Abstract
Accumulating evidence supports the notion that defective phagocytic clearance of dying cells, or defective “efferocytosis,” is causally linked to the progression of advanced atherosclerosis. In advanced atherosclerotic lesions, defective efferocytosis leads to post-apoptotic necrosis, expansion of plaque necrotic cores, and susceptibility to atherothrombosis. Both macrophages and DC-like efferocytes are juxtaposed near expanding necrotic cores, where they engage apoptotic cells. In this Viewpoint, we discuss how reduced efferocytosis by macrophages and CD11cHI DC-like cells may combine to reduce overall plaque stability and therefore promote susceptibility to acute atherothrombosis.
Keywords: DC, Efferocytosis, Macrophage
Relevance of efferocytosis by macrophage and DC-like cells in advanced atherosclerosis
Atherosclerosis is the leading cause of death in industrialized societies [1]. Atherosclerosis is characterized by infiltration of leukocytes into the arterial intima, which lies between the endothelium and the media. The recruited leukocytes include phagocytes such as monocyte/macrophages and CD11c+ DC-like cells. Theses phagocytes are initially recruited in response to apolipoprotein B-100 lipoproteins retained in the intima [2]. Once embedded within the vascular intima, macrophages internalize cholesterol-rich lipoproteins, giving rise to the characteristic foam cells of early atherogenesis. Smooth muscle cells of the medial vascular wall become activated and transmigrate towards the lumen as they act to fortify the endothelial lining by generating a fibrous cap. These reactive processes begin to degenerate as lesions mature from early stable lesions to advanced rupture-prone inflammatory plaque. In advanced atherosclerosis, inflammation fails to subside and this promotes lesion destabilization and susceptibility to heart attack and stroke [3, 4].
Failure to resolve inflammation in atherosclerotic lesions is due to the continuing and amplified presence of the inciting process, subendothelial retention of apolipoprotein B-containing lipoproteins, and to the tandem processes of accelerated apoptosis of macrophages and smooth muscle cells and defective apoptotic cell (AC) clearance [5]. Apoptosis in atherosclerosis is driven by a combination of factors, including cholesterol, bioactive lipids, and ER stress, and pattern-recognition receptor activation [6]. These factors synergize to suppress adaptive cell survival pathways and reinforce intracellular signaling that promotes programmed cell death [7]. In the intima of early lesions, clearance of ACs by phagocytes, termed “efferocytosis,” is rapid and nonphlogistic. As lesions mature, however, there is an accumulation of TUNEL-positive, non-phagocytosed ACs [8]. Non-cleared ACs lose membrane integrity and become secondarily necrotic, contributing over time to an expanding region of tissue necrosis that destabilizes plaque and is linked to occlusive thrombin deposition in the overlying arterial lumen and acute myocardial infarction [9]. Evidence in humans and experimental mice suggests that one mechanism behind post-apoptotic necrosis in cardiovascular disease is defective efferocytosis [5, 8]. The reasons for defective efferocytosis in plaque are unclear. These defects may be manifest at multiple levels, including improper presentation of AC ligands, failure to secrete “come find me” recruitment signals, or a defect at the level of the phagocyte itself, as discussed in this Viewpoint [10].
During the maturation of atherosclerotic lesions, efferocytes are recruited by chemokines and “come find me” signals [11]. These efferocytes include BM- and spleen-derived monocytes, differentiated and polarized macrophages, and CD11cHI DC-like cells [12]. Though macrophages are the predominant phagocyte within the intima, recent studies also highlight the presence of DCs [13, 14]. The overall role of DCs in atheromata, including their contribution to clearance efficiency, is less understood relative to their macrophage counterparts. In fact, whether macrophages and DCs are indeed distinct populations within plaque is not entirely clear [15]. Nevertheless, gene expression profiling of lesion cell types by laser-capture micro-dissection and RT-QPCR, combined with careful immunohistochemistry, clearly indicate phagocyte heterogeneity [16]. Within this diversity, there are cells that exhibit phenotypic and functional traits of DCs, including the expression of DC maturation markers and the ability to present antigen and stimulate T-cell activation [17]. In the following sections, we discuss candidate mechanisms of macrophage and DC efferocytosis in plaque and how suppression of these mechanisms could promote plaque destabilization.
Mechanisms of macrophage efferocytosis in atheromata
In the intimal space of advanced atheromata, macrophages outnumber all other phagocytes. Therefore, the efficiency, or lack thereof, of AC clearance in atherosclerotic lesions is likely to be affected by the integrity of macrophage-mediated clearance mechanisms. Careful histologic examination of human atherosclerotic plaque, combined with more recent genetic causation tests in experimental animals, suggest that macrophage efferocytosis signaling pathways in atheromata are both required and later compromised [18]. For example, in humans, atherosclerotic lesions contain considerable numbers of ACs that are not engulfed by nearby CD68+ phagocytes [8]. This finding is most striking when compared to non-diseased tissues, such as the tonsils and thymus, where cell turnover is relatively high, yet free, i.e. phagocyte-unassociated, and ACs are rarely detected due to efficient clearance [19]. These data are consistent with defective efferocytosis in advanced human atherosclerosis, but they do not address the critical issues of causation.
What are the molecular mechanisms of macrophage efferocytosis in atheromata?
Macrophage efferocytosis in atherosclerosis requires an interplay between AC ligands, phagocyte receptors, and extracellular bridging molecules that link phagocytes to ACs [20] (Fig. 1). These interactions only align after recruitment factors, called “find me” signals, attract the phagocyte to its AC prey [21]. Studies of macrophage efferocytosis in vitro that model the in vivo milieu suggest that the mechanisms required for clearance in atheromata may indeed be unique. For example, in a cell culture model of cholesterol-laden atherosclerotic lesions, Li et al. showed that interrupting the interactions of many prototypic efferocytosis receptors, such as CD36, had minimal effects on uptake of ACs that had been killed by free cholesterol. On the other hand, when phagocytes were deficient for the MERTK engulfment receptor, ingestion of these cholesterol-loaded ACs was markedly suppressed [22]. Mice deficient in MERTK show evidence of defective efferocytosis and susceptibility to a lupus-like autoimmune syndrome [23]. In advanced atherosclerotic lesions, mice lacking MERTK also had a defect in macrophage efferocytosis and this correlated with an increase in plaque inflammation and plaque necrosis [24, 25]. MERTK expression is much more abundant in macrophages relative to their DC counterparts [26], suggesting that macrophages and, particularly macrophage MERTK, is critical for the clearance of ACs in advanced atheromata.
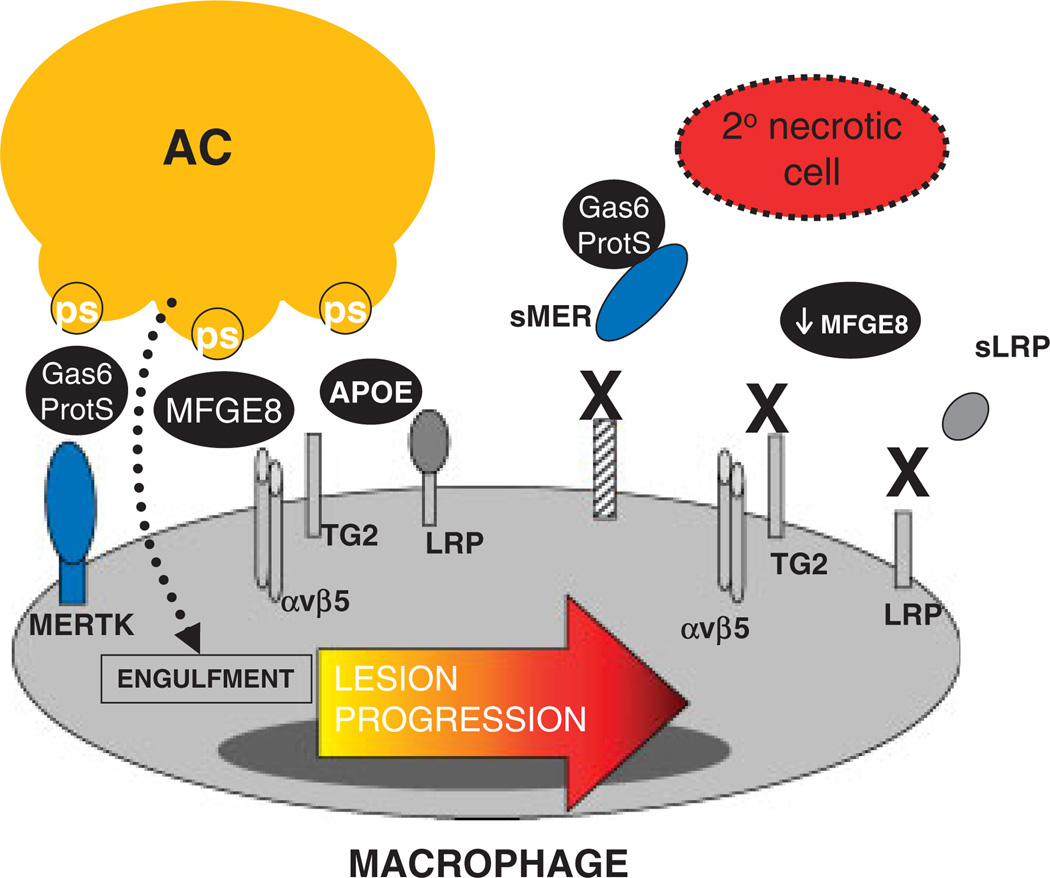
Figure 1.
Possible mechanisms of defective efferocytosis by macrophages in advanced atherosclerosis. Depicted here are several molecules that have been shown to a play a role in macrophage efferocytosis in atherosclerosis. These include the efferocytosis receptors MERTK, αvβ5 integrin, TG2, and LRP (low density lipoprotein related protein). AC receptors can engage bridging molecules such as Gas6/Protein S or MFGE8, which facilitate binding to and phosphatidylserine (PS). There are several hypotheses as to why efferocytosis loses efficiency in advanced plaques, including dysfunction of the molecules as a result of cleavage (MERTK and LRP leading to the soluble isoforms sMER and sLRP respectively), decreased expression (MFGE8), or competitive inhibition by other plaque molecules (sLRP).
Mouse studies have revealed roles for several other macrophage efferocytosis receptors and their ligands in advanced atherosclerosis. Fazio and colleagues showed that clearance of apoptotic macrophages was significantly reduced in Lrp1−/− lesions relative to controls. Compared with wild-type lesions, Lrp1−/− lesions exhibited larger necrotic cores with more dead cells not associated with antibody-stained macrophages [27]. Another efferocytosis receptor, the cell-surface and protein cross-linking transglutaminase-2 (TG2), is expressed in lesional macrophages and participates in recognition and engulfment of ACs. In vivo, atherogenic Ldlr−/− mice engrafted with Tg2−/− BM cells exhibit larger aortic root lesions and expanded necrotic cores relative to controls [28]. TG2, in cooperation with the αvβ3 integrin, can engage lactadherin-opsonized ACs and promote engulfment [29]. Lactadherin (MFG-E8) is expressed in atherosclerotic lesions and promotes efferocytosis in vitro and in vivo. Mallat and colleagues showed that lesions in mice lacking Mfge8 in BM were larger, more necrotic, and contained an increased amount of apoptotic cellular debris [30].
What goes wrong with macrophage efferocytosis in advanced atherosclerosis?
Studies, such as those described in the previous section, test the genetic deficiency of candidate efferocytosis molecules and help us understand the causal role of a molecule in vivo. However, we need to elucidate how efferocytosis mechanisms are naturally compromised in the setting of actual pathology, in this case advanced atherosclerosis. As one example, recent studies [24, 25] indicate that the macrophage efferocytosis receptor MERTK can become inactivated under some inflammatory conditions. The possibility that MERTK function is defective is intriguing in view of the fact that this molecule undergoes cleavage by one or more plasma membrane sheddases under inflammatory conditions [31]. The cleavage of MERTK suppresses efferocytosis by both destroying the receptor and by creating soluble MER, which competes for the efferocytosis bridging molecules Gas6 and Protein S. Whether MERTK cleavage accounts for the defective efferocytosis in advanced atherosclerosis remains to be tested experimentally. In our own hands [32] and work of others [33], evidence for MERTK cleavage has been identified in advanced plaques. Similar mechanisms may come into play for LRP. LRP is highly expressed in intimal macrophages and, like MERTK, can be cleaved [34, 35].
Besides post-translational mechanisms, changes in gene expression may also come into play. MFG-E8 was found to be down-regulated in splenic macrophages in a mouse model of sepsis [36]. Concomitant with this down-regulation, which was TLR4-dependent, was a decrease in efferocytosis and an increase in apoptotic bodies in the spleen. Given that advanced atheromata are at a heightened state of inflammation and have functional TLR4 signaling [37], a similar process might contribute to phagocytic inefficiency in advanced lesions. Finally, defective efferocytosis by F4/80-positive cells was observed in atherosclerotic lesions of genetically obese ob/obLdlr−/− mice, and this defect was reversed by feeding the mice a fish oil-rich diet [38]. These findings, together with in vitro mechanistic data, suggest that in obesity and type 2 diabetes, elevated levels of saturated fatty acids and/or decreased levels of ω-3 fatty acids contribute to decreased macrophage efferocytosis.
The case for DC efferocytosis in plaque
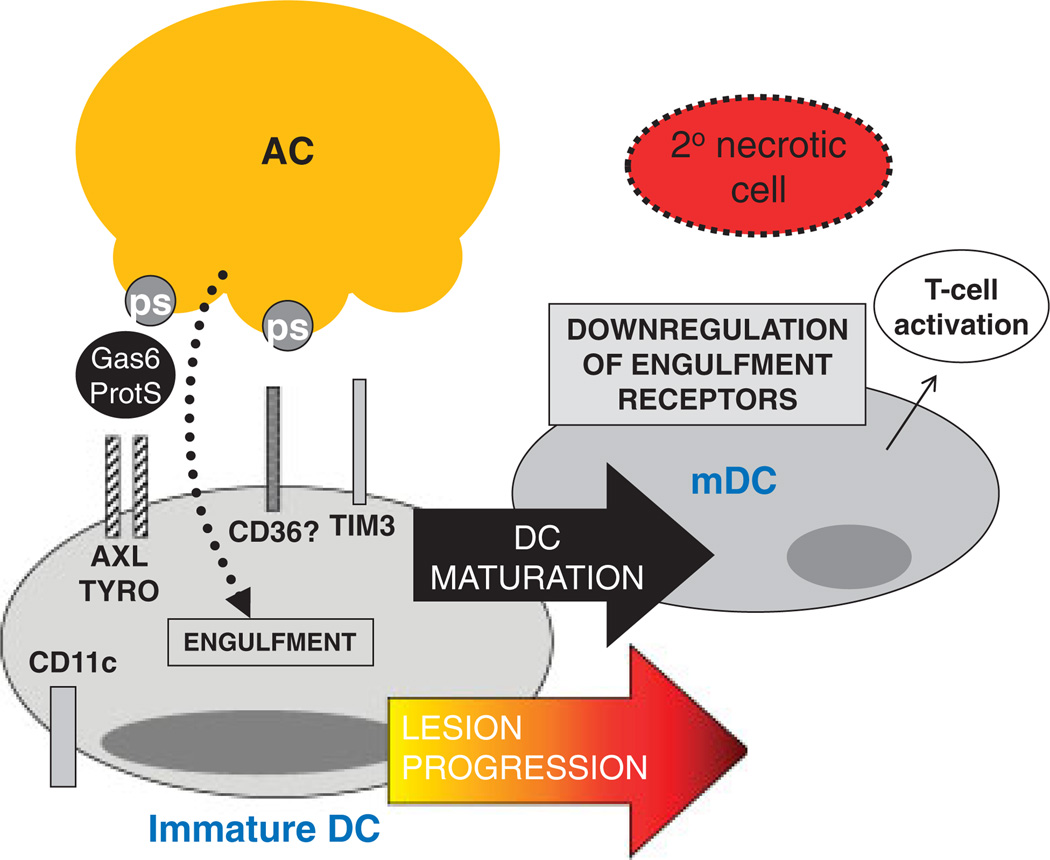
DCs are characterized by high expression of CD11c and MHC II and can comprise approximately 10–30% of the intima of the atherosclerotic plaque [39]. The overall impact of these antigen-presenting cells within the complex milieu of the plaque is unknown; however, recent findings suggest that they play a role in the formation of foam cells in nascent atherosclerotic lesions [14], as well as promoting Th1-driven plaque immune responses [40]. Recent evidence in humans indicates that the number of DCs increases with the progression of lesions [41] and that an accumulation of mature DCs is observed in rupture-prone vulnerable plaques [41]. The nature of the stimulus that results in DC maturation within a plaque is not known, however, maturation could be mediated by inflammatory cytokines, as well as by undigested components of necrotic cells [42]. Whatever the mechanism, it is known that DCs markedly lose their efferocytic capacity upon maturation [43]. Thus, it is possible that pockets of advanced lesions with a high content of mature DCs are a focus for plaque necrosis, i.e. due to locally poor clearance of ACs. The mechanism of decreased efferocytosis in mature DCs is not known, although there is a correlation in vitro with down-regulation of the efferocytosis receptors CD36 and αv integrins [43] and the bridging molecule MFGE-8 [44]. In immature DCs in vitro, several receptors including AXL/TYRO [26], CD36, αvβ5 and TIM-3 [45] have been implicated in efferocytosis (Fig. 2), but the roles of these receptors in vivo is not known.
Figure 2.
Speculation as to how the accumulation of mature DCs in advanced atherosclerosis could promote defective efferocytosis. Although the molecules required for efferocytosis by immature DCs in vivo are not well defined, shown here are several molecules that have been implicated in vitro, namely AXL/TYRO, CD36, and TIM3. These receptors recognize bridging molecules, such as Gas6/Protein S (ProtS) or phosphatidylserine (PS) on ACs. In advanced atheromata, the accumulation of mature DCs (mDCs), which are known to be very poor efferocytes, could contribute to decreased efferocytosis, and thus secondary necrosis.
In vivo several different subsets of DCs can be identified based on cell-surface expression markers. The CD8α+ DC subset selectively engulfs dying cells in culture, as well as in vivo [46] and cross-presents dead cell-associated antigens to T-cells to induce tolerance. On the other hand, CD8α− DCs are relatively poor efferocytes and so, as might be the case with mature DCs, focal areas of necrosis could develop if pockets of advanced plaques accumulated this DC subset. In this context, it is interesting to note that DCs expressing 33D1, a selective marker for CD8α− splenic DCs, have been identified in the intima of atherosclerotic lesions [14]. Interestingly, interactions between the CD8α+ DC subset and macrophages may affect efferocytosis. For example, splenic marginal zone macrophages direct the uptake of dying cells selectively by CD8α+ DCs, thereby inducing tolerance. However, deletion of these marginal zone macrophages by experimental means results in the anomalous uptake of dying cells by the normally poorly efferocytoic CD8α− DCs, which results in activation of the immune response [47]. The mechanisms of these effects and their possible relevance to defective efferocytosis in advanced atherosclerosis remain to be determined. Moreover, alterations in DC biology in advanced plaques might affect lesional T cell activation [48], which in turn could affect efferocytosis by macrophages through T cell cytokine-mediated effects [49].
Concluding remarks
Understanding the mechanisms and roles of macrophage and DC efferocytes in advanced atherosclerotic lesions may provide unique therapeutic targets for the prevention of plaque progression. We imagine that the adverse effect profile of efferocytosis-enhancing therapy would be minimal, because it would be naturally buffered by cellular “don’t eat me” signals, which should prevent removal of viable cells. To achieve the steps necessary to conceive, test, and evaluate therapy of this nature, we must first elucidate which receptors and mediators are involved in efferocytosis by lesional macrophages and DCs before the defects set in and which processes go awry as the lesions progress. Then, genetically altered models based on these discoveries can evaluate causation in vivo and provide proof-of-concept for future drug development and testing. The challenges are significant, but as more data support a key role for defective efferocytosis in plaque necrosis, the gains could be substantial.
Acknowledgement
Work described herein was supported by NIH grants HL54591 and HL75662 (to I. T.) and 1K99HL097021-01 and an Irving Institute for Clinical and Translational Research Pilot Grant (to E. T.).
Abbreviations
- AC
apoptotic cell
- LRP
low density lipoprotein related protein
- TG2
transglutaminase-2
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Lloyd-Jones D, et al. Circulation. 2010;121:e46. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 2.Tabas I, et al. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 4.Tabas I. Nat. Rev. Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabas I. Arterioscler. Thromb. Vasc. Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I. Circ. Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seimon T, Tabas I. J. Lipid Res. 2009;50:S382–S387. doi: 10.1194/jlr.R800032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrijvers DM, et al. Arterioscler. Thromb. Vasc. Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 9.Virmani R, et al. J. Interv. Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 10.Vandivier RW, et al. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 11.Tacke F, et al. J. Clin. Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P, et al. Circulation. 2008;117:3168–3170. doi: 10.1161/CIRCULATIONAHA.108.783068. [DOI] [PubMed] [Google Scholar]
- 13.Choi JH, et al. J. Exp. Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulson KE, et al. Circ. Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 15.Gordon S, Taylor PR. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 16.Trogan E, et al. Proc. Natl. Acad. Sci. USA. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packard RR, et al. Circ. Res. 2008;103:965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabas I. Nat. Rev. Immunol. 2010;10:34–36. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surh CD, Sprent J. Nature. 1994;372:100–103. [PubMed] [Google Scholar]
- 20.Elliott MR, Ravichandran KS. J. Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravichandran KS. Cell. 2003;113:817–820. doi: 10.1016/s0092-8674(03)00471-9. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, et al. J. Biol. Chem. 2006;281:6707–6717. doi: 10.1074/jbc.M510579200. [DOI] [PubMed] [Google Scholar]
- 23.Scott RS, et al. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 24.Thorp E, et al. Arterioscler. Thromb. Vasc. Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ait-Oufella H, et al. Arterioscler. Thromb. Vasc. Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 26.Seitz HM, et al. J. Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 27.Yancey PG, et al. Arterioscler. Thromb. Vasc. Biol. 2010;30:787–795. doi: 10.1161/ATVBAHA.109.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boisvert WA, et al. Arterioscler. Thromb. Vasc. Biol. 2006;26:563–569. doi: 10.1161/01.ATV.0000203503.82693.c1. [DOI] [PubMed] [Google Scholar]
- 29.Toth B, et al. J. Immunol. 2009;182:2084–2092. doi: 10.4049/jimmunol.0803444. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Oufella H, et al. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 31.Sather S, et al. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorp E, et al. Arterioscler. Thromb. Vasc. Biol. 2010;30:e186. [Google Scholar]
- 33.Hurtado B, et al. Thromb. Haemost. 2011;105:873–882. doi: 10.1160/TH10-10-0630. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, et al. J. Biol. Chem. 2008;283:35507–35516. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- 35.Quinn KA, et al. J. Biol. Chem. 1997;272:23946–23951. doi: 10.1074/jbc.272.38.23946. [DOI] [PubMed] [Google Scholar]
- 36.Komura H, et al. J. Immunol. 2009;182:581–587. doi: 10.4049/jimmunol.182.1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curtiss LK, Tobias PSJ. Lipid Res. 2009;50:S340–S345. doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, et al. Circ. Res. 2009;105:1072–1082. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaposhnik Z, et al. Arterioscler. Thromb. Vasc. Biol. 2007;27:621–627. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gautier EL, et al. Circulation. 2009;119:2367–2375. doi: 10.1161/CIRCULATIONAHA.108.807537. [DOI] [PubMed] [Google Scholar]
- 41.Yilmaz A, et al. Atherosclerosis. 2004;172:85–93. doi: 10.1016/j.atherosclerosis.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, et al. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 43.Albert ML, et al. J. Exp. Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miksa M, et al. Int. J. Mol. Med. 2008;22:743–748. [PMC free article] [PubMed] [Google Scholar]
- 45.Nakayama M, et al. Blood. 2009;113:3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 46.Iyoda T, et al. J. Exp. Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyake Y, et al. J. Clin. Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansson GK, Hermansson A. Nat. Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 49.Odegaard JI, Chawla A. Annu. Rev. Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]