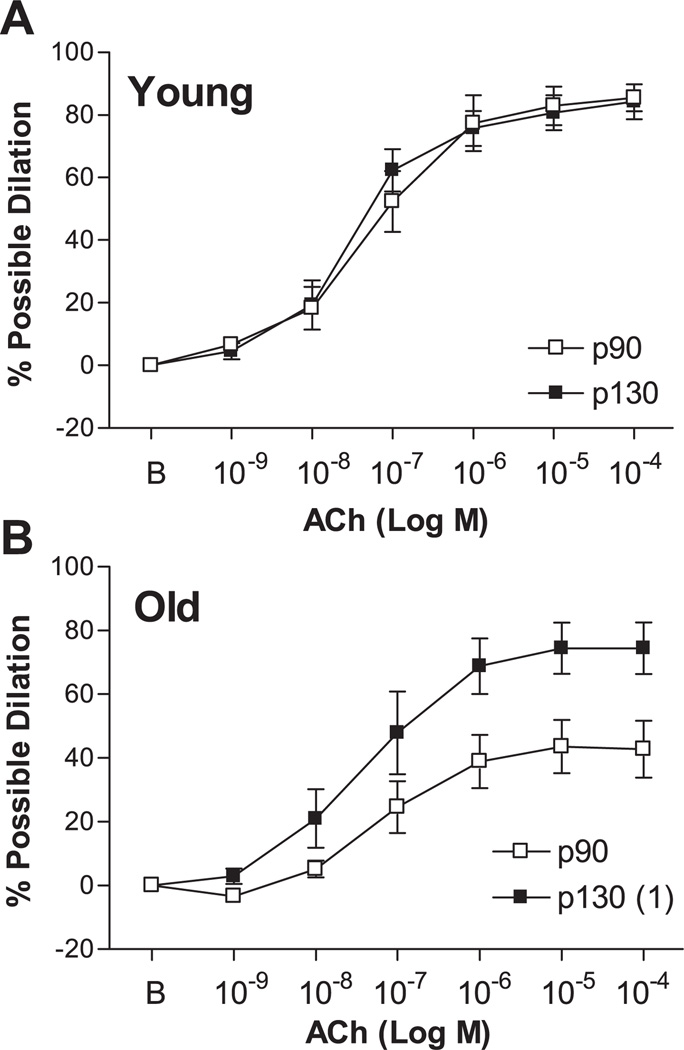
ACh elicited dose-dependent dilation of young and old p90 SFA; however, the vasodilator response to ACh was significantly blunted in old p90 SFA (Fig. 4). After 4 h of pretreatment with increased intraluminal pressure, ACh-induced dilation was improved in old (not young) SFA, such that ACh-induced dilation was greater in old p130 SFA than in old p90 SFA (Fig. 5). In addition, ACh-induced dilation of old p130 SFA was not different from that of young p90 or young p130 SFA (Fig. 5). In young p130 SFA, ACh-induced dilation was inhibited by l-NNA and l-NNA + Indo (Fig. 6A). In old p130 SFA, ACh-induced dilation was not significantly inhibited by l-NNA (Fig. 6B); however, double blockade with l-NNA + Indo inhibited ACh-induced dilation, such that the vasodilator response was no longer greater than in old p90 SFA.
Fig. 4.
Effect of aging on ACh-induced dilation in SFA. All SFA were pressurized to 90 cmH2O for 4 h before assessment of endothelial function. B, baseline diameter before the 1st dose of ACh. Values are means ± SE; n = 8–9 rats/group. 1Significantly different from young p90, P ≤ 0.05.
Fig. 5.
Effect of a short-term increase in intraluminal pressure on ACh-induced dilation in young (A) and old (B) SFA pressurized to 90 or 130 cmH2O for 4 h. At the end of the 4-h treatment period, intraluminal pressure in p130 SFA was reset to 90 cmH2O. ACh-induced dilation was assessed in all SFA at an intraluminal pressure of 90 cmH2O. B, baseline diameter before the 1st dose of ACh. Values are means ± SE; n = 8–9 rats/group. 1Significantly different from p90, P ≤ 0.05.
Fig. 6.
ACh-induced dilation in young (A) and old (B) SFA pressurized to 90 or 130 cmH2O for 4 h. Vasodilator responses were assessed in the absence of enzyme inhibitors, in the presence of 300 µM l-NNA, or in the presence of l-NNA + 5 µM Indo. B, baseline diameter before the 1st dose of ACh. Values are means ± SE; n = 8–9 rats/group. Significantly different from 1p90 and 2p130, P ≤ 0.05.
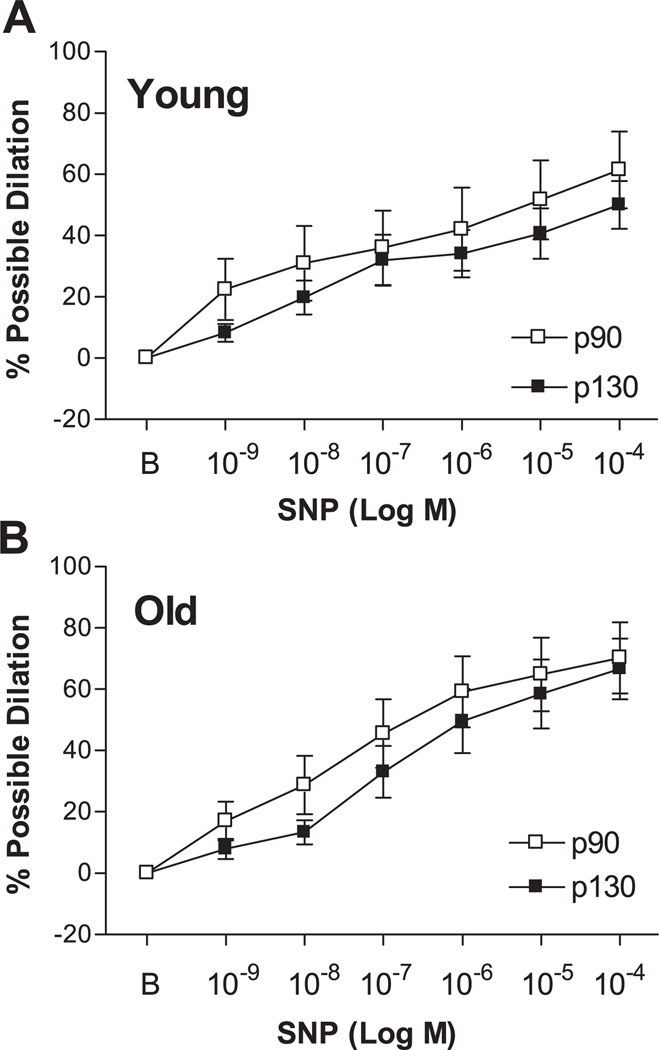
SNP-Induced Dilation
SNP elicited a dose-dependent dilation of young p90, young p130, old p90, and old p130 SFA (Fig. 7). Statistical analysis revealed no significant between-group differences.
Fig. 7.
Sodium nitroprusside (SNP)-induced dilation in young (A) and old (B) SFA pressurized to 90 or 130 cmH2O for 4 h. B, baseline diameter before the 1st dose of SNP. Values are means ± SE; n = 8–9 rats/group. Repeated-measures ANOVA revealed no significant between-group differences.
eNOS and SOD-1 mRNA Content
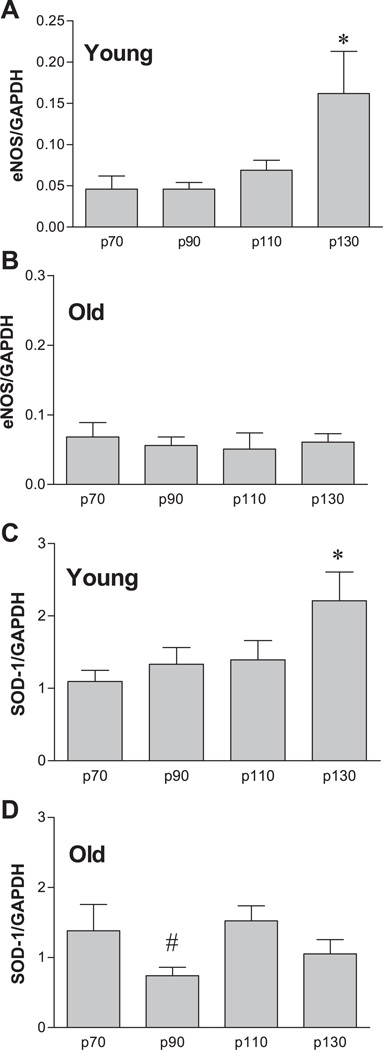
RT-PCR analysis revealed that increased intraluminal pressure increased eNOS (Fig. 8A) and SOD-1 (Fig. 8C) mRNA content in young SFA, such that the level of expression was significantly greater in p130 SFA than in p70, p90, and p110 SFA. In old SFA, eNOS mRNA expression was not altered by changes in pressure (Fig. 8B), whereas SOD-1 mRNA content was significantly greater in p110 SFA than in p90 SFA (Fig. 8D).
Fig. 8.
Influence of intraluminal pressure on endothelial nitric oxide synthase (eNOS; A and B) and SOD-1 (C and D) mRNA expression in SFA isolated from young and old Fischer 344 rats. Values are means ± SE; n = 8 rats per group. SFA were pressurized at 70 (p70), 90 (p90), 110 (p110), or 130 (p130) cmH2O for 4 h. *Significantly different from all other groups, P ≤ 0.05. #Significantly different from p70 and p110, P ≤ 0.05.
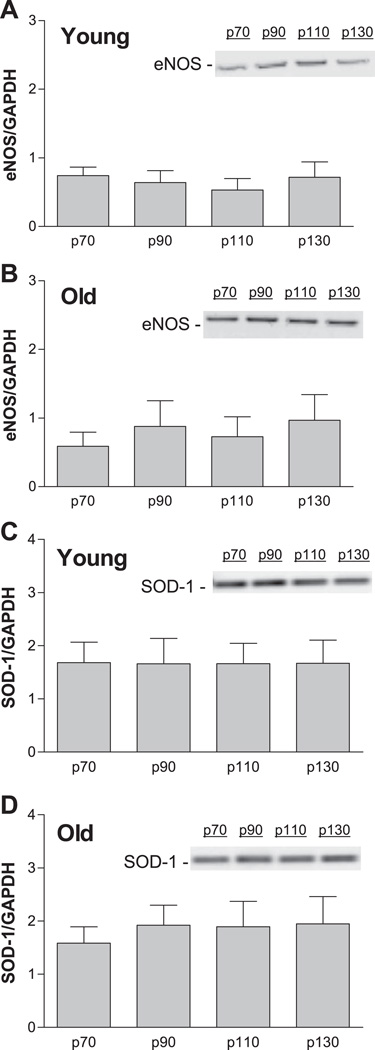
eNOS and SOD-1 Protein Content
Immunoblot analysis revealed that eNOS and SOD-1 protein content were not altered by increased intraluminal pressure in young or old SFA (Fig. 9).
Fig. 9.
Influence of intraluminal pressure on eNOS (A and B) and SOD-1 (C and D) protein content in SFA isolated from young and old Fischer 344 rats. Values are means ± SE; n = 9 rats per group. SFA were pressurized at 70, 90, 110, or 130 cmH2O for 4 h. ANOVA revealed no significant differences.
DISCUSSION
The purpose of the present study was to test the hypothesis that short-term increases in intraluminal pressure improve endothelium-dependent dilation and induce eNOS expression in senescent SFA. SFA were studied, because aging impairs NO-mediated, endothelium-dependent dilation in these arteries (22, 23). In addition, SFA play an important role in regulating soleus muscle blood flow at rest and during soleus muscle contractions (21). The primary findings of the present study were as follows. 1) Flow- and ACh-induced dilations were significantly impaired in old p90 SFA. 2) Pretreatment with increased intraluminal pressure (p130) improved flow- and ACh-induced dilations in old SFA, such that the vasodilator responses were not different from those in young SFA. 3) Flow-induced dilation was inhibited by l-NNA and l-NNA + Indo in old p130 SFA, resulting in a response that was not greater than the response in old p90 SFA. 4) ACh-induced dilation was inhibited by l-NNA + Indo (not l-NNA alone) in old p130 SFA, resulting in a response that was not greater than the response in old p90 SFA. 5) eNOS and SOD-1 mRNA expression was induced by increased intraluminal pressure in young (not old) SFA, such that the level of expression was significantly greater in young p130 SFA than in p70, p90, and p110 SFA. 6) eNOS and SOD-1 protein content were not altered by changes in intraluminal pressure in young or old SFA. Collectively, these results indicate that treatment of senescent SFA with short-term increases in intraluminal pressure improves endothelium-dependent dilation, in part by enhancing NO bioavailability; however, the beneficial effect was not associated with increased eNOS or SOD-1 expression.
Previous studies indicate that endurance exercise training attenuates or reverses the detrimental effects of aging on endothelium-dependent dilation in arteries perfusing skeletal muscle (9, 16–19). In aged arteries, where exercise improves endothelium-dependent dilation, enhanced vasodilator function is associated with increased eNOS expression (18) and enhanced NO mediation of endothelium-dependent dilation (18, 19). The mechanism(s) accounting for the beneficial effect of exercise training on eNOS expression and vasodilator responsiveness is not known; however, it has been proposed that short-term increases in arterial pressure and subsequent mechanical deformation of vascular endothelial cells during individual bouts of exercise may play an integral role (9). This speculation is supported by experimental evidence indicating that mechanical deformation of vascular endothelial cells induces eNOS mRNA expression and enhances NO production in cultured endothelial cells (1, 2). During exercise, skeletal muscle arteries dilate and perfusion pressure increases to facilitate the delivery of blood flow to actively contracting muscle. Therefore, we wished to determine whether treatment of senescent SFA with a short-term increase in intraluminal pressure, within a range believed to be present in these arteries during soleus muscle contractions, has the potential to induce eNOS expression and improve NO-mediated, endothelium-dependent dilation.
The finding that flow- and ACh-induced dilations were less in old p90 SFA than in young p90 SFA (Figs. 1 and 4) is in accord with previous studies and indicated that endothelial function was impaired in the old arteries (22–24). Pretreatment with high intraluminal pressure improved flow- and ACh-induced dilations in old (not young) SFA, such that the response was no longer different from the response in young SFA (Figs. 2 and 5). Thus, increasing intraluminal pressure in SFA to levels believed to be present during soleus muscle contractions improved endothelium-dependent dilation in old SFA to levels seen in young SFA.
To test the hypothesis that short-term increases in intraluminal pressure improve endothelial function in SFA by enhancing NO-mediated dilation, we assessed flow- and ACh-induced dilations in the absence and presence of l-NNA to inhibit NOS. The finding that l-NNA inhibited flow-induced dilation in old p130 SFA, such that the response was no longer greater than in old p90 SFA, is consistent with the interpretation that increased pressure improved flow-induced dilation in senescent SFA by enhancing NO production (Fig. 3). In addition, the improvement in flow-induced dilation could not be attributed to an enhanced ability of vascular smooth muscle to respond to NO, since vasodilator responses to SNP (an NO donor) were similar in old p130 SFA and old p90 SFA (Fig. 7). Interestingly, ACh-induced dilation of old p130 SFA was not significantly inhibited by l-NNA but was inhibited in the presence of double blockade with l-NNA + Indo (Fig. 6). These results suggest that enhanced ACh-induced dilation in old p130 SFA was mediated by a vasodilator other than NO, possibly prostacyclin.
On the basis of results indicating that pretreatment of old SFA with increased pressure improved NO mediation of flow-induced dilation, we tested the hypothesis that short-term increases in intraluminal pressure increased eNOS expression. Contrary to our hypothesis, a short-term increase in intraluminal pressure did not induce eNOS mRNA or protein content in old SFA (Figs. 8 and 9). Similarly, increased pressure did not increase the expression of SOD-1, an enzyme that scavenges superoxide anion and prolongs the biological half-life of NO. Thus, although pretreatment with increased pressure improved NO-mediated, flow-induced dilation in old SFA, the improvement in endothelial function was not associated with changes in eNOS or SOD-1 expression.
Pretreatment with increased intraluminal pressure did not improve endothelium-dependent dilation in young SFA, despite increased eNOS and SOD-1 mRNA expression (Fig. 8). In addition, the mechanism underlying the increase in SOD-1 mRNA expression in young SFA exposed to 4 h of reduced pressure is not clear. However, eNOS and SOD-1 protein contents were not altered by changes in pressure in the young SFA (Fig. 9). Thus it is not surprising that endothelial function was not improved in young SFA treated with increased pressure. The mechanism accounting for the age-related difference in the ability of short-term increases in pressure to improve endothelial function in SFA is not known; however, these results are consistent with previously published findings indicating that treatment of SFA with increased intraluminal shear stress in vitro enhances endothelium-dependent dilation in old (not young) SFA (24). In addition, endurance exercise training enhances endothelium-dependent dilation in old skeletal muscle arteries (18) but does not improve endothelium-dependent dilation in young SFA (12).
Limitations of the Study
In the present study, young and old SFA were treated with 4 h of increased intraluminal pressure. Central to the rationale for the study is our belief that physical inactivity underlies age-induced endothelial dysfunction in skeletal muscle arteries. Specifically, we believe that an age-related decline in the frequency and/or intensity of exercise reduces an important signal (i.e., increased perfusion pressure) for the maintenance of eNOS expression and/or activity, resulting in reduced NO bioavailability. The 4-h treatment protocol was selected on the basis of previous studies indicating that pretreatment of isolated SFA with 4 h of increased intraluminal shear stress induces eNOS expression and improves NO-mediated dilation in senescent SFA (24). We recognize that increases in intraluminal pressure associated with exercise would rarely last for 4 h, nor would they be static; however, the purpose of the present study was to determine whether short-term increases in intraluminal pressure, within a range of pressures believed to be present in these arteries during muscle contractions, have the potential to induce eNOS expression and improve endothelium- dependent dilation. Future studies are needed to determine the time course of the response to pressure as well as the efficacy of pulsatile increases in pressure on endothelial function in senescent arteries.
Conclusion
The results of the present study indicate that exposure of SFA to short-term increases in intraluminal pressure improves endothelium-dependent vasodilator responses in senescent SFA. The beneficial effect of increased pressure was characterized, in part, by enhanced NO bioavailability. Contrary to our hypothesis, increased intraluminal pressure did not induce eNOS expression in senescent SFA. Nor did it increase SOD-1 expression. These results suggest that increased perfusion pressure is one signal by which exercise training improves NO-mediated, endothelium-dependent vasodilator function in senescent arteries; however, further study is needed to determine the mechanism by which short-term increases in pressure enhance NO bioavailability in senescent arteries.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the expert technical assistance of Pam Thorne and Miles Tanner.
GRANTS
This work was supported by National Institutes of Health Grants AG-00988 (C. R. Woodman) and HL-36088 (M. H. Laughlin).
REFERENCES
- 1.Awolesi MA, Sessa WC, Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest. 1995;96:1449–1454. doi: 10.1172/JCI118181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awolesi MA, Widman MD, Sessa WC, Sumpio BE. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery. 1994;116:439–445. [PubMed] [Google Scholar]
- 3.Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Luscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgapoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan A, More RS, Mullins PA, Taylor G, Petch MC, Schofield PM. Aging-associated endothelial dysfunction in humans is reversed by l-arginine. J Am Coll Cardiol. 1996;28:1796–1804. doi: 10.1016/s0735-1097(96)00394-4. [DOI] [PubMed] [Google Scholar]
- 6.Corretti MC, Plotnick GD, Vogel RA. The effects of age and gender on brachial artery endothelium-dependent vasoactivity are stimulus dependent. Clin Cardiol. 1995;18:471–476. doi: 10.1002/clc.4960180810. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 8.Delp MD, Evans MV, Duan C. Effects of aging on cardiac output, regional blood flow, and body composition in Fischer 344 rats. J Appl Physiol. 1998;85:1813–1822. doi: 10.1152/jappl.1998.85.5.1813. [DOI] [PubMed] [Google Scholar]
- 9.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 10.Egashiro K, Inou T, Hirooka Y, Hisashi K, Sugimachi M, Suzuki S, Kuga T, Urabe Y, Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine. Circulation. 1993;88:77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 12.Jasperse JL, Laughlin MH. Vasomotor responses of soleus feed arteries from sedentary and exercise-trained rats. J Appl Physiol. 1999;86:441–449. doi: 10.1152/jappl.1999.86.2.441. [DOI] [PubMed] [Google Scholar]
- 13.Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol Heart Circ Physiol. 1991;261:H1706–H1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- 14.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 15.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- 16.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol. 2000;88:761–766. doi: 10.1152/jappl.2000.88.2.761. [DOI] [PubMed] [Google Scholar]
- 17.Rywick TM, Blackman MR, Yataco AR, Vaitkevicious PV, Zink RC, Cottrell EH, Wright JG, Katzel LI, Fleg JL. Enhanced endothelial vasoreactivity in endurance-trained older men. J Appl Physiol. 1999;87:2136–2142. doi: 10.1152/jappl.1999.87.6.2136. [DOI] [PubMed] [Google Scholar]
- 18.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taddei S, Galetta F, Virdis Agostino Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly adults. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 20.Taddei S, Viridis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 21.Williams DA, Segal SS. Feed artery role in blood flow to rat hindlimb skeletal muscles. J Physiol. 1993;463:631–646. doi: 10.1113/jphysiol.1993.sp019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- 23.Woodman CR, Price EM, Laughlin MH. Aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol. 2003;95:2164–2170. doi: 10.1152/japplphysiol.01073.2002. [DOI] [PubMed] [Google Scholar]
- 24.Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol. 2005;98:940–946. doi: 10.1152/japplphysiol.00408.2004. [DOI] [PubMed] [Google Scholar]