Abstract
Objective
We conducted a combined observational cohort and case-control study in patients with Alzheimer’s disease (AD) to assess the effects of acetylcholinesterase inhibitor (ChEI) treatment on cognitive functions important for driving.
Methods
Performance of twenty-four outpatients with newly diagnosed (untreated) early stage AD was compared prior to beginning ChEI (Pre-ChEI) and after 3 months of therapy (Post-ChEI) on a set of computerized tests of visual attention and executive function administered under both single-task and dual-task conditions. In order to address the limitation of a lack of an untreated control group in this observational cohort study, performance of thirty-five outpatients with newly diagnosed (untreated) early stage AD (ChEI Non-Users) were also compared to a demographically-matched group of AD patients treated with stable doses of a ChEI (ChEI Users) on these tasks.
Results
Performance was consistently worse under dual-task than single-task conditions regardless of ChEI treatment status. However, ChEI treatment consistently affected specific components of attention within each test across both sets of comparisons: ChEI treatment enhanced simulated driving accuracy, and was associated with significantly better visual search target detection accuracy and response time in both Pre/Post ChEI and Users/Non-Users treatment comparisons. ChEI treatment also improved overall time to complete a set of mazes while not affecting accuracy of completion.
Conclusion
ChEI treatment was associated with improvements in tests of executive function and visual attention. These findings could have important implications for patients who continue to drive in the early stages of AD.
Keywords: All cognitive disorders/dementia, Alzheimer’s disease, Cholinesterase inhibitors, Attention, Driving
Introduction
A substantial number of patients will continue to drive for varying time periods after receiving a diagnosis of Alzheimer’s disease (AD). Current and past driving research has focused on driving safety in dementia, clinical assessment of fitness to drive, and the development of office-based tools to aid in the evaluation process. Although practice guidelines recommend a trial of a cholinesterase inhibitor (ChEI) for all patients diagnosed with mild-moderate AD,1 the effects of this class of commonly prescribed medications on driving performance in AD has not been explored. Moreover, cholinergically mediated impairments in attention that can impact the skills necessary to drive safely appear early in AD. Since AD-related impairments in attention, visuospatial abilities, and reaction time increase crash risk, it is important to gain clearer understanding of ChEI effects on these domains.
A growing body of literature has linked poor neuropsychological test performance in the cholinergically mediated domains of attention and visuospatial abilities to impaired on-road driving performance in patients with AD.2 The cholinergic system plays an essential role in modulating attention, memory, and learning; the decline of the cholinergic system in AD is associated with early cognitive impairment and in later stage disease, with dementia severity.3
Of interest, cholinergic augmentation with donepezil was reported to improve the flight simulator measures of emergency detection and landing approach in a small group of cognitively normal pilots.4, 5 These flight tasks place a high demand on divided and sustained visual attention. Similarly, operating a motor vehicle requires prompt response to quickly changing situations and the ability to monitor and shift between multiple demands on attention.6 Therefore, cholinergic augmentation may improve those aspects of attention that may critically impact the driving ability of patients with AD.
The objective of this study was to examine the effects of ChEI treatment on computerized tests of simulated driving, visual attention, and executive function in drivers with AD. The effects of ChEI treatment were assessed in two ways: First, of primary interest, an observational cohort study was conducted comparing the performance of newly diagnosed, untreated AD patients at pre-treatment baseline (Pre-ChEI) and after three months of ChEI therapy (Post-ChEI). Since practice guidelines recommend offering ChEI therapy to all individuals upon diagnosis of mild-moderate AD,7 use of an untreated control group for comparison in the pre/post study was not only unethical, but unfeasible. Therefore, to address the limitation of a lack of an untreated control group, a secondary case control study comparing the baseline test performance of patients participating in the prospective study prior to initiating ChEI treatment (ChEI Non-Users) to that of a comparable demographically-matched group of AD patients treated with stable doses of a ChEI (ChEI Users) was also conducted. Demonstration of similar effects of ChEI across the prospective and case control studies would provide converging evidence for the impact of ChEI therapy on driving-related attentional abilities in AD patients.
MATERIALS AND METHODS
Design
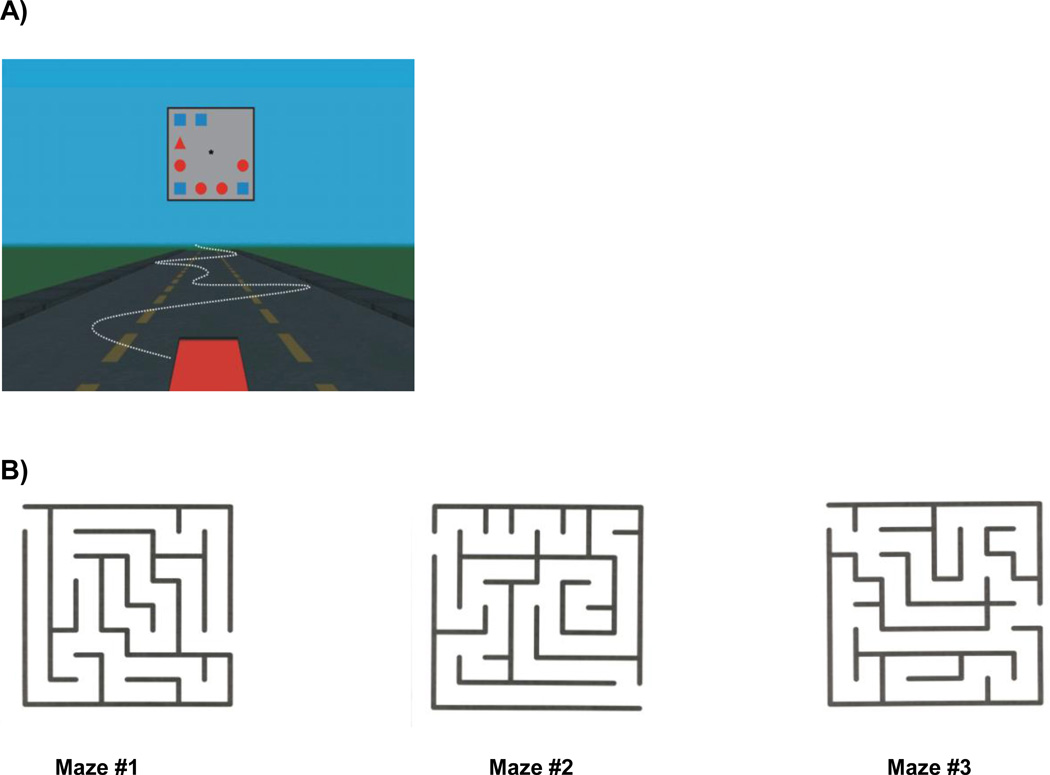
For the prospective, open-label cohort component of this study, patients with early AD completed tests prior to beginning the first dose of a ChEI (Pre-ChEI), which were then repeated after 3 months of treatment with a ChEI (Post-ChEI) titrated to a therapeutic dose. Participants were administered a computerized maze test and computerized tests of visuomotor tracking and visual search first separately (i.e., single-task condition) and then in combination (i.e., dual-task condition) in order to simulate typical situations encountered in on-road driving that demand effective use of sustained, selective, and divided attention. (Figure 1)
Figure 1.
Illustrations of the displays for (A) the simulated driving with visual search task and (B) the maze navigation task.
Visuomotor tracking accuracy and visual search accuracy were chosen as the primary outcome measures based upon previous work highlighting the link between impairments in visual attention and motor vehicle accidents in older drivers12 with aspects of visual search performance predicting on-road driving performance in AD. 11,12 Response time in the visual task served as a secondary measure reflecting the efficiency of selective attention. Maze completion time and performance scores were also included as secondary outcome measures based upon studies reporting a significant relationship between maze test performance and scores from on-road driving examination in drivers with AD.8–11.
In order to provide a ChEI-treated control group (ChEI Users) for direct comparison with the untreated prospective study cohort at baseline (ChEI Non-Users), a set of de-identified baseline demographic and computerized test data was selected from a cohort of patients diagnosed with probable or possible early AD who had participated in a longitudinal driving study conducted at the ADMDC.13 As part of this longitudinal study, the patients had completed the same computerized tests of simulated driving and visual search used in the current study protocol and were being treated with stable doses of a ChEI. These ChEI Users were matched to the ChEI Non-Users 1:1 on age and baseline CDR rating.
Participants
For the prospective cohort study, thirty-five drivers newly diagnosed with probable or possible early stage AD were recruited from the Alzheimer's Disease and Memory Disorders Center (ADMDC) of Rhode Island Hospital and completed baseline assessments. Participants underwent a standard dementia diagnostic workup including neurologic examination, laboratory testing (serum cobalamin, chemistry panel, thyroid function tests, complete blood count), and brain imaging (computed tomography or magnetic resonance imaging). Alzheimer’s disease diagnosis was based on the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable or possible AD.14 Dementia severity was assessed with the Clinical Dementia Rating (CDR) scale15; of those entering the study, 23 were rated as having very mild dementia (CDR 0.5) and 12 had mild dementia (CDR 1.0). All participants had received a new prescription for a ChEI during their clinic visit, were licensed drivers who were actively driving, and had a study partner who accompanied the participant on at least one monthly car trip prior to and throughout the study period.
Participants were not excluded on the basis of medical illness or physical impairment as long as they were medically stable and the impairment did not compromise the ability to drive safely. Corrected visual acuity of at least 20/40 on eye chart testing was required. History of severe psychiatric illness, developmental disorder, or history of alcohol or substance abuse within the past 2 years was exclusionary. Individuals with a diagnosis of depression being treated with a stable dose of an antidepressant lacking anticholinergic properties were permitted to enroll if their baseline score on the Cornell Scale for Depression in Dementia16 at entry was <8.
Use of a ChEI or memantine in the three months prior to enrollment, planned initiation of memantine during the study, and routine use of centrally acting anticholinergic medications was exclusionary. Individuals taking benzodiazepines, sedative hypnotics, antipsychotics, or supplements known to affect cognition such as estrogen, gingko biloba, or omega-3 fatty acids could enroll if the dose was stable at least 4 weeks prior to entry. Use of benzodiazepines or sedative hypnotic medications was not permitted within 24 hours of testing. Individuals with a history of medication nonadherence or without means to ensure adherence to the ChEI regimen were not enrolled.
Reasons for withdrawal were intolerance of ChEI therapy (20.0%), death (2.9%), non-adherence to ChEI regimen (5.7%) and refusal to perform one or more of the computerized tests (2.9%). Those who did not complete the study were older (mean [SD] age in years, 83.2 [7.5] vs. 75.8 [7.7]; t(33)= −2.66, p=0.01), had fewer years of education (mean years, 12.1 [2.7] vs. 14.5 [2.93.0]; t(33)= 2.28, p=0.03), drove fewer miles (mean miles/week, 23.4 [22.1] vs. 100.6 [107]; t(33)= 2.34, p=0.03), and reported fewer driving trips per week (mean trips, 3.7 [2.5] vs. 8.9 [7.4] (t(33)= 2.30, p=0.03). There were no differences between completers and discontinuers in baseline disease severity (CDR) (Fishers exact =0.13, p=0.09) and MMSE scores (t(33)= 1.73, p=0.09). Of the participants who completed baseline evaluations, twenty-four (68.6%) completed computerized tests at the second study visit after 3 months of ChEI treatment and were included in the Pre-/Post-CHEI analysis.
Choice of ChEI was not specified in the prospective study; physicians were permitted to prescribe donepezil, galantamine, or rivastigmine. Donepezil was the most frequently used ChEI, prescribed for the majority of the participants (N=22) at a mean daily dose of 9.5mg. Two participants received galantamine ER, 16mg daily. Rivastigmine was not prescribed for any participants in the study. All participants received at least 10 weeks of ChEI therapy within the therapeutic dose range (donepezil 5–10mg; galantamine 16–24mg) prior to the post-ChEI evaluations. Duration of therapy was similar for donepezil [mean, weeks (SD); 12.8 (1.9)] and galantamine-treated [mean, weeks (SD); 11.5 (1.7)] participants.
For the secondary case control analysis, the participants in the prospective cohort study who completed the pre-treatment baseline visit (ChEI Non-Users; N=35) were compared to a matched group of ChEI-treated AD subjects (CHEI Users; N=35) who had participated in a previous longitudinal driving study conducted at the ADMDC. The diagnostic, inclusion, and exclusion criteria were identical for both studies, with exception that the ChEI-treated cohort had been receiving stable doses of a ChEI for at least six weeks prior to entering the study. Donepezil was prescribed for 75% of these subjects (N=27) with a mean daily dose of 9.3mg; the remaining subjects received galantamine at mean daily dose of 18mg.
The matched sample of ChEI Users and ChEI Non-Users was similar except for duration of dementia symptoms, with ChEI Users exhibiting longer duration of illness at baseline (mean [SD] duration in years, 3.1 [1.6] vs. 2.2 [1.6]; t(67)= 2.32, p=0.02). Use of concommitant medications associated with cognitive adverse effects was infrequent; no participants received antipsychotic medication and low-dose benzodiazepines (< 5mg diazepam equivalents/day) were prescribed “as needed” for two participants in each group. Demographics and other information for Pre-/Post-ChEI treatment group and the matched sample of ChEI Users and ChEI Non-Users are presented in Table 1.
Table 1.
Demographic characteristics of ChEI comparison groups
| Pre/Post ChEI | ChEI Non-Users | ChEI Users | |
|---|---|---|---|
| Participants, n | 24 | 35 | 35 |
| Age (mean, SD) | 75.8 (7.7) | 78.1 (8.3) | 77.3 (6.3) |
| Education, years (mean, SD) | 14.5 (2.9) | 13.7 (3.0) | 14.0 (3.6) |
| Gender, % | |||
| Male | 54.2 | 51.4 | 62.9 |
| Female | 45.8 | 48.6 | 37.1 |
| CDR, n | |||
| CDR 0.5 | 18 | 23 | 23 |
| CDR 1.0 | 6 | 12 | 12 |
| MMSE (mean, SD) | 24.8 (2.8) | 24.3 (2.9) | 25.2 (2.9) |
| Illness duration, years* (mean, SD) | 2.6 (1.9) | 2.2 (1.6) | 3.1 (1.6) |
| Years driving (mean, SD) | 58.4 (6.0) | 60.1 (7) | 57.5 (9.1) |
| Miles/week (mean, SD) | 100.6 (107.4) | 73.6 (96.3) | 73.6 (73.1) |
| Trips/week (mean, SD) | 9.1 (7.3) | 7.3 (6.7) | 9.7 (8.0) |
| Motor vehicle accident past 3 years, % | 17 | 17 | 20 |
Note: Values in parentheses are standard deviation of the mean values.
ChEI Users vs Non-Users; p=0.02
The institutional review boards at Rhode Island Hospital and Brown University approved the study protocol. Participants were enrolled between February 2007 and April 2008. The last study visit was in July 2008.
Procedures and Outcome Measures
Simulated Driving Task
Participants performed a visuomotor tracking task in which they used a steering wheel to maintain the position of a car hood within the center lane of a straight, three-lane highway displayed on a computer monitor. The position of the car hood moved continuously and unpredictably to the left and right across the highway at the bottom of the display. Direction and relative magnitude of the force applied to the car hood was determined by a complex low frequency signal composed of three superimposed sine waves (3.75F, 7.5F, 15F) with different base amplitudes (0.1°, 0.5°, 0.2° of visual angle, respectively) at randomized phases (0–360°). The initial 30 seconds of each trial served as practice and the trial score was calculated from the amount of time the car was successfully maintained in the center lane during the final 60 seconds of the trial.
During the single-task condition, the forcing function was individually adjusted across a series of 8 driving trials to determine the optimal threshold at which each participant could maintain the car in the center lane 88–92% of the time. The largest amplitude at which the participants could successfully maintain the time-on-target accuracy within the criterion range was chosen as the threshold measure of each participant’s tracking ability, and was subsequently used in the dual-task condition in which the simulated driving task was performed in combination with the visual search task.
Visual Search Task
The search displays consisted of three, five, or nine colored geometric figures in random positions around the perimeter of a gray box centered at the horizon of the static three-lane highway. The items in the display were of three possible shapes (circle, square, triangle) in three possible colors (red, green, blue). The target item was always a red triangle; the distractor items varied across trials with half the items always matching the target in either shape or color but differing on the other dimension (e.g., blue triangles or red circles). The other half of the distractor items were unique from the target on both dimensions (e.g., green squares). Across all trials, the target was present in half of the search displays. Each trial consisted of a fixation cross appearing at the center of the gray box for 500 ms, which was followed by a search display presented for 1000 ms and then the blank box for 1500 ms.
Participants were instructed to press a response key on the back of the steering wheel only when the target appeared in the display. There was a maximum time limit of 2500 ms (the combined time of the presentation of the search display and the blank gray box) to respond. Each participant was given six practice trials, immediately followed by a single block of 96 test trials. Time to respond to target-present trials and overall accuracy in both target-present and target-absent trials was recorded. The overall accuracy rate in the visual search task was calculated by subtracting the false alarm rate (the number of times the subject incorrectly responded that the target was absent from the array) from the hit rate (the number of times the subject correctly responded to target in the array).
In the dual-task condition, participants were asked to maintain the car within the center lane while they simultaneously performed the visual search task. The forcing function was set to the participant’s previously-determined threshold value. During the initial 30 seconds, participants performed the driving task alone, after which the visual search task began. Visuomotor tracking accuracy for the driving task as well as time to respond to target-present trials and overall accuracy across both target-present and target-absent trials were recorded throughout the duration of the combined visual search and visuomotor tracking tasks.
Maze Task
The mazes used in the present study were chosen based on a previous study demonstrating their predictive value of driving competence of cognitively impaired and normal older drivers.8 The three selected mazes (MazeMaster version 1.01, The Flatirons Group) ranged in level of complexity, and were all highly correlated with onroad driving performance in participants with AD.
Participants were seated at a comfortable distance in front of an 18-inch Viewsonic touch-screen monitor in a softly lit room. To solve each of the mazes, participants were instructed to trace a path on the monitor using a rubber tipped stylus. Two randomly chosen practice mazes were used to familiarize participants with the procedures. Each participant then completed the three test mazes in order of decreasing complexity during each visit. There was no set time limit for completion, but the instructions were to complete each maze as quickly as possible.
The overall score for each maze was determined by calculating 100X the correct path length (range 1400–3300) minus 25X the number of dead ends. Three time measures (in seconds) were also recorded for each individual maze: 1) Planning Time (i.e., time from maze presentation to the initiation of drawing); 2) Drawing Time, and 3) Time to Completion (i.e., sum of planning and drawing time). The total score and combined time parameters for all three mazes were then calculated and used as outcome measures.
Analysis
The primary analysis consisted of the comparisons between the performance of the untreated AD patients at pre-treatment baseline (Pre-ChEI) and after three months of ChEI therapy (Post-ChEI) on the simulated driving, visual search, and maze tasks. For the secondary case-control study, the baseline test performance of the AD patients participating in the longitudinal study (ChEI Non-Users) was compared with the demographically-matched group of ChEI-treated AD patients (ChEI Users) on the simulated driving and visual search tasks. Maze data were not available for the ChEI Users, and therefore were not used in the between-group comparisons with ChEI Non- Users.
Statistical analyses were performed with SPSS Version 17.0. Between group differences were compared using Chi 2 or Fishers exact test for categorical data and Student’s t test for continuous data. Dependent measures from the visual search and tracking task were analyzed with mixed-model analyses of variance (ANOVAs) for the between-group baseline comparisons and repeated-measures ANOVAs for the within-group treatment comparisons.
RESULTS
Simulated Driving
Pre-ChEI versus Post-ChEI Treatment
A repeated measures ANOVA, using Treatment (Pre-ChEI, Post-ChEI) and Condition (Single-task, Dual-task) as factors, revealed a significant main effect of Treatment [F(1,23)= 5.97, p < 0.03, η2partial= .21], indicating that driving accuracy was significantly improved after ChEI treatment. No other effects were significant (ps > 0.21).
ChEI Users versus ChEI Non-Users
A two-way mixed-model ANOVA, using Group (ChEI Users, ChEI Non-Users) and Condition (Single-task, Dual-task) as factors, revealed a significant Group X Condition interaction [F(1,68) =5.67, p < 0.05, η2partial = .08] indicating that the two groups performed differently across the single-task and dual-task conditions. Pairwise group comparisons examining this interaction indicated that the accuracy rates were significantly better for the ChEI Users than the ChEI Non-Users under single-task conditions [t(68) = 2.45, p < 0.02], but not under dual-task conditions [t(68) = 1.18, p = 0.24]. Matched-pairs t tests further indicated that accuracy rates were significantly reduced from single-task to dual-task conditions for the ChEI Users [t(34) = 4.24, p < 0.001] but not for the ChEI Non-Users [t(34) = 0.96, p = 0.34].
Visual Search Task
Pre-ChEI versus Post-ChEI Treatment
Accuracy
A repeated measures ANOVA, with Treatment (Pre-ChEI, Post-ChEI) and Condition (Single-task, Dual-task) as factors, revealed significant main effects of Treatment [F(1,23) = 4.65, p < 0.05, η2 = .17] and Condition [F(1,23) = 4.83, p < 0.04, η2partial = .17], but no Treatment X Condition interaction (p = .76). These analyses indicated that while accuracy was consistently worse under dual-task than single task conditions, ChEI treatment significantly improved detection accuracy under both conditions.
Response Time
A repeated measures ANOVA on response times, with Treatment (Pre-ChEI, Post-ChEI) and Condition (Single-task, Dual-task) as factors, revealed only a significant main effect of Condition [F(1,23) = 9.48, p < 0.01, η2partial = .29] and a marginally significant main effect of Treatment [F(1,23) = 3.14, p = 0.09, η2partial = .12], indicating that response times increased from single-task to dual-task conditions, and improved marginally following the ChEI treatment.
ChEI Users versus ChEI Non-Users
Accuracy
A two-way mixed model ANOVA on these accuracy rates, with Group (ChEI Users, ChEI Non-Users) and Condition (Single-task, Dual-task) as factors, revealed significant main effects of Condition [F(1,68) = 9.94, p < 0.005, η2partial = .13] and Group [F(1,68) = 10.14, p < 0.005, η2partial = .13], but no Group X Condition interaction (p = 0.20). These analyses indicated that while target detection accuracy decreased from single-task to dual-task conditions for both groups, accuracy was significantly better for the ChEI Users than the ChEI Non-Users across both conditions.
Response Time
A two-way mixed model ANOVA with Group (ChEI Users, ChEI Non-Users) and Condition (Single-task, Dual- task) as factors, revealed a significant main effect of Condition [F(1,68) = 16.24, p < 0.001, η2partial = .19] and a marginally significant main effect of Group [F(1,68) = 3.81, p = 0.055, η2partial = .05], but no Group X Condition interaction (p = 0.43). These analyses indicated that while overall response times increased from single-task to dual-task conditions for both groups, the overall response times were faster for the ChEI Users than the ChEI Non-Users.
Maze Performance
Pre-ChEI versus Post-ChEI Treatment
Comparison of pre-treatment baseline to post-ChEI treatment maze performance revealed that after treatment, subjects completed the maze task more efficiently. Although all twenty-four participants in the longitudinal study completed the maze test, the data for one participant was lost due to equipment failure, and were therefore not included in the analyses. Although ChEI treatment did not significantly improve accuracy of maze completion as measured by mean total maze score (Mean score [SD], Pre-ChEI: 6314.1 [582.2] vs. Post-ChEI: 6392.4 [477.9]; t(22)= −0.67, p=.51), ChEI treatment did significantly improve the mean total time to complete all three mazes (mean time [SD] (seconds), Pre-ChEI: 166.0 [184.0] vs. Post-ChEI: 105.9 [80.1]; t(22)=1.99, p=.03, one-tailed). The decrease in total time to execute the mazes was achieved by decreases in both total maze drawing time (mean time [SD] (seconds), Pre-ChEI:123.7 [163.5] vs. Post-ChEI: 73.1 [66.9]; t(22)= 1.96, p=.03, one-tailed), and total planning time (mean time (seconds), Pre-ChEI:42.3 [25.2] vs. Post-ChEI: 33.8 [20.4]; t(22)= 1.70, p=.05, one-tailed).
Discussion
This study is the first combined longitudinal and cross-sectional investigation of how ChEI treatment might impact cognitive domains critical to driving safely in AD patients. Specifically, we investigated changes in performance across a set of computerized tests designed to assess simulated driving, attention, and executive processes with ChEI treatment; these tests were administered first separately (i.e., single-task condition) and then in combination (i.e., dual-task condition) in order to simulate typical situations encountered in on-road driving that demand effective use of sustained, selective, and divided attention.
In the present study, ChEI treatment was found to affect cognitive function consistently across the longitudinal and cross-sectional comparisons on the primary outcome measures. First, ChEI treatment was associated with better tracking accuracy within the simulated driving task, with this improvement observed as a main effect of treatment in the Pre/Post treatment comparison and as an effect of ChEI user status under single-task conditions in the Users/Non-Users comparison. The failure to also observe a significant effect of ChEI status under dual-task conditions in the cross-sectional comparison may be due in part to group differences in their prioritization in performing the two simultaneous tasks, since only the ChEI Users displayed a significant decrement in tracking under dual-task conditions. That is, the ChEI Non-Users may have weighted accurate performance of the tracking task more heavily under dual-task conditions than did the ChEI Users, which may in turn have compensated for any effect of ChEI treatment status. Second, ChEI treatment was associated both with better target detection accuracy and with decreased search response times within the visual search task for both the Pre/Post treatment and the Users/Non-Users comparisons. Finally, ChEI treatment significantly improved overall time to complete the mazes while not affecting accuracy of completion.
Taken together, the demonstration of similar effects of ChEI across these two comparison studies provides converging evidence for the impact of ChEI therapy on driving-related attentional abilities in AD patients. The vital role that acetylcholine plays in normal attention and executive function has been well documented; these cognitive processes are mediated through cortical projections arising from the basal forebrain cholinergic system.20, 21 Disruption of cholinergic pathways is thought to underlie the very early appearance of attentional deficits in AD, which often precede decrements in other domains such as language and visuospatial functioning. ChEIs increase the availability of acetylcholine at pre-synaptic cholinergic nerve terminals which may mitigate the pathological effects of AD on the cholinergic system in the mild to moderate stages of the illness.
Relatively little is known about the therapeutic effects of this class of drugs on cognitive domains in AD other than memory as the ChEI pivotal trials did not include measures of attention. However, positive treatment effects of ChEI on various aspects of attention (divided, sustained, and selective attention) and executive function have been demonstrated in animal models22, 23 and in a limited number of investigations in subjects with mild cognitive impairment (MCI), AD, and cognitive normals.5, 24–29 The results of these exploratory cross-sectional studies in patients with MCI or AD indicated that improvement in either attentional processes or executive control was associated with ChEI treatment.24–26 The present study confirmed these conclusions within a casecontrol cross sectional study, and also provided the first longitudinal investigation of the effects of ChEI treatment on these cognitive processes within the same individual.
Do substantive differences exist between the individual ChEIs in respect to their effects on attention? Of the four ChEI approved for the treatment of AD in the United States, galantamine is distinguished by its ability to modulate nicotinic acetylcholine receptors via allosteric potentiation, in addition to inhibiting acetylcholinesterase.30 While differences in efficacy on cognitive and functional outcomes in AD clinical trials have not been demonstrated for galantamine compared to the other CHEIs,31 it is possible that nicotinic receptor modulation might have additional therapeutic effects on attention in AD. Both muscarinic and nicotinic acetylcholine systems contribute to attentional task performance; the complimentary roles of muscarinic and nicotinic cholinergic receptors in visual processing have been demonstrated in a small group of healthy elderly subjects using physostigmine and scopolamine probes and positron emission tomography (PET) imaging.32
Although some researchers have theorized that the contribution of nicotinic cholinergic transmission to attentional functioning may be of even greater importance with increasing cognitive impairment, very little is known about the differences between galantamine and the other ChEIs on attention in AD.28 In one report, both donepezil and galantamine improved some aspects of attention in subjects with mild-moderate AD, but those treated with galantamine improved more quickly relative to baseline than those treated with donepezil.28 We were unable to evaluate potential pharmacological differences between ChEIs on test performance in our study. The majority of participants in the longitudinal and cross-sectional studies were prescribed donepezil, so the extent to which our findings can be generalized to AD patients treated with other CHEIs is unknown.
Although provocative, these results of this study cannot be directly extrapolated to predict the effects of ChEI on actual on-road driving performance in AD. While computerized maze and visual search tasks have been shown to correlate with road test performance in drivers with AD in previous studies8–11, 33, this study lacks a direct measure of on-road driving performance. Another limitation of this study is the lack of an untreated comparison group. The case-control comparison made between the ChEI users and ChEI non-users, however, suggests that the improvements seen in the simulated driving, attention and executive control measures post-ChEI treatment in the prospective study are not due simply to practice effects.
Despite the limitations, these preliminary findings warrant further investigation. Although many early stage patients with AD continue to drive safely for some period after diagnosis, the eventual suspension of driving privileges is an inevitable milestone for all. Attentionally demanding, complicated situations occur frequently while driving, so it is not surprising that impairments in visual attention have been linked to increased crash risk in older adults. 34–36 Individuals with AD have greater difficulty performing adequately in complex visual environments compared with cognitively normal elders and are at higher risk of driving unsafely.33 Potential compensatory strategies (including the use of cognitive enhancing medications) aimed at improving driver safety and decreasing motor vehicle crashes in drivers with AD have not been a research focus to date, but should be studied according to a recent review.37 In the absence of a cure for AD, these types of studies are of critical importance as the prevalence of cognitively impaired drivers will continue to rise as the population ages.
Table 2.
Mean values of simulated driving and visual search measures for pre-ChEI and post-ChEI treatment.
| Pre-ChEI (N=24) |
Post-ChEI (N=24) |
|
|---|---|---|
| Tracking Accuracy | ||
| Single-task | .89 (.08) | .91 (.03) |
| Dual-task* | .86 (.09) | .91 (.06) |
| Search Accuracy | ||
| Single-task* | .89 (.13) | .94 (.04) |
| Dual-task | .82 (.20) | .88 (.10) |
| Response Time (ms) | ||
| Single-task | 918.2 (145.8) | 881.4 (130.2) |
| Dual-task | 991.8 (154.2) | 942.1 (158.6) |
Note: Values in parentheses are standard deviation of the mean values;
p<.05 for paired-sample t-tests of pre-ChEI vs. post-ChEI measures
Table 3.
Mean values of simulated driving and visual search measures for ChEI Non-Users and ChEI-Users.
| ChEI Non-Users (N=35) |
ChEI Users (N=35) |
|
|---|---|---|
| Tracking Accuracy | ||
| Single-task* | .88 (.07) | .91 (.03) |
| Dual-task | .86 (.09) | .83 (.11) |
| Search Accuracy | ||
| Single-task* | .84 (.21) | .93 (.05) |
| Dual-task* | .74 (.27) | .89 (.08) |
| Response Time (ms) | ||
| Single-task | 975 (208) | 917 (108) |
| Dual-task* | 1066 (214) | 977 (150) |
Note: Values in parentheses are standard deviation of the mean values;
p<.05 for independent sample t-test comparisons of ChEI Non-Users vs. ChEI Users
Acknowledgments
This research was supported by grant #T32 AG020498-03 to Dr. Daiello from the National Institutes of Health and grant #AG16335 to Dr. Ott from the National Institute on Aging
Footnotes
Disclosure: The authors report no conflicts of interest.
Dr. Daiello and Dr. Festa performed the statistical analyses.
REFERENCES
- 1.Dubinsky RM, Stein AC, Lyons K. Practice parameter: risk of driving and Alzheimer's disease (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2000;54:2205–2211. doi: 10.1212/wnl.54.12.2205. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman L, McDowd JM, Atchley P, et al. The role of visual attention in predicting driving impairment in older adults. Psychol Aging. 2005;20:610–622. doi: 10.1037/0882-7974.20.4.610. [DOI] [PubMed] [Google Scholar]
- 3.Wezenberg E, Verkes RJ, Sabbe BG, et al. Modulation of memory and visuospatial processes by biperiden and rivastigmine in elderly healthy subjects. Psychopharmacology (Berl) 2005;181:582–594. doi: 10.1007/s00213-005-0083-7. [DOI] [PubMed] [Google Scholar]
- 4.Yesavage JA, Mumenthaler MS, Taylor JL, et al. Donepezil and flight simulator performance: effects on retention of complex skills. Neurology. 2002;59:123–125. doi: 10.1212/wnl.59.1.123. [DOI] [PubMed] [Google Scholar]
- 5.Mumenthaler MS, Yesavage JA, Taylor JL, et al. Psychoactive drugs and pilot performance: a comparison of nicotine, donepezil, and alcohol effects. Neuropsychopharmacology. 2003;28:1366–1373. doi: 10.1038/sj.npp.1300202. [DOI] [PubMed] [Google Scholar]
- 6.Duchek JM, Hunt L, Ball K, et al. The role of selective attention in driving and dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 1997 Suppl 1:48–56. doi: 10.1097/00002093-199706001-00011. [DOI] [PubMed] [Google Scholar]
- 7.Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 8.Ott BR, Festa EK, Amick MM, et al. Computerized maze navigation and on-road performance by drivers with dementia. J Geriatr Psychiatry Neurol. 2008;21:18–25. doi: 10.1177/0891988707311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ott BR, Heindel WC, Whelihan WM, et al. Maze test performance and reported driving ability in early dementia. J Geriatr Psychiatry Neurol. 2003;16:151–155. doi: 10.1177/0891988703255688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whelihan WM, DiCarlo MA, Paul RH. The relationship of neuropsychological functioning to driving competence in older persons with early cognitive decline. Arch Clin Neuropsychol. 2005;20:217–228. doi: 10.1016/j.acn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Snellgrove C. Cognitive screening for the safe driving competence of older people with mild cognitive impairment or early dementia. http://www.infrastructure.gov.au/roads/safety/publications/2005/pdf/cog_screen_old.pdf.
- 12.Ball K, Owsley C, Sloane ME, et al. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34:3110–3123. [PubMed] [Google Scholar]
- 13.Ott BR, Heindel WC, Papandonatos GD, et al. A longitudinal study of drivers with Alzheimer disease. Neurology. 2008;70:1171–1178. doi: 10.1212/01.wnl.0000294469.27156.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 16.Alexopoulos GS, Abrams RC, Young RC, et al. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 17.Baddeley AD, Baddeley HA, Bucks RS, et al. Attentional control in Alzheimer's disease. Brain. 2001;124:1492–1508. doi: 10.1093/brain/124.8.1492. [DOI] [PubMed] [Google Scholar]
- 18.Logie RH, Cocchini G, Delia Sala S, et al. Is there a specific executive capacity for dual task coordination? Evidence from Alzheimer's disease. Neuropsychology. 2004;18:504–513. doi: 10.1037/0894-4105.18.3.504. [DOI] [PubMed] [Google Scholar]
- 19.Dannhauser TM, Walker Z, Stevens T, et al. The functional anatomy of divided attention in amnestic mild cognitive impairment. Brain. 2005;128:1418–1427. doi: 10.1093/brain/awh413. [DOI] [PubMed] [Google Scholar]
- 20.Coulthard E, Singh-Curry V, Husain M. Treatment of attention deficits in neurological disorders. Curr Opin Neurol. 2006;19:613–618. doi: 10.1097/01.wco.0000247605.57567.9a. [DOI] [PubMed] [Google Scholar]
- 21.Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 22.Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Brain Res Cogn Brain Res. 2000;9:313–325. doi: 10.1016/s0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 24.Saykin AJ, Wishart HA, Rabin LA, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- 25.Wylie SA, Ridderinkhof KR, Eckerle MK, et al. Inefficient response inhibition in individuals with mild cognitive impairment. Neuropsychologia. 2007;45:1408–1419. doi: 10.1016/j.neuropsychologia.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Foldi NS, White RE, Schaefer LA. Detecting effects of donepezil on visual selective attention using signal detection parameters in Alzheimer's disease. Int J Geriatr Psychiatry. 2005;20:485–488. doi: 10.1002/gps.1319. [DOI] [PubMed] [Google Scholar]
- 27.Gorus E, Lambert M, De Raedt R, et al. The influence of galantamine on reaction time, attention processes, and performance variability in elderly Alzheimer patients. J Clin Psychopharmacol. 2007;27:182–187. doi: 10.1097/JCP.0b013e318032eadb. [DOI] [PubMed] [Google Scholar]
- 28.Galvin JE, Cornblatt B, Newhouse P, et al. Effects of galantamine on measures of attention: results from 2 clinical trials in Alzheimer disease patients with comparisons to donepezil. Alzheimer Dis Assoc Disord. 2008;22:30–38. doi: 10.1097/WAD.0b013e3181630b81. [DOI] [PubMed] [Google Scholar]
- 29.Caramelli P, Chaves ML, Engelhardt E, et al. Effects of galantamine on attention and memory in Alzheimer's disease measured by computerized neuropsychological tests: results of the Brazilian Multi-Center Galantamine Study (GAL-BRA-01) Arq Neuropsiquiatr. 2004;62:379–384. doi: 10.1590/s0004-282x2004000300001. [DOI] [PubMed] [Google Scholar]
- 30.Villarroya M, Garcia AG, Marco-Contelles J, et al. An update on the pharmacology of galantamine. Expert Opin Investig Drugs. 2007;16:1987–1998. doi: 10.1517/13543784.16.12.1987. [DOI] [PubMed] [Google Scholar]
- 31.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mentis MJ, Sunderland T, Lai J, et al. Muscarinic versus nicotinic modulation of a visual task. a pet study using drug probes. Neuropsychopharmacology. 2001;25:555–564. doi: 10.1016/S0893-133X(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 33.Duchek JM, Hunt L, Ball K, et al. Attention and driving performance in Alzheimer's disease. J Gerontol B Psychol Sci Soc Sci. 1998;53:P130–P141. doi: 10.1093/geronb/53b.2.p130. [DOI] [PubMed] [Google Scholar]
- 34.Rubin GS, Ng ES, Bandeen-Roche K, et al. A prospective, population-based study of the role of visual impairment in motor vehicle crashes among older drivers: the SEE study. Invest Ophthalmol Vis Sci. 2007;48:1483–1491. doi: 10.1167/iovs.06-0474. [DOI] [PubMed] [Google Scholar]
- 35.Owsley C, Ball K, Sloane ME, et al. Visual/cognitive correlates of vehicle accidents in older drivers. Psychol Aging. 1991;6:403–415. doi: 10.1037//0882-7974.6.3.403. [DOI] [PubMed] [Google Scholar]
- 36.Owsley C, Ball K, McGwin G, Jr., et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279:1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 37.Man-Son-Hing M, Marshall SC, Molnar FJ, et al. Systematic review of driving risk and the efficacy of compensatory strategies in persons with dementia. J Am Geriatr Soc. 2007;55:878–884. doi: 10.1111/j.1532-5415.2007.01177.x. [DOI] [PubMed] [Google Scholar]