Abstract
CD36 fatty acid translocase plays a key role in supplying heart with its major energy substrate, long-chain fatty acids (FA). Previously, we found that the spontaneously hypertensive rat (SHR) harbors a deletion variant of Cd36 gene that results in reduced transport of long-chain FA into cardiomyocytes and predisposes the SHR to cardiac hypertrophy. In the current study, we analyzed the effects of mutant Cd36 on susceptibility to ischemic ventricular arrhythmias and myocardial infarction in adult SHR-Cd36 transgenic rats with wild-type Cd36 compared with age-matched SHR controls. Using an open-chest model of coronary artery occlusion, we found that SHR-Cd36 transgenic rats showed profound arrhythmogenesis resulting in significantly increased duration of tachyarrhythmias (207 ± 48 s vs. 55 ± 21 s, P < 0.05), total number of premature ventricular complexes (2,623 ± 517 vs. 849 ± 250, P < 0.05) and arrhythmia score (3.86 ± 0.18 vs. 3.13 ± 0.13, P < 0.001). On the other hand, transgenic SHR compared with SHR controls showed significantly reduced infarct size (52.6 ± 4.3% vs. 72.4 ± 2.9% of area at risk, P < 0.001). Similar differences were observed in isolated perfused hearts, and the increased susceptibility of transgenic SHR to arrhythmias was abolished by reserpine, suggesting the involvement of catecholamines. To further search for possible molecular mechanisms of altered ischemic tolerance, we compared gene expression profiles in left ventricles dissected from 6-wk-old transgenic SHR vs. age-matched controls using Illumina-based sequencing. Circadian rhythms and oxidative phosphorylation were identified as the top KEGG pathways, while circadian rhythms, VDR/RXR activation, IGF1 signaling, and HMGB1 signaling were the top IPA canonical pathways potentially important for Cd36-mediated effects on ischemic tolerance. It can be concluded that transgenic expression of Cd36 plays an important role in modulating the incidence and severity of ischemic and reperfusion ventricular arrhythmias and myocardial infarct size induced by coronary artery occlusion. The proarrhythmic effect of Cd36 transgene appears to be dependent on adrenergic stimulation.
Keywords: myocardial infarction
cardiovascular diseases, specifically coronary heart disease and myocardial infarction (MI), are the leading cause of morbidity and mortality in developed countries. Most cardiac phenotypes including cardiac mass, arrhythmias, infarct size, and susceptibility to heart failure are quantitative traits determined by genetic and environmental factors such as diet or stress. Additional “extracardiac” factors that cause a predisposition to coronary heart disease include proatherogenic factors such as high blood pressure, abnormal lipid profiles, or insulin resistance. Despite advances in the treatment of conventional MI risk factors mentioned above, many facets of the pathophysiology of MI are poorly understood. Ventricular arrhythmias are common affecting 20% of patients treated with reperfusion therapy and ventricular tachycardia/fibrillation remains the primary cause of death in patients during and immediately after an MI. Identification of the factors regulating cardiac hypertrophy, ischemic and reperfusion arrhythmias, and infarct size would greatly improve our understanding and treatment of patients with MI. The importance of genetic determinants of cardiac complex traits has been demonstrated by several epidemiological studies (41, 44, 45, 49) and twin analyses (6, 18). Recently, genome-wide association studies (GWAS) identified many polymorphisms that confer increased risk of MI. Despite great progress during several last years, genetic determinants identified by GWAS explain only ∼10% of MI heritability, and most of these findings are based only on statistical evidence (14). Animal models of human diseases represent useful tools for identification of genetic determinants of complex cardiovascular disorders including MI because of the possibility to use mechanistic studies that are not possible in humans.
The spontaneously hypertensive rat (SHR) is the most widely studied animal model of human essential hypertension. In addition, compared with control strains, the SHR exhibits differences in a number of important cardiac phenotypes including increased cardiac mass (5, 28, 42), impaired cardiac ischemic tolerance (10, 47, 56), and susceptibility to ventricular arrhythmias (24, 27, 34) or heart failure (12). Numerous genetic linkage studies have shown the location, but not the identity, of major predisposing quantitative trait loci (QTL). Despite the wealth of physiological and genetic data available in the SHR, the pathogenic events leading to the development of hypertension and related cardiovascular and metabolic phenotypes in this model are still largely unclear. Our previous studies showed that the SHR harbors a deletion variant of Cd36 gene that results in reduced transport of long-chain fatty acids (FA) into cardiomyocytes and predisposes the SHR to cardiac hypertrophy (17). In addition, knockout mice with Cd36 deficiency exhibited reduced tolerance to myocardial ischemia/reperfusion injury compared with wild-type controls (20). These findings suggested that disturbances in cellular energy production associated with reduced Cd36-mediated long-chain FA uptake might play an important role in ischemic tolerance of the heart. In humans, CD36 mutations were also associated with significantly reduced uptake of long-chain FA into the heart and with a variety of heart diseases including hypertrophic cardiomyopathy, dilated cardiomyopathy, or coronary heart disease (52). In the current study, we analyzed the effects of mutant Cd36 on the incidence and severity of ischemic and reperfusion ventricular arrhythmias and MI size induced by coronary artery occlusion in SHR-Cd36 transgenic rats with wild-type Cd36 vs. SHR with mutant Cd36 and used analyses of gene expression profiles in left ventricles (LV) to search for potential underlying molecular mechanisms.
MATERIALS AND METHODS
Animals.
We used SHR/Ola and Cd36 transgenic SHR/Ola-TgN(EF1aCd36)19Ipcv line (hereafter referred to as SHR-Cd36 transgenic) that was prepared by microinjecting SHR/Ola zygotes with wild-type Cd36 cDNA isolated from fat tissue of WKY rat (43). Adult male rats with a body weight of 200–220 g were used. Gene expression profiles were determined in LVs dissected from 6-wk-old SHR and SHR-Cd36 transgenic males. Rats were kept at a 12 h-12 h light/dark period with free access to standard laboratory chow and water. The experiments were performed in agreement with the Animal Protection Law of the Czech Republic (311/1997) and were approved by the Ethics Committee of the Institute of Physiology, Academy of Sciences of the Czech Republic.
Echocardiography.
Evaluation of geometrical and functional parameters of the heart (12 animals in each group) was performed using echocardiographic system GE Vingmed System Seven with 14 MHz linear matrix probe. Animals were anesthetized by the inhalation of 2% isoflurane. Within the baseline echocardiographic evaluation, the following diastolic and systolic dimensions of the LV were measured: posterior and anterior wall thickness (PWTd, PWTs, AWTd, AWTs) and left ventricular cavity diameter (LVDd, LVDs). From these dimensions, the main functional echo parameter was derived; fractional shortening (FS%) by the formula FS% = (LVDd − LVDs)/LVDd. After the measurement of baseline parameters, the functional reserve with dobutamine infusion in progressively increasing rates was assessed as previously described (31).
Infarct size and arrhythmias in open-chest rats.
Anesthetized rats (pentobarbital sodium, 60 mg/kg ip; Sigma-Aldrich) were ventilated (rodent ventilator 7026, Ugo Basile) via tracheal cannula with room air at 68 strokes/min (tidal volume of 1.2 ml/100 g body wt); the number of examined animals was 8 and 12 for SHR and SHR-Cd36, respectively. Both blood pressure in the left carotid artery and a single-lead electrocardiogram (ECG) were continually recorded. The rectal temperature was maintained between 36.5 and 37.5°C by a heated table throughout the experiment. Left thoracotomy was performed and after 10-min stabilization, regional myocardial ischemia was induced by tightening a polyester ligature 6/0 (Ethicon, Edinburgh, UK) placed around the left anterior descending coronary (LAD) artery ∼1–2 mm distal to its origin. Characteristic changes in the configuration of the ECG and a transient decrease in blood pressure verified the coronary artery occlusion. After a 20-min occlusion period, the ligature was released and reperfusion of previously ischemic tissue continued.
At the end of 3 h reperfusion, the hearts were excised and washed with 20 ml saline through the cannulated aorta. The area at risk and the infarct size were determined as described earlier (33) by staining with 5% potassium permanganate (Pliva-Lachema, Brno, Czech Republic) and 1% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich), respectively. The hearts were cut perpendicularly to the LV long axis into slices 1 mm thick and stored overnight in 10% neutral formaldehyde solution. Next day, the RV free wall was separated and both sides of LV slices were photographed. The size of the infarct area (IS), the size of the area at risk (AR), and the size of the LV were determined by a computerized planimetric method using the software Ellipse (ViDiTo) with a grid of ∼400 points per slice. The IS was normalized to the AR (IS/AR), and the AR was normalized to the LV (AR/LV).
The incidence and severity of ventricular arrhythmias during ischemic insult and the first 5 min of reperfusion were assessed. Premature ventricular complexes (PVCs) occurring as singles, salvos, or tachycardia (a run of 4 or more consecutive PVCs) were counted separately. The incidence of ventricular tachycardia (VT) and fibrillation (VF) was also evaluated. VF lasting >2 min was considered as sustained (VFs). The severity of arrhythmias in each individual heart was evaluated by means of a 5-point arrhythmia score as described elsewhere (4). Scores were used for group analysis of severity of arrhythmias.
Infarct size and arrhythmias in isolated perfused hearts.
Animals were anesthetized as above. Hearts were rapidly excised and perfused at constant flow (∼10 ml·min−1·g−1) and temperature (37°C) according to Langendorff under nonrecirculating conditions by a modified Krebs-Henseleit solution (mmol/l: NaCl 118.0, KCl 3.2, CaCl2 1.25, MgSO4 1.2, NaHCO3 25.0, KH2PO4 1.2, glucose 7.0). The medium was gassed with 95% O2 and 5% CO2 (pH 7.4). The expected heart weights were calculated from regression equations established on the basis of our previous data for heart weight-to-body weight ratio of SHR and SHR-Cd36.
Epicardial electrograms were continuously recorded and subsequently analyzed by custom-designed software. After 20-min stabilization, regional no-flow ischemia was induced by the LAD occlusion for 40 min followed by 1 h reperfusion. Ventricular arrhythmias during ischemia and the first 10 min of reperfusion were counted, and the infarct size determined similarly as in open-chest experiments.
The involvement of catecholamines in cardiac ischemic tolerance of SHR and transgenic SHR-Cd36 hearts was examined in rats pretreated with reserpine (0.15 mg/kg dissolved in a mixture of glacial acetic acid and saline 1:50, 9 and 6 hearts per group) administered ip 24 h before ischemia according to Oxman et al. (37). Control rats (7 hearts in each group) received the same volume of the vehicle.
Blood pressure measurement and biochemical analysis.
In separate groups of rats, systolic and diastolic arterial blood pressures (SAP, DAP) were recorded by radiotelemetric technique. Animals were killed by decapitation. Sera and hearts were collected and stored at −80°C until analyses. Nonesterified FA (NEFA) levels were determined using an acyl-CoA oxidase-based colorimetric kit (Roche Diagnostics, Mannheim, Germany). Serum triglyceride concentrations were measured by standard enzymatic methods (Pliva-Lachema).
FA composition.
Serum and LV phospholipids from six animals in each group were extracted according to Folch et al. (15). Lipid classes were separated by thin layer chromatography using hexane-diethylether-acetic acid (85:15:1, vol/vol) as a solvent system. FA in serum and ventricular phospholipids were converted to methyl esters using 1% solution of NaCl in methanol, and the FA methyl esters were eluted with hexane. Gas chromatography analysis of the FA methyl esters was performed on a gas chromatograph GC-17A (Shimadzu, Kyoto, Japan) equipped with a flame-ionization detector and automatic sampler AOC-20i (Shimadzu). A fused silica capillary column (30 m × 0.32 mm ID) with chemically bonded stationary phase DB-WAXETR from J&W Scientific (Folsom) was used. The oven temperature was maintained for 1 min at 80°C, then raised to 150°C by 20 K/min, then by 3 K/min to 250°C, and kept for 5 min; hydrogen was used as the carrier gas. Injector and detector temperatures were set at 250°C. Individual peaks of FA methyl esters were identified by comparing the retention times with those of authentic standards (Nu-Chek Prep, Elysian, MN). The composition of serum and ventricular FA (spectrum of 27 main FA) was analyzed. The product/precursor ratios of the serum FA were used to calculate indices reflecting the activities of enzymes involved in hepatic FA metabolism: elongase (18:0/16:0), Δ6 desaturase (18:3n8/18:2n6), Δ5 desaturase (20:4n6/20:3n6), and Δ9 desaturase (16:1n7/16:0).
Heart triglyceride measurement.
For determination of triglycerides, LV myocardium was powdered under liquid nitrogen and extracted for 16 h in chloroform-methanol, after which 2% KH2PO4 was added and the solution was centrifuged. The organic phase was removed and evaporated under N2. The resulting pellet was dissolved in isopropyl alcohol and triglyceride content was determined by enzymatic assay (Pliva-Lachema).
Parameters of oxidative stress.
The activities of antioxidant enzymes and of lipoperoxidation products were determined as previously described by Malínská et al. (30).
Gene expression profiles.
For Illumina analysis, RNA was extracted from LV isolated from 6-wk-old SHR/Ola and SHR-Cd36 transgenic rats (5 males in each group). Tissue was crushed to a fine powder by pestle and mortar in liquid nitrogen. Approximately 150 mg of powdered sample was homogenized in 1.5 ml of TRIzol (Invitrogen, Paisley, UK) and subsequently purified using the RNeasy Mini Plus Kit (Qiagen). Library preparation (each constructed from 10 micrograms of total RNA) and sequencing was carried out essentially as described previously (21), however, oligonucleotide primers were modified to accommodate sequencing on the Illumina Genome Analyzer I (Wakimoto H, Seidman CE, and Seidman JG, unpublished). Tag sequences were mapped back to the genome using customized software (Wakimoto H and Seidman JG, unpublished). To exclude tags containing SNPs between SHR and SHR-Cd36 and to ensure that sequenced tags are likely to represent a single locus, tags which were observed zero times in any strain and/or were found to align more than four times to the genome were removed from the data set. The number of tags was normalized across both strains. For the SHR-Cd36 transgenic strain, the tag count fold change compared with SHR was calculated. Raw P values were calculated by Fisher's exact test for those tags that had an absolute fold change of tag abundance ≥1.5. P values were corrected for multiple testing using the Benjamini and Hochberg false discovery rate method. Tags showing both tag abundance ratios ≥1.5 and P < 0.05 were considered to be robustly differentially expressed. Functional analysis of KEGG pathways (Kyoto Encyclopedia of Genes and Genomes) was performed using the DAVID online software (19). In addition, gene pathways were analyzed using the Ingenuity Pathways Analysis (IPA). The significance of the association between the data set and the canonical pathway was determined based on two parameters: 1) a ratio of the number of genes from the data set that map to the pathway divided by the total number of genes that map to the canonical pathway and 2) a P value calculated using Fischer's exact test determining the probability that the association between the genes in the data set and the canonical pathway is due to chance alone (Ingenuity Systems, http://www.ingenuity.com).
Gene expression determined by real-time PCR.
Total RNA was extracted from LV using TRIzol reagent (Invitrogen), and cDNA was prepared and analyzed by real-time PCR using QuantiTect SYBR Green reagents (Qiagen) on an Opticon 2 continuous fluorescence detector (MJ Research). Gene expression levels were normalized relative to the expression of peptidylprolyl isomerase A (Ppia) (cyclophilin) gene, which served as the internal control, with results being determined in triplicate. Primers used for validation of differentially expressed genes selected from significant pathways were: Ppia - agc ata cag gtc ctg gca t and tca cct tcc caa aga cca c, Per1 - gct gct cct acc agc aaa tc and gag gca cat tta cgc ttg gt, Per2 - aga tca gct gcc tgg aca gt and tgg gaa ctc gca ctt tct tt, Arntl - cca cag cac agg cta ctt ga and gtg gaa cca tgt gtg agt gc, Cox5a - tag tgc tgt tcg cat ctt gg and cac ttt gtc aag gcc cag tt, Rhob - ccc agt gtc tgt gtg tgt cc and ggg tag ggg tgt agg gag tc, Klf4 - cca aag agg gga aga agg tc and ctg tgt gag ttc gca ggt gt.
Mitochondrial parameters.
High-resolution oxygraphy was performed on LV heart homogenate (40), which was prepared in H medium (0.25 M sucrose, 10 mM Tris·HCl, 2 mM EDTA, pH 7.4). The minced heart tissue (4 in each group) was homogenized with Teflon-glass homogenizer (clearance 0.25 mm, 7 strokes, 750 rpm) and filtered through a polyamide screen (mesh 56 μm). Oxygen consumption was measured at 30°C as described before (39) using Oxygraph-2k (Oroboros). The homogenate (0.1 mg/ml) was suspended in 2 ml of KCl medium (80 mM KCl, 10 mM Tris·HCl, 3 mM MgCl2, 1 mM EDTA, 5 mM K-Pi, 2.5 mM malate, 0.5 mg/ml BSA, pH 7.4) and for measurements, 3 mM malate, 1.5 mM ADP, 20 μM palmitoyl carnitine, and 10 mM glutamate were used. The oxygen consumption was expressed in pmol oxygen·s−1·mg protein−1.
Statistical analysis.
Data are expressed as means ± SE. Differences in parametric variables between the groups were compared by unpaired Student's t-test. Differences in the number of PVCs were compared by the Mann-Whitney U-test. The incidence of tachyarrhythmias was examined by Fisher's exact test. Statistical significance was defined as P < 0.05.
RESULTS
Blood pressure and heart function.
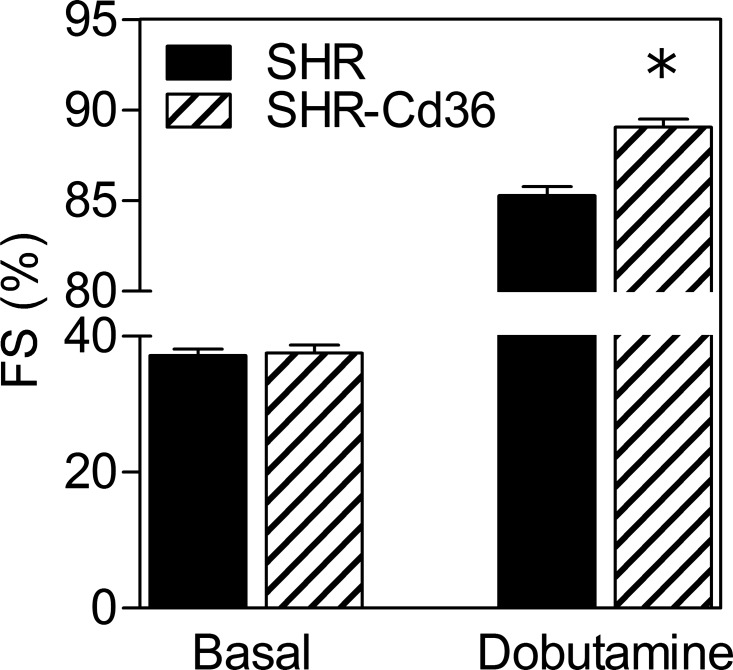
Table 1 summarizes values of blood pressure and ECG parameters. We confirmed that the expression of Cd36 transgene is associated with lower SAP and DAP compared with wild-type SHR. No significant differences were found in baseline systolic and diastolic dimensions of the LV and FS between the two strains (Table 1, Fig. 1). Nevertheless, the dobutamine stress ECG revealed significantly higher maximal FS in SHR-Cd36 rats (89.1 ± 0.4%) compared with SHR (85.3 ± 0.5%) as shown in Fig. 1.
Table 1.
Blood pressure and echocardiographic parameters of SHR and SHR-Cd36
| Strain | SAP, mmHg | DAP, mmHg | AWTd, mm | LVDd, mm | PWTd, mm | AWTs, mm | LVDs, mm | PWTs, mm |
|---|---|---|---|---|---|---|---|---|
| SHR | 198 ± 3 | 146 ± 2 | 2.05 ± 0.04 | 7.67 ± 0.08 | 2.00 ± 0.06 | 3.11 ± 0.06 | 4.82 ± 0.09 | 2.99 ± 0.10 |
| SHR-Cd36 | 186 ± 2* | 128 ± 3* | 2.10 ± 0.04 | 7.72 ± 0.14 | 1.93 ± 0.03 | 3.14 ± 0.05 | 4.83 ± 0.13 | 2.96 ± 0.07 |
Systolic arterial pressure (SAP); diastolic arterial pressure (DAP); diastolic anterior wall thickness (AWTd); diastolic left ventricle diameter (LVDd); diastolic posterior wall thickness (PWTd); systolic anterior wall thickness (AWTd); systolic left ventricle diameter (LVDs); systolic posterior wall thickness (PWTd);
P < 0.05 vs. SHR.
Fig. 1.
Baseline values of left ventricular fractional shortening (FS) and maximal values of FS determined by dobutamine stress echocardiography in the control spontaneously hypertensive rats (SHR) and transgenic SHR-Cd36. Values are means ± SE; *P < 0.05 vs. SHR.
IS and arrhythmias in open-chest rats.
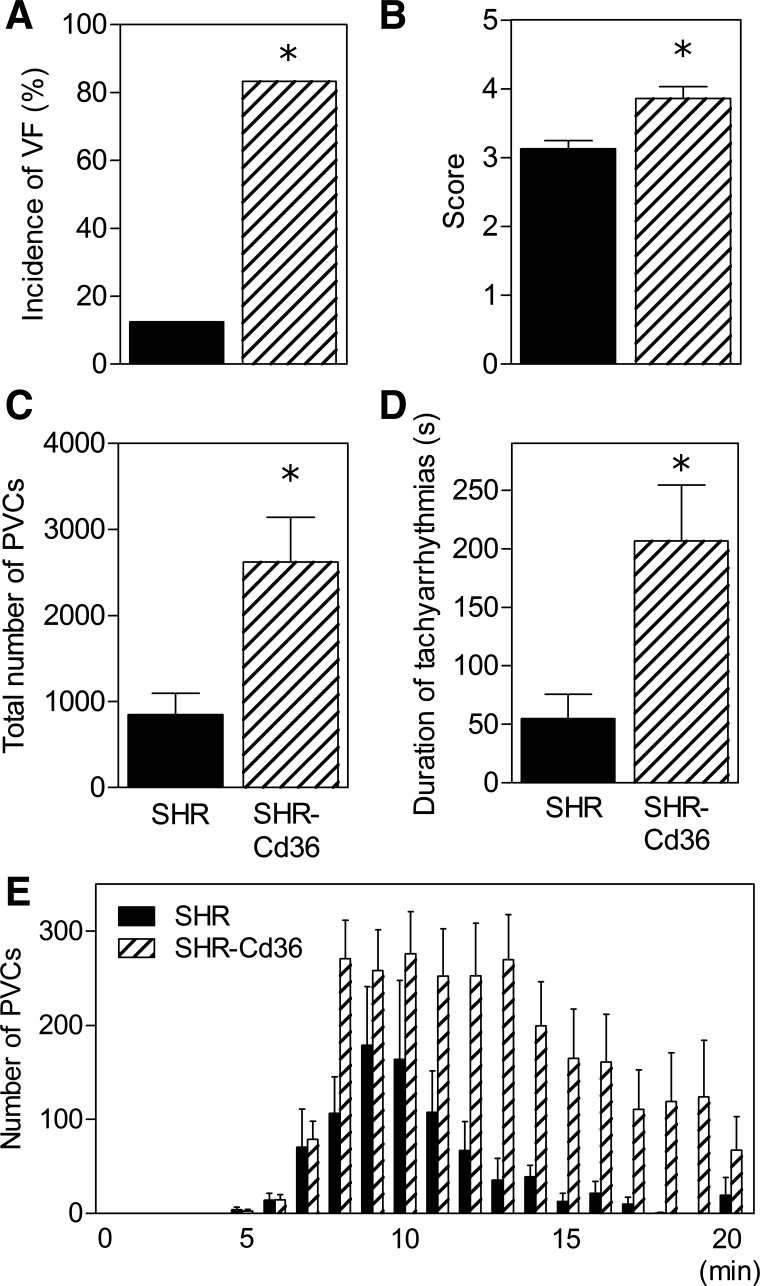
As can be seen in Fig. 2, transgenic expression of Cd36 was associated with a marked increase in the incidence and severity of ischemic ventricular arrhythmias. The incidence of VF reached 83% in SHR-Cd36 rats (two animals exhibited sustained VF) compared with 13% in SHR controls (Fig. 2A). Consequently, the arrhythmia score was significantly higher in the transgenic group (Fig. 2B). In both groups, VT was the predominant form of ischemic arrhythmias. Expression of Cd36 transgene dramatically increased the total duration of VT episodes (196 ± 47 s vs. 55 ± 21 s, P = 0.019) and the number of PVCs occurring as VT (2,136 ± 508 vs. 527 ± 191, P = 0.023) compared with SHR controls. This greater susceptibility to VT substantially contributed to the increase in total number of PVCs and duration of tachyarrhythmias (VT + VF) during the ischemic insult (Fig. 2, C and D). Figure 2E shows the time profile of PVCs distribution during 20 min of coronary artery occlusion. While the onset of ectopic activity did not differ between the groups, it lasted longer in transgenic rats than in SHR controls.
Fig. 2.
The incidence of ventricular fibrillation (VF; A), arrhythmia score (B), total number of premature ventricular complexes (PVCs; C), duration of tachyarrhythmias (D), and distribution of PVCs (E) over 20-min coronary artery occlusion in open-chest control SHR and transgenic SHR-Cd36. Values are means ± SE; *P < 0.05 vs. SHR.
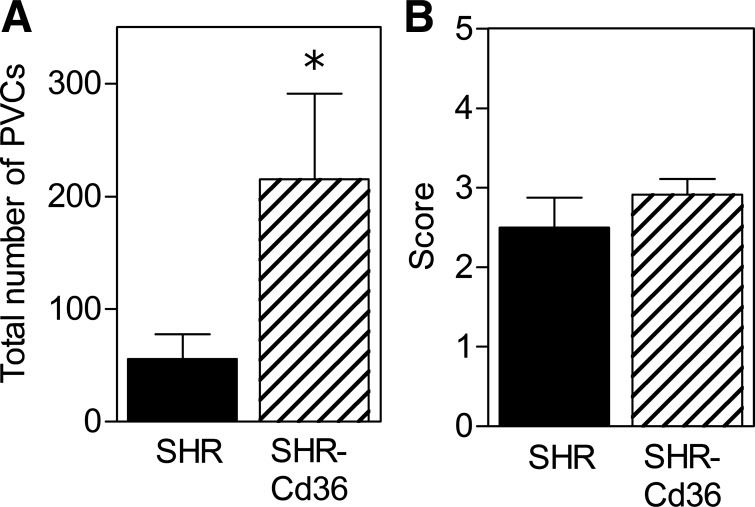
Ventricular arrhythmias occurring in the early phase of reperfusion were also significantly promoted in the SHR-Cd36 group as indicated by the increased number of total PVCs (Fig. 3A). However, the reperfusion arrhythmia score did not differ between the groups (Fig. 3B).
Fig. 3.
The total number of PVCs (A) and arrhythmia score (B) at the beginning (5 min) of reperfusion in open-chest control SHR and transgenic SHR-Cd36. Values are means ± SE; *P < 0.05 vs. SHR.
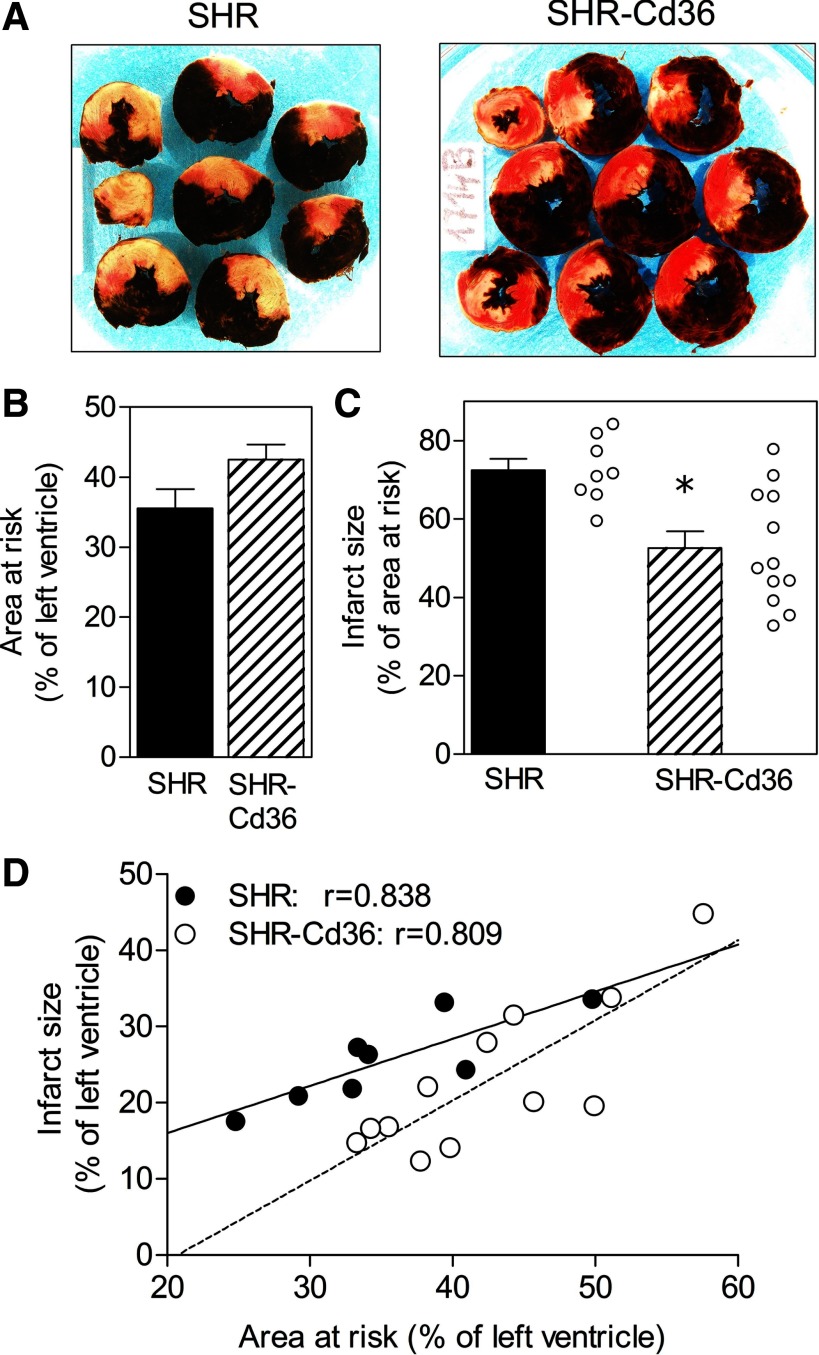
Figure 4A shows the representative examples of MI in SHR and SHR-Cd36 rats. The normalized AR did not differ between the groups (Fig. 4B). In contrast to the proarrhythmic effect, the expression of Cd36 transgene was associated with a significant reduction of IS to 52.6 ± 4.3% of AR compared with 72.4 ± 2.9% in SHR controls (Fig. 4C). Analysis of the relationship between IS and the size of AR revealed that the cardioprotective effect of Cd36 transgene manifested itself only in a lower range of ischemic areas (Fig. 4D).
Fig. 4.
Typical examples of myocardial infarction (A), size of area at risk expressed as percent of the left ventricle (B), myocardial infarct size expressed as percent of the area at risk (C), and relationship between area at risk and infarct size, both expressed as percent of the left ventricle (D) in control SHR and transgenic SHR-Cd36. Values are means ± SE; r, correlation coefficient; *P < 0.05 vs. SHR.
IS and arrhythmias in isolated perfused hearts.
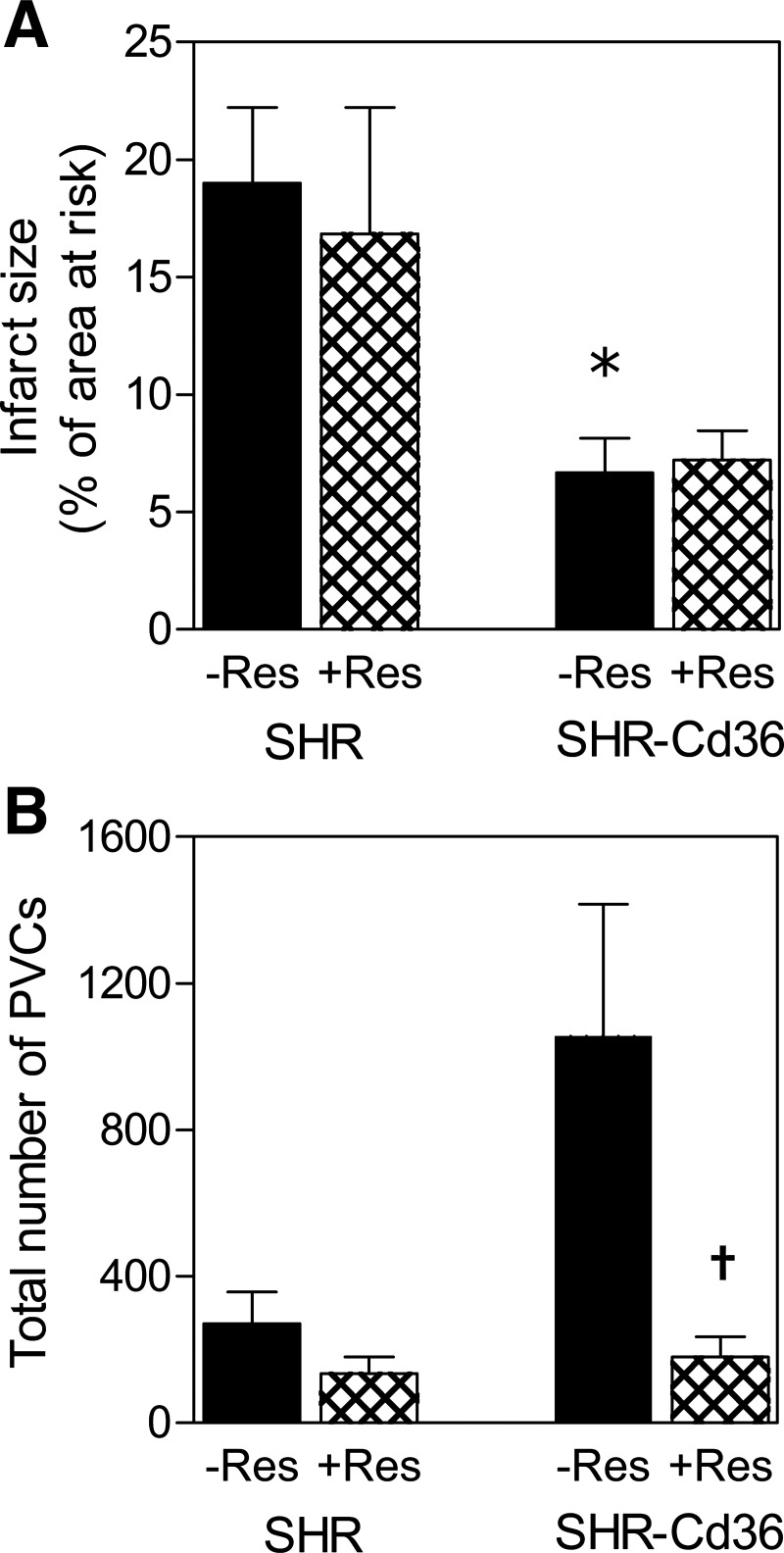
Proarrhythmic and IS limiting phenotype of transgenic rats was confirmed also in isolated perfused hearts. The IS/AR reached 19.0 ± 3.2% in SHR and was significantly smaller in SHR-Cd36 (6.7 ± 1.4%, Fig. 5A). On the other hand, the total number of PVCs was markedly increased in transgenic rats (1,057 ± 360) compared with SHR (271 ± 87) although the difference did not reach statistical significance due to a high individual variability (Fig. 5B). Depletion of catecholamines by reserpine had no effect on IS but completely abolished the increased susceptibility to ischemic arrhythmias in SHR-Cd36 as documented by a reduced total number of PVCs (179 ± 56; Fig. 5, A and B).
Fig. 5.
Myocardial infarct size expressed as percent of the area at risk (A) and the total number of PVCs (B) in isolated perfused hearts of untreated (−Res) and reserpine-pretreated (+Res) control SHR and transgenic SHR-Cd36. Values are means ± SE; *P < 0.05 vs. SHR, †P < 0.05 vs. corresponding untreated group.
Metabolic traits.
As can be seen in Table 2, expression of the Cd36 transgene was associated with significantly lower levels of serum NEFA and triglycerides. In addition, heart triglyceride concentration was also significantly lower in transgenic rats compared with SHR controls. The two strains did not differ in the composition of major FA of heart phospholipids (data not shown).
Table 2.
The levels of serum NEFA and triglycerides and cardiac triglycerides in SHR and SHR-Cd36
| Serum |
Cardiac | ||
|---|---|---|---|
| Strain | NEFA, mmol/l | Triglycerides, mmol/l | Triglycerides, μmol/g |
| SHR | 0.540 ± 0.047 | 0.776 ± 0.058 | 2.63 ± 0.26 |
| SHR-Cd36 | 0.419 ± 0.028* | 0.601 ± 0.047* | 1.88 ± 0.24* |
NEFA, nonesterified fatty acids.
P < 0.05 vs. SHR.
Parameters of oxidative stress.
The activities of antioxidant enzymes and levels of lipoperoxidation products, conjugated dienes and TBARS, in hearts isolated from SHR and Cd36 transgenic rats were similar (data not shown) except for reduced glutathione (GSH), which was significantly higher in SHR compared with Cd36 transgenic rats (29.0 ± 2.5 vs. 23 ± 0.6 mM/g protein, P < 0.05).
Changes in gene expression profiles associated with cardiac Cd36 transgene expression.
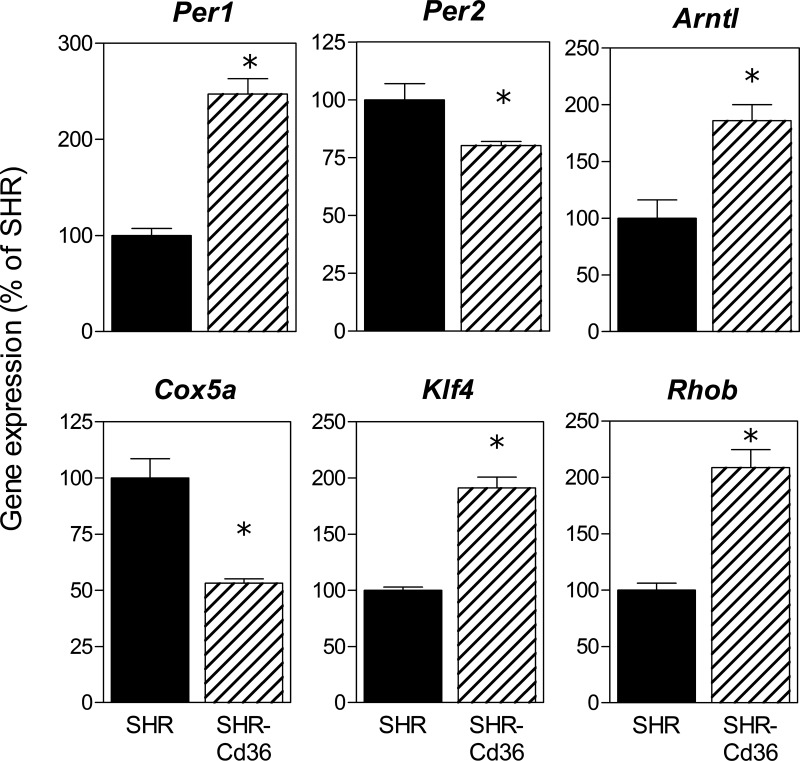
To search for potential molecular mechanisms that link expression of the Cd36 transgene to arrhythmias and IS, we compared gene expression profiles in LVs isolated from transgenic SHR vs. age-matched controls using Illumina-based sequencing. Altogether, we found 387 genes with significantly (adjusted P value <0.001) up- or downregulated expression (Supplementary Table S2).1 Directional differences in the expression of selected genes were confirmed by quantitative RT-PCR (Fig. 6). Table 3 shows circadian rhythms and oxidative phosphorylation as top KEGG pathways identified by DAVID online software analysis of differentially expressed genes. IPA identified circadian rhythms, VDR/RXR activation, IGF1 signaling, and HMGB1 signaling as top canonical pathways potentially important for Cd36-mediated effects on cardiac function and ischemic tolerance (Table 4).
Fig. 6.
Validation of gene expression profiles obtained by digital transcriptional profiling by qRT-PCR for 6 transcripts of differentially expressed genes. SHR expression values have been normalized to a relative value of 1. *P < 0.01.
Table 3.
List of genes from top KEGG pathways
| Pathway | P Value | Genes |
|---|---|---|
| Circadian rhythms | 0.04 | Dbp↓, Arntl↑, Per1↑, Per2↓, Bhlhb2↓, Bhlhb3↓, Nr1d1↓, Npas2↑, Tef↓, Cry1↑ |
| Oxidative phosphorylation | 0.03 | mt-Nu5m↑, mt-Cox2↑, mt-Atp8↑, mt-Cox1↑, mt-Nu3m↓, mt-Nu4m↑, mt-Atp6↓, mt-Cyb↑, mt-Cox3↓, mt-Nu2m↑, Atp5e↓, Cox17↓, Atp5 g3↓, Ndufs2↓, Uqcrh↑, Cox7b↓, Cox6a2↓, Cox8 h↓, Cox5a↓, Ndufb7↑, Atp5o↓, Atp5f1↑, Sdhc↑, Uqcrc1↑, Cox4i1↓, Ndufa10↓ |
↑ and ↓ denote up- and downregulated, respectively, in SHR-Cd36 transgenic vs. SHR controls; P value is Benjamini corrected.
Table 4.
List of top IPA canonical pathways
| Pathway | P Value | Genes |
|---|---|---|
| Circadian rhythms | 1.9E-08 | Arntl↑, Per1↑, Per2↓, Bhlhb2↓, Bhlhb3↓ |
| VDR/RXR activation | 9.8E-05 | Klf4↑, Cebpb↑, Cdkn1a↑, Wt1↑, Igfbp3↑ |
| IGF1 signaling | 1.9E-04 | Fos↑, Jun↑, Igf1r↑, Igfbp4↓, Igfbp3↑ |
| HMGB1 signaling | 2.0E-04 | Rhob↑, Fos↑, Jun↑, Plat↑, Rhoj↑ |
↑ and ↓ denote up- and downregulated, respectively, in SHR-Cd36 transgenic vs. SHR controls; P value is Benjamini corrected.
Mitochondrial oxidation.
We tested the functional effects of differentially expressed genes from the KEGG oxidative phosphorylation pathway in LV on oxidation of various substrates. As can be seen in Table 5, transgenic expression of Cd36 was associated with increased state 3-ADP respiration in heart homogenate when we tested oxidation of different substrates at conditions of coupled respiration; it is when ATP is produced by oxidative phosphorylation at the expense of mitochondrial proton gradient (ΔμÇ+) generated by substrate oxidation. As shown by respiration measurements using the substrates malate, palmityl carnitine, succinate, and glutamate, significantly higher rates of respiration were found in SHR-Cd36 rats compared with SHR controls with ADP + malate (lowest respiratory rate) and with ADP + malate + palmityl carnitine + succinate + glutamate (highest respiratory rate). These data show that mitochondrial energy provision can operate better in heart of SHR-Cd36 rats compared with SHR controls.
Table 5.
Respiration of rat heart homogenate of SHR and SHR-Cd36
| Substrates | SHR, pmol oxygen·s−1·mg protein−1 | SHR-Cd36, pmol oxygen·s−1·mg protein−1 | SHR-Cd36 in % of SHR |
|---|---|---|---|
| ADP + malate | 327 ± 11 | 514 ± 40 | 157† |
| ADP + malate + palmityl carnitine +succinate+ glutamate | 2,039 ± 84 | 2,458 ± 156 | 121* |
Number of animals (n);
P ≤ 0.05 vs. SHR,
P ≤ 0.005 vs. SHR.
DISCUSSION
In the current study, we found that transgenic expression of Cd36 in the SHR was associated with markedly increased susceptibility to ventricular arrhythmias induced by ischemia/reperfusion and reduced MI size. Differential susceptibility to cardiac ischemia/reperfusion was preceded by significantly altered expression of almost 400 genes in LVs isolated from SHR-Cd36 transgenic vs. SHR control rats.
It is well known that CD36 contributes markedly to the regulation of FA uptake, oxidation, and esterification in the heart. More than 50% of long-chain FA uptake by the heart occurs via sarcolemmal CD36-mediated transport (25, 29). It has been also suggested that increased uptake of FA by the heart after MI might represent a metabolic cause leading to fatal VF during acute myocardial ischemia (35). Accordingly, it is possible that transgenic SHR with wild-type Cd36 are predisposed to arrhythmias due to increased FA uptake. The mechanisms of these FA associated proarrhythmogenic effects are not fully understood but might include an increased requirement of oxygen for FA catabolism (26, 36), an accumulation of potentially toxic intermediates of FA metabolism such as long chain acylcarnitine and long chain acyl coenzyme A, or an FA-mediated inhibition of glucose utilization by myocardium (7, 9, 36, 53). On the other hand, SHR rats with mutant Cd36, with reduced FA transport and oxidation in the heart and with increased glucose oxidation for ATP production are relatively protected from adverse metabolic effects of increased FA levels.
However, we found that isolated hearts of SHR-Cd36 perfused with a crystalloid solution devoid of FA were also more susceptible to ischemic ventricular arrhythmias compared with the SHR. This finding suggests that the proarrhythmic effect of SHR-Cd36 transgene is an intrinsic myocardial property independent of FA uptake during acute ischemic insult. Moreover, the complete elimination of increased arrhythmogenesis in hearts of SHR-Cd36 pretreated with reserpine points to an important role of myocardial catecholamines in triggering ischemia-induced arrhythmias in this group. Indeed, it seems that transgenic animals are more sensitive to β-adrenergic stimulation than the SHR. This view is supported by our observation of higher maximum values of LV FS revealed by dobutamine stress ECG (Fig. 1) and by preliminary data demonstrating higher forskolin-stimulated activity of myocardial adenylyl cyclase in SHR-Cd36 compared with wild-type SHR (Novotny J and Kagan D, unpublished). In addition, Cd36 was originally identified as a quantitative trait gene regulating isoproterenol-induced lipolysis in isolated adipocytes when the SHR mutant allele was associated with reduced sensitivity to catecholamines (2, 32). These findings strongly suggest that mutated Cd36 is associated with reduced β-adrenergic stimulation in several tissues.
Contrary to increased numbers of arrhythmias in transgenic rats, expression of wild-type Cd36 was associated with significantly reduced IS determined in both open-chest rats and isolated perfused hearts. Opposite changes of the two endpoints of ischemia/reperfusion injury can be explained by different factors that are involved in their pathogenic mechanisms. In agreement with our observation, Irie et al. (20) found that FAT/CD36-null mice were energetically deficient and more sensitive to acute ischemic insult, similarly to SHR. However, the impaired cardiac ischemic tolerance was not confirmed in another study using the same mouse strain (25). In view of the limited data available, it is currently impossible to explain these contradictory results.
To search for possible molecular mechanisms, responsible for Cd36 associated cardiac traits we compared gene expression profiles in LV dissected from 6-wk-old transgenic SHR vs. age-matched controls. Circadian rhythms were identified as the most prominent KEGG and IPA canonical pathways of differentially expressed genes between SHR and transgenic SHR-Cd36 (Tables 3 and 4). It has been demonstrated that a profound time-of-day dependence of myocardial ischemia/reperfusion injury is mediated by the cardiomyocyte circadian clock (13). Genes coding for the cardiomyocyte circadian clock modify the expression of myocardial genes regulating β-adrenergic signaling, FA, triglyceride and glucose metabolism, and affect heart rate as well as ischemia/reperfusion tolerance. Analyses of gene expression profiles revealed that >10% of myocardial genes exhibit circadian rhythms (58). Recently, Virag et al. (54) reported that functional deletion of the Per2 gene in knockout mice significantly reduced the size of MI. In the current study, we observed that expression of Per2 gene is downregulated, while Arntl gene expression is increased in transgenic Cd36-SHR compared with SHR controls, which suggests that the repressor activity of Per2 is reduced and thus its targets might be upregulated. These targets include endothelin-1, vascular endothelial factor (VEGF), and some other factors that play important role in hypertrophy and angiogenesis. As can be seen in Supplementary Table S2, we observed significantly increased expression of the Vegf gene in the heart of transgenic SHR suggesting that Cd36 overexpression might exert its effects through activation of the Vegf pathway. Members of the VEGF family play an important role in regulating vasculogenesis and angiogenesis. It has been shown recently, using adeno-virus-mediated Vegf gene delivery, that increased cardiac expression of Vegf transgene is associated with significant improvement in cardiac function after MI in rats, by promoting cardiac contractility, preserving viable cardiac tissue, and preventing remodeling of the LV over time (60).
Transgenic SHR-Cd36 also showed significantly increased expression of genes involved in oxidative phosphorylation, especially of genes coded by mitochondrial DNA (Table 3). These findings suggest that overexpression of Cd36 is associated with increased capacity of FA oxidation in mitochondria compared with control SHR. Adverse proarrhythmogenic effects of increased utilization of FA vs. glucose are discussed above. In SHR-Cd36, five overexpressed mitochondrial genes coding for components of complexes I and III were identified. Both complexes produce reactive oxygen species (ROS) on the matrix side and β-oxidation of FA can lead to greater release of ROS, mainly from complex I (50). Although the involvement of ROS in protective signaling has been well documented (23, 57), it is unknown whether it may play a role in the infarct size reduction in SHR-Cd36.
The final reaction of mitochondrial electron transport chain is mediated by cytochrome c oxidase (COX). This complex is formed by 13 subunits, three of them being of mitochondrial origin (COX I–III). Wu et al. (55) proposed that COX activity is determined by the COX I-to-COX III ratio; overexpression of COX III results in a decreased level of COX I, decreased COX activity, and impaired cell viability. It has been shown that prolonged ischemia downregulated COX I and upregulated COX III, particularly in interfibrillar mitochondria (48). Ischemic preconditioning attenuated ischemia-induced reciprocal changes in COX I and COX III levels (59). Moreover, chronic administration of thyroid hormone affected COX I and III expression (46) and protected the heart against lethal ischemic injury (38). In line with these reports, the present study demonstrates increased COX I and decreased COX III gene expression and the improved cardiac ischemic tolerance in SHR-Cd36. Interestingly, our analysis of the mitochondrial oxidative phosphorylation system by substrate oxidation revealed that mitochondrial energy provision can operate better in SHR-Cd36 rats compared with SHR controls. Nevertheless, the causal relationships between abnormalities in the expression of these mitochondrial genes and cardiac ischemic tolerance have yet to be elucidated.
Genes from the VDR/RXR (vitamin D receptor/retinoid X receptor) canonical pathway were identified as significant by the IPA. It has been demonstrated that deficiency in vitamin D is associated with arrhythmias and MI. For instance, proteomic analysis revealed a significant increase in serum VDR levels in patients with MI (16). In a recent report, the correction of vitamin D deficiency and hypocalcemia resulted in control of incessant ventricular tachycardia and cardiomyopathy (8). In an animal study, rats fed a vitamin D-deficient diet for 12 wk developed significant QT-interval shortening despite normal serum calcium levels compared with normal rats (48). These findings suggest a possible role for vitamin D deficiency as a causal factor for arrhythmia and the need for further exploration.
One of the most overexpressed genes in SHR-Cd36 transgenic rats was cellular proto-oncogene Fos. Fos gene family members dimerize with proteins of the Jun family (Jun was also overexpressed in SHR-Cd36) and have a high affinity for transcription factor AP-1. Upregulation of Fos and Jun genes was demonstrated in preconditioned hearts (11, 22). IPA identified increased expression of Fos and Jun in the IGF1 and HMGB1 canonical pathways (Table 4). Upregulation of insulin-like growth factor signaling may contribute to the improved ischemic tolerance (51).
HMGB1 (high mobility group box 1) has been found to play a pivotal role in the pathogenesis of acute and chronic inflammatory disorders. Recently, HMGB1 was characterized as an early mediator of tissue injury caused by myocardial ischemia/reperfusion (3). On the other hand, low postischemic doses of HMGB1 improve myocardial function and decrease infarct size in association with suppressed myocardial inflammation. These results suggest a potential role for exogenous HMGB1 therapy in the acute postischemic period (1).
As with most gene expression profiling studies, it is difficult to determine which particular pathways are actually mediating the functional effects observed in vivo and which are simply reflecting secondary or coincidental phenomena. In the current study, we attempted to minimize this problem by identifying changes in gene expression in cardiac tissue prior to the development of hypertension, metabolic disturbances, and the ischemia/reperfusion injury experiment. Nevertheless, it will be important to next link the observed changes in gene expression to functional changes in vivo in more than a speculative fashion.
In conclusion, results of the current study provide strong evidence for the important role of Cd36 in modifying the incidence and severity of ischemic and reperfusion ventricular arrhythmias and myocardial infarct size induced by coronary artery occlusion. The proarrhythmic effect of Cd36 transgene appears to be dependent on adrenergic stimulation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-35018, HL-56028, and HL-63709 to T. W. Kurtz; the European Community's Seventh Framework Program (FP7/2007-2013) under grant agreement HEALTH-F4-2010-24,1504 (EURATRANS); grants ME08006, 1M6837805002 and 0C0801 to M. Pravenec and J. Houštěk; MSM 0021620858 to F. Novák and M. Klevstig; and 1M0510 to F. Kolář from the Ministry of Education of the Czech Republic; grants MZ000023001, NR9359-3, NR9387-3, NS9757-3, and NS10504-3 from the Ministry of Health of the Czech Republic to L. Kazdová and M. Pravenec; grants IAA500110805, IAAX01110901, and KAN 200520703 from the Grant Agency of the Academy of Sciences of the Czech Republic to V. Zídek, J. Neckář, and F. Kolář, respectively; grant GAUK 429611 from Grant Agency of Charles University in Prague to M. Klevstig; grant 305/08/H037 and P301/10/0756 from the Grant Agency of the Czech Republic to M. Klevstig and V. Landa; and research project AV0Z 50110509.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.N., J.S., V.Z., V.L., P.M., M.S., L.K., M.K., M.V., F.P., and Z.D. performed experiments; J.G.S., C.E.S., L.K., F.N., J.H., T.W.K., F.K., and M.P. analyzed data; T.W.K. and M.P. drafted manuscript; T.W.K., F.K., and M.P. edited and revised manuscript; F.K. and M.P. approved final version of manuscript.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Abarbanell AM, Hartley JA, Herrmann JL, Weil BR, Wang Y, Manukyan MC, Poynter JA, Meldrum DR. Exogenous high-mobility group box 1 improves myocardial recovery after acute global ischemia/reperfusion injury. Surgery 149: 329–335, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Aitman TJ, Gotoda T, Evans AL, Imrie H, Heath KE, Trembling PM, Truman H, Wallace CA, Rahman A, Doré C, Flint J, Kren V, Zidek V, Kurtz TW, Pravenec M, Scott J. Quantitative trait loci for cellular defects in glucose and fatty acid metabolism in hypertensive rats. Nat Genet 16: 197–201, 1997. [DOI] [PubMed] [Google Scholar]
- 3. Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117: 3216–3226, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Asemu G, Neckář J, Szárszoi O, Papoušek F, Ošt'ádal B, Kolář F. Effects of adaptation to intermittent high altitude hypoxia on ischemic ventricular arrhythmias in rats. Physiol Res 49: 597–606, 2000. [PubMed] [Google Scholar]
- 5. Bélichard P, Pruneau D, Rochette L. Influence of spontaneous hypertension and cardiac hypertrophy on the severity of ischemic arrhythmias in the rat. Basic Res Cardiol 83: 560–566, 1988. [DOI] [PubMed] [Google Scholar]
- 6. Bielen E, Fagard R, Amery A. The inheritance of left ventricular structure and function assessed by imaging and Doppler echocardiography. Am Heart J 121: 1743–1749, 1991. [DOI] [PubMed] [Google Scholar]
- 7. Bonnet D, Martin D, de Lonlay P, Villain E, Jouvet P, Rabier D, Brivet M, Saudubray JM. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation 100: 2248–2253, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Chavan CB, Sharada K, Rao HB, Narsimhan C. Hypocalcemia has been suggested as a cause of reversible cardiomyopathy with ventricular tachycardia. Ann Intern Med 146: 541–542, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Corr PB, Creer MH, Yamada KA, Saffitz JE, Sobel BE. Prophylaxis of early ventricular fibrillation by inhibition of acylcarnitine accumulation. J Clin Invest 83: 927–936, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dai W, Simkhovich BZ, Kloner RA. Ischemic preconditioning maintains cardioprotection in aging normotensive and spontaneously hypertensive rats. Exp Gerontol 44: 344–349, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Dawn B, Guo Y, Rezazadeh A, Wang OL, Stein AB, Hunt G, Varma J, Xuan YT, Wu WJ, Tan W, Zhu X, Bolli R. Tumor necrosis factor-α does not modulate ischemia/reperfusion injury in naïve myocardium but is essential for the development of late preconditioning. J Mol Cell Cardiol 37: 51–61, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 39: 89–105, 1998. [DOI] [PubMed] [Google Scholar]
- 13. Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JRB, Young ME. Ischemia/reperfusion tolerance is time-of-day-dependent. Mediation by the cardiomyocyte circadian clock. Circ Res 106: 546–550, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erdmann J, Linsel-Nitschke P, Schunkert H. Genetic causes of myocardial infarction: new insights from genome-wide association studies. Dtsch Arztebl Int 107: 694–699, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Folch J, Lees M, Sloan-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 16. Gasparri C, Curcio A, Torella D, Gaspari M, Celi V, Salituri F, Boncompagni D, Torella M, Gulletta E, Cuda G, Indolfi C. Proteomics reveals high levels of vitamin D binding protein in myocardial infarction. Front Biosci (Elite Ed) 2: 796–804, 2010. [DOI] [PubMed] [Google Scholar]
- 17. Hajri T, Ibrahimi A, Coburn CT, Knapp FF, Jr, Kurtz T, Pravenec M, Abumrad NA. Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J Biol Chem 276: 23661–6, 2001. [DOI] [PubMed] [Google Scholar]
- 18. Harshfield GA, Grim CE, Hwang C, Savage DD, Anderson SJ. Genetic and environmental influences on echocardiographically determined left ventricular mass in black twins. Am J Hypertens 3: 538–543, 1990. [DOI] [PubMed] [Google Scholar]
- 19. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Irie H, Krukenkamp IB, Brinkmann JF, Gaudette GR, Saltman AE, Jou W, Glatz JF, Abumrad NA, Ibrahimi A. Myocardial recovery from ischemia is impaired in CD36-null mice and restored by myocyte CD36 expression or medium-chain fatty acids. Proc Natl Acad Sci USA 100: 6819–6824, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim JB, Porreca GJ, Song L, Greenway SC, Gorham JM, Church GM, Seidman CE, Seidman JG. Polony multiplex analysis of gene expression (PMAGE) in mouse hypertrophic cardiomyopathy. Science 316: 1481–1484, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Kingma JG., Jr Cardiac adaptation to ischemia-reperfusion injury. Ann NY Acad Sci 874: 236–253, 1999. [DOI] [PubMed] [Google Scholar]
- 23. Kolář F, Ježková J, Balková P, Břeh J, Neckář J, Novák F, Nováková O, Tomášová H, Srbová M, Ošt'ádal B, Wilhelm J, Herget J. Role of oxidative stress in PKC-delta upregulation and cardioprotection induced by chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 292: H224–H230, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Kolář F, Parratt JR. Antiarrhythmic effect of ischemic preconditioning in hearts of spontaneously hypertensive rats. Exp Clin Cardiol 2: 124–127, 1997. [Google Scholar]
- 25. Kuang M, Febbraio M, Wagg C, Lopaschuk GD, Dyck JRB. Fatty acid translocase/CD36 deficiency does not energetically of functionally compromise hearts before or after ischemia. Circulation 109: 1550–1557, 2004. [DOI] [PubMed] [Google Scholar]
- 26. Lam A, Lopaschuk GD. Anti-anginal effects of partial fatty acid oxidation inhibitors. Curr Opin Pharmacol 7: 179–185, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Leenen FH, Yuan B. Mortality after coronary artery occlusion in different models of cardiac hypertrophy in rats. Hypertension 37: 209–15, 2001. [DOI] [PubMed] [Google Scholar]
- 28. Lindpaintner K, Lee M, Larson MG, Rao VS, Pfeffer MA, Ordovas JM, Schaefer EJ, Wilson AF, Wilson PW, Vasan RS, Myers RH, Levy D. Absence of association or genetic linkage between the angiotensin-converting-enzyme gene and left ventricular mass. N Engl J Med 334: 1023–1028, 1996. [DOI] [PubMed] [Google Scholar]
- 29. Luiken JJFP, Coort SLM, Koonen DPY, van der Horst DJ, Bonen A, Glatz JFC. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflügers Arch 448: 1–15, 2004. [DOI] [PubMed] [Google Scholar]
- 30. Malínská H, Oliyarnyk O, Hubová M, Zídek V, Landa V, Šimáková M, Mlejnek P, Kazdová L, Kurtz TW, Pravenec M. Increased liver oxidative stress and altered PUFA metabolism precede development of non-alcoholic steatohepatitis in SREBP-1a transgenic spontaneously hypertensive rats with genetic predisposition to hepatic steatosis. Mol Cell Biochem 335: 119–125, 2010. [DOI] [PubMed] [Google Scholar]
- 31. McDermot-Roe C, Ye J, Ahmed R, Sun XM, Serafín A, Ware J, Bottolo L, Muckett P, Cañas X, Zhang J, Rowe GC, Buchan R, Lu H, Braithwaite A, Mancini M, Hauton D, Martí R, García-Arumí E, Hubner N, Jacob H, Serikawa S, Zidek V, Papousek F, Kolar F, Cardona M, Ruiz-Meana M, García-Dorado D, Comella JX, Felkin LE, Barton PJR, Arany Z, Pravenec M, Petretto E, Sanchis D, Cook SA. Endonuclease G is a novel determinant of cardiac hypertrophy and mitochondrial function. Nature 478, 114–118, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mlejnek P, Kren V, Liska F, Zídek V, Landa V, Kurtz TW, Pravenec M. The CD36 protein functions as an immunogenic domain of the RT8 alloantigen. Eur J Immunogenet 30: 325–327, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Neckář J, Papoušek F, Nováková O, Ošt'ádal B, Kolář F. Cardioprotective effects of chronic hypoxia and ischemic preconditioning are not additive. Basic Res Cardiol 97: 161–167, 2002. [DOI] [PubMed] [Google Scholar]
- 34. Nguyen T, El Salibi E, Rouleau JL. Postinfarction survival and inducibility of ventricular arrhythmias in the spontaneously hypertensive rat: effects of ramipril and hydralazine. Circulation 98: 2074–2080, 1998. [DOI] [PubMed] [Google Scholar]
- 35. Oliver MF. Prevention of ventricular fibrillation during acute myocardial ischemia: control of free fatty acids. J Cardiovasc Pharmacol Ther 6: 213–217, 2001. [DOI] [PubMed] [Google Scholar]
- 36. Oliver MF. Sudden cardiac death: the lost fatty acid hypothesis. Q J Med 99: 701–709, 2006. [DOI] [PubMed] [Google Scholar]
- 37. Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol Heart Circ Physiol 273: H1707–H1712, 1997. [DOI] [PubMed] [Google Scholar]
- 38. Pantos CI, Malliopoulou VA, Mourouzis IS, Karamanoli EP, Paizis IA, Steimberg N, Varonos DD, Cokkinos DV. Long-term tyroxine administration protects the heart in a pattern similar to ischemic preconditioning. Thyroid 12: 325–329, 2002. [DOI] [PubMed] [Google Scholar]
- 39. Pecina P, Čapková M, Chowdhury SKR, Drahota Z, Dubot A, Vojtíšková A, Hansíková H, Houšt'ková H, Zeman J, Godinot C, Houštěk J. Functional alteration of cytochrome c oxidase by SURF1 mutations in Leigh syndrome. Biochim Biophys Acta 1639: 53–63, 2003. [DOI] [PubMed] [Google Scholar]
- 40. Pecinová A, Drahota Z, Nusková A, Pecina P, Houštěk J. Evaluation of basic mitochondrial functions using rat tissue homogenates. Mitochondrion 11: 722–728, 2011. [DOI] [PubMed] [Google Scholar]
- 41. Post WS, Larson MG, Myers RH, Galderisi M, Levy D. Heritability of left ventricular mass: the Framingham heart study. Hypertension 30: 1025–1028, 1997. [DOI] [PubMed] [Google Scholar]
- 42. Pravenec M, Gauguier D, Schott JJ, Buard J, Křen V, Bílá V, Szpirer C, Szpirer J, Wang JM, Huang H, St Lezin E, Spence MA, Flodman P, Printz M, Lathrop GM, Vergnaud G, Kurtz TW. Mapping of quantitative trait loci for blood pressure and cardiac mass in the rat by genome scanning of recombinant inbred strains. J Clin Invest 96: 1973–1978, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pravenec M, Landa V, Zídek V, Musilová A, Křen V, Kazdová L, Aitman TJ, Glazier AM, Ibrahimi A, Abumrad NA, Qi N, Wang JM, St Lezin EM, Kurtz TW. Transgenic rescue of defective Cd36 ameliorates insulin resistance in spontaneously hypertensive rats. Nat Genet 27:156–158, 2001. [DOI] [PubMed] [Google Scholar]
- 44. Samani NJ, Thompson JR, O'Toole L, Channer K, Woods KL. A meta-analysis of the association of the deletion allele of the angiotensin-converting enzyme gene with myocardial infarction. Circulation 94: 708–712, 1996. [DOI] [PubMed] [Google Scholar]
- 45. Schunkert H, Brockel U, Hengstenberg C, Luchner A, Muscholl MW, Kurzidim K, Kuch B, Doring A, Riegger GA, Hense HW. Familial predisposition of left ventricular hypertrophy. J Am Coll Cardiol 33: 1685–1691, 1999. [DOI] [PubMed] [Google Scholar]
- 46. Sheehan TE, Kumar PA, Hood DA. Tissue-specific regulation of cytochrome c oxidase subunit expression by thyroid hormone. Am J Physiol Endocrinol Metab 286: E968–E974, 2004. [DOI] [PubMed] [Google Scholar]
- 47. Snoeckx LH, van der Vusse GJ, Coumans WA, Willemsen PH, van der Nagel T, Reneman RS. Myocardial function in normal and spontaneously hypertensive rats during reperfusion after a period of global ischemia. Cardiovasc Res 20: 67–75, 1986. [DOI] [PubMed] [Google Scholar]
- 48. Sood S, Reghunandanan R, Reghunandanan V, Gopinathan K, Sood AK. Effect of vitamin D deficiency on electrocardiogram of rats. Indian J Exp Biol 33: 61–63, 1995. [PubMed] [Google Scholar]
- 49. Splawski I, Timothy KW, Tateyama M, Clancy CE, Malhotra A, Beggs AH, Cappuccio FP, Sagnella GA, Kass RS, Keating MT. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science 297: 1333–1336, 2002. [DOI] [PubMed] [Google Scholar]
- 50. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002. [DOI] [PubMed] [Google Scholar]
- 51. Suleiman MS, Singh RJR, Stewart CEH. Apoptosis and the cardiac action of insulin-like growth factor 1. Pharmacol Therapeut 114: 278–294, 2007. [DOI] [PubMed] [Google Scholar]
- 52. Tanaka T, Nakata T, Oka T, Ogawa T, Okamoto F, Kusaka Y, Sohmiya K, Shimamoto K, Itakura K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J Lipid Res 42: 751–759, 2001. [PubMed] [Google Scholar]
- 53. Turer AT, Stevens RD, Bain JR, Muehlbauer MJ, van der Westhuizen J, Mathew JP, Schwinn DA, Glower DD, Newgard CB, Podgoreanu MV. Metabolomic profiling reveals distinct patterns of myocardial substrate use in humans with coronary artery disease or left ventricular dysfunction during surgical ischemia/reperfusion. Circulation 119: 1736–1746, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Virag JA, Dries JL, Easton PR, Friesland AM, Deantonio JH, Chintalgattu V, Cozzi E, Lehmann BD, Ding JM, Lust RM. Attenuation of myocardial injury in mice with functional deletion of the circadian rhythm gene mPer2. Am J Physiol Heart Circ Physiol 298: H1088–H1095, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu C, Yan L, Depre C, Dhar SK, Shen YT, Sadoshima J, Vatner SF, Vatner DE. Cytochrome c oxidase III as a mechanism for apoptosis in heart failure following myocardial infarction. Am J Physiol Cell Physiol 297: C928–C934, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yano T, Miki T, Tanno M, Kuno A, Itoh T, Takada A, Sato T, Kouzu H, Shimamoto K, Miura T. Hypertensive hypertrophied myocardium is vulnerable to infarction and refractory to erythropoietin-induced protection. Hypertension 57: 110–115, 2011. [DOI] [PubMed] [Google Scholar]
- 57. Yao Z, Tong J, Tan X, Li C, Shao Z, Kim WC, Vanden Hoek TL, Becker LB, Head CA, Schumacker PT. Role of reactive oxygen species in acetylcholine-induced preconditioning in cardiomyocytes. Am J Physiol Heart Circ Physiol 277: H2504–H2509, 1999. [DOI] [PubMed] [Google Scholar]
- 58. Young ME. Anticipating anticipation: pursuing identification of cardiomyocyte circadian clock function. J Appl Physiol 107: 1339–1347, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yu Q, Nguyen T, Ogbi M, Caldwell RW, Johnson JA. Differential loss of cytochrome-c oxidase subunits in ischemia-reperfusion injury: exacerbation of COI subunit loss by PKC-ϵ inhibition. Am J Physiol Heart Circ Physiol 294: H2637–H2646, 2008. [DOI] [PubMed] [Google Scholar]
- 60. Zentilin L, Puligadda U, Lionetti V, Zacchigna S, Collesi C, Pattarini L, Ruozi G, Camporesi S, Sinagra G, Pepe M, Recchia FA, Giacca M. Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J 24: 1467–1478, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.