Abstract
Behavioral studies have shown that verbal information is better retained when it is self-generated rather than read (learned passively). We used fMRI and a paired associates task to examine brain networks underlying self-generated memory encoding. Subjects were 49 healthy English speakers ages 19–62 (30 female). In the fMRI task, related word pairs were presented in a “read” condition, where subjects viewed both words and read the second word aloud, or a “generate” condition, where the second word was presented with only the first letter and the subject was required to generate the word. Thirty word pairs were presented in each condition. After the fMRI scan, words that were read or generated were presented, each with two foils, in a forced-choice recognition task. On the recognition post-test, words from the “generate” condition were more correctly recognized than from the “read” condition (80.0% for generated words versus 72.0% for read words; t(48) = 5.17, p < 0.001). FMRI revealed increased activation for generate>read in inferior/middle frontal gyri bilaterally (L>R), anterior cingulate, and caudate nucleus and the temporo-parietal-occipital junction bilaterally. For the “read” condition, better subsequent memory performance across individual subjects was positively correlated with activation in the cuneus bilaterally. In the “generate” condition, better subsequent memory performance was positively correlated with activation in the left superior temporal gyrus. These results suggest that self-generation improves memory performance, that enhanced cortical activation accompanies self-generated encoding, and that recruitment of a specific brain network underlies self-generated encoding. The findings may have implications for the development of procedures to enhance memory performance.
Keywords: Verbal Memory, Memory Encoding, Paired Associates, Self-Generation
1. INTRODUCTION
Behavioral studies have shown that verbal information is better retained when it is self-generated rather than received passively (Kanfer & Schefft 1988, McDaniel, et al. 1988, Schefft & Biederman 1990, Olofsson & Nilsson 1992, Basso, et al. 1994, Craik 2002). In the clinical setting, the application of self-generation procedures has been found to improve memory in both nondemented elderly patients and individuals with Alzheimer’s disease (Lipinska, et al. 1994, Souliez, et al. 1996, Multhaup & Balota 1997, Barrett, et al. 2000), frontal lobe dementia (Souliez, et al. 1996), multiple sclerosis (Chiaravalloti & Deluca 2002), Parkinson’s disease (Barrett et al., 2000), schizophrenia (Vinogradov, et al. 1997), temporal or frontal lobectomy (Smith 1996), traumatic brain-injury (Schefft, et al. 2008a), epilepsy (Schefft, et al. 2008b) and aphasia (Marshall, et al. 1992). In particular, we recently demonstrated that self-generated encoding of verbal paired associates enhanced memory performance in patients with epilepsy by 11–20% and that patients with the greatest memory impairment benefitted the most from the use of self-generation strategies (Schefft, et al. 2008b). Overall, these clinical studies support the notion that active participation during verbal encoding engages memory mechanisms that supplement those used during passive observation, leading to improvements in memory performance. Furthermore, research on learning has demonstrated that an active learning process leads to improved mood state, better self esteem, more spontaneous use of various learning strategies, and greater generalization of new knowledge (Schefft & Biederman 1990, Basso, et al. 1994, Walsh, et al. 1995). Hence, improved performance related to self-generation vis-à-vis passive reading is expected and has been confirmed in previous studies (Lipinska, et al. 1994, Souliez, et al. 1996, Multhaup & Balota 1997, Barrett, et al. 2000, Schefft, et al. 2008a, Schefft, et al. 2008b).
The efficacy of self-generation encoding procedures likely lies in the fact that the individual takes an active role in producing material to be remembered rather than passively responding to stimuli provided. This may be explained by several conceptualizations: (1) that memories are enhanced as a result of self-generation of information because there is an increase in distinctiveness in the to-be-remembered items (McDaniel, et al. 1988); (2) individuals may be more likely to remember information because they feel self-empowered by participating in the creation of information (Olofsson & Nilsson 1992); (3) increased relevance of information leads to increased motivation and, as a result, enhances learning (Walsh, et al. 1995); and (4) the belief that information that is processed at a deeper level of meaning will be better remembered because the strategy enforces a certain type and depth of processing (Craik 2002, Lespinet-Najib, et al. 2004). Indeed, the depth-of-processing literature has well-documented the behavioral finding that verbal information is more effectively remembered when it is encoded with greater involvement of its semantics (Craik 2002).
Active learning and semantic processing are associated with the self-generation process. Therefore, several memory-specific cortical areas need to be involved in order for individuals to successfully encode new information. These cortical and subcortical regions include bilateral lingual and fusiform gyri, bilateral hippocampi and parahippocampal gyri, and predominantly left and medial parietal cortices with additional involvement of the left insula, prefrontal cortex, thalami, anterior cingulate cortex (attention), inferior frontal gyrus and premotor cortices (Kapur, et al. 1995, Fletcher & Henson 2001, Szaflarski, et al. 2004b, Aggleton, et al. 2005, Kim 2011). Neuroimaging data are also consistent with the behavioral pattern that semantic, “deeper” processing (relative to perceptual, “shallow” processing) leads to better memory performance. This deeper processing may be associated with a frontal and medial temporal encoding network (Otten, et al. 2001, Nyberg 2002).
Specific to encoding and retrieval of paired verbal associates, studies have found involvement of parahippocampal regions, visual integration areas, bilateral prefrontal cortex and cingulate gyrus in both encoding and retrieval (Krause, et al. 1999, Mottaghy, et al. 1999). Stronger activation in hippocampi and parahippocampal gyri during encoding is associated with better subsequent memory performance at the single-subject level (Meltzer & Constable 2005). More specifically, Kirchhoff and Buckner (2006) related fMRI activation patterns during a paired-object encoding task to the encoding strategies that subjects reported (Kirchhoff & Buckner 2006). Prefrontal activation was associated with a verbal elaboration strategy while activation in the left temporo-occipital junction was associated with a visual inspection strategy. Both of these strategies were related to improvements in subsequent memory performance, which also correlated with increased activation in the supporting brain regions. Qin et al. found that successful encoding of triplets (one visual and two verbal items) was associated with prefrontal and medial temporal fMRI activation, and that a wider set of brain regions including dorsolateral prefrontal cortex, the basal ganglia, posterior cingulate, and middle temporal cortex was engaged when successfully associating discontinuous members of the triplet (Qin, et al. 2007). A recent meta-analysis of similar studies of “subsequent memory effects” (activation during encoding of information that is later successfully remembered) suggested that for verbal associative tasks, left inferior frontal cortex/insula, bilateral fusiform cortex, and left medial temporal regions were most often engaged during successful encoding (Kim 2011).
While there is an abundance of research examining memory encoding, there has been little focus on active encoding methods. It becomes of interest to investigate active encoding inherent in self-generation memory methods, with regard to both memory enhancement effects and the neural activation patterns that may accompany them. Therefore, the main aim of this study was to evaluate the neural circuits involved in successful encoding of paired-associates with self-generated responses using fMRI in order to investigate the neural basis of the improvement in memory performance associated with self-generation.
2. RESULTS
2.1. Performance Data
In-scanner performance data during the encoding task were recorded and scored. More words were correctly read than correctly generated (t(48) = 15.07, p < 0.001), though performance levels were very high in both conditions (99.4% correct for read words, 80.3% for generated words). On the recognition post-test, more “generated” words were correctly recognized than read words (80.0% correct recognition for generated words, 72.0% for read words; t(48) = 5.17, p < 0.001). In-scanner word generation performance score was a highly significant predictor of post-test recognition of generated words in a linear regression analysis (r = 0.49, p < 0.001).
2.2. Functional MRI results
2.2.1. Group Composite: “generate” vs. ”read”
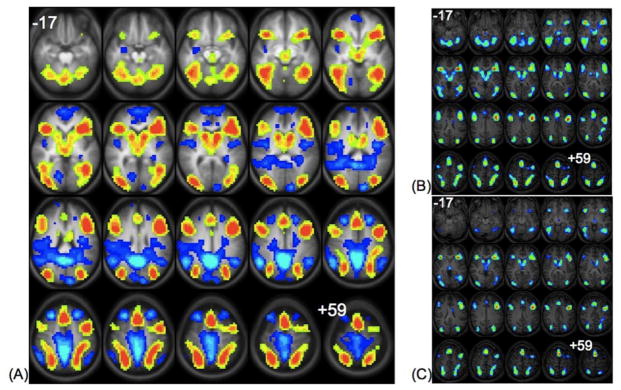
The statistical map for the group composite is shown in Figure 1A. In this map, six clusters in the group composite are showing regions where the “generate” condition activated more strongly than the “read” condition (Table 1A). There are two midline clusters, the more inferior encompassing bilateral caudate nuclei and thalamic nuclei, and the more superior in anterior cingulate and medial frontal gyri. Two sets of large lateral clusters were observed, with one pair anterior and one pair posterior. The anterior cluster pair encompasses the insula and inferior and medial frontal gyri on the right and left, while the posterior cluster pair encompasses inferior, medial, and superior occipital gyri, extending into the supramarginal and angular gyri on the right and left. There are also a number of regions that were more active for the “read” condition relative to the “generate” condition (Figure 1A). These include left medial frontal cortex, bilateral insula, ventromedial prefrontal cortex, inferior parietal cortex, and precuneus, along with left cuneus and right lingual gyrus. Figure 1 also contains the group composite images for “generate” > “read” in younger and older adults (Figures 1B and 1C). Similar to other fMRI studies of cognition, this analysis was conducted in order to examine whether the differences between conditions were driven by younger or older adults (Maguire & Frith 2003, Grady, et al. 2005, Nielson, et al. 2006). After halving the dataset into older (Figure 1B; ages 35–62) and younger (Figure 1C; ages 19–34) groups we show that the activation patterns in these two groups are similar with only a slightly greater extent of activation in the older group.
Figure 1.
Left Panel (A): Statistical map showing group composite activations in the “generate” (hot colors) versus the “read” condition (cool colors). Statistics for the clusters are listed in Table 1A. Top Right Panel (B): Statistical map showing group composite activations in the “generate” relative to the “read” condition in older adults ages 35–62. Bottom Right Panel (C): Statistical map showing group composite activations in the “generate” relative to the “read” condition in younger adults ages 19–34. All images are in radiological convention (right on the image corresponds to left in the brain), and span z-coordinates −17 to +59 in 4mm increments.
Table 1.
Location (Talairach Coordinates), Brodmann Areas, and extent in voxels for all clusters of activation for (A) Group composite, Generate > Read and Read > Generate; (B) Positive and negative correlations with post-scan memory performance for the Read condition and (C) the Generate condition.
| X | Y | Z | BA | Extent in Voxels | |
|---|---|---|---|---|---|
| A. Group Composite: Generate>Read (Figure 1A, hot colors) | |||||
| Anterior Cingulate/Medial Frontal G. | 1.875 | 22.5 | 43 | 8, 32 | 536 |
| Right Caudate | 13.125 | 7.5 | 11 | 219 | |
| Left Caudate | −9.375 | 3.75 | 11 | 296 | |
| Right Insula, Inferior/Middle Frontal G. | 35.625 | 22.5 | 3 | 43,44,6,8,9 | 662 |
| Left Insula, Inferior/Middle Frontal G. | −35.625 | 22.5 | 11 | 43,44,6,8,9 | 1062 |
| Right Inferior/Middle/Superior Occipital, Supramarginal/Angular G. | 39.75 | −52.5 | −5 | 18,19,37,39, 40 | 1222 |
| Left Inferior/Middle/Superior Occipital, Supramarginal/Angular G. | −39.375 | −52.5 | −1 | 18,19,37,39, 40 | 1256 |
| Group Composite: Read >Generate (Figure 1A, cool colors) | |||||
| Left Medial Frontal G. | −0.24 | 54.31 | 8.79 | 10 | 273 |
| Left Superior Frontal G. | −22.18 | 28.37 | 43.23 | 8 | 159 |
| Right Middle Frontal G. | 27.49 | 26.07 | 46.49 | 8 | 165 |
| Left Insula/Caudate Tail | −29.64 | −24.99 | 21.475 | 13 | 412 |
| Right Insula | 45.14 | −15.44 | 18.44 | 13 | 357 |
| Right Inferior Parietal Lobule | 46.06 | −47.64 | 27.95 | 40 | 410 |
| Left Inferior Parietal Lobule | −44.38 | −54.15 | 34.7 | 40 | 159 |
| Right Precuneus | 4.95 | −35.87 | 43.55 | 7 | 1617 |
| Right Lingual G. | 13.28 | −70.05 | 3.85 | 18 | 47 |
| Left Cuneus | −2.86 | −86.98 | 23.92 | 18 | 179 |
| B. Correlation w/post-scan memory performance: Read (Figure 1B) | |||||
| Positive Correlation (hot colors) | |||||
| Right Cuneus | 4.94 | −70.45 | 12.58 | 18 | 66 |
| Right Precuneus | 16.65 | −80.37 | 37.43 | 19 | 51 |
| Left Cuneus | −9.97 | −89.51 | 27.21 | 19 | 38 |
| Negative Correlation (cool colors) | |||||
| Right Medial Frontal G. | 7.41 | 39.73 | 35.39 | 6 | 227 |
| Right Middle Frontal G. | 43.49 | 19.58 | 28.83 | 9 | 72 |
| Left Postcentral G. | −31.42 | −35.06 | 52.61 | 40 | 149 |
| Right Cingulate G. | 3.66 | −38.44 | 28.2 | 31 | 40 |
| Left Middle Temporal G. | −38.74 | −49.85 | 7.66 | 39 | 195 |
| C. Correlation w/post-scan memory performance: Generate (Fig. 1C) | |||||
| Positive Correlation (hot colors) | |||||
| Left Middle/Superior Temporal G. | −43.32 | −47.74 | 0.9 | 37 | 78 |
| Negative Correlation (cool colors) | |||||
| Right Superior Frontal G. | 26.03 | 48.17 | 29.38 | 9 | 52 |
| Right Middle Frontal G. | 25.98 | 21 | 45.86 | 8 | 35 |
| Left Anterior Cingulate | −9.72 | 33.49 | 20.29 | 32 | 87 |
| Right Anterior Cingulate | 15.07 | 33.49 | 13.05 | 32 | 129 |
| Left Posterior Cingulate | −2.6 | −56.11 | 16.7 | 23 | 108 |
| Right Cingulate G. | 9.61 | 5.79 | 39.23 | 32 | 211 |
| Right Insula | 40.66 | −12.22 | 24.47 | 13 | 158 |
| Right Paracentral Lobule | 9.2 | −34.28 | 54.45 | 5 | 232 |
2.2.2. Correlations with post-scan performance
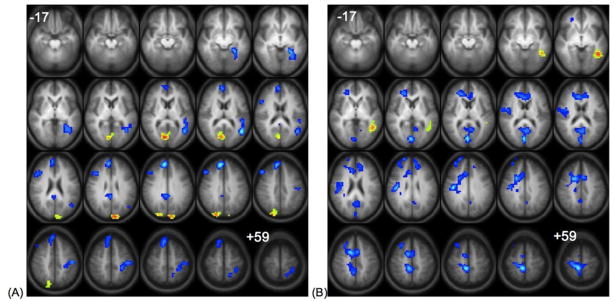
Two statistical maps show correlation with post-scan recognition performance in the “read” and “generate” conditions respectively. For the “read” condition (Figure 2A, Table 1B), better subsequent memory performance was positively correlated with activation in the cuneus bilaterally and right precuneus. Left middle/superior temporal gyrus, right medial frontal, cingulate and lateral prefrontal regions, and left postcentral gyrus showed negative correlations. For the “generate” condition (Figure 2B, Table 1C), better subsequent memory performance was positively correlated with activation in the left middle/superior temporal gyri. Activation in the bilateral middle frontal gyrus, anterior and posterior cingulate cortex, and right insula showed a negative correlation with performance.
Figure 2.
Left Panel (A): Correlation of “read” > ”generate” activation with post-test recognition performance for words presented during the “read” condition. Right Panel (B): Correlation of “generate” > ”read” activation with post-test recognition performance for words presented during the “generate” condition. Regions of positive correlation are shown in hot colors and negative correlation in cool colors. All images are in radiological convention (right on the image corresponds to left in the brain), and span z-coordinates −17 to +59 in 4mm increments. Statistics for the clusters are listed in Table 1, B, C, respectively.
2.2.3. Group Composite: Remembered vs. Forgotten
The contrast between subsequently remembered versus subsequently forgotten items for the “generate” condition is illustrated in Figure 3 with activation locations presented in Table 2. For the “read” condition, there were no significant differences, even when a more lenient threshold of p < 0.05 was used. For the “generate” condition, there was more involvement of the left superior temporal and supramarginal gyri and left posterior insula and medial frontal region in successful encoding of words that were subsequently remembered. The right insula/inferior frontal region was more active during encoding of words that were subsequently forgotten (i.e., unsuccessful encoding).
Figure 3.
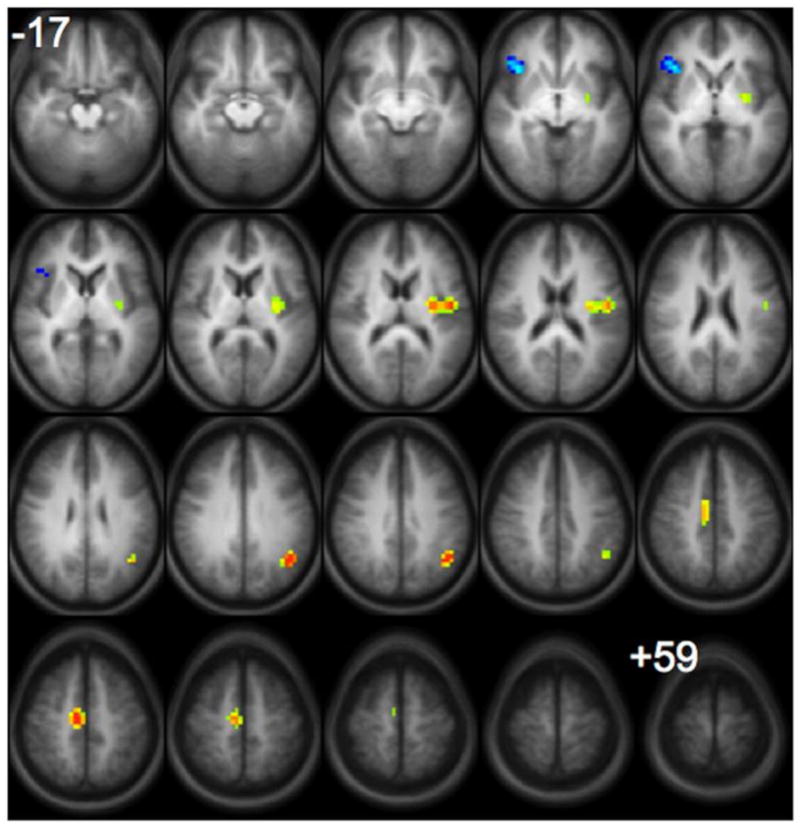
Statistical map showing group composite activation that is greater for subsequently remembered (hot colors) versus subsequently forgotten (cool colors) words for the “generate” condition. (There were no significant differences for this comparison in the “read” condition). All images are in radiological convention (right on the image corresponds to left in the brain) and span z-coordinates −17 to +59 in 4mm increments. Statistics for the clusters are listed in Table 2.
Table 2.
Location (Talairach Coordinates), Brodmann Areas, and extent in voxels for all clusters of activation for the group comparison of subsequently remembered vs. forgotten words in Figure 2.
| X | Y | Z | BA | Extent in Voxels | |
|---|---|---|---|---|---|
| Remembered>Forgotten (hot colors) | |||||
| Left Superior Temporal/Supramarginal G. | −43.125 | −56.25 | 31 | 39 | 28 |
| Medial Frontal G. | 9.375 | −15 | 47 | 6 | 40 |
| Left Insula | −39.375 | −7.5 | 15 | 13 | 68 |
| Forgotten>Remembered (cool colors) | |||||
| Right Insula/Inferior Frontal G. | 35.625 | 22.5 | −1 | 13, 44 | 30 |
3. DISCUSSION
As in previous behavioral studies, our results show that words that were generated during encoding were more accurately recognized in post-testing (McDaniel, et al. 1988, Schefft & Biederman 1990, Olofsson & Nilsson 1992, Basso, et al. 1994, Craik 2002) and that more frequent errors in generation were associated with poorer recognition. Results from the present study are also consistent with predictions from the self-regulation model (Schefft, et al. 1997), which underscores the utility of active participation, subject involvement and perceived choice and control, all of which are associated with positive learning effects. Further, using fMRI we documented that there is a wide network of regions involved in active word generation relative to passive reading aloud, including inferior and medial frontal gyri, insula, temporo-occipital regions bilaterally, anterior cingulate and bilateral subcortical regions. These findings not only confirm the utility of this task for intra-scanner testing of verbal paired associate learning but also identify areas associated with this process itself as well as the areas associated with improved performance that could potentially serve as targets for intervention in patients with cognitive or language deficits e.g., with rTMS (Boyd & Lindsell 2009, Szaflarski, et al. 2011).
Frontal activations greater for the “generate” compared to the “read” condition (Figure 1A, Table 1A) reflect semantic retrieval processes (Damasio, et al. 1996, Thompson-Schill, et al. 1997, Badre, et al. 2005) as well as speech production (Soros, et al. 2006), and these are consistent with other studies examining verbal encoding (Buckner, et al. 1999, Mottaghy, et al. 1999, Bassett, et al. 2006, Murray & Ranganath 2007, Eyler, et al. 2008), or encoding of images using a verbal elaboration strategy (Kirchhoff & Buckner 2006). Furthermore, studies examining increased depth-of-processing during encoding found that inferior frontal regions were involved when to-be-encoded information was more deeply processed (Otten, et al. 2001, Fliessbach, et al. 2010). It is also of interest that left frontal engagement has long been associated with novel problem solving (Petrides & Milner 1982), controlled (versus automatic) cognitive processing (Kanfer & Schefft 1988), organizational strategies used in memory encoding (Stuss, et al. 1994) and effective self-regulatory behavior (Luria 1973).
In studies of verbal encoding with visually presented stimuli, the occipital regions we found to be more active during the “generate” than the “read” condition (Figure 1A, Table 1A) have been implicated in the processing of visual word forms (Krause, et al. 1999, Mottaghy, et al. 1999); these regions may be especially engaged in the process of matching the number of asterisks presented and the number of letters in a generated word. These activations associated with processing the visually presented stimuli extended into parietal regions that may support mental imagery and working memory (see (Mesulam 1998) for discussion), as well as middle temporal cortex, supporting semantic processing including processing of metaphors (Bottini, et al. 1994, Bookheimer 2002).
Cingulate cortex, also active during word generation (Figure 1A, Table 1A), has been implicated in a number of fMRI studies of attention (Hahn, et al. 2006), stimulus/response compatibility (Botvinick, et al. 2004) and self-monitoring (Botvinick, et al. 2004). In the case of our neurocognitive task, subjects may be self-monitoring their responses as they generate words in an effort to perform accurately. Participation of this node of the attentional network may also be, at least in part, related to the increased demands placed on the network by the constantly changing rules of the paired associates task (Pashler et al., 2000). Further, we recently observed differences in the participation of anterior cingulate cortex in the process of verbal memory retrieval between healthy controls (decreased BOLD signal) and epilepsy patients (increased BOLD signal) suggestive of a possible interaction between the brain’s resting state and the level of a task’s attentional demands (Eliassen, et al. 2008). Cingulate cortex has also been suggested to play a major role in the brain’s “default mode” (Greicius, et al. 2003, Fox, et al. 2005, Difrancesco, et al. 2008).
Comparison of older and younger participants revealed vastly similar activation patterns with the exception of a slightly greater extent of activation in older participants. These results are consistent with other studies of memory and executive function in that they have shown more widespread activation associated with successful task performance in older adults (see Guidotti Breting, et al. 2011). Overall, the average age of the older group included in our study is 48.04 years. Thus, since cognitive decline and associated with it fMRI and DTI changes are usually noted in subjects in their late 60’s and older, lack of differences between the groups is likely related to the fact that the “older” group is in fact much younger than the older subjects in studies which showed age-related fMRI and DTI changes (Maguire & Frith 2003, Grady, et al. 2005, Nielson, et al. 2006).
In contrast, other regions associated with the “default mode” network were more active for the “read” versus the “generate” condition, including medial frontal cortex, ventromedial prefrontal cortex, inferior parietal cortex, cuneus and precuneus. (Greicius, et al. 2003, Fox, et al. 2005, Buckner, et al. 2008, Difrancesco, et al. 2008). While performing the less cognitively demanding “read” task, the default network may be only partially inhibited by task-related cognitive processes. Activation in insula may involve different processes in the right and left hemispheres – right insula has been associated with self-awareness (Critchley, et al. 2004) and emotional processing (Van Dillen, et al. 2009). Left insula has been associated with both overt (Wise, et al. 1999, Riecker, et al. 2005) and internal speech (Marvel & Desmond 2011).
Correlation analyses revealed the relationship between the regions involved in each encoding condition and subsequent memory performance. For the “read” condition, better subsequent memory performance was positively correlated with activation in the cuneus bilaterally, suggesting that increased visual processing of the words during reading supports more effective encoding; this region also overlaps with activation for the “read” condition in the group composite map discussed above. Right lateral prefrontal, medial frontal, and cingulate regions, and left postcentral gyrus, and left middle/superior temporal gyrus showed negative correlations. The relationship between left temporal activation and poorer memory performance suggests that increased lexical-phonological processing in this region (Hickok 2009) makes “read” words more difficult to encode, perhaps due to interference from related words in the lexicon.
For the “generate” condition, better subsequent memory performance was supported by increases in the left middle/superior temporal gyrus, an area that was more active for the “generate” condition in the group analysis. This relationship suggests that increased lexical-phonological processing (Hickok 2009) leads to more effective encoding, in contrast to the “read” condition. Right dorsal prefrontal regions, anterior and posterior cingulate cortex, precuneus and right insula showed a negative correlation. These regions of negative correlation suggest there is increased activity in default mode network regions when encoding is less successful (Kim, et al. 2010); increased activation in the insula has also previously been associated with unsuccessful encoding (Daselaar, et al. 2004).
When words were self-generated, a number of regions were more active during encoding when directly comparing subsequently remembered words to forgotten ones (Figure 3, Table 2). All of these regions have been previously observed in studies of subsequent memory effects. First, the left supramarginal and superior temporal gyri have been suggested to support attention during encoding (Uncapher & Wagner 2009), and may interact with the adjacent middle/superior temporal region found to be associated with more effective encoding in the correlation analysis above. The left insula may be engaged by more complex semantic processing during self-generation since it is more active during associative encoding relative to encoding of simple verbal material (Kirwan, et al. 2008) as well as overt and covert speech (Marvel & Desmond 2011). Premotor (medial frontal) regions have also been suggested to modulate attention, particularly during successful encoding of verbal relative to non-verbal material (Kim 2011). However, there were no regions more active for subsequently remembered words than forgotten ones when the words were simply passively encoded. The low amount of cognitive effort and minimal processing of words in this condition may lead to the lack of unique activation (i.e., significant engagement of specific brain regions) for subsequently remembered words and is also the reason for increased activation in the default mode network (Binder, et al. 1999, Fox, et al. 2005).
The right inferior frontal region was more active when encoding was unsuccessful. In previous studies this region was more often associated with successful encoding (Kim 2011), though Park et al. (2008) suggested this region may be engaged specifically when encoding phonological information. In the self-generation task employed here, increased focus on phonological information during encoding may have been detrimental to memory performance, since most of the relationships between the word pairs were semantic in nature rather than phonological (category members, opposites, etc.); some of these relationships may be less efficiently encoded. A limitation of the study was that the low number of word pairs for each specific relationship among the paired associates did not allow for separate analysis of each relationship in the fMRI data. In addition, there were relatively few “forgotten” items, so this comparison may lack statistical power to identify the full network of regions more engaged when items are unsuccessfully encoded.
The paired-associates generation task requires that subjects draw on their knowledge of multiple semantic properties of the words they are generating, since the members of the word pairs are related in various ways. Accessing this variety of semantic knowledge leads to deeper processing and may also activate a wider network of brain regions that support such knowledge. Our results demonstrate that when verbal information is self-generated rather than simply read aloud, this leads to better recognition memory for the self-generated words and associated increases in neural activity that may involve a wider range of semantic knowledge being accessed. This may make individual items more distinctive in their neural representation, and together with depth of processing effects may underlie the improved memory performance that is seen in both healthy individuals and patients with neurological disorders (Craik 2002, Schefft, et al. 2008a, Schefft, et al. 2008b). This underscores the effectiveness of self-generation procedures in memory compensation training in patients with various neurological illnesses or injury affecting the network of brain regions supporting encoding and semantic processing (Marshall, et al. 1992, Lipinska, et al. 1994, Smith 1996, Souliez, et al. 1996, Multhaup & Balota 1997, Vinogradov, et al. 1997, Barrett, et al. 2000, Chiaravalloti & Deluca 2002, Schefft, et al. 2008a, Schefft, et al. 2008b).
4. METHODS
4.1. Subjects
Subjects were 49 healthy right-handed adults (30 females) ages 19–62 (mean age 33), native speakers of English with no history of neurological or psychiatric disorders. Their level of education was 10–25 years (mean 16.5). Subjects were recruited from the local Cincinnati community via print and word-of-mouth advertising as part of a larger study ongoing study (NIH R01-NS04828). The project was reviewed and approved by the Institutional Review Boards at the University of Cincinnati and the Cincinnati Children’s Hospital Medical Center, and all subjects provided written informed consent.
4.2. Materials
60 pairs of related words were selected from the paired associates used in previous studies (Basso, et al. 1994, Schefft, et al. 2008a, Schefft, et al. 2008b). These word pairs were made up of familiar words 6 letters or less in length. Relationships between members of a pair were evenly distributed across 5 rules (12 pairs per rule): associates (e.g., lock – key), category members (e.g., saucer - bowl), synonyms (e.g., street – road), antonyms (e.g., hot – cold) and rhymes (e.g., care – dare). Half of the pairs in each rule group were assigned to the didactic learning condition (“read” condition) and half to the self-generation condition (“generate” condition).
4.3. Procedure – Encoding phase
FMRI scanning was preceded by a 15-item practice session that included three pairs from each of the 5 rules described above (8 “generate” trials and 7 “read” trials). Subjects were instructed to generate (or read) the second word in the pair. Word pairs were presented on a computer screen for 5000 ms (using DirectRT software, www.empirisoft.com) centered in 36-point white font on a black background. In the “read” condition, both words were fully presented, but in the “generate” condition, only the first letter of the second word was presented, with the remaining letters replaced by asterisks (e.g., salt – p*****). During the fMRI scanning, the same procedure was used with the 60 word pairs (30 “read”, 30 “generate”) described above. Subjects were told that they would be expected to later recognize the second word of each pair. Pairs were presented in random order in an event-related design. As in the practice session, each pair was visually presented for 5000 ms, and subjects could make their verbal response anytime during the visual presentation; their response was recorded via in-scanner microphone. A sparse acquisition approach (Schmithorst & Holland 2004, Vannest, et al. 2009, Allendorfer, et al. 2011) was used so that verbal responses could be given and recorded without scanner noise. Specifically, after the 5000 ms visual presentation of the word pair, the word STOP appeared on the screen for 6000 ms at which time fMRI images were acquired. This approach allows for image acquisition at the peak of the hemodynamic response for the preceding stimulus-response event.
4.4. MRI acquisition methods
FMRI scanning was performed on a 3T Philips Achieva MRI scanner with the following parameters: TR/TE = 2000/38 ms, FOV 24.0 × 24.0 cm, matrix 64 × 64, slice thickness = 4 mm. This resulted in a voxel size of 4×4×4 mm and 32 axial slices. High-resolution T1-weighted anatomical images were also obtained using the following parameters: TR/TE=8.1/3.7 ms, FOV 25.0 × 21.1 × 18.0 cm, matrix 252 × 211, slice thickness = 1mm. Three brain volumes were collected during each acquisition following stimulus presentation for the total of 120 volumes; three additional volumes collected prior to starting the proper data collection were discarded.
4.5. Recognition post-test
After the fMRI acquisition, subjects’ recognition memory was evaluated outside of the scanner. All 60 of the “read” or “generated” words (second word in each pair, 30 in each condition) were presented in a three-item forced-choice recognition task. The target item and two foils were presented simultaneously on the computer screen, and subjects responded with a key press to indicate which of the three words they recognized from the preceding task. Responses were self-paced.
4.6. Functional MRI data analysis
Processing of 3D anatomical and fMRI image data was done using routines written in-house in the Interactive Data Language (IDL; ITT Visual Information Solutions, Boulder, CO) programming language and the Cincinnati Children’s Hospital Image Processing Software (CCHIPS©) using methods similar to those used in our previous studies (Schmithorst, et al. 2000, Szaflarski, et al. 2004a, Szaflarski, et al. 2008). Briefly, several pre-processing steps were performed including removal of ghosting and geometric distortion artifacts using a multi-echo reference technique (Schmithorst, et al. 2001), motion correction using a pyramid iterative algorithm (Thevenaz, et al. 1998), and affine spatial transformation of the anatomical and functional images to align them with the standard Talairach coordinate reference frame (Talairach 1988). The statistical parametric maps for the t statistics were computed using the GLM (Generalized Linear Model) algorithm implemented within CCHIPS (Worsley & Friston 1995). Sets of cosine basis functions were used as covariates to account for possible signal drift and aliased respiratory and cardiac signals. Statistical maps were computed for each subject showing those brain regions that activated more strongly during the “generate” condition than the “read” condition.
4.7. Group Composite: “generate” vs. “read”
The single-subject statistical maps underwent a second-level analysis to create a group composite activation map, spatially smoothed using a 4 mm Gaussian filter, thresholded at a t ≥ 7.5, and limited to clusters of 30 voxels or greater, which resulted in a voxel-wise corrected p-value of ≤ 0.01.
4.7.1. Group Composites: Older and Younger Participants
In order to examine whether the differences between the “read” and “generate” condition were driven by younger or older adults, we halved the dataset into older (ages 35–62; N=25; mean age 26.71) and younger (ages 19–34; N=24; mean age 48.04) groups. For each group, a group composite map was created with the same statistical threshold and cluster correction (t ≥ 7.5, cluster size 30, corrected p≤ 0.05; Figures 1B and 1C).
4.8. Correlation Analysis
A map of the brain regions where there was increased activity in the “read” condition was correlated with each subject’s post-test score on words that were previously read. Similarly, a map of the brain regions where there was increased activity in the “generate” condition was correlated with each subject’s post-test score on words that were previously generated. Subject age was included as a covariate of no interest in each analysis to assure that these significantly correlated regions were associated only with performance improvement and not with age. These correlation maps were thresholded at a t ≥ 7.0 and limited to clusters of 30 voxels or greater, which also resulted in a voxel-wise corrected p-value of ≤ 0.01.
4.9. Group Composite: Remembered vs. Forgotten
A second GLM analysis was conducted to examine differences between words that were subsequently remembered versus those that were subsequently forgotten. Each trial in each subject’s fMRI data was categorized depending on whether the “read” or “generated” word was successfully recognized on the subsequent recognition post-test and only brain volumes corresponding to correct responses for either condition were used in subsequent analyses. Individual statistical maps were computed to identify brain regions that differed for successful and unsuccessful encoding separately for each of the “generate” and “read” conditions. Group composite activation maps were then constructed for each of these conditions, spatially smoothed using a 4mm Gaussian filter, thresholded at a t ≥ 7.0, and limited to clusters of 30 voxels or greater, which resulted in a voxel-wise corrected p-value of ≤ 0.01.
Highlights.
Self-generated information is better retained than information learned passively.
This fMRI study examines brain networks for self-generated memory encoding.
For self-generation vs. reading, several cortical and subcortical regions were active.
Subsets of these regions were more active with improved memory performance.
The findings have implications for procedures to enhance memory performance.
Acknowledgments
This study was presented in part at the 38th Annual Meeting of the International Neuropsychological Society. This study was supported by R01 NS048281 (JPS).
Footnotes
The authors do not report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43 (12):1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Allendorfer JB, Lindsell CJ, Siegel M, Banks CL, Vannest J, Holland SK, Szaflarski JP. Females and males are highly similar in language performance and cortical activation patterns during verb generation. Cortex. 2011 doi: 10.1016/j.cortex.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47 (6):907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Crucian GP, Schwartz RL, Heilman KM. Testing memory for self-generated items in dementia: method makes a difference. Neurology. 2000;54 (6):1258–1264. doi: 10.1212/wnl.54.6.1258. [DOI] [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL. Familial risk for Alzheimer’s disease alters fMRI activation patterns. Brain. 2006;129 (Pt 5):1229–1239. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MR, Schefft BK, Hoffmann RG. Mood-moderating effects of affect intensity on cognition: sometimes euphoria is not beneficial and dysphoria is not detrimental. J Pers Soc Psychol. 1994;66 (2):363–368. doi: 10.1037//0022-3514.66.2.363. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11 (1):80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RS, Frith CD. The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain. 1994;117(Pt 6):1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8 (12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Lindsell MA. Excitatory repetitive transcranial magnetic stimulation to left dorsal premotor cortex enhances motor consolidation of new skills. BMC Neuroscience. 2009;10(72) doi: 10.1186/1471-2202-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2 (4):311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, Deluca J. Self-generation as a means of maximizing learning in multiple sclerosis: an application of the generation effect. Arch Phys Med Rehabil. 2002;83 (8):1070–1079. doi: 10.1053/apmr.2002.33729. [DOI] [PubMed] [Google Scholar]
- Craik FI. Levels of processing: past, present. and future? Memory. 2002;10 (5–6):305–318. doi: 10.1080/09658210244000135. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7 (2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380 (6574):499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23 (3):921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Difrancesco MW, Holland SK, Szaflarski JP. Simultaneous EEG/functional magnetic resonance imaging at 4 Tesla: correlates of brain activity to spontaneous alpha rhythm during relaxation. J Clin Neurophysiol. 2008;25 (5):255–264. doi: 10.1097/WNP.0b013e3181879d56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliassen JC, Holland SK, Szaflarski JP. Compensatory brain activation for recognition memory in patients with medication-resistant epilepsy. Epilepsy Behav. 2008;13 (3):463–469. doi: 10.1016/j.yebeh.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Jeste DV, Brown GG. Brain response abnormalities during verbal learning among patients with schizophrenia. Psychiatry Res. 2008;162 (1):11–25. doi: 10.1016/j.pscychresns.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124 (Pt 5):849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fliessbach K, Buerger C, Trautner P, Elger CE, Weber B. Differential effects of semantic processing on memory encoding. Hum Brain Mapp. 2010 doi: 10.1002/hbm.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102 (27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43 (10):1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100 (1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti Breting LM, Tuminello ER, Duke Han S. Functional Neuroimaging Studies in Normal Aging. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2011_139. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Stein EA. Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage. 2006;32 (2):842–853. doi: 10.1016/j.neuroimage.2006.04.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. The functional neuroanatomy of language. Phys Life Rev. 2009;6 (3):121–143. doi: 10.1016/j.plrev.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfer FH, Schefft BK. Guiding the process of therapeutic change. Research Press; Champaign, IL: 1988. [Google Scholar]
- Kapur N, Friston KJ, Young A, Frith CD, Frackowiak RS. Activation of human hippocampal formation during memory for faces: a PET study. Cortex. 1995;31 (1):99–108. doi: 10.1016/s0010-9452(13)80108-6. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54 (3):2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kim H, Daselaar SM, Cabeza R. Overlapping brain activity between episodic memory encoding and retrieval: roles of the task-positive and task-negative networks. Neuroimage. 2010;49 (1):1045–1054. doi: 10.1016/j.neuroimage.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron. 2006;51 (2):263–274. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Wixted JT, Squire LR. Activity in the medial temporal lobe predicts memory strength, whereas activity in the prefrontal cortex predicts recollection. J Neurosci. 2008;28 (42):10541–10548. doi: 10.1523/JNEUROSCI.3456-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause BJ, Horwitz B, Taylor JG, Schmidt D, Mottaghy FM, Herzog H, Halsband U, Muller-Gartner H. Network analysis in episodic encoding and retrieval of word-pair associates: a PET study. Eur J Neurosci. 1999;11 (9):3293–3301. doi: 10.1046/j.1460-9568.1999.00723.x. [DOI] [PubMed] [Google Scholar]
- Lespinet-Najib V, N’Kaoua B, Sauzeon H, Bresson C, Rougier A, Claverie B. Levels of processing with free and cued recall and unilateral temporal lobe epilepsy. Brain Lang. 2004;89 (1):83–90. doi: 10.1016/S0093-934X(03)00303-1. [DOI] [PubMed] [Google Scholar]
- Lipinska B, Backman L, Mantyla T, Viitanen M. Effectiveness of self-generated cues in early Alzheimer’s disease. J Clin Exp Neuropsychol. 1994;16 (6):809–819. doi: 10.1080/01688639408402695. [DOI] [PubMed] [Google Scholar]
- Luria A. The working brain: An introduction to neuropsychology. Basic Books; New York: 1973. [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126 (Pt 7):1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Marshall RC, Neuburger SI, Phillips DS. Effects of facilitation and cueing on labeling of novel stimuli by aphasic subjects. Aphasiology. 1992;6:567–583. [Google Scholar]
- Marvel CL, Desmond JE. From storage to manipulation: How the neural correlates of verbal working memory reflect varying demands on inner speech. Brain Lang. 2011 doi: 10.1016/j.bandl.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Waddill PJ, Einstein GO. A contextual account of the generation effect: A three-factor theory. Journal of Memory and Language. 1988;27:521–536. [Google Scholar]
- Meltzer JA, Constable RT. Activation of human hippocampal formation reflects success in both encoding and cued recall of paired associates. Neuroimage. 2005;24 (2):384–397. doi: 10.1016/j.neuroimage.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Shah NJ, Krause BJ, Schmidt D, Halsband U, Jancke L, Muller-Gartner HW. Neuronal correlates of encoding and retrieval in episodic memory during a paired-word association learning task: a functional magnetic resonance imaging study. Exp Brain Res. 1999;128 (3):332–342. doi: 10.1007/s002210050853. [DOI] [PubMed] [Google Scholar]
- Multhaup KS, Balota DA. Generation effects and source memory in healthy older adults and in adults with dementia of the Alzheimer type. Neuropsychology. 1997;11 (3):382–391. doi: 10.1037//0894-4105.11.3.382. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci. 2007;27 (20):5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson KA, Douville KL, Seidenberg M, Woodard JL, Miller SK, Franczak M, Antuono P, Rao SM. Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiol Aging. 2006;27 (10):1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L. Levels of processing: a view from functional brain imaging. Memory. 2002;10 (5–6):345–348. doi: 10.1080/09658210244000171. [DOI] [PubMed] [Google Scholar]
- Olofsson U, Nilsson L. Mobilization of cognitive resources and the generation effect. Psychological Research. 1992;54:103–109. doi: 10.1007/BF00937138. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124 (Pt 2):399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20 (3):249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Qin S, Piekema C, Petersson KM, Han B, Luo J, Fernandez G. Probing the transformation of discontinuous associations into episodic memory: an event-related fMRI study. Neuroimage. 2007;38 (1):212–222. doi: 10.1016/j.neuroimage.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64 (4):700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Schefft B, Malec J, Lehr B, Kanfer F, JAM MM. Clinical neuropsychology: Theoretical foundations for practitioners. Lawrence Erlbaum; New York: 1997. The role of self-regulation therapy with the brain-injured patient; pp. 237–282. [Google Scholar]
- Schefft BK, Biederman J. Emotional effects of self- generated behavior and the influence of resourcefulness and depressed mood. Journal of Social and Clinical Psychology. 1990;9:354–366. [Google Scholar]
- Schefft BK, Dulay MF, Fargo JD. The use of a self-generation memory encoding strategy to improve verbal memory and learning in patients with traumatic brain injury. Appl Neuropsychol. 2008a;15 (1):61–68. doi: 10.1080/09084280801917806. [DOI] [PubMed] [Google Scholar]
- Schefft BK, Dulay MF, Fargo JD, Szaflarski JP, Yeh HS, Privitera MD. The use of self-generation procedures facilitates verbal memory in individuals with seizure disorders. Epilepsy Behav. 2008b;13 (1):162–168. doi: 10.1016/j.yebeh.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Schmithorst V, Holland SK, Dardzinski BJ. CCHIPS: Cincinnati Children’s Hospital Imaging Processing Software. 2000 http://www.irc.chmcc.org/chips.htm.
- Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20 (6):535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Event-related fMRI technique for auditory processing with hemodynamics unrelated to acoustic gradient noise. Magn Reson Med. 2004;51 (2):399–402. doi: 10.1002/mrm.10706. [DOI] [PubMed] [Google Scholar]
- Smith ML. Recall of frequency of occurrence of self-generated and examiner-provided words after frontal or temporal lobectomy. Neuropsychologia. 1996;34 (6):553–563. doi: 10.1016/0028-3932(95)00139-5. [DOI] [PubMed] [Google Scholar]
- Soros P, Sokoloff LG, Bose A, McIntosh AR, Graham SJ, Stuss DT. Clustered functional MRI of overt speech production. Neuroimage. 2006;32 (1):376–387. doi: 10.1016/j.neuroimage.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Souliez L, Pasquier F, Lebert F, Leconte P, Petit H. Generation effect in short-term verbal and visuospatial memory: comparisons between dementia of Alzheimer type and dementia of frontal lobe type. Cortex. 1996;32 (2):347–356. doi: 10.1016/s0010-9452(96)80056-6. [DOI] [PubMed] [Google Scholar]
- Stuss D, Alexander M, Palumbo C, Buckle L, Sayer L, Pogue J. Organizational strategies of patients with unilateral or bilateral frontal lobe injury in word list learning tasks. Neuropsychology. 1994;8:355–373. [Google Scholar]
- Szaflarski J, Vannest J, Wu S, DiFrancesco M, Banks C, Gilbert D. Excitatory repetitive transcranial magnetic stimulation induces improvements in chronic post-stroke aphasia. Med Sci Monit. 2011;17 (3):132–139. doi: 10.12659/MSM.881446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008;12 (1):74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Dunn RS. High-resolution fMRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy and Behavior. 2004a;5 (2):244–252. doi: 10.1016/j.yebeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Dunn RS, Privitera MD. High-resolution functional MRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy Behav. 2004b;5 (2):244–252. doi: 10.1016/j.yebeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7 (1):27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94 (26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91 (2):139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest JJ, Karunanayaka PR, Altaye M, Schmithorst VJ, Plante EM, Eaton KJ, Rasmussen JM, Holland SK. Comparison of fMRI data from passive listening and active-response story processing tasks in children. J Magn Reson Imaging. 2009;29 (4):971–976. doi: 10.1002/jmri.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Willis-Shore J, Poole JH, Marten E, Ober BA, Shenaut GK. Clinical and neurocognitive aspects of source monitoring errors in schizophrenia. Am J Psychiatry. 1997;154 (11):1530–1537. doi: 10.1176/ajp.154.11.1530. [DOI] [PubMed] [Google Scholar]
- Walsh K, Briggs R, Ayoub J, Vanderboom C. Teaching with GSS: techniques for enabling student participation. First Americas Conference on Information Systems; 1995. pp. 621–623. [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK. Brain regions involved in articulation. Lancet. 1999;353 (9158):1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2 (3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]