Abstract
Src is a nonreceptor tyrosine kinase essential for the activation of osteoclasts, the cells that degrade bone. Src also regulates normal cell functions, cancer cell growth and metastasis to organs, including bone where tumor cells induce bone destruction by osteoclasts. Src inhibitors prevent bone destruction and tumor cell growth in animal models of metastatic bone disease, and some are being investigated in clinical trials, particularly in patients with prostate cancer, which has high bone metastatic potential. Here, we review how Src regulates osteoclast formation, activation and survival and the results of preclinical and clinical trials of Src inhibitors, which show some promise in inhibiting the effects of tumor cells on the skeleton.
Keywords: bone metastasis, bone resorption, osteoclasts, Src tyrosine kinase
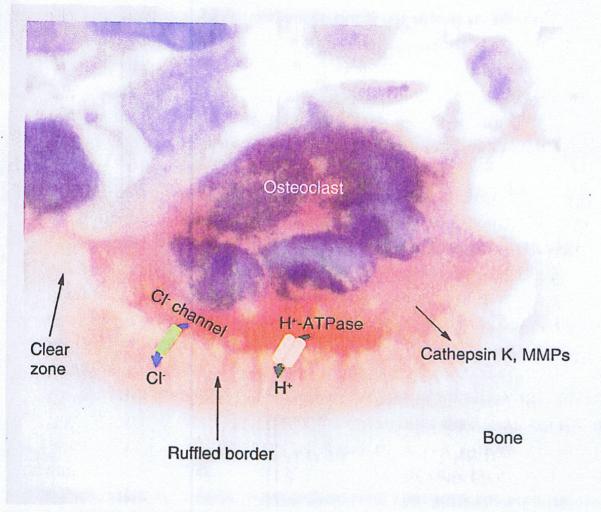
Osteoclasts are multinucleated cells derived from bone marrow precursors in the monocyte/macrophage lineage. They are formed by fusion of precursors and are the only cells that resorb bone. Bone is composed of organic matrix and nonorganic mineral, which consists mainly of hydroxyapatite crystals that can be solubilized only at low pH. Osteoclastic bone resorption occurs in two phases; solubilization of bone mineral and degradation of bone matrix [1-3]. To resorb bone, osteoclasts attach to the bone surface and form a ruffled border to their cell membrane inside a ‘clear zone’ of organelle-poor cytoplasm that seals the area that will be resorbed, and protects the rest of the cell and adjacent cells from the destructive action of resorption. Within the ruffled borders there is a V-type ATPase, a proton pump that transfers protons (H+) into the extracellular space beneath the clear zone, and a Cl−/H+ antiport channel through which chloride ions pass. Protons and chloride ions form hydrochloric acid, which can solubilize hydroxyapatite crystals. After solubilization of the nonorganic mineral, the organic matrix, which consists predominantly of collagen fibers, is degraded through a process carried out by proteolytic enzymes, including lysosomal proteinases and matrix metalloproteinases (Figure 1). The ruffled border increases the surface area of the cell membrane available for secretion and thus enhances the resorptive process. During bone resorption, growth factors stored in the bone matrix are released into the bone marrow cavity where they can affect the functions of adjacent cells, including metastatic cancer cells. Cancer cells, in turn, can release factors that stimulate osteoclast formation and activity. Thus, metastatic cancer cells can initiate a vicious cycle within the bone marrow cavity that encourages their growth and in which osteoclasts play critical supportive roles (Figure 2) [4].
Figure 1. Osteoclast resorbing bone.
The ruffled border formed on the underside of the osteoclast facing the bone increases the surface area of the cell. The clear zone surrounds the ruffled border and seals the resorbing compartment. Hydrochloric acid is formed in the cavity following active transfer of H+ by a proton pump (the vacuolar-type H+-ATPase) and passage of Cl− via a Cl− channel, and proteolytic enzymes are released passively from the osteoclast into the cavity to dissolve the mineral and matrix components of bone, respectively. (Section of calvarial bone from a mouse treated with IL-1 and stained for tartrate-resistant acid phosphatase activity with hematoxylin counterstaining).
MMP: Matrix metalloproteinase.
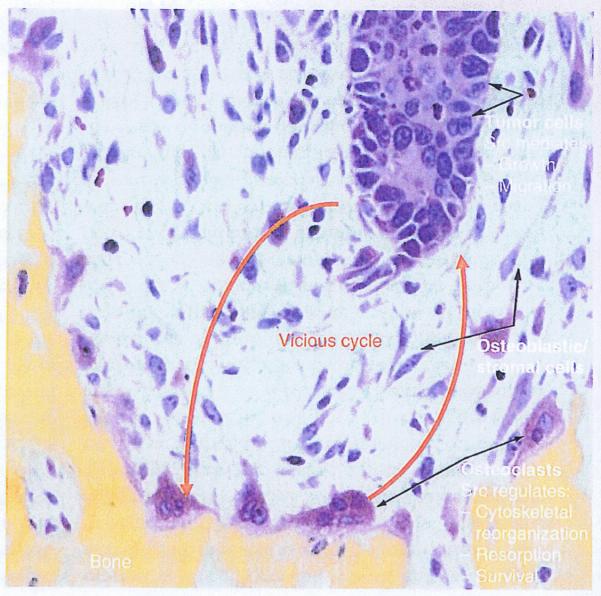
Figure 2. A vicious cycle of tumor cell-induced resorption and resorption-induced tumor growth.
Tumor cells metastasize to the bone marrow where Src signaling mediates their growth and movement within the marrow cavity. The tumor cells secrete osteoclast-stimulating factors, including PTHrP, which stimulate osteoclast formation and activation by inducing expression of RANKL by osteoblastic stromal cells within the marrow. Osteoclasts resorb the bone, releasing growth factors, including TGF-β and FGFs, which stimulate tumor cell proliferation. TGF-β also induces expression of PTHrP by the tumor cells. Src regulates various functions of cancer cells and osteoclasts, and Src inhibition could reduce growth of metastatic cancer cells in bone by disrupting this vicious cycle.
Understanding of the mechanisms that regulate osteoclast formation, activation and survival has increased significantly over the past 20 years, since the identification of a role for Src tyrosine kinase [4] in osteoclast activation and ruffled border formation [5]. This includes identification of the RANK and its ligand (RANKL) as the key regulatory molecules for osteoclast formation and activation [6-9]. RANKL is expressed on the surface of osteoblastic cells in the marrow cavity and by osteocytes embedded within bone [10,11] and it interacts with its receptor RANK, located on the surface of osteoclasts and osteoclast precursors (OCPs). RANKL/RANK interaction leads to recruitment ofTNF receptor (TNFR)-associated factors (TRAFs), adaptor proteins to which enzymes, including MAP kinases and Src tyrosine kinase, bind and are activated to mediate downstream signaling, since RANK lacks intrinsic kinase activity. TRAF-mediated signaling leads to activation of NF-κB, c-Fos, and NFATc1 [6-9], transcription factors that induce the expression of genes involved in the early stages of OCP differentiation. Later signaling results in activation of the enzymes that mediate the degradation of the matrix of bone and the secretion of protons [12] and chloride ions [13], which form hydrochloric acid for dissolution of the mineral component of bone [14,15] (reviewed by Boyle et al. [16]). Subsequently, a number of small molecules that specifically inhibit these enzymes, including vacuolar adenosine triphosphatase, cathepsin K, and Src tyrosine kinase, have been developed and are in preclinical and clinical trials in patients with increased bone resorption and/or malignancies. In addition, denosumab, a monoclonal antibody that prevents RANKL from binding to RANK [17,18] has been approved by the US FDA for the treatment of patients with osteoporosis or metastatic bone disease. Here, we review current understanding of the mechanisms whereby Src tyrosine kinase regulates osteoclast activation and the results of preclinical and clinical trials of Src inhibitors that have been developed to inhibit bone resorption in patients with metastatic bone disease.
Src tyrosine kinase
Src (pronounced sarc) is the prototypic member of a family of nonreceptor tyrosine kinases, which includes Yes, Fyn, Hck. Lck, Fgr, Blk, Lyn and Frk [19]. Nonreceptor tyrosine kinases bind directly or indirectly to cell surface receptors, such as the TNFR and RANK that lack intrinsic tyrosine kinase activity to mediate downstream signaling by phosphorylating target signaling proteins. Src was named following the discovery that its viral counterpart (v-Src) was responsible for inducing sarcomas in soft tissues of chickens [20]. Since then, numerous studies have identified roles for cellular Src in mediating changes in cell morphology, adhesion, migration, invasion, proliferation, differentiation and survival. These functions involve activation of integrin cell adhesion receptors, growth factor receptors (e.g., EGFR and HER2), steroid hormone and G-protein coupled receptors, focal adhesion kinase and cytoskeletal proteins [21,22].
Src functions in osteoclasts
In 1990, Soriano et al. generated src−/− mice [4] assuming they would identify essential roles for Src in neurologic functions or hemostasis because Src is expressed at particularly high levels in the brain and platelets [23]. Surprisingly, they found that the mice developed normally until around 10 days after birth, at which time their incisor teeth did not erupt and subsequently they failed to thrive unless they were fed a liquid chow diet [4]. Radiographs indicated that the mutant mice had osteopetrosis. Osteopetrosis is a rare genetic disease characterized by an increase in bone mass due to a failure of osteoclast formation, or of formed osteoclasts to resorb bone [24,25]. Histologic sections of bones from the src−/− mice showed that osteoclasts had formed. Thus, the osteopetrosis and failure of tooth eruption resulted from a defect in the osteoclast function [4]. Later studies showed that src−/− mice actually formed increased numbers of osteoclasts, but the cells were smaller than normal and failed to resorb bone because they did not form ruffled borders [5]. As Src did not appear to have necessary functions in any other cell types, these initial studies laid the foundation for efforts by a number of biotechnology and pharmaceutical companies to develop inhibitors that would target Src specifically in osteoclasts.
Src is expressed highly in the ruffled border membrane of osteoclasts, but it is also expressed throughout the cells in numerous organelles, suggesting that it is involved in multiple cellular functions [5]. Src is activated in osteoclasts after the cells bind to integrins in bone matrix during the initial stages of bone resorption [26]. This initiates signaling that leads to the complex intracellular cytoskeletal reorganization that is required for polarization of the cytoplasm and ruffled border formation. Src is recruited to the adapter protein TRAF6 [27], on the intracellular domain of RANK to mediate signaling downstream in osteoclasts OCPs [27]. Src recruits a number of signaling proteins to TRAF6, including Cbl [28], Pyk-2 [29] and cortactin [30], which mediate polarization of the cell and formation of podosome-rich actin rings and the ruffled border membrane in an, as yet, incompletely understood process.
Podosomes are microscopic structures formed on the cell membranes of highly motile cells, such as lymphocytes and osteoclasts, to allow them to interact transiently with and move along matrices in soft tissue and bone, respectively [31]. Podosomes consist of an actin core to which several focal adhesion proteins, including integrins, vinculin, paxillin and talin are attached. They are assembled and disassembled rapidly in belts that help form the sealing zone between the osteoclast cell membrane and bone matrix, and inside which osteoclasts secrete H+, Cl− and enzymes to degrade the bone. Podosomes formed by osteoclasts on plastic are different to those formed on bone [31]. A critical role for Src to regulate the organization and dynamics of podosomes was revealed from studies of src−/− osteoclasts in which podosome number and the podosome-associated cytoskeleton-regulating proteins are decreased [32]. Overexpression of mutant forms of Src in src−/− osteoclasts showed that both the kinase activity and either the SH2 or SH3 domains of Src are required for normal podosome function [32]. The GTPase dynamin is associated with podosomes by binding Pyk-2 in osteoclasts [33]. Overexpression of dynamin reduces growth factor- and adhesion-induced Pyk-2 phosphorylation, which is potentiated by Src through its kinase domain. Therefore, Src may activate a negative-feedback loop by promoting dynamin-mediated dephosphorylation of Pyk-2, ultimately leading to the dissociation of Src from Pyk-2 [34].
αvβ3 integrin plays a central role in osteoclast cytoskeletal organization. β3−/− mice develop progressive mild osteopetrosis due to defective ruffled border and actin ring formation [35]. Src associates constitutively with β3, an interaction that requires Src kinase activity [36]. β3/Src binding brings in a secondary tyrosine kinase, Syk, to the β3/Src complex, an interaction mediated by Src-phosphorylated tyrosine residues on the immunoreceptor tyrosine-based activation motif proteins, including Dap12 and FcRγ1, which play essential functions in osteoclast formation [37,38]. This interaction also causes Syk phosphorylation by Src and brings the Syk interacting protein SLP-76 into the complex [39]. Thus, the αvβ3-stimulated signaling complex consists of at least Src, Syk, immunoreceptor tyrosine-based activation motif-containing proteins and SLP-76. Temporal and spatial interaction and interdependency of these signaling proteins allow the formation of the highly motile osteoclast cytoskeleton required for normal bone resorption. SLP-76−/− mice form osteoclasts in response to PTH treatment in vivo, but the cells have reduced spreading in vitro and reduced resorptive capacity in vivo, indicating that SLP-76 expression is necessary for the full resorptive function of osteoclasts [40]. These processes and podosome assembly and disassembly are similar to those used by malignant cells, which form lamellipodia to invade and move through soft tissues as they invade and prepare for metastasis [41].
Following ligand binding and receptor engagement, membrane lipid rafts play a critical role in cell activation by recruiting some, and excluding other, specific signaling components of cell surface receptors. In osteoclasts, the disruption of lipid rafts by agents that extract and sequester cholesterol, and thereby disrupt rafts, blocks translocation of TRAF6 and subsequent Akt activation induced by RANKL, leading to reduced cell survival, actin ring formation and bone resorption [42]. A recent study reported that the expression levels in osteoclasts of C-terminal c-Src kinase (Csk), which negatively regulates Src kinase activity, was comparable with that in other tissues. However, in osteoclasts, Csk was expressed at very low levels in lipid rafts, where Src expression is high. This low level of expression of Csk in osteoclast lipid rafts was attributed to very low levels of Cbp, a Csk binding protein that recruits Csk into lipid rafts through direct physical interaction [43,44]. Overexpression of Cbp in osteoclasts brought Csk into lipid rafts and suppressed Src activity, actin ring formation and bone resorption. In contrast, RANKL treatment or overexpression of either TRAF6 or NFATc1 all inhibit Cbp expression. Thus, low Cbp expression maintains high c-Src activity in osteoclasts [45].
Src also appears to mediate osteoclast survival in response to RANKL, at least in vitro. For example, when RANKL and macrophage colony-stimulating factor (M-CSF) were withdrawn from src−/− or wild-type osteoclasts, the wild-type cells survived when RANKL alone was re-added, while src−/− osteoclasts died by apoptosis [27,46]. However, treatment of src−/− osteoclasts with M-CSF alone did not lead to apoptosis, indicating that Src is not necessary for M-CSF-mediated survival signaling. This anti-apoptotic effect of Src appears to be mediated by PI3-kinase, which is recruited to the intracellular domain of RANK along with TRAF6 [27]. PI3-kinase activates the Akt/mTOR pathway, leading to phosphorylation and thus inactivation of caspase 3, which induces apoptosis in response to many stimuli [47]. However, other studies have questioned this role for Akt [48]. Src inhibitors, such as the pyrrolopyrimidines CGP-76775 and CGP-76030, have been shown to influence osteoclast survival in vitro and in vivo. CGP-77675 and CGP-76030 inhibited osteoclastogenesis and resorption in osteoclast cultures, impaired adhesion ability and actin ring organization, and induced apoptosis of mature osteoclasts. In vivo, they reduced osteoclast numbers and induced osteoclast cytoskeletal disruption. The mechanism of apoptosis is associated with changes in the phosphorylation status of extracellular signal regulated kinase (ERK1/2). Blockage of ERK1/2 rephosphorylation by PD98059 reduced CGP77675- and CGP76030-induced osteoclast apoptosis [49]. In contrast to these in vitro findings, src−/− mice do not have increased osteoclast apoptosis in vivo, and indeed they actually have more osteoclasts than wild-type mice [46]. These in vitro versus in vivo differences remain unexplained, but it is possible that M-CSF signaling or perhaps other Src family kinase members substitute for Src in vivo in the src−/− mice to maintain their survival.
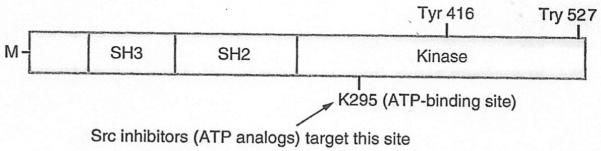
The Src protein has three major structural domains that mediate its functions [50]: the Src homology 2 (SH2) domain, to which molecules with tyrosine residues bind; the SH3 domain, which binds proline-rich portions of interacting molecules; and the kinase domain, which phosphorylates tyrosines on target proteins. Two critical phosphorylation sites in Src are tyrosine 416, which is activated through autophosphorylation, and tyrosine 527 whose phosphorylation maintains Src in an inactive state. In the inactive conformation, the tyrosine 527 is phosphorylated by C-terminal Src kinase, resulting in formation of a bridge with the SH2 domain, and the SH3 domain binds to the kinase domain to prevent its activation. Phosphorylation of Tyr 416 restructures the Src protein conformation, leading to kinase activation (Figure 3) [51]. Schwartzberg et al. designed studies to determine which domain(s) of Src mediates its resorptive function in osteoclasts [52]. They used the tartrate-resistant acid phosphatase promoter [53] to drive expression of wild-type and various mutated src genes in transgenic mice and crossed the mice with src+/− mice to generate src−/− mice that expressed these transgenes in their osteoclasts [52]. As anticipated, src−/− transgenic mice expressing wild-type Src had normal osteoclast function, bone volume and tooth eruption [52]. Surprisingly, however, 25% of the src−/− mice expressing the kinase-inactive mutant gene, K295M, had normal bone volume, tooth eruption and ruffled border formation. The ATP-binding site of Src is mutated in K295M to prevent it from phosphorylating tyrosine residues on interacting molecules. It was also surprising that 25% of the mice had more pronounced osteopetrosis than src−/− mice, associated with significantly increased osteoclast apoptosis, while the remaining 50% of the mice had no change in bone volume or osteoclast survival [52]. These divergent findings remain unexplained, but they may reflect the effects of optimal (rescue of the osteopetrosis) versus high (more marked osteoporosis) expression levels of K295M proteins as the severity of the osteopetrosis correlated with the number of apoptotic osteoclasts in bone sections from the mice [52]. Furthermore, expression of the SH2 domain of the molecule, without the kinase domain (termed Src-251), also induced more pronounced osteopetrosis in src−/− transgenic mice and in wild-type mice due to a dominant negative function of the protein to increase osteoclast apoptosis. Osteoclasts from these mice had reduced phospho-Akt and PI3 kinase levels, consistent with a failure of Src activation of the survival pathway [46]. A recent study indicates that Src-251 mediates degradation of NF-κB essential modulator, thereby inhibiting RANKL-induced activation of NF-κB signaling. This process also requires appropriate cellular localization of Src-251, because deletion of its myristoylation domain ablated its degradation capacity. This study provides a new molecular mechanism for reduced osteoclast function in Src-251 transgenic mice [54].
Figure 3. Src tyrosine kinase structural domains.
The Src protein has three major structural domains that mediate its functions: the SH2 domain to which molecules with tyrosine residues bind; the SH3 domain, which binds proline-rich portions of interacting molecules, and the kinase domain, which phosphorylates tyrosines on target proteins. Autophosphorylation of tyrosine 416 keeps the Src kinase in an active conformation while phosphorylation of tyrosine 527 has the opposite effect. Tyrosine 295 (K295) is in the ATP-binding site and is essential for Src kinase activity. The N-terminal region of Src is M, which allows it to be associated with the cell membrane.
M: Myristoylated; SH: Src homology.
Src functions in malignancy
Src tyrosine kinase also has important regulatory functions in malignant cells, including cell division, movement through tissues and growth factor signaling [21]. Its activity is increased in many cancers, including some that metastasize to bone, such as breast and prostate cancer [55]. Similar to human EGFRs (e.g., HER2), Src is overexpressed in more than 70% of breast cancers [56] and mediates downstream signaling from these receptors [57]. HER2 is commonly overexpressed in hormone-resistant breast cancers and confers a poorer prognosis. Trastuzumab is an FDA-approved HER2 inhibitor, which has been reported to increase survival of patients with HER2-positive metastatic breast cancers [58]. However, approximately half of patients with HER2-overexpressing breast cancer do not respond to trastuzumab-based treatment regimens because the tumor cells develop resistance to treatment. Src appears to be involved in the development of resistance to trastuzumab treatment of breast cancers [59], based on a study that found that Src activation not only mediated trastuzumab resistance in breast cancer cells in vitro, but also correlated with both lower response to trastuzumab-based therapy and poorer survival of patients who received therapy. These data suggest that a combination of a Src inhibitor plus trastuzumab could be useful clinically for overcoming trastuzumab resistance in patients with Her-2 overexpressing breast cancers.
Overexpression of a constitutively active Src in MDA-MB-231 breast cancer cells increased, while a kinase-inactive Src construct decreased the area of osteolytic metastases [60]. The increased osteolysis was associated with increased production of PTHrP and PTHrP promoter activity in MDA-MB-231 cells overexpressing constitutively active Src kinase. A more recent study reported that Src knockdown in MDA-MB-231 breast cancer cells reduced MDA-MB-231 cell metastasis to bone, and growth of the cells in and out of bone, and also enhanced survival of mice after day 21 following intracardiac tumor cell inoculation [61]. Knockdown of two other Src family kinases, Fyn and Yes, did not inhibit the bone metastatic activity of MDA-MB-231 cells, indicating specificity for Src versus these other kinases. Src overexpression did not promote growth of MDA-MB-231 cells injected into the mammary gland or metastasis of the cells to lung, suggesting that effects of Src expression are selective for bone in this mouse model [61]. These investigators also reported that bioinformatics analyses identified a strong association between late-onset bone metastasis and Src activity in more than 600 breast cancer patients, which they called a Src response signature. Interestingly, a TGF-β response signature in primary tumors was associated with lung, but not bone metastasis in these patients. Most breast cancers that metastasize to bone are ER+/PR− [62]. Zhang et al. also found that their Src response signature defined a subset of ER− patients that develop delayed bone metastases [61]. Thus, Src inhibiting drugs could potentially inhibit not only osteoclasts, but also tumor cells and their interactions with osteoclasts in patients with metastatic bone disease. The involvement of Src in the regulation of both osteoclasts and breast cancer cells highlights the potential importance of Src as a target in the treatment of bone metastases. Preclinical studies in which genetically modified cancer cells are transplanted to nude mice demonstrate a ‘vicious cycle’ among cancer and bone cells (especially osteoclasts and bone matrix) [63]. Tumor cells metastasize to the bone marrow where Src signaling mediates their growth and movement within the marrow cavity. The tumor cells secrete osteoclast-stimulating factors, including PTHrP, which stimulate osteoclast formation and activation by inducing expression of RANKL by osteoblastic stromal cells within the marrow. Osteoclasts resorb the bone, releasing growth factors, which stimulate tumor cell proliferation and their expression of PTHrP. Since Src regulates various functions of cancer cells and osteoclasts, Src inhibitors could reduce the growth of bone metastases by disrupting the vicious cycle (Figure 2).
Src tyrosine kinase inhibitors
Several pharmaceutical companies have developed Src inhibitors as potential therapeutic agents for use in diseases with increased resorption, such as osteoporosis and osteolytic bone disease. Examples of such Src inhibitors (Table 1) include the ATP-based compounds NVP-AAK980[64], AZD0530 (Saracatinib) [65], AP23451 [66], the pyrazolopyrimidines PP1 and PP2 [67], the pyrrolopyrimidines CGP-76775 and CGP-76030 [68], the pyridopyrimidinones PD166326 and PD18097 [69], the quinazoline AZM475271 [70], the quinoline SKI-606 [71], the indolinone SU6656 [72] and BMS-354825 (dasatinib), a small-molecule multitargeted kinase inhibitor that potently inhibits BCR-ABL, SRC family and other kinases by binding to the ATP-binding site of the enzymes [67]. Among these, saracatinib and dasatinib have been used in clinical trials to treat patients with some forms of metastatic or antidrug-resistant cancers (Table 2), details of which are summarized below.
Table 1.
Src inhibitors and their utilization.
| Name | Utilization | Ref. |
|---|---|---|
| ATP-based | ||
| NVP-AAK980 | Preclinical | [64] |
| AZD0530 (saracatinib) | Preclinical | [59,73,74] |
| Clinical | [65,75–77] | |
| AP23451 | Preclinical | [66,88–91] |
| Pyrazolopyrimidines (PP1, PP2) | Preclinical | [67] |
| Pyrrolopyrimidines (CGP-77675, CGP-76030) | Preclinical | [49,68] |
| Pyridopyrimidinones (PD166326, PD18097) | Preclinical | [69] |
| Quinazoline (AZM475271) | Preclinical | [70] |
| Quinolin (SKI-606) | Preclinical | [71] |
| Indolinone | ||
| SU6656 | Preclinical | [72] |
| BMS-354825 (Sprycel®) | Preclinical | [61,67,80–83,85] |
| Clinical | [78,79,86,87] |
Table 2.
Src inhibitors and their clinical applications.
| Name | Company | Type of cancers | Ref. |
|---|---|---|---|
| Saracatinib (AZD0530) | AstraZeneca | Phase I, normal subjects | [65] |
| Phase II, CRPC | [75] | ||
| Phase II ER−/PR− breast cancer | [76] | ||
| Phase II, gastric cancer, gastroesophageal junction adenocarcinoma | [77] | ||
| Dasatinib-Spycel | Bristol-Myers Squibb | Chronic myeloid leukemia Philadelphia chromosome and acute lymphoblastic leukemia |
[78,79] |
| Phase II, CRPC | [86,87] | ||
| Metastatic colorectal cancer | ASCO 2011 | ||
| Hormone-refractory prostate cancer | ASCO 2011 | ||
| Metastatic melanoma | ASCO 2011 | ||
| Imatinib-resistant gastrointestinal stromal tumor | ASCO 2011 |
ASCO: American Society of Clinical Oncology; CRPC: Castration-resistant prostate cancer.
Saracatinib (AZD0530) is an orally administered, highly selective, small molecule with dual-specific inhibition of Src kinase and Bcr-Abl, which was developed by AstraZeneca [65]. Saracatinib inhibited bone resorption of mature osteoclasts in cultures of isolated rabbit osteoclasts and mouse calvarial explants. In co-cultures of human osteoblasts and PBMCs, it reduced actin ring formation and the formation of osteoclasts and their resorptive activity [73]. It also inhibited osteoclast numbers and osteolysis induced by PC-3 human prostate cancer cells injected into the tibiae of nude mice [74]. Saracatinib sensitized trastuzumab-resistant BT474 breast cancer cells to trastuzumab in vivo, in part through induction of apoptosis of the tumor cells [59]. Furthermore, a combination of saracatinib and trastuzumab caused subcutaneously injected tumors to become barely detectable 21 days after treatment, an effect that was not observed with either treatment alone [59]. In two randomized, double-blind, placebo-controlled, Phase I clinical trials, saracatinib significantly decreased mean serum and urine levels of the bone resorption markers, serum cross-linked C-telopeptides and urinary cross-linked N-telopeptides, from baseline. In one study, the 1000 mg dose in a single ascending dose (2.5–1000 mg) study was effective [65], and in the other multiple ascending dose (60–250 mg) study in healthy, male volunteers the highest dose was effective (88% decrease in C-telopeptide levels) [65]. There were no significant effects on levels of bone formation markers and no significant adverse events were reported. However, saracatinib had little efficacy in a Phase II trial of 28 patients, with a median age of 67 years with advanced castration-resistant prostate cancer (CRPC) given 175 mg orally, once-daily. The median progression-free survival time was 8 weeks. Treatment was generally well tolerated, but showed little clinical efficacy as monotherapy [75]. The authors concluded that it warrants further investigation in earlier-stage prostate cancer or as combination therapy. A more recent study reports that saracatinib did not appear to have significant single-agent activity for the treatment of patients with ER−/PR− metastatic breast cancer [76]. The study was terminated prematurely because the results were not sufficiently promising to justify continued accrual. Only nine patients were recruited; common adverse events included fatigue, elevated liver enzymes, nausea, hyponatremia, dyspnea, cough and adrenal insufficiency.
Saracatinib also has been used in the treatment of patients with other types of solid tumors. In a recent Phase II trial, saracatinib was tested in ten patients with gastric adenocarcinoma and in 11 cases of gastroesophageal junction adenocarcinoma. Objective response and prolonged stable disease rates were used as outcome measures. No significant benefits were observed, suggesting that saracatinib as a single agent is not sufficient to treat patients with advanced gastric adenocarcinoma [77].
Bristol-Myers Squibb has developed dasatinib (BMS-354825, Sprycel®), a potent, orally active inhibitor of several oncogenic protein tyrosine kinases (active in the low nm range), including members of the Src tyrosine kinase family [78,79]. It is an FDA-approved drug for the treatment of chronic myeloid leukemia, Philadelphia chromosome and acute lymphoblastic leukemia [78,79]. Dasatinib inhibits osteoclast formation and bone resorption in vitro, in part by inhibiting Src activity [80] and osteoclastogenic signaling mediated by M-CSF and its receptor, c-fms [81]. Dasatinib has also been found to accelerate the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts [82] and to suppress proliferation and enhance differentiation of osteoblasts [83]. This is in keeping with previous studies that reported that Src negatively regulates osteoblast functions based on the observation that src−/− mice have increased bone formation [84].
Dasatinib (2.5–20 nM) inhibited resorption by osteoclasts derived from human CD14+ mononuclear cells [81]. Dasatinib inhibited the growth of MDA-231 cells injected into the tibial bone marrow cavity when given with or after tumor cell injection [61]. Surprisingly, in these studies dasatinib induced apoptosis of MDA-231 cells in the tibial bone marrow cavity, but did not affect osteoclast numbers, suggesting that its effects may primarily be directed at inhibition of survival-related functions of kinases in the tumor cells. In contrast, dasatinib alone, or in combination with docetaxel, not only inhibited increases in serum PSA levels in SCID mice given intra-tibial injections of C4–2B prostatic cancer cells, but it also prevented the osteolysis seen in control mice, associated with an increase in bone mineral density [85]. Neither osteoclast numbers nor markers of bone resorption were assessed in this study in which the C4–2B cell line used has osteoblastic properties, making it difficult to be certain that dasatinib had an inhibitory effect in vivo on bone resorption. However, the radiographs illustrated in the paper show that control mice had osteolysis in their tibiae, suggesting that the tumor cells induced osteolysis in these experiments and that dasatinib prevented it, but docetaxel alone did not.
In clinical studies, dasatinib decreased bone turnover in two Phase II studies in 48 patients with CRPC, the response rates were similar, but modest for two dosing regimens; urinary N-telopeptide decreased by >40% in 21 out of 43 evaluable patients; serum bone-specific alkaline phosphatase, a marker of osteoblast activity, decreased in 25 out of 44 patients [86]. This study suggests that dasatinib may be effective at stabilizing metastatic prostate cancer disease and decreasing bone turnover. In another study, dasatinib plus docetaxel reduced bone turnover in 46 patients with CRPC: serum PSA values decreased in 13 out of 32 patients; 12 out of 21 patients had reduced response evaluation criteria in solid tumors; nine patients had stable disease (four at >21 weeks and five at >6 weeks); serum urinary N-telopeptide decreased by >35% in 12 out of 26 patients; bone-specific alkaline phosphatase decreased by >35% in 17 out of 24 patients [87]. These are encouraging results, but further studies will be required to identify optimal doses of dasatinib in combination studies.
The potential utility of Src inhibitors extends beyond metastatic bone disease, as seen in the programs of the recent American Society of Clinical Oncology meetings (17–19 February 2011, Orlando, FL, USA and 4–8 June 2011, Chicago, IL, USA). Numerous clinical studies have reported the results of dasatinib given alone or in combination with other chemotherapeutic agents in the treatment of patients with a variety of cancers, including chronic myeloid leukemia, metastatic colorectal cancer, hormone-refractory prostate cancer, metastatic melanoma and imatinib-resistant gastrointestinal stromal tumor [88,89].
Since Src and other Src tyrosine kinases are so widely expressed and have overlapping functions, development of inhibitors that target Src specifically in bone without having adverse effects on other cells is likely to be challenging. The ARIAD pharmaceutical company initiated a program in the 1990s to develop a series of Src kinase inhibitors, including some directed at the kinase domain with varying ATP-related templates (Figure 3) and others directed at the SH2 domain [90]. They targeted these inhibitors to bone by incorporating a bisphosphonate group into them that lacked intrinsic anti-osteoclast activity, thereby conferring bone-targeting and tissue selectivity properties to potentially minimize adverse effects in vivo [91]. One of these, AP23451 is a purine-based Src tyrosine kinase inhibitor that inhibited osteoclast formation and survival in vitro in the 0.1 to 1 μM range and following daily sub-subcutaneous injections dose-dependently prevented PTH-induced bone resorption and ovariectomy-induced bone loss [92]. AP23451 also prevented osteolysis induced by metastatic MDA-MB-231 breast cancer cells, similar to the bisphosphonate zoledronic acid [93], and reduced tumor cell volume in the marrow cavities of the mice, which was not observed in mice treated with zoledronic acid. These findings suggest that this Src inhibitor may have had inhibitory effects on tumor cells distinct from its effects on osteoclasts. Since AP23451 inhibited resorption similarly to zoledronic acid, it is unlikely that differences in the amount of growth factors released from the bone, and thus available locally to stimulate tumor cell growth, could explain the reduced numbers of tumor cells [94]. Since many cancer cells express Src, these findings support the possibility that Src inhibitors could target tumor cells and osteoclasts in patients with bone metastases. Despite these promising initial findings, adverse effects observed in later preclinical toxicity studies led to termination of this Src inhibitor program.
Future perspective
It has been known for decades that Src activity is increased and plays important pathogenetic roles in many common cancers and that it is required for osteoclastic bone resorption. Despite this knowledge, only two Src inhibitors (saracatinib and dasatinib) have been investigated in clinical trials for the treatment of metastatic bone disease. Neither of these drugs is a Src-specific inhibitor and in that respect they are similar to bosutinib (SKI-606; Wyeth), a dual Src/Abl kinase inhibitor that has not been studied in clinical trials and probably will not be investigated further for drug development at this time. Other pharmaceutical companies have developed Src inhibitors, but have not pursued their development into clinical trials, in part because of adverse effects observed in preclinical toxicity studies. Saracatinib and dasatinib are being investigated in ongoing Phase II and III clinical trials in a variety of clinical settings, including metastatic bone disease, alone and in combination with standard chemotherapy.
So what does the future hold for Src inhibitors in the setting of metastatic bone disease? As patients with cancer live longer, the number of individuals with metastatic bone disease is likely to increase, along with a growing need to prevent the so-called skeletal-related events (SREs) that accompany bone metastases. SREs include bone pain, hypercalcemia, the need for radiation therapy or surgery to prevent fractures, pathologic and radiologic fractures and progression of bone metastases. Intravenously administered bisphosphonates have been the standard of care for the prevention of skeletal complications in breast and prostate cancer patients with bone metastases for several years, and typically are given along with standard adjuvant chemotherapy [95]. Denosumab, a monoclonal antibody against RANKL, has recently been approved by the FDA as another antiresorptive agent in patients with metastatic bone disease [96,97]. To date, studies have not proven that either of these types of drugs significantly affect tumor cell growth or survival in vivo in humans, despite their ability to reduce SREs. Thus, there remains a need for agents that will not only inhibit bone resorption, but also tumor cell growth and/or survival. Src inhibitors should theoretically be able to fill this gap in treatment options, but their development could be halted if adverse effects limit their use in long-term clinical trials with efficacious doses. Treatment of some patients with metastatic bone disease with intravenously administered bisphosphonates, along with other standard chemotherapeutic agents, has been associated with the development of osteonecrosis of the jaw (ONJ) [98]. More recent studies have reported ONJ occurring in some patients with metastatic bone disease given denosumab along with chemotherapy [99], adding to the controversy surrounding whether there may be a causal link between the use of resorption inhibitors and ONJ in this setting [100,101]. ONJ has not been reported in patients given the Src inhibitors, dasatinib or saracatanib. However, the number of patients treated with these inhibitors alone or in combination with standard chemotherapeutic agents has been relatively small and the duration of therapy has been limited. Further studies will be required to determine if long-term treatment with Src inihitors is associated with the development of ONJ. Ongoing clinical trials should determine the safety profiles of saracatinib and dasatinib in patients with metastatic bone disease in the next few years, as well as the most effective combination therapies. Since the number of patients that need to be enrolled in such trials is much lower than those required to demonstrate fracture reduction in patients with osteoporosis, for example, the cost and duration of these studies is significantly less. Thus, it is likely that in the next 5–10 years a Src inhibitor will be approved for administration to patients with metastatic bone disease to reduce SREs.
Executive summary.
■ Src tyrosine kinase has been implicated in tumor cell growth and metastasis for decades, and in osteoclast function for over 20 years.
■ Src expression is required in osteoclasts for effective bone resorption.
■ Src interacts with a number of key molecules on cell surface receptors in osteoclasts to mediate signaling involved in osteoclast activation and survival.
■ Pharmaceutical companies have developed several Src inhibitors.
■ Preclinical studies with small-molecule Src inhibitors have demonstrated efficacy in reducing progression of osteolytic bone metastases.
■ Clinical studies have shown that orally active Src inhibitors can reduce serum levels of markers of bone resorption in healthy individuals and in patients with metastatic prostate cancer.
■ Studies to date have not reported serious adverse effects of Src inhibitors in clinical studies of patients with metastatic bone disease, but further studies will be required to identify optimal doses for these inhibitors in combination studies with established chemotherapeutic agents.
Acknowledgments
This work was partially supported by Grants R01-43510 to BF Boyce and R01-AR48697 to LX from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Ethical conduct of research The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Boyce BF, Yao Z, Xing L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit. Rev. Eukaryot. Gene Expr. 2009;19(3):171–180. doi: 10.1615/critreveukargeneexpr.v19.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Ann. Rev. Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003;4(8):638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 4.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-Src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. ■■ First study using a genetic approach to eliminate Src expression in mice to determine the necessary functions of Src in vivo. Unexpectedly, the only major phenotype in the Src−/− was osteopetrosis, which can occur due to a defect in osteoclast formation or function.
- 5.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J. Clin. Invest. 1992;90(4):1622–1627. doi: 10.1172/JCI116032. ■■ Examines the potential mechanisms whereby Src expression in osteoclasts was required for bone resorption. It reported that formation of the ruffled border membrane, a characteristic morphologic feature of activated osteoclasts, requires Src expression in osteoclasts.
- 6.Iotsova V, Caamano J, Loy J, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-B1 and NF-B2. Nat. Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 7.Franzoso G, Carlson L, Xing L, et al. Requirement for NF-κB in osteoclast and B cell development. Genes Dev. 1997;11(24):3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong YY, Boyle WJ, Penninger JM. Osteoprotegerin ligand: a common link between osteoclastogenesis, lymph node formation and lymphocyte development. Immunol. Cell Biol. 1999;77(2):188–193. doi: 10.1046/j.1440-1711.1999.00815.x. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011;17(10):1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 11.Xiong J, Onal M, Jilka RL, et al. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011;17(10):1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YP, Chen W, Liang Y, et al. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat. Genet. 2011;23(4):447–451. doi: 10.1038/70563. [DOI] [PubMed] [Google Scholar]
- 13.Kornak U, Kasper D, Bosl MR, et al. Loss of the C1C-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104(2):205–215. doi: 10.1016/s0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 14.Gowen M, Lazner F, Dodds R, et al. Cathepsin κ knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J. Bone Miner. Res. 1999;14(10):1654–1663. doi: 10.1359/jbmr.1999.14.10.1654. [DOI] [PubMed] [Google Scholar]
- 15.Hofbauer LC, Heufelder AE. Osteopetrosis in cathepsin κ-deficient mice. Eur. J. Endocrinol. 1999;140(5):376–377. doi: 10.1530/eje.0.1400376. [DOI] [PubMed] [Google Scholar]
- 16.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 17.Lipton A. Denosumab in breast cancer. Curr. Oncol. Rep. 2011;13(1):1–4. doi: 10.1007/s11912-010-0135-y. [DOI] [PubMed] [Google Scholar]
- 18.Charopoulos I, Orme S, Giannoudis PV. The role and efficacy of denosumab in the treatment of osteoporosis: an update. Expert Opin Drug Saf. 2011;10(2):205–217. doi: 10.1517/14740338.2010.516249. [DOI] [PubMed] [Google Scholar]
- 19.Erpel T, Courtneidge SA. Src family protein tyrosine kinases and cellular signal transduction pathways. Curr. Opin Cell Biol. 1995;7(2):176–182. doi: 10.1016/0955-0674(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 20.Golden A, Brugge JS. The cellular and viral src gene. In: Reddy EP, Skalka AM, Curran T, editors. The Oncongen Handbook. Volume 7. Elsevier Sceince Publishers; Amsterdam, The Netherlands: 1986. [Google Scholar]
- 21.Schwartzberg PL. The many faces of Src: multiple functions of a prototypical tyrosine kinase. Oncogene. 1998;17(11):1463–1468. doi: 10.1038/sj.onc.1202176. [DOI] [PubMed] [Google Scholar]
- 22.Guarino M. Src signaling in cancer invasion. J. Cell Physiol. 2010;223(1):14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 23.Golden A, Nemeth SP, Brugge JS. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc. Natl Acad. Sci. USA. 1986;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teti A, Migliaccio S, Taranta A, et al. Mechanisms of osteoclast dysfunction in human osteopetrosis: abnormal osteoclastogenesis and lack of osteoclast-specific adhesion structures. J. Bone Miner. Res. 1999;14(12):2107–2117. doi: 10.1359/jbmr.1999.14.12.2107. [DOI] [PubMed] [Google Scholar]
- 25.Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42(1):19–29. doi: 10.1016/j.bone.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 26.Insogna KL, Sahni M, Grey AB, et al. Colony-stimulating factor-1 induces cytoskeletal reorganization and c-src-dependent tyrosine phosphorylation of selected cellular proteins in rodent osteoclasts. J. Clin. Invest. 1997;100(10):2476–2485. doi: 10.1172/JCI119790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong BR, Besser D, Kim N, et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell. 1999;4(6):1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka S, Amling M, Neff L, et al. c-Cbl is downstream of c-Src in a signaling pathway necessary for bone resorption. Nature. 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- 29.Sanjay A, Houghton A, Neff L, et al. Cbl associates with Pyk2 and Src to regulate Src kinase activity, α(v)β(3) integrin-mediated signaling, cell adhesion, and osteoclast motility. J. Cell Biol. 2001;152(1):181–195. doi: 10.1083/jcb.152.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trubert CL, Bernard F, Hivroz C, et al. PP60c-src expression in osteoclasts from osteopetrotic children and in giant tumor cells. Eur. J. Histochem. 1997;41(3):169–176. [PubMed] [Google Scholar]
- 31.Saltel F, Chabadel A, Bonnelye E, Jurdic P. Actin cytoskeletal organisation in osteoclasts: a model to decipher transmigration and matrix degradation. Eur. J. Cell Biol. 2008;87(8–9):459–468. doi: 10.1016/j.ejcb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Destaing O, Sanjay A, Itzstein C, et al. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol. Biol. Cell. 2008;19(1):394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruzzaniti A, Neff L, Sanjay A, et al. Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity. Mol. Biol. Cell. 2005;16(7):3301–3313. doi: 10.1091/mbc.E04-12-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruzzaniti A, Neff L, Sandoval A, Du L, Horne WC, Baron R. Dynamin reduces Pyk2 Y402 phosphorylation and SRC binding in osteoclasts. Mol. Cell Biol. 2009;29(13):3644–3656. doi: 10.1128/MCB.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McHugh KP, Hodivala-Dilke K, Zheng MH, et al. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 2000;105(4):433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou W, Kitaura H, Reeve J, et al. Syk, c-Src, the αvβ3 integrin and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J. Cell Biol. 2007;176(6):877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou W, Reeve JL, Liu Y, et al. DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Mol. Cell. 2008;31(3):422–431. doi: 10.1016/j.molcel.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat. Rev. Rheumatol. 2009;5(12):667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 39.Zou W, Reeve JL, Zhao H, et al. Syk tyrosine 317 negatively regulates osteoclast function via the ubiquitin-protein isopeptide ligase activity of Cbl. J. Biol. Chem. 2009;284(28):18833–18839. doi: 10.1074/jbc.M109.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeve JL, Zou W, Liu Y, et al. SLP-76 couples Syk to the osteoclast cytoskeleton. J. Immunol. 2009;183(3):1804–1812. doi: 10.4049/jimmunol.0804206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi H, Pixley F, Condeelis J. Invadopodia and podosomes in tumor invasion. Eur. J. Cell Biol. 2006;85(3–4):213–218. doi: 10.1016/j.ejcb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Ha H, Kwak HB, Lee SK, et al. Membrane rafts play a crucial role in receptor activator of nuclear factor κB signaling and osteoclast function. J. Biol. Chem. 2003;278(20):18573–18580. doi: 10.1074/jbc.M212626200. [DOI] [PubMed] [Google Scholar]
- 43.Kawabuchi M, Satomi Y, Takao T, et al. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404(6781):999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 44.Brdicka T, Pavlistova D, Leo A, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 2000;191(9):1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsubara T, Ikeda F, Hata K, et al. Cbp recruitment of Csk into lipid rafts is critical to c-Src kinase activity and bone resorption in osteoclasts. J. Bone Miner. Res. 2010;25(5):1068–1076. doi: 10.1359/jbmr.091039. [DOI] [PubMed] [Google Scholar]
- 46.Xing L, Venegas AM, Chen A, et al. Genetic evidence for a role for Src family kinases in TNF family receptor signaling and cell survival. Genes Dev. 2001;15(2):241–253. doi: 10.1101/gad.840301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glantschnig H, Fisher JE, Wesolowski G, et al. M-CSF, TNFα and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10(10):1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 48.Sugatani T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J. Biol. Chem. 2005;280(5):3583–3589. doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]
- 49.Recchia I, Rucci N, Funari A, et al. Reduction of c-Src activity by substituted 5,7-diphenyl-pyrrolo[2,3-d]-pyrimidines induces osteoclast apoptosis in vivo and in vitro. Involvement of ERK1/2 pathway. Bone. 2004;34(1):65–79. doi: 10.1016/j.bone.2003.06.004. ■■ Reports the effects of dasatinib, one of two Src inhibitors currently under investigation in clinical trials, on osteoclast apoptosis and the molecular mechanisms involved.
- 50.Pawson T, Gish GD. SH2 and SH3 domains: from structure to function. Cell. 1992;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- 51.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23(48):7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 52.Schwartzberg PL, Xing L, Hoffmann O, et al. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 1997;11(21):2835–2844. doi: 10.1101/gad.11.21.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyce BF, Wright K, Reddy SV, et al. Targeting simian virus 40 T antigen to the osteoclast in transgenic mice causes osteoclast tumors and transformation and apoptosis of osteoclasts. Endocrinology. 1995;136(12):5751–5759. doi: 10.1210/endo.136.12.7588333. [DOI] [PubMed] [Google Scholar]
- 54.Dai S, Abu-Amer W, Karuppaiah K, et al. Evidence that the kinase-truncated c-Src regulates NF-κB signaling by targeting NEMO. J. Cell Biochem. 2011;112(9):2463–2470. doi: 10.1002/jcb.23170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Araujo J, Logothetis C. Targeting Src signaling in metastatic bone disease. Int. J. Cancer. 2011;124(1):1–6. doi: 10.1002/ijc.23998. 2009. [DOI] [PubMed] [Google Scholar]
- 56.Belsches-Jablonski AP, Biscardi JS, Peavy DR, et al. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene. 2001;20(12):1465–1475. doi: 10.1038/sj.onc.1204205. [DOI] [PubMed] [Google Scholar]
- 57.Ishizawar RC, Miyake T, Parsons SJ. c-Src modulates ErbB2 and ErbB3 heterocomplex formation and function. Oncogene. 2007;26(24):3503–3510. doi: 10.1038/sj.onc.1210138. [DOI] [PubMed] [Google Scholar]
- 58.Bartsch R, Rottenfusser A, Wenzel C, et al. Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J. Neurooncol. 2007;85(3):311–317. doi: 10.1007/s11060-007-9420-5. [DOI] [PubMed] [Google Scholar]
- 59.Zhang S, Huang WC, Li P, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat. Med. 2011;17(4):461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Myoui A, Nishimura R, Williams PJ, et al. C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res. 2003;63(16):5028–5033. [PubMed] [Google Scholar]
- 61.Zhang XH, Wang Q, Gerald W, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16(1):67–78. doi: 10.1016/j.ccr.2009.05.017. ■■ Reports that inhibition of Src in breast cancer cells reduced their metastasis to bone and enhanced survival of mice. Also demonstrated a strong association between late-onset bone metastasis and Src activity in more than 600 breast cancer patients. Thus, drugs inhibiting Src could potentially inhibit not only osteoclasts, but also tumor cells and their interactions with osteoclasts in patients with metastatic bone disease.
- 62.Wei B, Wang J, Bourne P, et al. Bone metastasis is strongly associated with estrogen receptor-positive/progesterone receptor-negative breast carcinomas. Hum. Pathol. 2008;39(12):1809–1815. doi: 10.1016/j.humpath.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 63.Casimiro S, Guise TA, Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Mol. Cell Endocrinol. 2009;310(1–2):71–81. doi: 10.1016/j.mce.2009.07.004. ■ Comprehesively covers current understanding of bone and cancer cell interactions in the bone microenviroment.
- 64.Missbach M, Altmann E, Susa M. Tyrosine kinase inhibition in bone metabolism. Curr. opin Drug Discov. Devel. 2000;3(5):541–548. [PubMed] [Google Scholar]
- 65.Hannon RA, Clack G, Rimmer M, et al. Effects of the Src kinase inhibitor saracatinib (AZD0530) on bone turnover in healthy men: a randomized, double-blind, placebo-controlled, multiple-ascending-dose Phase I trial. J. Bone Miner. Res. 2010;25(3):463–471. doi: 10.1359/jbmr.090830. [DOI] [PubMed] [Google Scholar]
- 66.Sundaramoorthi R, Kawahata N, Yang MG, et al. Structure-based design of novel nonpeptide inhibitors of the Src SH2 domain:phosphotyrosine mimetics exploiting multifunctional group replacement chemistry. Biopolymers. 2003;71(6):717–729. doi: 10.1002/bip.10600. [DOI] [PubMed] [Google Scholar]
- 67.Hanke JH, Gardner JP, Dow RL, et al. Discovery of a novel, potent and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 1996;271(2):695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 68.Missbach M, Jeschke M, Feyen J, et al. A novel inhibitor of the tyrosine kinase Src suppresses phosphorylation of its major cellular substrates and reduces bone resorption in vitro and in rodent models in vivo. Bone. 1999;24(5):437–449. doi: 10.1016/s8756-3282(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 69.Kraker AJ, Hartl BG, Amar AM, et al. Biochemical and cellular effects of c-Src kinase-selective pyrido[2, 3-d]pyrimidine tyrosine kinase inhibitors. Biochem. Pharmacol. 2000;60(7):885–898. doi: 10.1016/s0006-2952(00)00405-6. [DOI] [PubMed] [Google Scholar]
- 70.Ple PA, Green TP, Hennequin LF, et al. Discovery of a new class of anilinoquinazoline inhibitors with high affinity and specificity for the tyrosine kinase domain of c-Src. J. Med. Chem. 2004;47(4):871–887. doi: 10.1021/jm030317k. [DOI] [PubMed] [Google Scholar]
- 71.Boschelli DH. 4-anilino-3-quinoline-carbonitriles: an emerging class of kinase inhibitors. Curr. Top. Med. Chem. 2002;2(9):1051–1063. doi: 10.2174/1568026023393354. [DOI] [PubMed] [Google Scholar]
- 72.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol. Cell Biol. 2000;20(23):9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Vries TJ, Mullender MG, van Duin MA, et al. The Src inhibitor AZD0530 reversibly inhibits the formation and activity of human osteoclasts. Mol. Cancer Res. 2009;7(4):476–488. doi: 10.1158/1541-7786.MCR-08-0219. [DOI] [PubMed] [Google Scholar]
- 74.Yang JC, Bai L, Yap S, et al. Effect of the specific Src family kinase inhibitor saracatinib on osteolytic lesions using the PC-3 bone model. Mol. Cancer Ther. 2010;9(6):1629–1637. doi: 10.1158/1535-7163.MCT-09-1058. [DOI] [PubMed] [Google Scholar]
- 75.Lara PN, Longmate J, Evans CP, et al. A Phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study. Anticancer Drugs. 2009;20(3):179–184. doi: 10.1097/CAD.0b013e328325a867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gucalp A, Sparano JA, Caravelli J, et al. Phase II trial of saracatinib (AZD0530), an oral src-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clin. Breast Cancer. 2011;11(5):306–311. doi: 10.1016/j.clbc.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mackay HJ, Au HJ, McWhirter E, et al. A Phase II trial of the Src kinase inhibitor saracatinib (AZD0530) in patients with metastatic or locally advanced gastric or gastro esophageal junction adenocarcinoma: a trial of the PMH Phase II consortium. Invest. New Drugs. 2011 doi: 10.1007/s10637-011-9650-4. doi:10.1007/s10637-011-9650-4Online First™. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 78.Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Clin. Ther. 2011;29(11):2289–2308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Quintas-Cardama A, Kantarjian H, Cortes J. Targeting ABL and SRC kinases in chronic myeloid leukemia: experience with dasatinib. Future Oncol. 2006;2(6):655–665. doi: 10.2217/14796694.2.6.655. [DOI] [PubMed] [Google Scholar]
- 80.Araujo JC, Foblenz A, Corn P, et al. Dasatinib inhibits both osteoclast activation and prostate cancer PC-3-cell-induced osteoclast formation. Cancer Biol. Ther. 2009;8(22):2153–2159. doi: 10.4161/cbt.8.22.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vandyke K, Dewar AL, Farrugia AN, et al. Therapeutic concentrations of dasatinib inhibit in vitro osteoclastogenesis. Leukemia. 2009;23(5):994–997. doi: 10.1038/leu.2008.356. [DOI] [PubMed] [Google Scholar]
- 82.Id Boufker H, Lagneaux L, Najar M, et al. The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts. BMC Cancer. 2010;10:298. doi: 10.1186/1471-2407-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee YC, Huang CF, Murshed M, et al. Src family kinase/abl inhibitor dasatinib suppresses proliferation and enhances differentiation of osteoblasts. Oncogene. 2010;29(22):3196–3207. doi: 10.1038/onc.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marzia M, Sims NA, Voit S, et al. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J. Cell Biol. 2000;151(2):311–320. doi: 10.1083/jcb.151.2.311. ■■ Demonstrates that src−/− mice have increased bone formation rates and that osteoblast precursors from them had increased differentiation, providing the first evidence that inhibition of Src might also have an anabolic effect on bone.
- 85.Koreckij T, Nguyen H, Brown LG, et al. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br. J. Cancer. 2009;101(2):263–268. doi: 10.1038/sj.bjc.6605178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu EY, Massard C, Gross ME, et al. Once-daily dasatinib: expansion ofPhase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology. 2011;77(5):1166–1171. doi: 10.1016/j.urology.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Araujo J, Logothetis C. Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010;36:492–500. doi: 10.1016/j.ctrv.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gnoni A, Marech I, Silvestris N, et al. Dasatinib: an antitumour agent via Src inhibition. Curr. Drug Targets. 2011;12(4):563–578. doi: 10.2174/138945011794751591. [DOI] [PubMed] [Google Scholar]
- 89.Montero JG, Ruzo S Seoane, Ocana A, Pandiella A. Inhibition of Src family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin. Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2616. doi:10.1158/1078-0432.CCR-10-2616. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 90.Shakespeare W, Yang M, Bohacek R, et al. Structure-based design of an osteoclast-selective, nonpeptide src homology 2 inhibitor with in vivo antiresorptive activity. Proc. Natl Acad. Sci. USA. 2011;97(17):9373–9378. doi: 10.1073/pnas.97.17.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sundaramoorthi R, Shakespeare WC, Keenan TP, et al. Bone-targeted Src kinase inhibitors: novel pyrrolo- and pyrazolopyrimidine analogues. Bioorg. Med. Chem. Lett. 2003;13(18):3063–3066. doi: 10.1016/s0960-894x(03)00647-4. [DOI] [PubMed] [Google Scholar]
- 92.Boyce BF, Shakespeare W, Xing L, et al. Development of a novel bone-targeted Src tyrosine kinase inhibitor AP23451 having potent in vitro and in vivo antiresorptive properties. J. Bone Miner. Res. 2002;17:S159. [Google Scholar]
- 93.Boyce BF, Xing L, Shakespeare W, et al. Regulation of bone remodeling and emerging breakthrough drugs for osteoporosis and osteolytic bone metastases. Kidney Int. Suppl. 2003;(85):S2–S5. doi: 10.1046/j.1523-1755.63.s85.2.x. [DOI] [PubMed] [Google Scholar]
- 94.Yin JJ, Selander K, Chirgwin JM, et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 1999;103(2):197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coleman RE, McCloskey EV. Bisphosphonates in oncology. Bone. 2011;49(1):71–76. doi: 10.1016/j.bone.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 96.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scott LJ, Muir VJ. Denosumab: in the prevention of skeletal-related events in patients with bone metastases from solid tumours. Drugs. 2011;71(8):1059–1069. doi: 10.2165/11207370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 98.Melo MD, Obeid G. Osteonecrosis of the jaws in patients with a history of receiving bisphosphonate therapy: strategies for prevention and early recognition. J. Am. Dent. Assoc. 2005;136(12):1675–1681. doi: 10.14219/jada.archive.2005.0110. [DOI] [PubMed] [Google Scholar]
- 99.Aghaloo TL, Felsenfeld AL, Tetradis S. Osteonecrosis of the jaw in a patient on denosumab. J. Oral Maxillofac. Surg. 2010;68(5):959–963. doi: 10.1016/j.joms.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rizzoli R, Burlet N, Cahall D, et al. Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone. 2008;42(5):841–847. doi: 10.1016/j.bone.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 101.Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann. NY Acad. Sci. 2011;1218:38–46. doi: 10.1111/j.1749-6632.2010.05768.x. [DOI] [PubMed] [Google Scholar]